Abstract
Bofutsushosan (BOF), an oriental herbal medicine, has been used as an anti-obesity drug in overweight patients. In the present study, to evaluate the anti-obesity and anti-diabetic effects of BOF, we investigated the effects of BOF on the white adipose tissue (WAT) weight, the size of adipocytes, adiponectin expression, and oral glucose tolerance test results in high-fat diet-fed male KK/Ta mice. In addition, the mRNA expression levels of uncoupling protein 1 (UCP1) and UCP2 mRNA in WAT and brown adipose tissue (BAT) were measured. 6-week-old KK/Ta mice were divided into four groups and fed a purified powdered basal diet (the BD group), a purified high-fat (HF) powdered diet containing suet powder at 37.5 g/100 g diet (the HF group), a high-fat diet plus 1.0% bofutsushosan (BOF) treatment (the HF + BOF group), or a high-fat diet plus 1.0% daisaikoto (DAI) treatment (the HF + DAI group) for 4 weeks. The weight of WAT and the size of adipocytes were increased in the HF group compared with those in the BD group, and these increases in the HF group were significantly inhibited in the HF + BOF group, but not affected in the HF + DAI group. There were no statistically significant differences in plasma levels and tissue mRNA levels of adiponectin among the four groups. There were no significant differences in UCP1 mRNA expression of BAT among the four groups. The expression of UCP1 mRNA in WAT was found in the HF + BOF group, but little expression was seen in the WAT of the BD, HF, or HF + DAI groups. The elevated plasma glucose levels and responses after the glucose loading in the HF group tended to decrease in the HF + BOF group. These results suggest that BOF decreases the weight and size gains of WAT along with up-regulating UCP1 mRNA in WAT in high-fat diet-fed mice.
Keywords: metabolic syndrome, high-fat diet, bofutsushosan, white adipose tissue, uncoupling protein 1
Introduction
Obesity, which is characterized by excess accumulation of adipose tissue, is the most common risk factor for metabolic syndrome, a cluster of several abnormalities including dyslipidemia, insulin resistance, type 2 diabetes, hypertension, and atherosclerosis [1–3]. Although the molecular pathways that link obesity with such a wide spectrum of conditions are not well understood, recent studies have indicated that adipose tissue plays a central role of in the development of this syndrome [4–6]. Accumulating evidence suggests that adipose tissue is not simply an inert energy storage depot but also functions as a major endocrine organ, producing and releasing a variety of bioactive substances, especially adiponectin, into the bloodstream [7].
Recently, we developed a mouse model of metabolic syndrome, in which an increase in visceral adipose tissue precedes the development of fatty liver and insulin resistance in high-fat diet-fed male KK/Ta mice [8]. Using this experimental model, we sought to investigate the efficacy of traditional Chinese drugs and formulations (Kampo formulations) in the prevention of obesity. The first aim of the present study was to investigate the preventive effects of Bofutsushosan (BOF) and Daisaikoto (DAI) on the contents and functions of visceral adipose tissues. BOF contains 18 components (Table 1) and has been used for the prevention of obesity, hypertension, and insulin resistance [9]. The anti-obesity action of BOF was first reported in monosodium-L-glutamate-induced obese mice [10]. Yoshida et al. [10] have demonstrated that white adipose tissue (WAT) weight was decreased and guanosine-5'-diphosphate (GDP) binding activity, an index of brown adipose tissue (BAT) activation, was increased in mice treated with BOF in a dose-dependent manner, and have concluded that BOF works by activating the BAT thermogenesis and inhibiting the phosphodiesterase activity in mice. DAI contains eight components and has been used as a hypocholesterolemic agent for liver disorders and as a therapeutic and preventive agent for cholesterol gallstone disease [11]. In addition, DAI does not contain ephedrine, a β-3 adrenoreceptor agonist, which is found in BOF [10]. Therefore, we compared the effects of these two Kampo medicines on the obesity in mice in the present study.
Table 1.
