Abstract
This study aimed to consider the significance of breast milk in preventing oxidative stress by comparing total antioxidant capacity (TAC) in breast milk and formula milk for premature infants, demonstrating the relationship between TAC in breast milk and postnatal age in days. We used the biological anti-oxidant potential test, a new method to measure TAC in breast milk. Breast milk for premature infants were stored at −20°C and thawed within 48 h of collection. We measured TAC in two types of formula milk in the same way. TAC was clearly higher in breast milk than formula milk. Although a negative correlation was observed between TAC in breast milk and age when collected, TAC was always higher than the average TAC in formula milk. TAC in breast milk is higher than TAC in formula milk. We suggest the importance of breast milk for preventing oxidative stress and starting breastfeeding early.
Keywords: breast milk, total antioxidant capacity, premature infant
Introduction
Oxidative stress tends to occur during the neonatal period because the younger the gestational age, the lower the antioxidant capacity (AC) and a lower AC tends to result in an increase in free radicals (FR) and reactive oxygen species (ROS) [1]. Cell damage due to oxidative stress during the neonatal period has been associated with disease [2]. For example, oxidative stress has been reported in association with necrotizing enterocolitis (NEC) if the site of the damage is in the digestive tract [3], with chronic lung disease (CLD) if in the lungs [4], retinopathy of prematurity (ROP) if in the eyes [5], and periventricular leukomalacia (PVL) and intraventricular hemorrhage (IVH) if in the brain [6, 7].
Determining how to avoid oxidative stress is an important factor for improving prognosis. Broadly, there are two ways to prevent oxidative stress. One is to avoid factors that trigger the production of FR and ROS. The other is to strengthen AC, which protects the body from cell damage by scavenging FR and ROS when they increase.
One study of preventing factors that trigger the production of FR and ROS reported that using 21% oxygen instead of 100% oxygen during resuscitation can keep oxidative stress low, since oxygen causes an increase in FR and ROS [8].
A number of studies of strengthening AC have investigated the oral administration of antioxidants (vitamins A, E, and D), but the studies have not reached a consensus of opinion [9–12].
Breast milk is a food that includes antioxidants. Although based on short-term studies, it is known that breast milk has greater AC than formula milk [13, 14]. Breastfeeding has been reported to be clinically significant when compared with formula feeding. For example, compared to formula feeding cohorts, breastfeeding cohorts have been reported to show a 1/10 decrease in risk for development of NEC [15], better neurodevelopment [16], and a decrease in the development of ROP [17]. These results strongly suggest that AC is higher in breast milk than formula milk. The present study was conducted to elucidate the significance of breastfeeding with a view toward preventing oxidative stress.
Thus far there have been no reports on long-term total antioxidant capacity (TAC) in breast milk. A new method to measure TAC was used to compare the AC in formula milk and breast milk and to clarify the relationship between AC in breast milk and the postnatal age in days when collected.
Materials and Methods
At our facility, we put breast milk provided by mothers for their babies into breast milk packs and freeze it at −20°C. Later, when an opportunity to give it to the babies comes, we slowly thaw the breast milk at 37°C and give it to the babies as nourishment. During the current study, we used thawed breast milk instead of fresh breast milk to measure the degree of AC supplied to babies. Since breast milk is an important source of neonatal nutrition, we used the excess portion of breast milk in the packs to ensure they have the necessary amount of milk, milk that would normally be destroyed. We obtained consent from the parents before thawing the milk, and took measurements. All samples were taken from breast milk that was thawed within 48 hours after being frozen following collection.
Extraction of defatted milk
Before taking measurements, we prepared defatted milk through a double centrifugal process. The breast milk was centrifuged at low speed (500 g for 25 min) to settle out the suspended cellular components. Next, the cellular components were removed and the decelled milk was centrifuged at high speed (5000 g for 30 min) to separate it into defatted milk and fat content. The defatted milk was used for taking the measurements. The same method was used to prepare defatted milk using two types of formula milk having a composition similar to the breast milk of Japanese women [18–20], and the measurements taken (Table 1).
Table 1.
