Abstract
In a placebo-controlled bupropion smoking cessation trial, we examined blind integrity, the link between blind integrity and quit rates, and whether side effects and changes in nicotine withdrawal symptoms or mood were mechanisms through which blind integrity is threatened. At a 12-month follow-up, 498 participants indicated whether they thought they received bupropion, placebo, or were not sure. Potential mediators of treatment effects on treatment arm guess (i.e., side effects, withdrawal, and mood) were measured during treatment and 7-day point prevalence cessation was assessed at the end of treatment (EOT) and at 6- and 12-months post quit date. Overall, 55% of participants guessed their randomization correctly. Compared to guessing not sure, participants who guessed they were taking bupropion were more than twice as likely to have been randomized to bupropion. Similarly, participants who guessed placebo were twice as likely to have been randomized to placebo. Treatment arm guess was associated with quit rates. Including treatment arm guess with actual treatment arm in models of quit rates significantly reduced the odds ratio for bupropion efficacy at EOT and at 6- and 12-months post quit date. There was no evidence for mediation. In bupropion smoking cessation trials, blind failure may occur and participant guess about treatment arm assignment is associated with quit rates.
Keywords: Study Blind, Bupropion, Smoking Cessation, Mediators, Blind Bias
1. Introduction
The double-blind procedure is used to determine if a therapeutic outcome is isolated from sources of bias such as experimenter and participant judgment (Goodwin, 1995). Although the double-blind study is the gold standard for scientific research, studies can have their blind integrity violated and this violation can bias results (Bang, Ni & Davis, 2004). Few studies, however, measure blind integrity, the potential effect of violations on study results, or factors that may facilitate blind violations (Desbiens, 2002; Mooney, White & Hatsukami, 2004).
Clinical trials of pharmacotherapies for nicotine dependence may be particularly susceptible to violations of blind integrity since these medications are psychoactive and, thus, may be discriminated from a placebo based on the experience of side effects (Henningfield, Fant, Buchhalter & Stitzer, 2005; Perkins et al., 1996). Further, since smoking cessation typically leads to a withdrawal syndrome (e.g., craving) and since medications for nicotine dependence are often designed to diminish these symptoms, participants may interpret their study arm assignment by whether or not the intensity of the withdrawal syndrome has been mitigated (Mooney et al., 2004). A meta-analysis of blind integrity in nicotine replacement therapy (NRT) double-blind clinical trials reported blind failure in 12 of 17 studies (71%), although blind failure was not related to quit rates (Mooney et al., 2004).
The anti-depressant bupropion (Zyban) for the treatment of nicotine dependence doubles quit rates versus placebo and outperforms NRT (Hughes, Stead & Lancaster, 2004; Jorenby et al., 1999). Yet, relatively few bupropion studies report data on blind integrity. Ahluwalia, Harris, Catley, Okuyemi & Mayo (2002) and Simon, Duncan, Carmody & Hudes (2004) reported that 58–59% of participants correctly judged they were receiving bupropion and 41–47% correctly guessed that they were receiving placebo (see also Hall et al., 2002; Killen et al., 2004, 2006; Wagena et al., 2005). These studies, however, did not assess if failure to maintain blind integrity was related to quit rates. Further, these studies did not evaluate whether bupropion’s impact on side effects or nicotine withdrawal serve as potential mechanisms through which study blind can be threatened. Finally, since bupropion is an anti-depressant, trial participants may discern their treatment arm via changes in positive and negative mood. Considering broad recommendations for the use of bupropion for the treatment of nicotine dependence (Fiore et al., 2000), more systematic study of blind integrity in bupropion clinical trials is needed.
To fill this gap, we examined blind integrity in a randomized, double-blind, placebo-controlled bupropion smoking cessation trial. To assess blind integrity, the rate at which participants correctly guessed they received bupropion or placebo was examined (Desbiens, 2002). To extend past studies of blind integrity within bupropion smoking cessation trials, we examined if: 1) correctly guessing treatment arm was related to the probability of cessation at the end of treatment (EOT), and at the 6- and 12-month assessment time-points, and 2) self-reported treatment side effects, and changes in nicotine withdrawal symptoms and positive and negative mood, served as mechanisms through which the study blind could be determined. The findings from this study could have implications for the evaluation of pharmacotherapies for nicotine dependence, further enhance knowledge concerning the use of the double-blind procedure in clinical trials, and highlight the relationship between the smoker’s beliefs about treatment and clinical outcome that could be considered in the context of smoking cessation treatment.
2. Materials and Methods
2.1. Participants
Participants who responded to flyers and newspaper advertisements for a free smoking cessation research program were enrolled in a double-blind pharmacogenetic bupropion smoking cessation trial approved by the Georgetown IRB between May 1999 and September 2001 (Collins et al., 2004; Epstein et al., 2004; Lerman, Berrettini, et al., 2004; Lerman, Jepson, et al., 2006; Lerman, Niaura et al., 2004; Lerman, Roth, et al., 2002; Lerman, Shields, et al., 2002; Lerman, Shields, et al., 2003; Wileyto et al., 2004; Wileyto et al., 2005). Participants were enrolled at Georgetown University in Washington, DC, and the State University of New York at Buffalo in Buffalo, NY, and received the same treatment across the two sites. Informed consent was ascertained. To be eligible, individuals must have been smoking ≥ 10 cigarettes a day for one year, could not be pregnant, have a past or current psychiatric disorder defined by the Diagnostic and Statistical Manual of Mental Disorders, and have a medical condition or use a medication contraindicated with bupropion (American Psychiatric Association, 1994). Initially, 670 people were randomized (341 bupropion, 329 placebo); 116 people withdrew pre-treatment (56 bupropion, 59 placebo), leaving 555 participants. Lastly, 51 subjects did not provide data at 12-months (when judgment about treatment group was assessed) and 6 subjects did not provide data on covariates. This resulted in a sample of 498 (254 bupropion, 244 placebo).