Components of Kampo medicines (Chinese herbal preparations)
| Bofu-tsusho-san (BOF): 4.5 g of a water extract of the following raw materialsa | ||
| 1 | Scutellariae Radix (Labiatae) | 2.0 |
| 2 | Glycyrrhizae Radix (Leguminosae) | 2.0 |
| 3 | Platycodi Radix (Campanulaceae) | 2.0 |
| 4 | Atractyloidis Lanceae Rhizoma (Compositae) | 2.0 |
| 5 | Rhei Rhizoma (Polygonaceae) | 1.5 |
| 6 | Schizonepetae Spica (Labiatae) | 1.2 |
| 7 | Gardeniae Fructus (Rubiaceae) | 1.2 |
| 8 | Paeonia Ragix (Paeoniaceae) | 1.2 |
| 9 | Cnidii Rhizoma (Umbelliferae) | 1.2 |
| 10 | Angelicae Radix (Umbelliferae) | 1.2 |
| 11 | Menthae Folium (Labiatae) | 1.2 |
| 12 | Saposhnikoviae Radix | 1.2 |
| 13 | Ephedrae herba (Ephedraceae) | 1.2 |
| 14 | Forsythiae Fructus | 1.2 |
| 15 | Zingiberis Rhizoma (Zingiberaceae) | 0.3 |
| 16 | Kaolinum | 3.0 |
| 17 | Gypsum Fibrosum | 2.0 |
| 18 | Natrium Sulfricum | 0.7 |
| Dai-saiko-to (DAI): 4.5 g of a water extract of the following raw materialsa | ||
| 1 | Bupleuri Radix (Umbelliferae) | 6.0 |
| 2 | Pinelliae Tuber (Araceae) | 4.0 |
| 3 | Scutellariae Radix (Labiatae) | 3.0 |
| 4 | Paeonia Ragix (Paeoniaceae) | 3.0 |
| 5 | Zizypus Fructus (Rhamnaceae) | 3.0 |
| 6 | Aurantii Fructus Immaturus (Rutaeae) | 2.0 |
| 7 | Zingiberis Rhizoma (Zingiberaceae) | 1.0 |
| 8 | Rhei Rhizoma (Polygonaceae) | 1.0 |
Each value (g) was represented as dry weight.
Recent investigations have focused on adaptive thermogenesis by uncoupling protein (UCP) families (UCP1, UCP2, and UCP3) as a physiological defense against obesity, hyperlipidemia, and diabetes [12–14]. UCP-1 expression in BAT is known to be a significant component of whole body energy expenditure and its dysfunction contributes to the development of obesity [15]. However, adult humans have very little BAT and most of their fat is stored in WAT. Therefore, several studies have shown that _3-adrenoreceptor agonists and some dietary constituents induce expression of UCP1 not only in BAT, but also in WAT [16–18]. Indeed, UCP1 expression in WAT can lead to an increase in energy expenditure via the generation of heat. Thus the second aim of the present study was to investigate the effect of BOF and DAI on the mRNA expression of UCP1 and UCP2 in the BAT and WAT in high-fat diet-fed mice.
Materials and Methods
Animals and treatment
Male KK/Ta Jcl mice (5 weeks old, 18–20 g body weight) were purchased from CLEA Japan Inc. (Tokyo, Japan). The mice were individually housed in plastic cages at 24 ± 2ºC and 45 ± 5% relative humidity with a 12/12-h light/dark cycle, and were given free access to food and water throughout the experimental period. After acclimation for 1 week by feeding with a basal diet (BD), 6-week-old KK/Ta mice were divided into four groups of five mice each by body weight and blood glucose level, and fed a purified powdered diet (the BD group), a purified high-fat (HF) powdered diet containing suet powder at 37.5 g/100 g diet (the HF group), a high-fat diet plus bofutsushosan (BOF) treatment (the HF + BOF group), or a high-fat diet plus daisaikoto (DAI) treatment (the HF + DAI group) (Table 2). After treatment with the experimental diet for 4 weeks, starved animals were sacrificed under anesthesia with intraperitoneal injection of sodium pentobarbital {Nembutal® (Dainippon Sumitomo Pharma Co., Ltd., Osaka, Japan), 50 mg/kg of body weight} to collect blood, BAT, perirenal, epididymal, mesenteric and inguinal WAT, and liver tissue. All animal experiments and care were conducted in conformity with the Guidelines of the Animal Care and Use Committee of Kyoto Prefectural University of Medicine.
Table 2.