Exogenous antioxidants in breast and formula milk in Japan
| Type of milk | Breast milk | Formula milk |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | ||||||||
| Day after delivery (days) |
3–5 |
6–10 |
11–15 |
16–30 |
31–60 |
61–120 |
121–240 |
||
| Cu (µg/dl) | 45.1 | 42 | 44.5 | 38.3 | 35.4 | 28.6 | 25.3 | 43 | 41 |
| Zn (µg/dl) | 487 | 411 | 352 | 280 | 207 | 125 | 80.9 | 410 | 340 |
| Vitamin A (µg/100 ml) | 192 | 145 | 109 | 77 | 76 | 62 | 64 | 53 | 59 |
| β-carotene (µg/100 ml) | 23 | 17 | 6 | 4 | 3 | 4 | 5 | 9.5 | 5.2 |
| α-tocopherol (µg/100 ml) | 1.2 | 0.9 | 0.5 | 0.4 | 0.4 | 0.4 | 0.4 | 0.84 | 0.51 |
| Vitamin C (mg/100 ml) | 7.4 | 6.3 | 7.5 | 7.1 | 6.7 | 6 | 5.7 | 6.8 | 6.2 |
Mesurements of TAC
TAC levels were determined using the biological anti-oxidant potential (BAP) test, which is based on the ability of a colored solution, containing a source of ferric (Fe3+) ions adequately bound to a special chromogenic substrate, to decolour when Fe3+ ions are reduced to ferrous ions (Fe2+), as it occurs by adding a reducing/antioxidant system [21]. Practically, 50 µL of ferric chloride reagent are transferred into the cuvette containing the thiocyanate derivative reagent. The resulting colored solution, after gently mixing by inversion, undergoes 550 nm photometric reading. Then, 10 µL of sample milk are added in the same cuvette and the obtained solution is gently mixed and incubated into the thermostatic block for 5 min at 37°C. After incubation, the sample can be read for absorbance of 550 nm. BAP test, by exploiting the same chemical principle of the well-known ferric reducing ability of plasma (FRAP) test—i. e. the reduction of ferric to ferrous ions—provides a reliable measure of biological antioxidant potential of blood plasma and food [22, 23].
Statistical analysis
An unpaired t test was used for comparison of numerical data between the two groups. All p values were based on two-sided tests. Statistics were compiled using SPSS10.0J (SPSS Inc, Chicago, IL) and Graph Pad Prism 4 (Graph Pad Software, Inc., San Diego, CA). Simple linear regression analysis was used to determine the correlation between different postnatal ages in days and TAC.
Results
The study was performed on 56 samples of breast milk collected from mothers of premature infants born with a mean ± SD gestational age of 33 ± 4 weeks (range: 24–37 weeks) that was collected at postnatal age 39 ± 43 days (range: age 4–145 days).
The compositions of formula milk A and formula milk B are shown in Table 1. No significant difference was observed (p = 0.51) between the TAC in formula milk A (n = 6) and formula milk B (n = 6), which were 2739 ± 126.1 µmol/L and 2602 ± 152.4 µmol/L, respectively. The average TAC in formula milk (formula milk A and formula milk B) was 2671 ± 96.0 µmol/L and the TAC (n = 56) in breast milk was 3807 ± 103.5 µmol/L, clearly revealing that TAC in breast milk is higher than TAC in formula milk (p<0.0001).
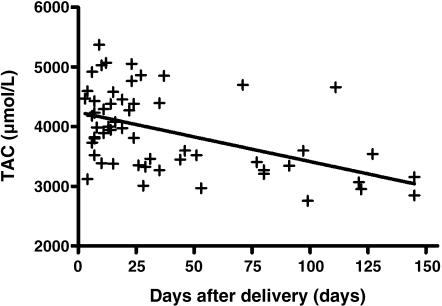
Fig. 1 shows the correlation between TAC and the postnatal age in days when the breast milk was collected. The TAC in breast milk was negatively correlated to the postnatal age in days when collected (r2 = 0.25, p<0.0001), while significantly higher than the average TAC in formula milk.
Fig. 1.