2.2. Procedure
Participants were randomly assigned to receive 10 weeks of placebo or bupropion, which was initiated at Week 1 and delivered according to standard therapeutic dose (150 mg/day for the first 3 days, followed by 300 mg/day; Lerman, Shields et al., 2002). Counselors provided 8 sessions of standardized group behavioral counseling focusing on self-monitoring and behavioral modification to all participants. Trained health educators delivered this counseling and weekly supervision and videotaping of sessions were performed to maintain the integrity of the structured intervention protocol. Initial sessions focused on preparing for quitting (e.g., gradual reduction, eliciting support from friends and family), whereas subsequent sessions focused on relapse prevention (e.g., understanding and avoiding tempting situations, reinforcement). A quit date for Week 3 was identified. At baseline, and then weekly from Week 2 to Week 6, participants completed assessments of withdrawal symptoms and mood. Side effects were assessed weekly from Week 2 to Week 8. Smoking status was assessed and biochemically confirmed (cotinine < 15ng/ml) at the EOT and at a 6- and 12-month follow-up. Guess about treatment arm assignment was assessed at the 12-month assessment.
2.3. Measures
Covariates
Demographic characteristics (e.g., age) and smoking history were assessed at baseline. Participants completed the Fagerström Test of Nicotine Dependence (FTND), a 6-item self-report measure of nicotine dependence (Heatherton, Kozlowski, Frecker & Fagerström, 1991). A score of ≥6 indicates a high level of nicotine dependence (Fagerström et al., 1996). Race/ethnicity was assessed with self-report, using categories defined by study personnel, to assess potential variation in treatment response due to this variable.
Predictors
As in past studies, guess about treatment arm assignment was assessed after treatments and assessments were completed by asking: “Which pills do you think you were taking during the smoking cessation program, Zyban or placebo (sugar pill)?” (Desbiens, 2002). Subjects could say “not sure”. Responses were coded: −1 (placebo), 0 (not sure), or +1 (Zyban).
Mediators
A withdrawal symptom checklist (Hughes et al., 1984; Piasecki et al., 2000), which consists of 18 items such as irritability, anxiety, and physical complaints (e.g., dizziness), assessed withdrawal. Responses to items (0 = not at all, to 3 = severe) were summed for a withdrawal index at each time point. The change score from Week 2 (1 week pre-quit) to Week 4 (1 week post-quit) was used since withdrawal symptoms peak in the first week post-quit (Ward, Swan and Jack, 2001). For side effects, a list of 17 physical complaints possibly related to bupropion (e.g., headache, dry mouth) was used. At each time-point, items were summed for a side effects index score. The average from Weeks 2 and 3 (i.e., first 2 weeks on medication) was used, since medication reaction would be most evident at drug initiation. The positive and negative affect schedule (PANAS) assessed mood. There are 10 items for each subscale (e.g., enthusiastic, distressed) and a 1-week time frame is used. The PANAS is internally consistent and valid (Watson, Clark and Tellegen, 1988). The change score from differences between Week 2 (1 week pre-quit) and Week 4 (1 week post-quit) was used.
Outcomes
Self-reported 7-day point prevalence abstinence at the EOT and at 6- and 12- months following the quit date, was biochemically verified with urine cotinine (Society for Research on Nicotine and Tobacco, Subcommittee on Biochemical Verification, 2002). Participants with a cotinine > 15 ng/ml were smokers (n = 34, 19, 16, respectively) (SRNT Subcommittee on Biochemical Verification, 2002). Those who provided self-report only (n = 307, 346, 392, respectively), or no data (n = 11, 18, 0, respectively) were considered smokers (intention-to-treat). The remaining participants (n = 146, 115, 90, respectively) were considered abstinent. The sample remained 498 across all time-points.
2.4. Data Analysis
Using STATA, we first determined whether participants remained blind to their treatment arm with contingency table analysis and chi-square. This was followed by multinomial logistic regression, with randomization predicting treatment arm guess, controlling for covariates. Next, a proportional odds model evaluated the degree to which actual assignment to bupropion affected the odds of being in the bupropion guess group, versus the placebo guess or not sure group. Quit rates across the guess groups were assessed using frequency distribution. To determine whether participant guess biased the estimate of the bupropion effect on quit rates across time-points, we assessed the effect of actual treatment arm on quit rate with and without the variable measuring treatment arm guess in the analysis. Logistic regression and Generalized Linear Models, controlling for covariates, was used for two models: Model A (only actual treatment arm) and Model B (actual treatment arm and treatment arm guess; Desbiens, 2002). Models were compared using seemingly unrelated estimation and the Wald (χ2) test. The robust variance estimate adjusted standard errors for correlation within-subjects.