Composition of the experimental diet
| Basal diet |
High-fat (HF) |
HF + BOF |
HF + DAI |
|
|---|---|---|---|---|
| w/w (%) | ||||
| Milk casein | 20.0000 | 24.4800 | 24.4800 | 24.4800 |
| DL-methionine | 0.3000 | 0.3672 | 0.3672 | 0.3672 |
| Corn starch | 14.6625 | 5.9294 | 5.6994 | 5.6994 |
| Granulated sugar | 49.0875 | 19.8506 | 19.0806 | 19.0806 |
| Cellulose | 5.0000 | 6.1200 | 6.1200 | 6.1200 |
| Suet powder | 6.2500 | 37.5000 | 37.5000 | 37.5000 |
| Vitamin mix (AIN-76) | 1.0000 | 1.2240 | 1.2240 | 1.2240 |
| Mineral mix (AIN-76) | 3.5000 | 4.2840 | 4.2840 | 4.2840 |
| Choline bitartrate | 0.2000 | 0.2448 | 0.2448 | 0.2448 |
| Bofutsusyo-san | 1.0000 | |||
| Daisaiko-to | 1.0000 | |||
Oral glucose tolerance test and determination of plasma glucose, insulin, lipids, and adiponectin
The oral glucose tolerance test (OGTT) was performed before and 4-, 8- and 16-weeks after the treatment after overnight fasting. The mice received a 20% glucose solution (2 g/kg). Blood samples were collected just before and 0.5, 1, and 2 h after glucose loading. The rise in plasma glucose concentrations was quantitated by the incremental area under the curve (AUC glucose) for plasma glucose [19]. Plasma glucose levels were determined with a Glutest Ace R (Sanwa Kagaku Kenkyusho Co., Ltd., Nagoya, Japan). Plasma adiponectin levels were measured using a mouse adiponectin enzyme-linked immunosorbent assay (ELISA) kit (Ohtsuka, Ltd., Tokyo, Japan). Asparate 2-oxoglutarate aminotransferase (AST), alanine 2-oxoglutarate aminotransferase (ALT), BUN, free cholesterol, triglyceride, non-esterified fatty acid, and LDL-cholesterol were measured with a Shimadzu CL8000 Clinical Chemistry Analyzer (Shimadzu, Ltd., Tokyo, Japan).
Histological analysis
Small pieces of epididymal white adipose tissues and liver were removed and rinsed with saline. The tissues were fixed with 10% formalin and embedded in paraffin. Tissue sections were cut at a thickness of 2.5 µm and stained with hematoxylin and eosin. To examine the size of the white adipocytes, the area of each adipocyte was measured in 40 cells of representative sections with Image J software (National Institutes of Health, Bethesda, MD). The mean value was designated as an index of the cell size.
mRNA analysis
Mouse BAT and epididymal WAT were taken and stored in an Isogen solution (Nippon Gene Co., Ltd., Tokyo, Japan). The mRNA expression of adiponectin and of GAPDH as an internal control in the adipose tissue was determined by real-time polymerase chain reaction (PCR). Total RNA was isolated from adipose tissue by the acid guanidinium phenol chloroform method with an Isogen kit (Nippon Gene Co., Ltd) and the concentration of RNA was determined by absorbance at 260 nm in relation to that at 280 nm. The isolated RNA was stored at 70°C until use in real-time PCR. For the real-time PCR for adiponectin, UCP-1, and -2, 1 µg of extracted RNA was reverse-transcribed into first-strand complementary DNA (cDNA) at 42°C for 40 min, using 200 U of M-MLV reverse-transcriptase (Promega Corporation, Madison, WI) and 0.5 µg of oligo (dT) 15 primer (Takara Bio Inc., Shiga, Japan) in a 20 µl reaction mixture. The real-time PCR for adiponectin, UCP-1, and -2 was carried out with a 7300 Real-Time PCR system (Applied Biosystems, Foster City, CA) using the DNA-binding dye SYBER Green I for the detection of PCR products. The reaction mixture contained 12.5 µl Premix Ex Taq and 0.5 µl ROX reference dye (Code RRO41A; Takara Bio Inc.), 1 µl custom-synthesized primers, and 2 µl cDNA (equivalent to 20 ng total RNA) to give a final reaction volume of 25 µl. The PCR settings were as follows: the initial denaturation for 15 s at 95ºC was followed by 40 cycles of amplification for 15 s at 95°C and 31 s at 60°C, with subsequent melting curve analysis increasing the temperature from 60 to 95°C.
The primers were designed using a Primer Express® software (Applied Biosystems) and had the following sequences: for adiponectin, sense 5'-CTGGCTTTCTTCTCTTCCATGATAC-3', and antisense 5'-GTGTCGACGTTCCATGATTCTC-3' (82-bp product); for UCP1, sense 5'-TGGAGGTGTGGCAGTGTTCA-3', and antisense 5'-ATGGCTCTGGGCTTGCATT-3' (70-bp product); for UCP2, sense 5'-CTGGTCGCCGGCCTGCAGCGC-3', and antisense 5'-GATCCCTTCCTCTCGTGCAAT-3' (273-bp product); and for β-actin, sense 5'-TAGCCACCTTCCAGCAGATGT-3', and antisense 5'-AGCTCAGTAACAGTCCGCCTA-3' (101-bp product). Relative quantification of gene expression with real-time PCR data was calculated relative to β-actin.
Data analysis
All statistical analysis was performed with the Statcel 2 software package (OMS publishing Inc., Saitama, Japan). We used one-way ANOVA with a Scheffe-type multiple comparison test to compare several parameters among four groups. All data are expressed as the means ± SE, and p values <0.05 were considered statistically significant.