Total antioxidant capacity in correlation with day after delivery
Discussion
Despite the fact that formula milk, which is made to simulate breast milk, has the same level of exogenous antioxidants as breast milk (Table 1), the TAC of breast milk is higher than that of formula milk. Moreover, although the TAC in breast milk was observed to decrease with the passage of time after birth, TAC in breast milk throughout the period of this study (postnatal days 4–145) was continually higher than the average TAC in formula milk. These are highly interesting results (Fig. 1). A conceivable reason for these results is the action of endogenous antioxidants, such as superoxide desmutase (SOD), glutathione peroxidase (GP), and catalase (CAT), which are present only in breast milk and not in formula milk [24, 25].
There are only two or three reports that measure both the endogenous and exogenous TAC in breast milk [13, 14]. The values in the common measurement method, which uses 2,2-azino-di-3-ethylbenzthiazolinesulph onate (ABTS), have a range of less than 1, and so are statistically limited if there are only a few samples and could therefore be biased. In that respect, the BAP test, which measures TAC using thiocyanate that we used in the present study, can become a very useful method to measure TAC in breast milk, since it has a very large range of values.
Endogenous antioxidants in breast milk such as CAT, GP, and SOD were thought to increase with the passage of days after birth [24, 25]. It was imagined, therefore, that the TAC in breast milk would increase as the postnatal age in days increased. However, our results showed that TAC tends to decrease with the passage of time. A possible reason why the TACs we measured in this study, which reflect both exogenous and endogenous antioxidants, decreased with the number of days after birth is that the exogenous antioxidants in breast milk decrease with the passage of time, as shown in Table 1. Accordingly, we feel that measuring the TAC in breast milk is useful for comprehensively evaluating the antioxidant capacity of the substances being measured.
It is essential to understand that TAC decreases with postnatal age in days. It has been reported that early breastfeeding is important in a number of respects, including the fact that the earlier breastfeeding begins, the lower mortality is [26]; the fact that 8-hydroxy-2'-deoxyguanosine in urine, which is a marker of DNA damage, is lower at two and four weeks after birth in babies who consume breast milk from early on when compared with those who are fed formula milk [27]; and the fact that in a comparison of preterm infants of formula milk versus breast milk in a large scale, multi center study, visual development at the chronological age of six months and language development at 14 months were higher in the cohort of babies who were breastfed for a mean period of 135 ± 118 days (the cohort in which babies’ consumption of milk before hospital discharge was ≥50% breast milk) than those who were fed only formula milk [28]. The possibility that the higher TAC in breast milk in early postnatal age neutralizes oxidative stress for younger babies suggests one reason for these findings.
Studies to date on the early oral administration of vitamins aimed at protection from ROS and FR have not produced a consensus on the efficacy of vitamin administration. This may have resulted from the differences between breastfeeding and formula feeding and differences in when the different types of feedings were started.
We learned that TAC, which is higher in breast milk than formula milk, decreases with the passage of days. A possible future research topic for improving the prognosis of neonates ( i.e. NEC, CLD, ROP, PVL, IVH and neurological development) could be how to prevent the TAC in breast milk from decreasing with time, as the current study shows that it does, including supplementation with exogenous antioxidants. Breast milk is important to the neonate, who is subjected to a number of FR and ROS. In particular, we believe that starting breastfeeding early is extremely important and useful in protecting against oxidative stress.
Acknowledgments
The first author expresses his gratitude to the late Dr. Yunosuke Ogawa and Dr. Akira Nishida for stimulating his interest in neonatology, and to Dr. Kazuo Itabashi for teaching him significance of nutrition in neonatal medicine. This study was financially supported by the Japanese Ministry of Health, Labour and Welfare.
