Abstract
In the head, neural crest cells generate ectomesenchymal derivatives: cartilage, bone and connective tissue. Indeed, these cells generate much of the cranial skeleton. There have, however, been few studies of how this lineage is established. Here, we show that neural crest cells stop expressing early neural crest markers upon entering the pharyngeal arches and switch to become ectomesenchymal. By contrast, those neural crest cells that do not enter the arches persist in their expression of early neural crest markers. We further show that FGF signalling is involved in directing neural crest cells to become ectomesenchymal. If neural crest cells are rendered insensitive to FGFs, they persist in their expression of early neural crest markers, even after entering the pharyngeal arches. However, our results further suggest that while FGF signalling is required for the realisation of the ectomesenchymal lineages, other cues from the pharyngeal epithelia are also likely to be involved.
Keywords: neural crest, ectomesenchyme, FGF, pharyngeal arches
Introduction
The cranial neural crest is a multipotent progenitor population that generates a broad range of derivatives, which can be grouped into two categories, ectomesenchymal and non-ectomesenchymal. Ectomesenchymal derivatives include bone, cartilage, connective tissue and dentine, while the non-ectomesenchymal derivatives consist of neurons, glia and pigment cells (Le Douarin and Kalcheim, 1999; Graham, 2003). In the embryonic head these two populations of the cranial crest are spatially segregated. The ectomesenchymal crest lie ventrally and are those that fill the pharyngeal arches and facial prominences, while the non-ectomesenchymal crest lie more dorsal, close to the neural tube (Lumsden et al., 1991; Baker et al., 1997). The establishment of the ectomesenchymal lineage within the cranial neural crest is of great significance as it is these cells that generate much of the head skeleton (Noden, 1988; Couly et al., 1993). The presence of neural crest-derived cranial skeletal tissues is also a defining feature of vertebrates (Donoghue and Sansom, 2002). Thus the emergence of the ectomesenchymal lineage is of profound importance for both the development and the evolution of the vertebrates.
It has been far from clear as to how the ectomesenchymal lineage is established within the cranial crest. It had long been held that the ability of cranial crest to generate ectomesenchymal derivatives was an intrinsic property of this population, and was not shared by trunk crest (Le Lievre and Le Douarin, 1975; Nakamura and Ayer-le Lievre, 1982). More recently, however, it has been shown that trunk neural crest cells do have the potential to generate ectomesenchymal derivatives. If trunk neural crest cells are cultured in media typically used to support skeletogenic differentiation, they will form bone and cartilage (McGonnell and Graham, 2002; Abzhanov et al., 2003). Additionally, if trunk crest cells are grafted into the facial primordial, the sites of skeletogenic differentiation, they will contribute to the skeletal elements forming within these structures (McGonnell and Graham, 2002). Hence, and as with other neural crest fates, the realisation of ectomesenchymal potential in the developing head must be the result of local spatial and/or temporal cues.
The natures of such cues, however, are ill defined and results from different vertebrates are conflicting. A fate map of the cranial neural crest in zebrafish has suggested that the mechanisms acting to promote ectomesenchymal fate may be acting at very early stages of development, and that the ectomesenchymal and the neuronal lineages are spatially segregated in the premigratory crest (Schilling and Kimmel, 1994). It was found that those crest cells that were located medially, close to the neural primordium, filled the pharyngeal arches and formed cartilage and connective tissue, while cells lying laterally gave rise to neurons in the trigeminal ganglia. By contrast, a study of the fate of cranial neural crest cells in the chick suggested that the fates of the cranial crest are determined by the environments encountered by these cells as they migrate (Baker et al., 1997). In that analysis, it was shown that early-migrating neural crest cells distributed along the entire dorsoventral axis, filling the pharyngeal arches as well as populating more dorsal regions closer to the neural tube, while late-migrating crest was restricted dorsally. Importantly, however, both early and late migrating crest formed neurons, glia, cartilage, bone and pigment cells, although the late migrating crest produced less cartilage and bone. Moreover, these heterochronic transplantations demonstrated that early- and late-migrating crest cells could substitute for each other.
In this paper, we move towards resolving these issues and we present evidence that peripheral signals, notably FGFs, instruct the establishment of the ectomesenchymal lineage in the cranial crest. We present evidence, from studies of both chick and zebrafish embryos that neural crest cells only assume an ectomesenchymal fate upon entering the pharyngeal arches, suggesting that this fate switch is directed by epithelial cues within the arches. The neural crest cells that lie dorsally and which will follow a non-ectomesenchymal fate, continue in their expression of early neural crest markers for a prolonged period after the cessation of migration. We further demonstrate that FGF signalling is required to direct neural crest cells to become ectomesenchymal. Thus if cranial neural crest are made insensitive to FGF signalling they do not switch to become ectomesenchymal, even after having entered the pharyngeal arches, but persist in their expression of early neural crest markers. These results demonstrate that the ectomesenchymal lineage is established within the cranial neural crest as a result of local cues and that this involves FGF signalling from the pharyngeal epithelia. However, we also find that newly emergent cranial neural crest do not deploy the downstream effectors of FGF signalling even though they migrate in close proximity to sources of FGF’s, suggesting that other factors within the pharyngeal environment are also required to act with the FGFs to promote the adoption of the ectomesenchymal lineage.