Finally, we used path analysis to assess changes in mood, side effects, and withdrawal as mediators of violations of blind integrity. Mediation of blind failure would be demonstrated if: 1) actual treatment arm predicted treatment arm guess; 2) actual treatment arm predicted changes in mediators; and 3) inclusion of the mediators in the analysis led to a significant reduction of effects of actual treatment arm on treatment arm guess (Baron & Kenny, 1986). Variables were standardized and multiple regression estimated standardized coefficients for the path model were used2. The products of the coefficients were used with each of the respective paths from actual treatment arm to treatment arm guess to determine the indirect path effects using seemingly unrelated estimation to assemble the full path model and the delta method to obtain confidence intervals on indirect effects. If mediators are identified, the direct effects should be about zero and one or more of the indirect paths should be relatively large.
3. Results
3.1. Sample Characteristics
Sample characteristics are shown in Table 1. The participants who did not complete the 12-month evaluation (n = 51) were compared to the 498 participants who did complete these measures. Non-completers were less likely to have quit smoking at EOT (7%), versus completers (29%; χ2 [1] = 12.9, p < .05).
Table 1.
Demographic and Smoking-related Variables (Covariates) Across Treatment Arms and Treatment Arm Guess Groups (N = 498).
| Actual Treatment Arms | |||||
|---|---|---|---|---|---|
| Covariates | Placebo | Bupropion | Overall | χ 2(1df) or t (495df) | p |
| Gender (% Female) | 55.56 | 57.87 | 56.74 | 0.27 | 0.6 |
| FTND (% Highly Dependent) | 46.50 | 41.34 | 43.86 | 1.34 | 0.3 |
| Race (% Non-White) | 23.87 | 16.93 | 20.32 | 3.69 | 0.1 |
| Depression Symptoms (% CESD > 16) | 22.22 | 28.35 | 25.35 | 2.46 | 0.1 |
| College(% Graduate) | 44.44 | 46.85 | 45.67 | 0.29 | 0.6 |
| Currently Married (%) | 48.97 | 42.13 | 45.47 | 2.35 | 0.1 |
| Age (Mean) | 45.66 | 44.77 | 45.21 | 0.89 | 0.8 |
| Treatment Guess Groups | ||||||
|---|---|---|---|---|---|---|
| Covariates | Guessed Placebo | Not Sure | Guessed Bupropion | Overall | χ2(2df) or F (2,494 df) | p |
| Gender (% Female) | 59.22 | 58.62 | 53.66 | 56.83 | 1.44 | 0.5 |
| FTND (% Highly Dependent) | 46.6 | 42.53 | 41.46 | 43.78 | 1.17 | 0.6 |
| Race (% Non-White) | 20.39 | 16.09 | 22.44 | 20.48 | 1.51 | 0.5 |
| Depression Symptoms (% CESD > 16) | 28.78 | 31.03 | 28.29 | 28.97 | 0.23 | 0.9 |
| College (% Graduate) | 41.75 | 47.13 | 49.27 | 45.78 | 2.42 | 0.3 |
| Currently Married (%) | 48.06 | 42.53 | 43.9 | 45.38 | 1.06 | 0.6 |
| Age (Mean) | 45.73 | 45.84 | 44.41 | 45.21 | 0.89 | 0.4 |
Note. FTND = Fagerström Test for Nicotine Dependence.
3.2. Assessment of Blind Integrity
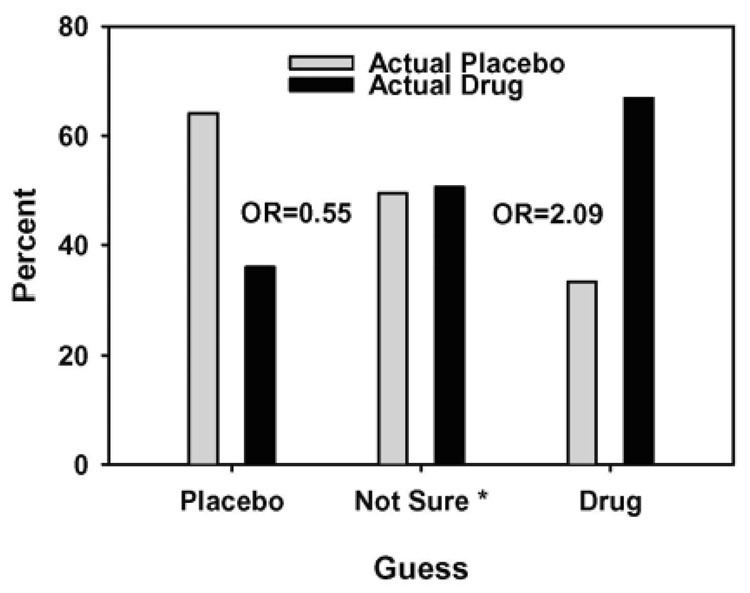
Table 2 shows the relationship between treatment arm guess and actual treatment arm (χ2 [2] = 39.3, p < .0001); 54% of participants randomized to bupropion indicated they were in the bupropion arm, versus 28% of placebo arm participants, and 54% of participants randomized to placebo indicated they were in the placebo arm, versus 29% of those randomized to bupropion. Using multinomial logistic regression (Figure 1), and treating “Not Sure” as the reference group, participants who indicated they were taking bupropion were more than twice as likely to have been randomized to bupropion (OR = 2.09 [1.24−3.50], p = 0.005), while participants who indicated placebo were half as likely to have been randomized to bupropion (OR = 0.54 [0.33−0.91], p = 0.02). A proportional-odds model showed that assignment to bupropion tripled the odds of guessing that one received bupropion, relative to placebo or not sure (OR = 3.10 [2.19−4.37], p < 0.0001).