References
- 1.Robles R., Palomino N., Robles A. Oxidative stress in the neonate. Early Hum. Dev. 2001;65:S75–81. doi: 10.1016/s0378-3782(01)00209-2. [DOI] [PubMed] [Google Scholar]
- 2.Saugstad O.D. Mechanisms of tissue injury by oxygen radicals: implications for neonatal disease. Acta. Paediatr. 1996;85:1–4. doi: 10.1111/j.1651-2227.1996.tb13880.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Y., Wang Q., Evers B.M., Chung D.H. Signal transduction pathways involved in oxidative stress-induced intestinal epithelial cell apoptosis. Pediatr. Res. 2005;58:1192–1197. doi: 10.1203/01.pdr.0000185133.65966.4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schock B.C., Sweet D.G., Halliday H.L., Young I.S., Ennis M. Oxidative stress in lavage fluid of preterm infants at risk of chronic lung desease. Am. J. Physiol. Lung Cell Physiol. 2001;281:L1386–L1391. doi: 10.1152/ajplung.2001.281.6.L1386. [DOI] [PubMed] [Google Scholar]
- 5.Papp A., Nemeth I., Karg E., Papp E. Glutathione status in retinopathy of prematurity. Free Radic. Biol. Med. 1999;27:738–743. doi: 10.1016/s0891-5849(99)00041-6. [DOI] [PubMed] [Google Scholar]
- 6.Inder T.E., Volpe J.J. Mechanisms of perinatal brain injury. Semin Neonatol. 2000;5:3–16. doi: 10.1053/siny.1999.0112. [DOI] [PubMed] [Google Scholar]
- 7.Volpe J.J. Brain injury in the premature infant—from pathogenesis to prevention. Brain Dev. 1997;19:519–534. doi: 10.1016/s0387-7604(97)00078-8. [DOI] [PubMed] [Google Scholar]
- 8.Vento M., Asensi M., Sastre J., Lloret A., Garcia-Sala F., Vina J. Oxidative stress in asphyxiated term infants resuscitated with 100% oxygen. J. Pediatr. 2003;142:240–246. doi: 10.1067/mpd.2003.91. [DOI] [PubMed] [Google Scholar]
- 9.Delvin E.E., Salle B.L., Claris O., Putet G., Hascoet J.M., Desnoulez L., Messai S., Levy E. Oral vitamin A, E and D supplementation of pre-term newborns either breast-fed or formula-fed: a 3-month longitudinal study. J. Pediatr. Gastroenterol. Nutr. 2005;40:43–47. doi: 10.1097/00005176-200501000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Raju T.N., Langenberg P., Bhutani V., Quinn G.E. Vitamin E prophylaxis to reduce retinopathy of prematurity: a reappraisal of published trials. J. Pediatr. 1997;131:844–850. doi: 10.1016/s0022-3476(97)70031-3. [DOI] [PubMed] [Google Scholar]
- 11.Darlow B.A., Buss H., McGill F., Fletcher L., Graham P., Winterbourn C.C. Vitamin C supplementation in very preterm infants: a randomised controlled trial. Arch. Dis. Child Fetal Neonatal Ed. 2005;90:F117–F122. doi: 10.1136/adc.2004.056440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson L., Schaffer D., Quinn G., Goldstein D., Mathis M.J., Otis C., Boggs T.R. Jr. Vitamin E supplementation and the retinopathy of prematurity. Ann. N.Y. Acad. Sci. 1982;393:473–495. doi: 10.1111/j.1749-6632.1982.tb31285.x. [DOI] [PubMed] [Google Scholar]
- 13.Hanna N., Ahmed K., Anwar M., Petrova A., Hiatt M., Hegyi T. Effect of storage on breast milk antioxidant activity. Arch. Dis. Child Fetal Neonatal Ed. 2004;89:F518–520. doi: 10.1136/adc.2004.049247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turoli D., Testolin G., Zanini R., Bellu R. Determination of oxidative status in breast and formula milk. Acta. Paediatr. 2004;93:1569–1574. doi: 10.1080/08035250410022495. [DOI] [PubMed] [Google Scholar]
- 15.Lucas A., Cole T.J. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336:1519–1523. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 16.Vohr B.R., Poindexter B.B., Dusick A.M., Mckinley L.T., Wright L.L., Langer J.C., Poole W.K., NICHD Neonatal Research Network Beneficial effects of breast milk in the neonatal intensive care unit on the developmental outcome of extremely low birth weight infants at 18 months of age. Pediatrics. 2006;118:e115–123. doi: 10.1542/peds.2005-2382. [DOI] [PubMed] [Google Scholar]