Results
The spatial and temporal development of the cranial neural crest can be dissected through the use of a range of molecular markers. In chick embryos by stage 15 of development the production of cranial neural crest by the neural tube has ceased and the neural crest cells have migrated into the periphery and have reached their destinations (Lumsden et al., 1991). At this stage the transcription factor AP2α is expressed by all of the cranial neural crest and highlights the three cranial neural crest streams: the trigeminal, hyoid and post-otic (Fig 1a). In each of these streams, cells can be found both within the pharyngeal arches and more dorsally between the arches and the neural tube (Fig 1 a, b). This pattern of expression is maintained at later stages of development. Importantly, however, within these cranial crest cells one can also detect non-overlapping expression of transcription factors that correlate with different crest fates. Thus at stage 15, the crest cells in each of the three streams that lie between the arches and the hindbrain express the transcription factors Sox10 and Foxd3 (Fig 1 c-f). Sox10 is also expressed in the otic vesicle (Fig 1 c). These dorsally located crest cells that express Sox10 and Foxd3 (Fig 1 d, f) are those that will have a non-ectomesenchymal fate (Baker et al., 1997). This population will eventually, between days 4 and 7 of embryogenesis, give rise to a proportion of the sensory neurons of the trigeminal ganglion, all of the neurons of the superior and jugular ganglia and to glia (D’Amico-Martel and Noden, 1983). Contrastingly, the crest cells that populate the pharyngeal arches and which will have an ectomesenchymal fate (Baker et al., 1997), do not express Sox10 or Foxd3, but rather express the homeobox gene, Dlx2 (Fig 1 g, h). At stage 15, the cells of the first four pharyngeal arches express this gene (Fig 1 g). Notably, it can be seen that the cells expressing Dlx2 within the arches are not those in the central portion but those in proximity to the epithelia, these are the neural crest cells (Fig 1 h). The centrally located cells are mesodermal (Graham et al., 2005).
Figure 1. Disposition of ectomesenchymal and non-ectomesenchymal crest within the cranial neural crest.

Ap2α is a general marker of the cranial neural crest, labelling neural crest within the pharyngeal arches as well as those lying between the arches and the hindbrain (a, b). Sox10 (c,d) and Foxd3 (e,f) are expressed by the crest that lie between the arches and the neural tube. Sox10 is additionally expressed in otic vesicle (c,d). The ectomsenchymal crest can be identified through their expression of Dlx2 (g,h). Dlx2 only labels the crest cells within the pharyngeal arches.
(a,c,e,g) Lateral view of the head of stage 15 chick embryos with anterior to the left and dorsal to the top. (b,d,f,h) Transverse sections (40μm), shown at the level of otic vesicle of stage 19 embryos., with dorsal to the top
ov, otic vesicle; I,II,III,IV the position of each pharyngeal arches.
We further characterised the expression profiles of these neural crest markers at earlier stages to gain insights into how the association, evident at stage 15 onwards, between ectomsenchymal fate and Dlx2 expression and non-ectomesenchymal fate and Sox10 and Foxd3 expression becomes established. Neural crest production initiates anteriorly and spreads posteriorly, and at anterior hindbrain levels, the production of neural crest cells is well underway by stage 10 (Lumsden et al., 1991). At this time, neural crest cells migrating from the midbrain and rhombomeres 1 and 2 of the hindbrain express Sox10 and Foxd3 (Fig 2 a, d). Sox10 expression is additionally found in the otic placode, which lies alongside rhombomeres 4, 5 and 6 (Fig 2 a). These newly emigrant neural crest cells do not, however, express Dlx2. The only site of Dlx2 expression in the developing head at this stage is within the posterior hindbrain (Fig 2 g). By stage 11 of development, neural crest cells are also emerging from rhombomere 4 and from rhombomere 6. These cells from the hyoid and post-otic crest streams, like the more anterior crest, express Sox10 and Foxd3 (Fig 2 b, e). Sox10 continues to be expressed by the otic placode (Fig 2 b). At this stage of development, Dlx2 is not yet expressed by the migrating crest cells. This gene is, however, expressed within the posterior hindbrain and within the pharyngeal endoderm (Fig 2 h). As development proceeds, the cranial crest migrate further ventrally, and by stage 14 of development, Dlx2 expression first becomes apparent in neural crest cells. It can be seen that Dlx2 is expressed only in crest cells populating the first and second pharyngeal arches; the ectomesenchyme (Fig 2 i). The more dorsal crest continues to expresses Sox10 and Foxd3 (Fig 2 c, f).
Figure 2. Emigrant neural crest cells are non-ectomesenchymal in nature.
Cranial neural crest cells emerging from the midbrain and anterior hindbrain at HH10 express Sox-10 (a) and Foxd3 (d). At this stage Dlx2 is not expressed by any neural crest cells, although this gene is expressed in rhombomere 5 of the hindbrain. At stage 11, neural crest cells emerge from rhombomeres 4 and 6 of the hindbrain, express Sox-10 (b) and Foxd3 (e). Dlx2 is still not expressed by neural crest cells, although there continues to be some expression in rhombomere 5 and also now in the pharyngeal endoderm. By stage 14 of development, Sox10 (c) and Foxd3 (f) expression is restricted to the dorsally located crest, while the more ventral crest that has started to populate the pharyngeal arches now expresses Dlx2 (i) (a,b,d,e,g,h) Dorsal views of the neural tube with anterior to the left and posterior to the right. (c,f,i) Lateral view of pharyngeal arches with anterior to the left, dorsal to the top.
op, otic placode; r2, rhombomere 2, r4, rhombomere 4, r5, rhombomere 5; other abbreviations as before.