Table 2.
Assessment of the Study Blind using Contingency Table Analysis (N = 498).
| Guessed Placebo N (%) | Guessed Bupropion N (%) | Not Sure N (%) | Total N (%) | χ2(df) | p | |
|---|---|---|---|---|---|---|
| Randomized to Placebo | 132 (54%) | 68 (28%) | 43 (18%) | 243 (100%) | 39.3 (2) | <0.0001 |
| Randomized to Bupropion | 74 (29%) | 137 (54%) | 44 (17%) | 255 (100%) | ||
| Total | 205 (41%) | 206 (41%) | 87 (18%) | 498 (100%) |
Figure 1. Percent Randomized to Actual Treatment Arm by Treatment Arm Guess Category (N = 498).
Note: * Odds ratios reflect odds of actual assignment to bupropion versus placebo based on participant judgment, using “not sure” as the reference group. Those judging that they received placebo were less likely to be in the bupropion group than those not sure (OR = 0.55, p = 0.02), and those judging that they received bupropion were more likely to be in the bupropion group than those not sure (OR = 2.09, p = 0.005).
3.3. Assessment of the Relationship between Treatment Arm Guess and Quit Rates
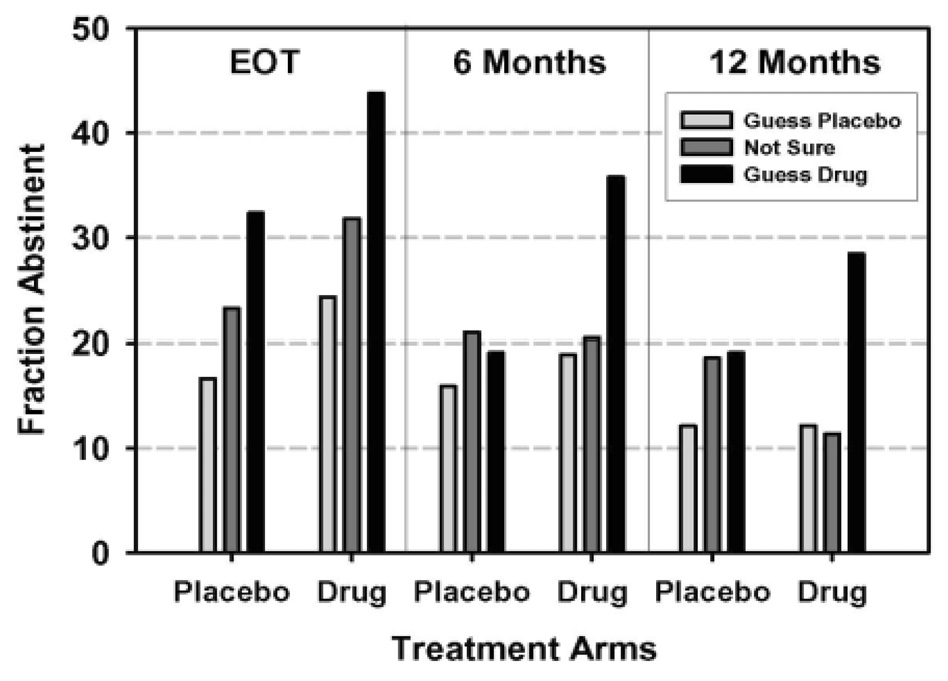
Figure 2 illustrates the quit rates for bupropion vs. placebo, stratified by treatment arm guess. At EOT, for placebo participants, guessing that one received bupropion nearly doubled quit rate, versus guessing that one received placebo (32% vs. 16%); for bupropion participants, guessing that one received bupropion increased the EOT quit rate by close to 70%, versus guessing that one received placebo (43% vs. 25%). At 6- and 12-months, placebo participants who indicated that they received bupropion showed higher quit rates (19% and 19%), versus placebo participants who indicated that they received placebo (15% and 12%). For bupropion participants, those who guessed that they received bupropion showed a 6-month quit rate that was close to double that of participants who guessed that they received placebo (36% vs. 19%). The 12-month quit rate for bupropion participants more than doubled if participants guessed that they received bupropion, versus placebo (29% vs. 12%).
Figure 2. Longitudinal View of Abstinence Rates by Actual Treatment Arm and Treatment Arm Guess Across Time (N = 498).
Note. While overall abstinence declined over time, both actual assignment to treatment arm and judgment about treatment arm assignment improve abstinence at all timepoints (see Table 3). Effects of judgment about treatment arm did not differ across timepoints.