- 17.Hylander M.A., Strobino D.M., Pezzullo J.C., Dhanireddy R. Association of human milk feedings with a reduction in retinopathy of prematurity among very low birthweight infants. J. Perinatol. 2001;21:356–362. doi: 10.1038/sj.jp.7210548. [DOI] [PubMed] [Google Scholar]
- 18.Idota T., Sakurai T., Ishiyama Y., Murakami Y., Kubota J., Ii N., Sakamoto T., Doki R., Shimoda K., Asai Y. The latest survey for the composition of human milk obtained from Japanese mothers. Part 1. The contents of gross components and minerals. Japanese Journal of Pediatric Gastroenterology and Nutrition. 1991;5:145–158. (in Japanese) [Google Scholar]
- 19.Yakabe T., Murakami Y., Idota T., Maeda T., Nakajima I. The latest for the composition of human milk obtained from Japanese mothers. Part VII. The contents of vitamin A, β-carotene and vitamin E. Japanese Journal of Pediatric Gastroenterology and Nutrition. 1995;9:8–15. (in Japanese) [Google Scholar]
- 20.Idota T., Sugawara M., Yakabe T., Sato N., Maeda T. The latest survey for the composition of human milk obtained from Japanese mothers. Part X. Content of water soluble vitamins. Japanese Journal of Pediatric Gastroenterology and Nutrition. 1996;10:11–20. (in Japanese) [Google Scholar]
- 21.Dohi K., Satoh K., Ohtaki H., Shioda S., Miyake Y., Shindo M., Aruga T. Elevated plasma levels of bilirubin in patients with neurotrauma reflect its pathophysiological role in free radical scavenging. In Vivo. 2005;19:855–860. [PubMed] [Google Scholar]
- 22.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 23.Szeto Y.T., Tomlinson B., Benzie I.F. Total antioxidant and ascorbic acid content of fresh fruits and vegetables: implications for dietary planning and food preservation. Br. J. Nutr. 2002;87:55–59. doi: 10.1079/BJN2001483. [DOI] [PubMed] [Google Scholar]
- 24.Friel J.K., Martin S.M., Langdon M., Herzberg G.R., Buettner G.R. Milk from mothers of both premature and full-term infants provides better antioxidant protection than does infant formula. Pediatr. Res. 2002;51:612–618. doi: 10.1203/00006450-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 25.L’Abbe M.R., Friel J.K. Superoxide dismutase and glutathione peroxidase content of human milk from mothers of premature and full-term infants during the first 3 months of lactation. J. Pediatr. Gastroenterol. Nutr. 2000;31:270–274. doi: 10.1097/00005176-200009000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Edmond K.M., Zandoh C., Quigley M.A., Amenga-Etego S., Owusu-Agyei S., Kirkwood B.R. Delayed breastfeeding initiation increases risk of neonatal mortality. Pediatrics. 2006;117:e380–e386. doi: 10.1542/peds.2005-1496. [DOI] [PubMed] [Google Scholar]
- 27.Shoji H., Shimizu T., Shinohara K., Oguchi S., Shiga S., Yamashiro Y. Suppressive effects of breast milk on oxidative DNA damage in very low birthweight infants. Arch. Dis. Child Fetal Neonatal Ed. 2004;89:F136–F138. doi: 10.1136/adc.2002.018390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Connor D.L., Jacobs J., Hall R., Adamkin D., Auestad N., Castillo M., Connor W.E., Connor S.L., Fitzgerald K., Groh-Wargo S., Hartmann E.E., Janowsky J., Lucas A., Margeson D., Mena P., Neuringer M., Ross G., Singer L., Stephenson T., Szabo J., Zemon V. Growth and development of premature infants fed predominantly human milk, predominantly premature infant formula, or a combination of human milk and premature formula. J. Pediatr. Gastroenterol. Nutr. 2003;37:437–446. doi: 10.1097/00005176-200310000-00008. [DOI] [PubMed] [Google Scholar]