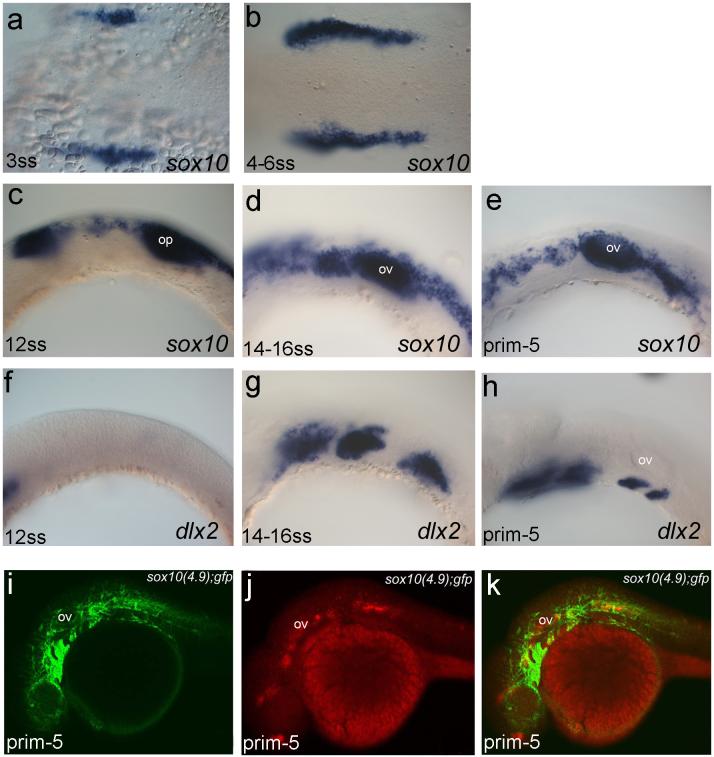
Previous work in zebrafish had suggested that ectomesenchymal crest were specified at very early stages, well before they populate the pharyngeal arches, and this would possibly suggest that the results observed here might not apply to zebrafish embryos (Schilling and Kimmel, 1994). To assess whether or not this was the case, we documented in detail the expression profiles of sox10 and dlx2 in zebrafish during the period of cranial neural crest production and migration. sox10 expression in neural crest cells in zebrafish is evident as early as the 3 somite stage at the presumptive midbrain level (Fig 3 a). As development proceeds, sox10 expression also becomes evident in neural crest cells at hindbrain levels (Fig 3 b). By the 12 somite stage of development, sox10 is expressed by the migrating cranial neural crest cells and in the otic placode (Fig 3 c). At this stage, dlx2 is expressed in the forebrain but not in the neural crest cells (Fig 3 f). By the 14 somite stage, however, dlx2 becomes expressed by the neural crest, clearly labelling three populations of ventrally located crest cells in the forming first, second and third pharyngeal arches (Fig 3 g). The more dorsally located neural crest cells, which lie close to the nervous system, express sox10, as does the otic vesicle (Fig 3 d). At slightly later stages, dlx2 is evident in the ectomesenchymal crest cells of the first four pharyngeal arches (Fig 3 h), while the more dorsal non-ectomesenchymal crest express sox10. Thus the expression dynamics of sox10 and dlx2 in zebrafish mirrors the expression pattern observed in chick, with premigratory and dorsally located post-migratory cells expressing sox10 while dlx2 expression is only apparent in post-migratory ectomesenchymal neural crest cells that have filled the pharyngeal arches.
Figure 3. Appearance of non-ectomesenchymal and ectomesenchymal crest in the zebrafish.
a: Neural crest cells can be first identified alongside the neural tube at the midbrain level at the 3 somite stage through their expression of sox10. b,c: As development proceeds, sox10 expression is associated with neural crest cells from more posterior regions. f: At the 12 somite stage, dlx2 is expressed in the forebrain but not in neural crest cells. d,g: At 14 somites, sox10 expression is restricted to those crest cells that lie dorsally, close to the neural tube (d), while dlx2 can now be seen in the ectomesenchymal crest that have filled the first three pharyngeal arches (g). e,h: By the prim-5 stage, sox10 expression is still restricted to the more dorsal crest (e) and dlx2 to the ectomesenchymal crest of the first four pharyngeal arches (h). c-e: From the 12 somite stage onwards, sox10 expression is associated with the developing inner ear. To test the hypothesis that ectomesenchymal crest emerge from within the ectomesenchymal crest, we have analysed the distribution of GFP protein and GFP RNA in a sox10 transgenic reporter line. i-k: GFP protein is found in all the cranial neural crest cells (i, k) but GFP RNA is only found in the non-ectomesenchymal crest (j). (k) Overlay GFP protein with GFP RNA. In all panels, anterior is to the left and dorsal to the top.
Abbreviations as before.
The analyses of sox10 and dlx2 expression patterns suggest that, prior to adoption of an ectomesenchymal fate, the crest cells that fill the arches were sox10 positive but once in the arches they have downregulated expression of this gene. Definitive proof of this comes from our analysis of a sox10 GFP transgenic reporter line. In these fish, 4.9 kb of the sox10 promoter region upstream of the translation start site is used to drive GFP expression. These fish express gfp RNA in neural crest cells in a pattern recapitulating normal sox10 expression. Thus in the head, at the prim-5 stage of development, gfp RNA is only expressed by the dorsally located non-ectomesenchymal crest (Fig 3 j). Importantly, however, Green Fluorescent Protein is found in these dorsal cells and additionally it is detected in the ectomesenchymal cells of the arches (Fig 3 i, k). Here the stability of the Green Fluorescent Protein is acting as a lineage tracer indicating that the cells of the arches previously expressed Sox10. These cells have, however, shut down expression of this gene and consequently do not express GFP RNA. These results suggest that the establishment of the ectomesenchymal lineage within the cranial neural crest is the result of crest cells entering the arches and responding to that environment, and switching their fate, downregulating sox10 and intiating dlx2 expression.
Much of the development of the pharyngeal arches is directed by the pharyngeal epithelia (Graham et al., 2005). Significantly, the pharyngeal epithelia express a number of signalling molecules, including members of the FGF family of signalling molecules and these have been implicated in aspects of arch development. Embryos treated with SU5402, an inhibitor of FGF receptor signalling, fail to develop pharyngeal cartilages (David et al., 2002; Walshe and Mason, 2003). More specifically, Fgf3 and Fgf8 have pronounced expression in the pharyngeal endoderm and ectoderm, and embryos injected with antisense morpholinos directed against the transcripts of these two genes fail to form pharyngeal cartilages (David et al., 2002; Walshe and Mason, 2003). Other studies have also noted that the mature pharyngeal arches are a prominent site of expression of members of the FGF synexpression group, including the transcriptional effectors pea3 and erm, as well as inhibitors of FGF signalling, such as Pyst1 (MKP3) and sprouty 2 (Munchberg et al., 1999; Chambers and Mason, 2000; Chotteau-Lelievre et al., 2001; Eblaghie et al., 2003; Walshe and Mason, 2003).