Table 3 shows the effect of bupropion on quit rates, with (Model B) and without (Model A) controlling for treatment arm guess. Adding guess about treatment arm to the model improved model fit (change in deviance = −28.99). The odds ratios for actual treatment arm at each time point differed significantly in Models A and B (Wald χ2[1] > 9.7, p < 0.002). In Model B, fitting a single effect for actual treatment arm across time (OR = 1.46 [0.98−2.18], p = 0.06) and treatment arm guess (OR = 1.48 [1.18−1.84], p = 0.001) improved quit rates to an almost equal extent.
Table 3.
Longitudinal Logistic Regression Models of Cessation Controlling for Covariates (N = 498).
| Prediction Models | Odds Ratio | 95% CI | p |
|---|---|---|---|
| Model A (Deviance = 1582.67) | |||
| Actual Treatment Arm (Bupropion) at EOT | 1.98* | 1.33–2.95 | 0.001 |
| At 6 Months | 1.83* | 1.19–2.83 | 0.006 |
| At 12 Months | 1.46* | 0.92–2.34 | 0.11 |
| Model B (Deviance = 1553.66) | |||
| Actual Treatment Arm (Bupropion) at EOT | 1.64* | 1.08 2.48 | 0.02 |
| At 6 Months | 1.51* | 0.97–2.37 | 0.07 |
| At 12 Months | 1.20* | 0.74–1.94 | 0.45 |
| Treatment Arm Guess | 1.48 | 1.18–1.84 | 0.001 |
Note. Model B was an improvement over model A, with a decrease in deviance of 29.01
indicates estimate of treatment effects differ significantly in Models A and B (all Wald χ2(1) > 9.70, p < 0.002)
sex, race, FTND, and depression were included in both models as covariates (for estimates, see Lerman, Shields et al., 2002).
3.4. Assessment of Side Effects, Withdrawal Symptoms, and Mood as Mediators of Study Blind
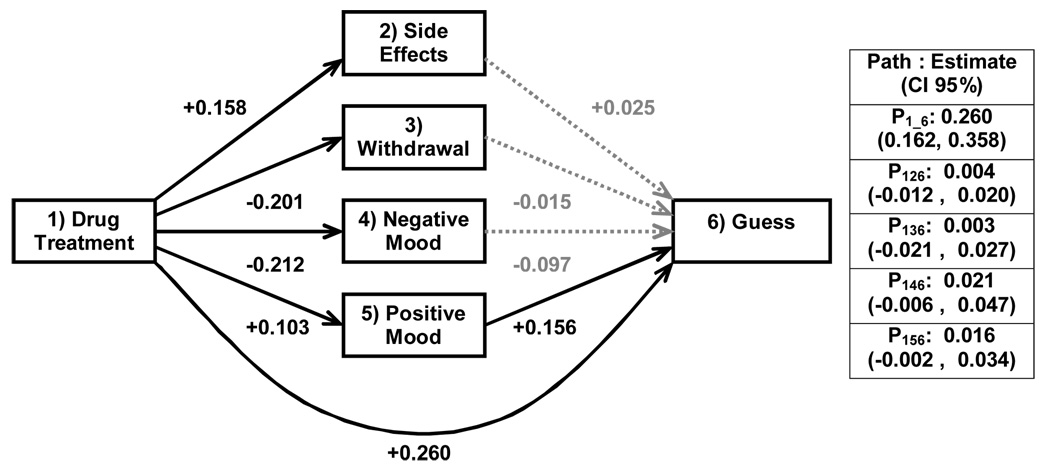
Since many subjects were missing one or another data point, the sample was reduced to 381 for these analyses. Figure 3 depicts the path analysis of the relationship between actual treatment arm, treatment arm guess, and the mediators. Actual treatment arm was a significant predictor of treatment arm guess, satisfying criterion one of mediation. Actual treatment arm predicted withdrawal symptoms and negative and positive mood (but not side effects), partially satisfying the second criterion of a mediation. However, the mediators were not better predictors of treatment arm guess than actual treatment arm, when controlling for actual treatment arm assignment. The total effect of the prediction model was 0.308. When this effect was partitioned into direct vs. indirect (i.e., mediating) effects, 0.260 was attributable to the direct effect of actual treatment arm, with all indirect pathways accounting for the remaining 0.048. Thus, these variables do not appear to represent mediators of the relationship between actual treatment arm and treatment arm guess.
Figure 3. Path Diagram of Suspected Mediators of Blind Failure (N = 381).
Note. Total effect of actual treatment arm on treatment arm guess is 0.308. Total direct effect of actual treatment arm is 0.260, while total indirect effect is 0.048. Dotted lines represent non-significant beta values.
4. Discussion
Many participants randomized to bupropion were able to guess that they were randomized to bupropion. When compared to the not sure category, those who guessed that they were randomized to bupropion were more than two times as likely to have actually been randomized to bupropion, while those who guessed that they were randomized to placebo were about half as likely to have actually been assigned to bupropion. The rate of correctly guessing that one received bupropion was lower than that reported in NRT trials (59%), perhaps because NRT more effectively reduces withdrawal symptoms than bupropion (Mooney et al., 2004). Our results also differed from previous bupropion trials, which showed that participants were far better at correctly guessing they were taking bupropion versus their ability to correctly guess that they were taking placebo (Ahluwalia et al., 2002; Simon et al., 2004). Subjects in this trial were as good at guessing either treatment arm. Thus, researchers may not be able to fully rely on the double-blind procedure to ensure that participants are unaware of treatment arm assignment in trials using bupropion for treating nicotine dependence.