Results
Effects of BOF and DAI on body weight and WAT weight
There was no significant difference in food intake among groups; the mean was 8.0 g/day/body at the first week of the experiment. Throughout the experimental period of 4 weeks, no significant differences in mean body weight were observed among the four groups of mice given the four different diets (Table 3). The weight of WAT, which was composed of perirenal, epididymal, mesenteric and inguinal adipose tissues, was higher in the HF group (high-fat diet-fed mice) than in the BD group (basal diet-fed normal mice). The wet weights of the perirenal, epididymal, mesenteric, and inguinal WAT of the HF + BOF group were 75%, 89%, 85%, and 70%, respectively, of those in the HF group. The ratio of WAT/body weight was significantly higher in the HF group compared with that in the BD group (Table 3). The increase in the WAT/body weight ratio in the HF group was significantly inhibited in the HF + BOF group, but not affected in the HF + DAI group.
Table 3.
Effects of bofu-tsusho-san (BOF) and dai-saiko-to (DAI) on body weight and white adipose tissue, liver, and kidney weights
| Group | Body weight (g) | White adipose tissue weight (g) |
WAT/BW × 100 (%) | liver weight (g) | Kidney weight (g) | |||
|---|---|---|---|---|---|---|---|---|
| perirenal | epididymal | mesenteric | inguinal | |||||
| Basal diet (BD) | 31.5 ± 0.5 | 0.55 ± 0.04 | 1.22 ± 0.10 | 0.97 ± 0.08 | 0.92 ± 0.03 | 11.6 ± 0.6 | 1.52 ± 0.06 | 0.40 ± 0.02 |
| High-fat (HF) diet | 32.2 ± 0.8 | 0.65 ± 0.05 | 1.33 ± 0.07 | 1.12 ± 0.08 | 1.23 ± 0.11 | 13.4 ± 0.7# | 1.20 ± 0.05 | 0.41 ± 0.02 |
| HF + BOF | 31.1 ± 0.6 | 0.49 ± 0.01 | 1.19 ± 0.07 | 0.95 ± 0.05 | 0.87 ± 0.04 | 11.2 ± 0.3+ | 1.18 ± 0.05 | 0.44 ± 0.02 |
| HF + DAI | 32.3 ± 0.6 | 0.62 ± 0.03 | 1.40 ± 0.09 | 1.11 ± 0.07 | 1.22 ± 0.08 | 13.4 ± 0.6 | 1.23 ± 0.04 | 0.43 ± 0.01 |
The data are expressed as the means ± SE of 5–6 mice.
#p<0.05 as compared with the BD group and +p<0.05 as compared with the HF group.
Effects of BOF and DAI on the size of white adipocytes
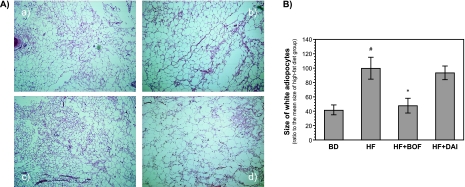
The histological findings of WAT in each group are shown in Fig. 1A. The adipocytes in the HF group were larger than those in the BD group. The average size of adipocytes in WAT was increased by >200% in the HF group. The increase in adipocyte size was significantly reduced in the HF + BOF group, but not in the HF + DAI group (Fig. 1B).
Fig. 1.
Representative histological findings of white adipose tissues (A) and the size of white adipocytes (B) of mice fed a purified powdered diet (BD), a purified high-fat powdered diet (HD), a high-fat diet plus bofutsushosan (HF + BOF), or a high-fat diet plus daisaikoto (HF + DAI). The data are expressed as the means ± SE of 5–6 mice. #p<0.05 compared with the BD group and +p<0.05 compared with the HF group.
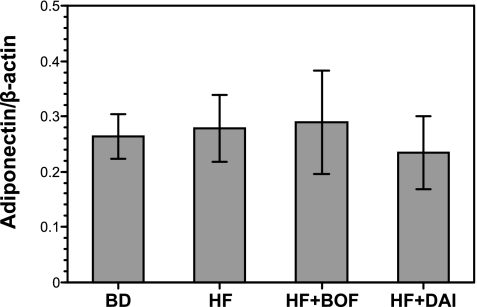
Effects of BOF and DAI on mRNA expression of adiponectin and its plasma levels
No significant differences were observed among the four groups of mice in adiponectin mRNA expression in WAT (Fig. 2). The plasma levels of adiponectin tended to decrease in the HF group compared to the BD group, but not significantly. There were no statistically significant differences in plasma adiponectin levels between the HF group and the HF + BOF group (Table 4).
Fig. 2.