We therefore hypothesised that FGF signalling might play a role in promoting a fate switch in the cranial neural crest. Our study of the expression dynamics of sox10 and dlx2 has shown that cells take up an ectomesenchymal fate from the 12 somite stage onwards as they enter the arches in an anteroposterior sequence (Fig 3). Moreover, at that stage dlx2 positive cells are closely associated with FGF expressing pharyngeal epithelia (Walshe and Mason, 2003). To specifically, inhibit FGF signalling during this period we treated zebrafish embryos with the FGF receptor (FGFR) inhibitor, SU5402, and then allowed them to develop to the prim-5 stage of development. We postulated that in this situation crest cells entering the arches might not switch their fate and assume ectomesenchymal identity. Thus, they might not turn off sox10 and turn on dlx2, but would persistently express sox10. We found, however, that while acute treatment at this stage did result in the failure to establish dlx2 expression (Fig 4 d), it did not produce ectopic sox10 expression in cells within the arches (Fig 4 b). Control embryos treated with DMSO showed normal sox10 and dlx2 expression (Fig 4 a, c). Yet, we noted that SU5402 treatment did result in an accumulation of sox10 expressing cells just dorsal of the presumptive arches (compare Fig 4 a and b). This observation was of interest as it has also been shown that FGF signalling is required for endodermal pouch formation (Crump et al., 2004), and if this process fails then the pharyngeal arches themselves do not form. A consequence of the failure of pharyngeal arches to form is that there will be no territory for the cells to populate and so they pile up on each other.
Figure 4. Dlx2 expression is absent from zebrafish embryos treated with an inhibitor of FGF signalling.
In embryos treated with DMSO alone, sox10 expression is found in the dorsally located crest (a) and dlx2 in the ectomesenchymal crest within the pharyngeal arches (c). In embryos treated with SU5402, sox10 expressing crest are still found in the more dorsal position (b), but there is a lack of dlx2 expression (d). There seems to be an abundance of post-otic sox10 expressing crest in the SU5402 treated embryos (c). (a-d) Lateral view of prim-5 embryos. In all panels, anterior is to the left and dorsal to the top.
Abbreviations as before.
To create a situation in which the crest cells are selectively insensitive to FGF signalling but FGF function within the pharyngeal epithelia themselves is unaffected, and thus the arches form, we returned to the chick embryo. It has been demonstrated that cranial neural crest cells express the FGF Receptors, in particular Fgfr1 (Wilke et al., 1997; Walshe and Mason, 2000), and we, therefore, used in vivo electroporation to introduce a dominant negative murine Fgfr1 construct into the neural crest to render them numb to FGF signalling. This construct has been shown to inhibit FGF signalling both in vivo and in vitro (JW, H. Maroon and IM unpublished). This vector also carried an IRES-GFP cassette allowing us to monitor the behaviour of the transfected crest cells. Neural crest cells fill the pharyngeal arches in an anteroposterior sequence and thus crest cells that populate different arches can be targeted by varying the timing of the electroporation. We found that electroporation into the anterior hindbrain at the 8 somite stage resulted in GFP-expressing cells within the first pharyngeal arch (Fig 5 f) and significantly in the ectopic expression of Sox10 within the arch (Fig 5 e, f). These ectopic Sox10 expressing cells within the arch were those that carried the dominant negative receptor construct as evidenced by their production of Green Fluorescent Protein (Fig 5 f). No such ectopic expression of Sox10 was seen on the contralateral side (Fig 5 d). Electroporation of this construct at the 9-somite stage resulted in GFP expressing cells within the second arch (Fig 5 i) and again in the ectopic expression of Sox10 within that arch (Fig 5 h). As before, the ectopic Sox10 expressing cells within the arch are also GFP positive (Fig 5 i). No Sox10 expressing cells were seen within the second arch on the control side (Fig 5 g). Thus ectopic Sox10 expression was seen in crest carrying the dominant negative FGF receptor in 17 out of 20 cases. To control for potential effects of the electroporation, we introduced a vector containing a chicken β-actin promoter driving eGFP into the neural crest, and although this also resulted in the presence of many GFP expressing cells within the arches (Fig 5 c), ectopic Sox10 expression was never observed (n=8/8) (Fig 5 b, c). We next analysed whether crest cells within the arch carrying the dominant negative FGF receptor also displayed ectopic Foxd3 expression. Again electroporation resulted in GFP expressing crest cells in the first and second arches (Fig 5l), and we found that these cells also ectopically expressed Foxd3 (n=9/12) (Fig 5 k, l). Control electroporation never resulted in ectopic Foxd3 expression (n=6/6). Finally, we assessed whether rendering crest cells insensitive to FGF signalling prevented them from expressing Dlx2. We found that the presence of neural crest cells carrying the dominant negative FGF receptor construct resulted in a dimunition in Dlx2 expression (n=6/11) (Fig 5 n, o). Thus in the second arch of the control side there is pronounced Dlx2 expresssion (Fig 5m), while on the electroprated side there is reduced expression in the region containing crest cells carrying this construct (Fig 5 n, o). In contrast normal Dlx2 expression was observed when cells were elecroprated with a construct containing a chicken β-actin promoter driving eGFP (n=6/6). Note that for both the control and the dominant negative FGFR electroporations GFP expressing cells were found distributed along the crest streams emanating from the hindbrain. That is GFP positive cells were found both within the arches and dorsal of the arches, demonstrating that the dominant negative FGFR construct does not affect the migratory behaviour of these cells (Fig 5 c, f, i, l. o). Thus if neural crest cells are rendered insensitive to FGF signalling, they do not switch to assume an ectomesenchymal identity upon entering the pharyngeal arches, but persist in their expression of early neural crest markers.
Figure 5. Neural crest cells insensitive to FGF signalling persist in their expression of non-ectomesenchymal markers even after they have entered the pharyngeal arches.