Guessing treatment arm correctly was associated with quit rates. In models of abstinence, model fit was significantly enhanced when guess about treatment arm assignment was added to the model, compared to when actual treatment arm alone was a predictor. Further, there was a significant decrease in the odds ratios for actual treatment arm when treatment arm guess was included in the models. The effect of treatment arm guess appears most pronounced at EOT, but may fade somewhat in the placebo arm over time, remaining evident only for bupropion participants in the long-term. It is tempting to speculate that, for bupropion participants, guessing correctly about treatment arm assignment may increase the probability for cessation and guessing incorrectly about treatment arm assignment may decrease the likelihood for cessation. Likewise, for placebo participants, guessing correctly may decrease the probability for cessation and guessing incorrectly may increase the likelihood for cessation. These results may converge with a long-line of research on placebo and anti-placebo effects (Carpenter, 1968; Guess, Kleinman, Kusek & Engel, 2002). Perkins, Sayette, Conklin and Caggiula (2003) described two sources of influence on participant beliefs that can lead to placebo and anti-placebo effects. First, stimulus expectancies are beliefs that people have about the medication being administered that can originate from previous experience with the medication or from the context in which it is introduced at the outset of the study. Second, response expectancies are beliefs that participants have regarding the likely effects that the study medication will have on their behavior, affect, and cognitions. Thus, participants may have entered this trial with expectancies about bupropion (or had these expectancies formed when the experimenters introduced the study) and these expectancies affected responsiveness to treatment. As such, the double-blind procedure may not adequately prevent bias in quit rates within placebo-controlled double-blind clinical trials arising from participant expectations and beliefs. However, it is also plausible that beliefs about treatment arm are influenced by quitting success. Participants who successfully quit simply may have subsequently concluded that they must have received bupropion while participants who were unable to quit smoking subsequently concluded that they received placebo.
Finally, contrary to our hypotheses, the mediational analyses failed to show that side effects, withdrawal symptoms, and mood were mechanisms through which participants guessed treatment arm assignment. Participants randomized to bupropion did report reduced withdrawal symptoms and negative mood and increased positive mood, versus placebo participants. And, reduced negative mood and increased positive mood was related to a greater likelihood that participants would guess that they were taking bupropion. However, after controlling for the amount of variability in treatment arm guess attributable to actual treatment arm, the proposed mediators accounted for a small and non-significant proportion of variance in treatment arm guess. The processes through which participants constructed their beliefs concerning which arm of the study they had been randomized to remains unclear and is an important area for future research. Participants in placebo-controlled bupropion smoking cessation trials may form beliefs about which treatment arm they have been selected for, independent of potential physical and psychological changes experienced following the initiation of study treatment, and these beliefs may influence cessation. However, which behaviors, cognitions, emotions, or physical processes enable this treatment arm guess remains poorly understood. The present analyses should be interpreted very cautiously given that guess about treatment arm assignment was assessed long after actual treatment arm assignment, the experience of side effects, and changes in withdrawal symptoms and mood (i.e., up to one year). The model tested here assumes that guess about treatment arm randomization is stable, which may not be the case.
Interpretation of these results must be considered in the context of important study limitations. Perhaps the most important study limitation is that we assessed guess about treatment arm assignment at the end of the trial, not during or after treatment. It is entirely plausible that abstinence informed guess, not guess informing abstinence. Thus, interpreting a predictive, cause-effect relationship between guess about treatment arm randomization and cessation rates would not be appropriate. Ongoing and future placebo-controlled bupropion smoking cessation trials need to utilize a more optimal study design to examine the temporal link between guess about treatment arm assignment and quitting success. Second, participant guess about their treatment arm assignment was assessed at one time point. This belief may vary over time as changes occur in side effects or success within the trial and ongoing and future trials should repeat assessments of participant guess regarding treatment arm assignment over time (Mooney et al., 2004). Third, we did not assess guess about treatment arm assignment among study personnel. This could have validated participant reports and may have provided information critical to understanding how study blind is threatened. Lastly, the measure of blind integrity may not have fully captured the concept. When the study was being designed, alternative measures of this variable could not be located. Thus, a face-valid question was used.
Despite these important shortcomings, the results from this study shed initial light on the issue of the double-blind procedure in the context of bupropion smoking cessation clinical trials and may have implications for future research. First, the results suggest that future studies should assess participant beliefs about treatment arm assignment, as this variable likely contributes to prediction of medication efficacy. However, since smokers’ expectations about their medications taken in the clinical setting contribute to efficacy, it may not be necessary to covary this effect out of the study’s analysis, but simply assess this effect and describe it in outcome studies. Second, meta-analysis across bupropion cessation trials can be used to assess the rate of maintaining the study blind and the degree to which participant guess independently predicts cessation outcome as done in the context of NRT (Mooney et al., 2004). Identifying the mechanisms through which participants are guessing their treatment arm is also an important direction for future research and this research should consider the temporal stability of participant guess about treatment arm assignment.