Adiponectin mRNA expression levels of the epididymis adipose tissue of mice fed a purified powdered diet (BD), a purified high-fat powdered diet (HD), a high-fat diet plus bofutsushosan (HF + BOF), or a high-fat diet plus daisaikoto (HF + DAI). The data are expressed as the means ± SE of 5–6 mice.
Table 4.
Plasma levels of adiponectin
| Group | Plasma adiponectin (µg/ml) |
|---|---|
| Basal diet (BD) | 19.43 ± 2.86 |
| High-fat (HF) diet | 17.20 ± 1.34 |
| HF + BOF | 19.33 ± 2.28 |
The data are expressed as the means ± SE of 5–6 mice.
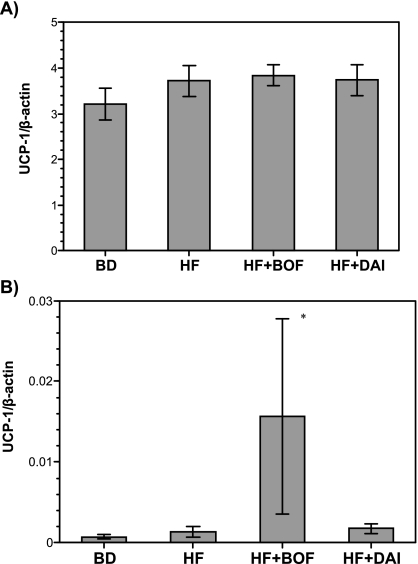
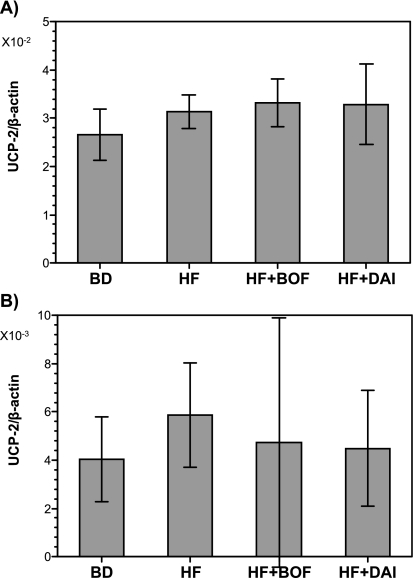
mRNA expression of UCP1 and UCP2 in WAT and BAT
There were no significant differences in UCP-1 mRNA expression of BAT among the four groups (Fig. 3A). The expression of UCP-1 mRNA was significantly increased in WAT in the HF + BOF group, but there was little expression of UCP-1 mRNA in the BD, HF, or HF + DAI groups (Fig. 3B). There were no significant differences in UCP2 mRNA expression in BAT or WAT among the four groups (Fig. 4).
Fig. 3.
Uncoupling protein 1 (UCP1) mRNA levels of brown adipose tissue (A) and white adipose tissue (B) of mice fed a purified powdered diet (BD), a purified high-fat powdered diet (HD), a high-fat diet plus bofutsushosan (HF + BOF), or a high-fat diet plus daisaikoto (HF + DAI). The data are expressed as the means ± SE of 5–6 mice.
Fig. 4.
Uncoupling protein 2 (UCP2) mRNA levels of brown adipose tissue (A)and white adipose tissue (B) of mice fed a purified powdered diet (BD), a purified high-fat powdered diet (HD), a high-fat diet plus bofutsushosan (HF + BOF), or a high-fat diet plus daisaikoto (HF + DAI). The data are expressed as the means ± SE of 5–6 mice. +p<0.05 compared with the HF group.
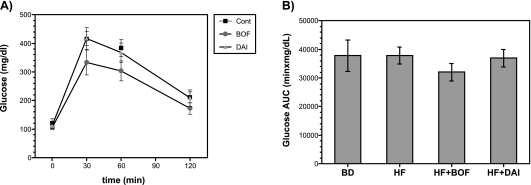
Effects of BOF and DAI on oral glucose tolerance test
Blood glucose levels at 30 min and 60 min after glucose loading were elevated to >200 mg/dl (Fig. 5A) in all groups. The elevated glucose levels and increased glucose AUC in the HF group tended to decrease in the HF + BOF group, but not significantly (Fig. 5A, B).
Fig. 5.
Oral glucose tolerance test (A) and area under the curve (AUC) glucose (B) of mice fed a purified powdered diet (BD), a purified high-fat powdered diet (HD), a high-fat diet plus bofutsushosan (HF + BOF), or a high-fat diet plus daisaikoto (HF + DAI). The data are expressed as the means ± SE of 5–6 mice.
Effects of BOF and DAI on serum lipids
The serum levels of lipids (LDL cholesterol, triglyceride, and non-esterified fatty acid) tended to increase in the HF group compared with those in the BD group. The treatment with BOF did not affect these lipid levels (Table 5).