(a-c) Embryos electroporated with pCAβ-eGFP at 9 somite stage (HH9+). Normal expression of Sox10 is seen on both the non-electroporated side (a) and also on the electroporated side (b). (c) Overlay with GFP positive cells. (d-f) Embryo electroporated with dnFGFR1-GFP at the 8 somite stage (HH9-). (d) non-electoporated/control side shows normal Sox10 expression. Contrstaingly, ectopic Sox10 expression is seen in cells that have entered the first arch on the electroporated side (e). (f) Overlay of the Sox10 expression with the dnFGFR1-GFP positive cells in green. (g-i) Embryos electroporated with dnFGFR1-GFP at the 9 somite stage (HH9+). (g) non-electroporated control side shows normal Sox10 expression. (h) On the electroporated side, ectopic Sox10 expression can be seen in the second arch. (i) Overlay of Sox10 expression with dnFGFR1-GFP positive cells in green. (j-l) Embryos electroporated with dnFGFR1-GFP at the 6 somite stage (HH9-). (j) non-electroporated control side shows normal Foxd3 expression. (k) On the electroporated side, ectopic Foxd3 expression can be seen in the second arch. (l) Overlay of Foxd3 expression with dnFGFR1-GFP positive cells in green. (m-o) Embryos electroporated with dnFGFR1-GFP at the 9 somite stage (HH9+). (m) non-electroporated control side shows normal Dlx2 expression. (n) On the electroporated side, diminished Dlx2 expression can be seen in the second arch. (i) Overlay of Dlx2 expression with dnFGFR1-GFP positive cells in green. Schematic illustration of cranial neural crest at the level of pharyngeal arches at HH15, normal distribution of non-ectomesenchymal crest (p). Ectopic non-ectomesenchymal crest cells are seen within the pharyngeal arches when crest cells carry the domninant negative FGF receptorin (q, r). This is shown in side view (q) and in transverse (r). In the tranverse view (r) ectopic ectomesenchymal crest cells are only seen in the electroprated right side. In panels (a - q), anterior is to the left and dorsal to the top. In (r), dorsal is to the top.
It is important to note, however, that the pharyngeal epithelia are not the first sources of FGF’s encountered by cranial neural crest cells. At earlier stages, the newly emergent crest cells, which express the FGF receptors (Wilke et al., 1997; Walshe and Mason, 2000), come into to close proximity to the isthmus, a prominent site of Fgf8 expression, and the central region of the hindbrain, which expresses Fgf3 (Crossley and Martin, 1995; Mahmood et al., 1995). Yet these cells clearly express Sox10 and Foxd3 and do not express Dlx2 (Fig 2). To determine if these newly emergent crest cells are responding to these sources of FGF signalling, we conducted an analysis of the expression profiles of members of the FGF synexpression group at stage 11, a time point at which Fgf expression in the isthmus and hindbrain is established and at which neural crest cells are migrating from the neural tube (see Fig 2). We find pronounced expression of pea3, erm, sprouty 2, and dpERK staining in the embryo at this stage (Fig 6). In particular, we find that these molecules are all expressed at known sites of Fgf expression: the anterior neuropore, the isthmus, the hindbrain, the otic placode and in the pharyngeal endoderm. However, the migrating cranial neural crest cells do not express pea3, erm, or sprouty 2 (members of the Fgf synexpression group) or exhibit pronounce dpERK staining (compare Fig 6 with Fig 2). These results suggest that the newly emergent cranial neural crest cells are insensitive to the to the sources of FGF signalling that they encounter. This has also been reported in zebrafish (Walshe and Mason, 2003). This would in turn suggest that while FGF signalling is required for neural crest cells to assume an ectomesenchymal fate, other factors produced by the pharyngeal epithelia are also likely to act to enable neural crest cells to respond to the FGFs.
Figure 6. Expression of members of the FGF synexpression group during the period of neural crest migration.
Dorsal views of stage 11 embryos, anterior to the left, showing (a) erm expression (b) pea3 expression (c) sprouty 2 expression and (d) dpERK immostaining. In all cases, expression of erm, pea3, sprouty2 and dpERK staining can be seen at the anterior neuropore, around the isthmus in the mesencephalon and anterior hindbrain, and, with the exception of sprouty 2, in the central region of the hindbrain and in the otic placode. Expression is not seen in the neural crest cells, which at this stage are in abundance lateral to the neural tube (see Fig 2). AN - anterior neuropore, Mes - mesencephalon, I - Isthmus, Hind - Hindbrain, Op - Otic placode.
Discussion
A number of important conclusions can be drawn from our study. We demonstrate that the ectomesenchymal lineage emerges as neural crest cells populate the pharyngeal arches. We show that the switch to an ectomesenchymal fate involves FGF signalling. If neural crest cells are rendered insensitive to FGF signalling, they will populate the pharyngeal arches but persist in their expression of early neural crest markers and will not adopt an ectomesenchymal fate. It is, however, important to note that other signals from the pharyngeal epithelia must also be involved in promoting this fate switch, as newly emergent crest cells encounter sources of FGFs but do respond to them. Finally, when considered with previous studies of neural crest potential our results support the suggestion that cranial neural crest are fundamentally gliogenic in nature.
Previous studies have suggested a number of routes through which ectomesenchymal fate is imparted to neural crest cells. It had been suggested, through an analysis in zebrafish, that the ectomesenchymal precursors are spatially segregated within the cranial neural crest (Schilling and Kimmel, 1994). However, analyses in chick had suggested that ectomesenchymal fate is assigned to neural crest cells as a result of environmental cues (Baker et al., 1997). Finally, it has also been argued that ectomesenchyme cells are not derived from the neural crest but are a separate population that derives from the lateral non-neural domain of the neural folds (Weston et al., 2004). Our study clearly support the view that that the ectomesenchymal lineage is imparted to the neural crest cells as a result of the environmental cues they encounter within the pharyngeal arches. We demonstrate that the ectomesenchymal cells of the pharyngeal arches, were Sox10 positive, and as such are not a separate population from the rest of the neural crest. We also show that neural crest cells that are deaf to the epithelial cues within the pharyngeal arches maintain their expression of early neural crest markers, and do not switch to assume an ectomesenchymal fate. Our results would also suggest that the assignment of a neuronal or glial fate upon crest cells is also not established at very early stages but is assigned to those crest cells that remained dorsally and do not enter the arches. The assignment of a neuronal lineage to these crest cells is likely to be the result of local cues, and in the case of the dorsally located cranial sensory ganglia these are likely to emanate from the neural tube.