Lastly, should the present findings affect what clinicians do with their patients to treat nicotine dependence? Importantly, given that the present findings are limited by the study design issues previously described, clinicians should not broadly question the literature on bupropion treatment for nicotine dependence. Bupropion is an effective treatment for nicotine dependence. Nevertheless, the present findings do suggest that some degree of the benefits of bupropion for treating nicotine dependence is related to the smoker’s beliefs about receiving the medication. As such, the present findings suggest that clinicians may want to discuss with their patients what their expectations are for treatment response and address negative expectancies using behavioral counseling techniques. Given the substantial difference in quit rates between smokers who guessed that they received bupropion and those who were not sure or guessed that they had received placebo (Figure 2), adjunctive assessment and treatment for negative expectancies in the context of bupropion treatment for nicotine dependence may have benefits for maintaining and advancing the effectiveness of bupropion for the treatment of nicotine dependence.
Acknowledgements
This research was supported by a grant from the National Cancer Institute (RO1 CA63562 to CL). Additional funding for this research was provided by a Transdisciplinary Tobacco Use Research Grant from the National Cancer Institute and the National Institute on Drug Abuse (P50 84718 to CL) and by a grant from the National Cancer Institute (RO1 CA95678 to RAS). The authors thank the following individuals for their contributions to study implementation: Angela Pinto, Susan Ware, and Susan Crystal-Mansour. A version of this study was presented at the 13th Annual Meeting of the Society for Research on Nicotine and Tobacco, Austin, TX, February, 2007.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Path analysis uses standardized variables, which keeps the regression outcome and all predictors in the same units. One of the properties resulting from this standardization is that a regression effect that needs explaining (e.g., actual treatment predicting judgment about treatment) may be partitioned into the fractions attributable to suspected mediating variables vs. a direct effect. The standardization makes these fractions of regression coefficients additive.
References
- Ahluwalia JS, Harris KJ, Catley D, Okuyemi KS, Mayo MS. Sustained release bupropion for smoking cessation in African Americans: a randomized controlled trial. Journal of the American Medical Association. 2002;288:468–474. doi: 10.1001/jama.288.4.468. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV. 4th ed. Washington, DC: 1994. [Google Scholar]
- Bang H, Ni L, Davis CE. Assessment of blinding in clinical trials. Controlled Clinical Trials. 2004;25:143–156. doi: 10.1016/j.cct.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Carpenter JA. Contributions from psychology to the study of drinking and driving. Quarterly Journal of Studies on Alcohol. 1968 Suppl 4:234–251. [PubMed] [Google Scholar]
- Collins B, Wileyto P, Patterson F, Rukstalis M, Audrain-McGovern A, Kaufmann V, Pinto A, Hawk L, Niaura R, Epstein LH, Lerman C. Gender differences in smoking cessation in a placebo-controlled trial of bupropion with behavioral counseling. Nicotine and Tobacco Research. 2004;6:27–37. doi: 10.1080/14622200310001656830. [DOI] [PubMed] [Google Scholar]
- Desbiens NA. In randomized controlled trials, should subjects in both placebo and drug groups be expected to guess that they are taking drug 50% of the time? Medical Hypotheses. 2002;59:227–232. doi: 10.1016/s0306-9877(02)00205-0. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Wright SM, Paluch RA, Leddy JJ, Hawk LW, Jr, Jaroni JL, Saad FG, Crystal-Mansour S, Shields PG, Lerman C. Relation between food reinforcement and dopamine genotypes and its effect on food intake in smokers. American Journal of Clinical Nutrition. 2004;80:82–88. doi: 10.1093/ajcn/80.1.82. [DOI] [PubMed] [Google Scholar]
- Fagerström KO, Kunze M, Schoberberger R, Breslau N, Hughes JR, Hurt RD, Puska P, Ramstrom L, Zatonski W. Nicotine dependence versus smoking prevalence: comparisons among countries and categories of smokers. Tobacco Control. 1996;5:52–56. doi: 10.1136/tc.5.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, Heyman RB, Jaen CR, Kottke TE, Lando HA, Mecklenburg RE, Mullen PD, Nett LM, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME. Clinical Practice Guideline. Rockville, MD: USDHHS; 2000. Treating tobacco use and dependence. [Google Scholar]
- Goodwin JC. Research in Psychology: Methods and Design. New York: John Wiley and Sons; 1995. [Google Scholar]
- Guess HA, Kleinman A, Kusek JW, Engel LW. The science of the placebo. London: BMJ Books; 2002. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Fant RV, Buchhalter A, Stitzer ML. Pharmacotherapy for nicotine dependence. CA: A Cancer Journal for Clinicians. 2005;55:281–299. doi: 10.3322/canjclin.55.5.281. quiz 322-283, 325. [DOI] [PubMed] [Google Scholar]