Table 5.
Effects of bofutsushosan (BOF) on serum levels of lipid, ALT, and BUN
| Basal diet | High-fat diet | High-fat diet + BOF | ||
|---|---|---|---|---|
| ALT | U/l | 26 ± 13 | 14 ± 8 | 22 ± 13 |
| Free cholesterol | mg/dl | 22 ± 4 | 18 ± 4 | 20 ± 0 |
| LDL cholesterol | mg/dl | 150 ± 36 | 218 ± 63 | 238 ± 52 |
| HDL cholesterol | mg/dl | 54 ± 25 | 93 ± 31 | 137 ± 39 |
| Triglyceride | mg/dl | 94 ± 39 | 122 ± 84 | 182 ± 23 |
| Non-esterified fatty acid | µeq/l | 1178 ± 583 | 1680 ± 358 | 2114 ± 757 |
| BUN | mg/dl | 19 ± 3 | 19 ± 3 | 18 ± 3 |
The data are expressed as the means ± SE of 5–6 mice.
Effects of BOF and DAI on blood pressure and heart rate
Because adrenergic pathway is involved in the reaction of Chinese medicine used, blood pressure and heart rate were measured. There were no significant differences in blood pressure or heart rate among groups (Table 6).
Table 6.
Blood pressure and heart rate of mice fed a purified powdered diet (BD), a purified high-fat (HF) powdered diet, a high-fat diet plus bofutsushosan (HF + BOF), or a high-fat diet plus daisaikoto (HF + DAI).
| Group | Blood pressure (mmHg) |
Heart rate (/min) | |
|---|---|---|---|
| Systolic | Mean | ||
| Basal diet (BD) | 120.4 ± 1.9 | 76.4 ± 2.4 | 716 ± 30 |
| High-fat (HF) diet | 115.4 ± 2.8 | 74.4 ± 3.1 | 715 ± 35 |
| HF + BOF | 113.5 ± 3.7 | 70.3 ± 3.7 | 735 ± 64 |
| HF + DAI | 110.0 ± 3.8 | 69.8 ± 3.9 | 735 ± 47 |
The data are expressed as the means ± SE of 5–6 mice.
Discussion
In the present study, the ratio of WAT/body and the size of adipocytes were significantly increased in the HF group compared with those in the BD group, and these increases were significantly inhibited by the treatment with BOF, but not DAI. This anti-obesity action of BOF was consistent with the previous reports using other models of obesity [10, 20]. In addition, no significant differences were observed among the four groups of mice given the different diets in the mean whole body, liver, or kidney weights throughout the experimental period of 4 weeks. There were no significant differences in plasma lipid profile, serum ALT level as an index of hepatic injury, or BUN as an index of renal injury between the HF group and the HF + BOF group. These data suggest the possibility that the fat mass-lowering effect of BOF is not due to the toxicity of the Kampo medicine but rather to a direct pharmacological action on adipose tissues.
Obesity is a common risk factor for type 2 diabetes and cardiovascular disease, because adipose tissue secretes several factors that contribute to the development of such pathologies. In the present study, we first measured the changes in serum adiponectin concentration and adiponectin mRNA expression in WAT, because adiponectin mRNA expression and adiponectin plasma levels was reported to be significantly reduced in obese/diabetic mice [21] and humans [22]. However, no significant differences were observed among the groups in serum adiponectin concentration or adiponectin mRNA expression in WAT. Our previous study using this model demonstrated that mRNA expression of adiponectin in WAT was markedly decreased beginning at 8 weeks in the high-fat diet-fed mice [8]. The present study also demonstrated that the BOF treatment tended to decrease the blood glucose levels after glucose loading and to decrease glucose AUC compared with that in the HF group. However, these differences did not reach the levels of statistical significance. In the future, it will be necessary to investigate the effects of BOF treatment on adiponectin expression or glucose tolerance in a study with a longer follow-up.