These results also raise concerns as to how the correct number of cells becomes allocated to an ectomesenchymal versus a non-ectomesenchymal fate. It is far from clear how this is achieved, but it has been previously shown that there is a progressive ventral to dorsal filling of the pharyngeal arches (Lumsden et al., 1991). Thus crest cells emerging at later times have an increased probability of assuming a non-ectomesenchymal fate. This would suggest that crest cells are, in part allocated to follow an ectomesenchymal fate versus a non-ectomesenchymal fate as a result of the time at which they migrate from the neural tube and therefore the environmental cues that they will subsequently perceive. Thus, once early migrating crest cells have filled the pharyngeal arches, the later migrating crest can no longer enter the arches and are therefore assume a non-ectomesenchymal fate. It is important to note, however, that while early versus late migrating neural crest cells may have different fates, there is no difference in potential between these populations (Baker et al., 1997). Late migrating crest cells, when grafted into a younger embryo, will fill the arches and generate ectomesenchymal derivatives.
The previous studies that established a role for FGF signalling in the formation of the pharyngeal cartilages found no evidence that FGFs promote a fate switch within the cranial neural crest (David et al., 2002; Walshe and Mason, 2003). These analyses in zebrafish using morpholino approaches or systemic treatment with the FGF receptor inhibitor, SU5402, suffered from the problem that FGF signalling is also required for the development of the pharyngeal pouches and thus the formation of the arches (Crump et al., 2004). Consequently, there was no territory for the migrating crest cells to enter, and as we note in our analysis the neural crest cells in zebrafish embryos exposed to SU5402 at the 12 somite stage pile up dorsally of the presumptive arch territory. During normal development pharyngeal segmentation is required before neural crest cells can move ventrally and populate the forming arches. The post-otic crest stream, which will fill the posterior pharyngeal arches, sits as a single mass dorsal of the presumptive arch territory, and is only split into separate arch population following the formation of pharyngeal pouches (Veitch et al., 1999; Quinlan et al., 2004). Failure in the formation of the pouches results in a failure in the segmentation of the crest (Piotrowski and Nusslein-Volhard, 2000).
By contrast, we were able to selectively render the neural crest cells insensitive to FGF signalling without compromising FGF function in the pharyngeal epithelia. Thus the arches could form, but the crest cells that populated these structures could not respond to the FGFs expressed by the pharyngeal epithelia. This approach allowed us to demonstrate that crest cells carrying the dominant negative FGFR construct would populate the pharyngeal arches but once in that environment they did not switch off early neural crest markers. Importantly, the introduction of the dominant negative FGFR construct did not affect the migration of the neural crest cells. There was no difference in the dorsoventral distribution of cells electroporated with the control GFP expression construct or with the dominant negative FGFR construct; in both situation GFP positive cells could be found within the arches and within the neural crest cells lying between the arches and the hindbrain. Thus, FGF signalling is require for neural crest cells to switch and assume an ectomesenchymal fate.
Significantly, the pharyngeal epithelia are not the first sources of FGFs encountered by migrating cranial neural crest cells. At earlier stages, some of these cells will be in close proximity to the isthmus, which expresses Fgf8, and others to the central region of the hindbrain, which expresses Fgf3. However, at these earlier stages the crest cells not only express Sox10 and Foxd3 but they also show no response to these sources of FGF signalling. Thus, other signals from the pharyngeal epithelia must also be involved in promoting this fate switch. The pharyngeal epithelia express numerous signalling molecules and future work will be aimed at determining which combinations of these acts with the FGFs to switch neural crest cells from their early status towards becoming ectomesenchymal.
This is the first demonstration of an involvement of FGF signalling in controlling neural crest cell fates, and our results, coupled with those from previous studies, suggest that FGFs play ongoing roles in the development of the ectomesenchyme. We show that FGFs are involved in directing cells to adopt this fate, while others have demonstrated a role for FGFs in promoting the proliferation and survival of the ectomesemchymal crest (Trumpp et al., 1999; Creuzet et al., 2004). Finally it has also been shown that FGFs promote the formation of ectomesenchymal derivatives. Absence of FGF function results in a failure in the formation of pharyngeal cartilages (David et al., 2002; Walshe and Mason, 2003), and the culturing of neural crest cells in FGFs promotes skeletogenesis (Sarkar et al., 2001; Ido and Ito, 2006).
Finally, the results we present here also support a previous suggestion that cranial neural crest cells are fundamentally gliogenic in nature (Le Douarin et al., 2004). We show here that all newly emergent neural crest cells express Sox10, a transcription factor that plays a key role in establishing the gliogenic lineage (Britsch et al., 2001; Paratore et al., 2001) and Foxd3 a gene associated with differentiating glia at later stages of development (Kelsh et al., 2000; Dottori et al., 2001). We show that the ectomesenchymal crest cells of the arches were Sox10 positive. We also show that FGF insensitive neural crest cells that populate the arches persist in their expression of Sox10 and Foxd3. These observations are in keeping with the results obtained from the clonal analysis of the potential of neural crest cells (Le Douarin et al., 2004). In these studies, it was noted that all pluripotential crest cell progenitors, including bipotential precursors, were able to yield glial cells besides any other derivatives. Thus it would seem that gliogenic differentiation potential is a fundamental feature of neural crest cells.
Experimental Procedures
In situ hybridisation and Immunostaining of chick embryos
Fertilised hen eggs were incubated in a humidified chamber at 37°C and staged according to (Hamburger and Hamilton, 1992). Whole mount in situ hybridisation was performed according to (Henrique et al., 1995). Immunostaining was done using standard whole mount imunostaining protocol (Mackenzie et al., 1998). To detect ectopic GFP rabbit anti-GFP serum (Molecular Probes, Eugene) was used at 1:500, followed by a fluorophore-conjugated anti-rabbit secondary antibody (Alexa Fluor 488, Molecular Probes).