- Hughes J, Hatsukami D, Pickens RW, Krahn D, Malin S, Luknic A. Effect of nicotine on the tobacco withdrawal syndrome. Psychopharmacology (Berl) 1984;83:82–87. doi: 10.1007/BF00427428. [DOI] [PubMed] [Google Scholar]
- Hughes J, Stead L, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews. 2004:CD000031. doi: 10.1002/14651858.CD000031.pub2. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, Fiore MC, Baker TB. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. New England Journal of Medicine. 1999;340:685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Murphy GM, Jr, Hayward C, Arredondo C, Cromp D. Extended Treatment With Bupropion SR for Cigarette Smoking Cessation. Journal of Consulting and Clinical Psychology. 2006;74:286–294. doi: 10.1037/0022-006X.74.2.286. [DOI] [PubMed] [Google Scholar]
- Killen JD, Robinson TN, Ammerman S, Hayward C, Rogers J, Stone C, Samuels D, Levin SK, Green S, Schatzberg AF. Randomized clinical trial of the efficacy of bupropion combined with nicotine patch in the treatment of adolescent smokers. Journal of Consulting and Clinical Psychology. 2004;72:729–735. doi: 10.1037/0022-006X.72.4.729. [DOI] [PubMed] [Google Scholar]
- Lerman C, Berrettini W, Pinto A, Patterson F, Crystal-Mansour S, Wileyto EP, Restine SL, Leonard DG, Shields PG, Epstein LH. Changes in food reward following smoking cessation: a pharmacogenetic investigation. Psychopharmacology (Berl) 2004;174:571–577. doi: 10.1007/s00213-004-1823-9. [DOI] [PubMed] [Google Scholar]
- Lerman C, Jepson C, Wileyto EP, Epstein LH, Rukstalis M, Patterson F, Kaufmann V, Restine S, Hawk L, Niaura R, Berrettini W. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: results of two randomized clinical trials. Neuropsychopharmacology. 2006;31:231–242. doi: 10.1038/sj.npp.1300861. [DOI] [PubMed] [Google Scholar]
- Lerman C, Niaura R, Collins BN, Wileyto P, Audrain-McGovern J, Pinto A, Hawk L, Epstein LH. Effect of bupropion on depression symptoms in a smoking cessation clinical trial. Psychology of Addictive Behaviors. 2004;18:362–366. doi: 10.1037/0893-164X.18.4.362. [DOI] [PubMed] [Google Scholar]
- Lerman C, Roth D, Kaufmann V, Audrain J, Hawk L, Liu A, Niaura R, Epstein L. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug and Alcohol Dependence. 2002;67:219–223. doi: 10.1016/s0376-8716(02)00067-4. [DOI] [PubMed] [Google Scholar]
- Lerman C, Shields PG, Wileyto EP, Audrain J, Pinto A, Hawk L, Krishnan S, Niaura R, Epstein L. Pharmacogenetic investigation of smoking cessation treatment. Pharmacogenetics. 2002;12:627–634. doi: 10.1097/00008571-200211000-00007. [DOI] [PubMed] [Google Scholar]
- Lerman C, Shields PG, Wileyto EP, Audrain J, Hawk LH, Pinto A, Kucharski S, Krishnan S, Niaura R, Epstein LH. Effects of dopamine transporter and receptor polymorphisms on smoking cessation in a bupropion clinical trial. Health Psychology. 2003;22:541–548. doi: 10.1037/0278-6133.22.5.541. [DOI] [PubMed] [Google Scholar]
- Mooney M, White T, Hatsukami D. The blind spot in the nicotine replacement therapy literature: assessment of the double-blind in clinical trials. Addictive Behaviors. 2004;29:673–684. doi: 10.1016/j.addbeh.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Perkins K, Grobe J, D'Amicao D, Fonte C, Wilson A, Stiller R. Low dose nicotine nasal spray use and effects during initial smoking cessation. Experimental and Clinical Psychopharmacology. 1996;4:157–165. [Google Scholar]
- Piasecki T, Niaura R, Shadel W, Abrams D, Goldstein M, Fiore M, Baker T. Smoking withdrawal dynamics in unaided quitters. Journal of Abnormal Psychology. 2000;109:74–86. doi: 10.1037//0021-843x.109.1.74. [DOI] [PubMed] [Google Scholar]
- Perkins K, Sayette M, Conklin C, Caggiula A. Placebo effects of tobacco smoking and other nicotine intake. Nicotine and Tobacco Research. 2003;5:695–709. doi: 10.1080/1462220031000158636. [DOI] [PubMed] [Google Scholar]
- Simon JA, Duncan C, Carmody TP, Hudes ES. Bupropion for smoking cessation: a randomized trial. Archives of Internal Medicine. 2004;164:1797–1803. doi: 10.1001/archinte.164.16.1797. [DOI] [PubMed] [Google Scholar]
- Society for Research on Nicotine and Tobacco. Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and smoking cessation. Nicotine and Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Ward MM, Swan GE, Jack LM. Self-reported abstinence effects in the first month after smoking cessation. Addictive Behaviors. 2001;26:311–327. doi: 10.1016/s0306-4603(00)00107-6. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark L, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wileyto EP, Patterson F, Niaura R, Epstein L, Brown R, Audrain-McGovern J, Hawk L, Lerman C, Patterson F. Do small lapses predict relapse to smoking behavior under bupropion treatment. Nicotine and Tobacco Research. 2004;6:357–366. doi: 10.1080/1462220042000202463. [DOI] [PubMed] [Google Scholar]
- Wileyto EP, Patterson F, Niaura R, Epstein LH, Brown RA, Audrain-McGovern J, Hawk LW, Jr, Lerman C. Recurrent event, analysis of lapse and recovery in a smoking cessation clinical trial utilizing bupropion. Nicotine and Tobacco Research. 2005;7:257–268. doi: 10.1080/14622200500055673. [DOI] [PubMed] [Google Scholar]