BAT has been implicated as an important site for facultative energy expenditure in small rodents, because of its capacity for uncoupled mitochondrial respiration. UCP1 expression in BAT is also known as a significant component of whole body energy expenditure and its dysfunction contributes to the development of obesity [15]. However, there was no significant difference in UCP1 mRNA expression of BAT among the four groups in this study. Thus, the decrease in WAT found in the HF + BOF group could not be explained only by the energy expenditure in BAT mitochondria by UCP1. However, if the expression of UCP1 can be activated in tissues other than BAT by Kampo ingredients, this could constitute a good therapy for obesity. In the present study, UCP1 mRNA in WAT was barely detectable in the BD and HF groups, whereas significant expression was found in the HF + BOF group. A number of studies have shown that β-3 adrenoreceptor agonists induce expression of UCP1 not only in BAT, but also WAT. Indeed, the appearance of brown adipocyte-like cells in WAT upon stimulation of β-3 adrenoreceptors has been reported [16, 23–26]. Inokuma et al. [16] have clearly demonstrated that the anti-obesity effect of β-3 adrenogenic stimulation is largely attributable to UCP1 in BAT and ectopically expressed in WAT, but is less attributable to UCP2 and UCP3, and thereby to UCP1-dependent degradation of fatty acids released from WAT. In addition, it has been suggested that the release of norepinephrine, a β-3 adrenoreceptor agonist, can be stimulated by the ephedrine [27] contained in Ephedrae Herba, which is found in BOF, not in DAI. It has been reported that 1 g of BOF contains 3.33 mg of l-ephedrine and 0.73 mg of d-pseudoephedrine [10], and it has also been demonstrated that ephedrine reduces the weight of WAT and increases energy expenditure in monosodium-L-glutamate-induced obese mice. Therefore, BOF containing ephedrine is considered to reduce the weight of WAT by enhancing the thermogenic anti-obesity effect of ephedrine.
In conclusion, we found that a diet supplemented with BOF inhibited the visceral fat accumulation in high-fat diet-fed mice, and that BOF induced gene expression of UCP1, which regulates energy metabolism in WAT. Our results suggest that BOF exerts anti-obesity properties. Recently, a randomized, double-blind clinical trial showed that BOF improved visceral adiposity and insulin resistance in obese women with impaired glucose tolerance [9]. Further studies are needed to evaluate the effect of BOF on thermogenesis and UCP1 expression in human WAT.
Acknowledgment
This study was supported by a Grant for the Research and Development Program for New Bio-industry Initiatives from Bio-oriented Technology Research Advancement Institution.
References
- 1.Moller D.E., Kaufman K.D. Metabolic syndrome: a clinical and molecular perspective. Annual Review of Medicine. 2005;56:45–62. doi: 10.1146/annurev.med.56.082103.104751. [DOI] [PubMed] [Google Scholar]
- 2.Zivkovic A.M., German J.B., Sanyal A.J. Comparative review of diets for the metabolic syndrome: implications for nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 2007;86:285–300. doi: 10.1093/ajcn/86.2.285. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe S., Hojo M., Nagahara A. Metabolic syndrome and gastrointestinal diseases. J. Gastroenterol. 2007;42:267–274. doi: 10.1007/s00535-007-2033-0. [DOI] [PubMed] [Google Scholar]
- 4.Tchernof A. Visceral adipocytes and the metabolic syndrome. Nutr. Rev. 2007;65:S24–29. doi: 10.1111/j.1753-4887.2007.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 5.Laclaustra M., Corella D., Ordovas J.M. Metabolic syndrome pathophysiology: the role of adipose tissue. Nutr. Metab. Cardiovasc. Dis. 2007;17:125–139. doi: 10.1016/j.numecd.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Bergman R.N., Kim S.P., Hsu I.R., Catalano K.J., Chiu J.D., Kabir M., Richey J.M., Ader M. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am. J. Med. 2007;120:S3–8. doi: 10.1016/j.amjmed.2006.11.012. discussion S29–32, [DOI] [PubMed] [Google Scholar]
- 7.Lara-Castro C., Fu Y., Chung B.H., Garvey W.T. Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease. Current Opinion in Lipidology. 2007;18:263–270. doi: 10.1097/MOL.0b013e32814a645f. [DOI] [PubMed] [Google Scholar]