In situ hybridisation on zebrafish embryos embryos
Staging of wild type (kwt) zebrafish embryos, Danio rerio, that were maintained at King’s College London was performed as described previously (Westerfield, 1995). Whole mount in situ hybridisation was carried out according to (Coutelle et al., 2001).
Electroporation
Electroporation of chick embryos at HH stages 6-10 was performed following the well established procedure (Itasaki et al., 1999). DNA was injected in the midbrain/rostral hindbrain at the concentration of 2μg/μl. 0.25mm Platinum/Iridium electrodes were placed on either side of the embryo, with the anode at the right side. Electroporation was performed at 50ms, 7V, 5 pulses for the HH stages 6-8, or at 8/9V for the stages 9 and 10 respectively. After electroporation embryos were incubated in a humidified chamber for additional 24h at 37°C.
Treatment of zebrafish with SU5402, FGFR Inhibitor
Once embryos reached 12-16 somite stage of development their chorion was removed manually in aquarium water containing methylene blue. Following this, embryos were transferred to Petri dishes coated with 1% agarose on their base and incubated in 50-100 μM SU5402 (Calbiochem) (Maroon et al., 2002)diluted in aquarium water from 10mM stock solution dissolved in DMSO until 5prim stage. Control embryos were treated with equivalent volume of DMSO added to the aquarium water.
Dominant negative FGFR1 construct
The extracellular and transmembrane encoding sequences of the IIIc, 3 Ig domain isoform of murine FGFR1 were amplified by RT-PCR with the 5′ primer modifying the initiation codon for translation to include an Nco1 restriction enzyme site. RNA transcribed from the clone generated the expected phenotype when injected into 1-4 cell zebrafish embryos. To generate the plasmid for electroporation, the β-gal sequences downstream of the CMV and chicken β-actin promoters in the vector, pCAβ (Fukuchi et al., 1994), were replaced with a linker containing EcoRV, EcoR1, Not1 and Cla1 restriction enzyme sites but leaving the downstream β-globin 3′ UTR intact (pCAlinker1 vector; C.Formstone and IM unpublished). The dominant negative FGFR1 sequences were directionally cloned into the EcoRV and EcoR1 sites and IRES-eGFP into the Not1 and Cla1 sites. The ability of the dominant negative construct to inhibit FGF signalling in chicken cells was demonstrated by its ability to inhibit FGF-stimulated proliferative effects on primary cultures of E9 chick embryo pectoral myoblasts and to support their differentiation and fusion in the presence of exogenous FGF (JW and IM data not shown).
Sox-10 GFP transgenic zebrafish
Generation and characterisation of the zebrafish sox10:egfp transgenics have been described elsewhere (Wada et al., 2005; Carney et al., 2006). egfp mRNA was detected by in situ hybridisation using Fast red fluorescence followed by immunofluorescent antibody detection of GFP. Imaging was performed on a Zeiss 510 confocal microscope
Acknowledgements
We would like to thank Malcolm Maden, Imelda McGonnell, Moya Smith and Hannah Thompson for helpful comments, and Jon Gilthorpe for the IRES-GFP vector. This work was supported by the Medical Research Council UK and The Wellcome Trust.
Grant Sponsors - The Medical Research Council (UK), The Wellcome Trust
References
- Abzhanov A, Tzahor E, Lassar AB, Tabin CJ. Dissimilar regulation of cell differentiation in mesencephalic (cranial) and sacral (trunk) neural crest cells in vitro. Development. 2003;130:4567–4579. doi: 10.1242/dev.00673. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M, Le Douarin NM, Teillet MA. Early- and late-migrating cranial neural crest cell populations have equivalent developmental potential in vivo. Development. 1997;124:3077–3087. doi: 10.1242/dev.124.16.3077. [DOI] [PubMed] [Google Scholar]
- Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave K-A, Birchmeier C, Wegner M. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney TJ, Dutton KA, Greenhill E, Delfino-Machin M, Dufourcq P, Blader P, Kelsh RN. A direct role for Sox10 in specification of neural crest-derived sensory neurons. Development. 2006 doi: 10.1242/dev.02668. [DOI] [PubMed] [Google Scholar]
- Chambers D, Mason I. Expression of sprouty2 during early development of the chick embryo is coincident with known sites of FGF signalling. Mech Dev. 2000;91:361–364. doi: 10.1016/s0925-4773(99)00288-9. [DOI] [PubMed] [Google Scholar]
- Chotteau-Lelievre A, Dolle P, Peronne V, Coutte L, de Launoit Y, Desbiens X. Expression patterns of the Ets transcription factors from the PEA3 group during early stages of mouse development. Mech Dev. 2001;108:191–195. doi: 10.1016/s0925-4773(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, Le Douarin NM. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development. 1993;117:409–429. doi: 10.1242/dev.117.2.409. [DOI] [PubMed] [Google Scholar]
- Coutelle O, Blagden CS, Hampson R, Halai C, Rigby PW, Hughes SM. Hedgehog signalling is required for maintenance of myf5 and myoD expression and timely terminal differentiation in zebrafish adaxial myogenesis. Dev Biol. 2001;236:136–150. doi: 10.1006/dbio.2001.0193. [DOI] [PubMed] [Google Scholar]
- Creuzet S, Schuler B, Couly G, Le Douarin NM. Reciprocal relationships between Fgf8 and neural crest cells in facial and forebrain development. Proc Natl Acad Sci U S A. 2004;101:4843–4847. doi: 10.1073/pnas.0400869101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- Crump JG, Maves L, Lawson ND, Weinstein BM, Kimmel CB. An essential role for Fgfs in endodermal pouch formation influences later craniofacial skeletal patterning. Development. 2004;131:5703–5716. doi: 10.1242/dev.01444. [DOI] [PubMed] [Google Scholar]