- 8.Akagiri S., Naito Y., Ichikawa H., Mizushima K., Takagi T., Handa O., Kokura S., Yoshikawa T. A mouse model of metabolic syndrome; the increase in visceral adipose tissue precedes the development of fatty liver and insulin resistance in high-fat diet-fed male KK/Ta mice. J. Clin. Biochem. Nutr. 2008;42:150–157. doi: 10.3164/jcbn.2008022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hioki C., Yoshimoto K., Yoshida T. Efficacy of bofu-tsusho-san, an oriental herbal medicine, in obese Japanese women with impaired glucose tolerance. Clin. Exp. Pharmacol. Physiol. 2004;31:614–619. doi: 10.1111/j.1440-1681.2004.04056.x. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida T., Sakane N., Wakabayashi Y., Umekawa T., Kondo M. Thermogenic, anti-obesity effects of bofu-tsusho-san in MSG-obese mice. Int. J. Obes. Relat. Metab. Disord. 1995;19:717–722. [PubMed] [Google Scholar]
- 11.Shoda J., Matsuzaki Y., Tanaka N., Miyamoto J., Osuga T. The inhibitory effects of dai-chai-hu-tang (dai-saiko-to) extract on supersaturated bile formation in cholesterol gallstone disease. Am. J. Gastroenterol. 1996;91:828–830. [PubMed] [Google Scholar]
- 12.Dulloo A.G., Samec S. Uncoupling proteins: their roles in adaptive thermogenesis and substrate metabolism reconsidered. Br. J. Nutr. 2001;86:123–139. doi: 10.1079/bjn2001412. [DOI] [PubMed] [Google Scholar]
- 13.Argiles J.M., Busquets S., Lopez-Soriano F.J. The role of uncoupling proteins in pathophysiological states. Biochem. Biophys. Res. Commun. 2002;293:1145–1152. doi: 10.1016/S0006-291X(02)00355-8. [DOI] [PubMed] [Google Scholar]
- 14.Argyropoulos G., Harper M.E. Uncoupling proteins and thermoregulation. J. Appl. Physiol. 2002;92:2187–2198. doi: 10.1152/japplphysiol.00994.2001. [DOI] [PubMed] [Google Scholar]
- 15.Lowell B.B., V S.S., Hamann A., Lawitts J.A., Himms-Hagen J., Boyer B.B., Kozak L.P., Flier J.S. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- 16.Inokuma K., Okamatsu-Ogura Y., Omachi A., Matsushita Y., Kimura K., Yamashita H., Saito M. Indispensable role of mitochondrial UCP1 for antiobesity effect of beta3-adrenergic stimulation. Am. J. Physiol. Endocrinol. Metab. 2006;290:E1014–1021. doi: 10.1152/ajpendo.00105.2005. [DOI] [PubMed] [Google Scholar]
- 17.Maeda H., Hosokawa M., Sashima T., Funayama K., Miyashita K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005;332:392–397. doi: 10.1016/j.bbrc.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y.C., Satoh K., Hasegawa Y. Feeding scallop shell powder induces the expression of uncoupling protein 1 (UCP1) in white adipose tissue of rats. Biosci. Biotechnol. Biochem. 2006;70:2733–2738. doi: 10.1271/bbb.60349. [DOI] [PubMed] [Google Scholar]
- 19.Wolever T.M., Vorster H.H., Bjorck I., Brand-Miller J., Brighenti F., Mann J.I., Ramdath D.D., Granfeldt Y., Holt S., Perry T.L., Venter C., Xiaomei W. Determination of the glycaemic index of foods: interlaboratory study. Eur. J. Clin. Nutr. 2003;57:475–482. doi: 10.1038/sj.ejcn.1601551. [DOI] [PubMed] [Google Scholar]
- 20.Morimoto Y., Sakata M., Ohno A., Maegawa T., Tajima S. Effects of bofu-tsusho-san, a traditional Chinese medicine, on body fat accumulation in fructose-loaded rats. Nippon Yakurigaku Zasshi. 2001;117:77–86. doi: 10.1254/fpj.117.77. (in Japanese) [DOI] [PubMed] [Google Scholar]
- 21.Hu E., Liang P., Spiegelman B.M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 22.Arita Y., Kihara S., Ouchi N., Takahashi M., Maeda K., Miyagawa J., Hotta K., Shimomura I., Nakamura T., Miyaoka K., Kuriyama H., Nishida M., Yamashita S., Okubo K., Matsubara K., Muraguchi M., Ohmoto Y., Funahashi T., Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 23.Ghorbani M., Claus T.H., Himms-Hagen J. Hypertrophy of brown adipocytes in brown and white adipose tissues and reversal of diet-induced obesity in rats treated with a beta3-adrenoceptor agonist. Biochem. Pharmacol. 1997;54:121–131. doi: 10.1016/s0006-2952(97)00162-7. [DOI] [PubMed] [Google Scholar]
- 24.Ghorbani M., Himms-Hagen J. Appearance of brown adipocytes in white adipose tissue during CL 316,243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int. J. Obes. Relat. Metab. Disord. 1997;21:465–475. doi: 10.1038/sj.ijo.0800432. [DOI] [PubMed] [Google Scholar]
- 25.Guerra C., Koza R.A., Yamashita H., Walsh K., Kozak L.P. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J. Clin. Invest. 1998;102:412–420. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozak L.P., Koza R.A. Mitochondria uncoupling proteins and obesity: molecular and genetic aspects of UCP1. Int. J. Obes. Relat. Metab. Disord. 1999;23 Suppl. 6:S33–37. doi: 10.1038/sj.ijo.0800941. [DOI] [PubMed] [Google Scholar]
- 27.Dulloo A.G., Seydoux J., Girardier L. Peripheral mechanisms of thermogenesis induced by ephedrine and caffeine in brown adipose tissue. Int. J. Obes. 1991;15:317–326. [PubMed] [Google Scholar]