- D’Amico-Martel A, Noden DM. Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. Am J Anat. 1983;166:445–468. doi: 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- David NB, Saint-Etienne L, Tsang M, Schilling TF, Rosa FM. Requirement for endoderm and FGF3 in ventral head skeleton formation. Development. 2002;129:4457–4468. doi: 10.1242/dev.129.19.4457. [DOI] [PubMed] [Google Scholar]
- Donoghue PC, Sansom IJ. Origin and early evolution of vertebrate skeletonization. Microsc Res Tech. 2002;59:352–372. doi: 10.1002/jemt.10217. [DOI] [PubMed] [Google Scholar]
- Dottori M, Gross MK, Labosky P, Goulding M. The winged-helix transcription factor Foxd3 suppresses interneuron differentiation and promotes neural crest cell fate. Development. 2001;128:4127–4138. doi: 10.1242/dev.128.21.4127. [DOI] [PubMed] [Google Scholar]
- Eblaghie MC, Lunn JS, Dickinson RJ, Munsterberg AE, Sanz-Ezquerro JJ, Farrell ER, Mathers J, Keyse SM, Storey K, Tickle C. Negative feedback regulation of FGF signaling levels by Pyst1/MKP3 in chick embryos. Curr Biol. 2003;13:1009–1018. doi: 10.1016/s0960-9822(03)00381-6. [DOI] [PubMed] [Google Scholar]
- Fukuchi K, Hearn MG, Deeb SS, Smith AC, Dang N, Miyazaki J, Bothwell M, Martin GM. Activity assays of nine heterogeneous promoters in neural and other cultured cells. In Vitro Cell Dev Biol Anim. 1994;30A:300–305. doi: 10.1007/BF02631450. [DOI] [PubMed] [Google Scholar]
- Graham A. The neural crest. Curr Biol. 2003;13:R381–384. doi: 10.1016/s0960-9822(03)00315-4. [DOI] [PubMed] [Google Scholar]
- Graham A, Okabe M, Quinlan R. The role of the endoderm in the development and evolution of the pharyngeal arches. J Anat. 2005;207:479–487. doi: 10.1111/j.1469-7580.2005.00472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. 1951. [DOI] [PubMed] [Google Scholar]
- Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- Ido A, Ito K. Expression of chondrogenic potential of mouse trunk neural crest cells by FGF2 treatment. Dev Dyn. 2006;235:361–367. doi: 10.1002/dvdy.20635. [DOI] [PubMed] [Google Scholar]
- Itasaki N, Bel-Vialar S, Krumlauf R. ‘Shocking’ developments in chick embryology: electroporation and in ovo gene expression. Nat Cell Biol. 1999;1:E203–207. doi: 10.1038/70231. [DOI] [PubMed] [Google Scholar]
- Kelsh RN, Dutton K, Medlin J, Eisen JS. Expression of zebrafish fkd6 in neural crest-derived glia. Mechanisms of Development. 2000;93:161–164. doi: 10.1016/s0925-4773(00)00250-1. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development. 2004;131:4637–4650. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Kalcheim C. The Neural crest. Cambridge, U.K.: Cambridge University Press; 1999. [Google Scholar]
- Le Lievre CS, Le Douarin NM. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol. 1975;34:125–154. [PubMed] [Google Scholar]
- Lumsden A, Sprawson N, Graham A. Segmental origin and migration of neural crest cells in the hindbrain region of the chick embryo. Development. 1991;113:1281–1291. doi: 10.1242/dev.113.4.1281. [DOI] [PubMed] [Google Scholar]
- Mackenzie S, Walsh FS, Graham A. Migration of hypoglossal myoblast precursors. Dev Dyn. 1998;213:349–358. doi: 10.1002/(SICI)1097-0177(199812)213:4<349::AID-AJA1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Mahmood R, Kiefer P, Guthrie S, Dickson C, Mason I. Multiple roles for FGF-3 during cranial neural development in the chicken. Development. 1995;121:1399–1410. doi: 10.1242/dev.121.5.1399. [DOI] [PubMed] [Google Scholar]
- Maroon H, Walshe J, Mahmood R, Kiefer P, Dickson C, Mason I. Fgf3 and Fgf8 are required together for formation of the otic placode and vesicle. Development. 2002;129:2099–2108. doi: 10.1242/dev.129.9.2099. [DOI] [PubMed] [Google Scholar]
- McGonnell IM, Graham A. Trunk neural crest has skeletogenic potential. Curr Biol. 2002;12:767–771. doi: 10.1016/s0960-9822(02)00818-7. [DOI] [PubMed] [Google Scholar]
- Munchberg SR, Ober EA, Steinbeisser H. Expression of the Ets transcription factors erm and pea3 in early zebrafish development. Mech Dev. 1999;88:233–236. doi: 10.1016/s0925-4773(99)00179-3. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Ayer-le Lievre CS. Mesectodermal capabilities of the trunk neural crest of birds. J Embryol Exp Morphol. 1982;70:1–18. [PubMed] [Google Scholar]
- Noden DM. Interactions and fates of avian craniofacial mesenchyme. Development. 1988;103(Suppl):121–140. doi: 10.1242/dev.103.Supplement.121. [DOI] [PubMed] [Google Scholar]
- Paratore C, Goerich DE, Suter U, Wegner M, Sommer L. Survival and glial fate acquisition of neural crest cells are regulated by an interplay between the transcription factor Sox10 and extrinsic combinatorial signaling. Development. 2001;128:3949–3961. doi: 10.1242/dev.128.20.3949. [DOI] [PubMed] [Google Scholar]
- Piotrowski T, Nusslein-Volhard C. The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (Danio rerio) Dev Biol. 2000;225:339–356. doi: 10.1006/dbio.2000.9842. [DOI] [PubMed] [Google Scholar]
- Quinlan R, Martin P, Graham A. The role of actin cables in directing the morphogenesis of the pharyngeal pouches. Development. 2004;131:593–599. doi: 10.1242/dev.00950. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Petiot A, Copp A, Ferretti P, Thorogood P. FGF2 promotes skeletogenic differentiation of cranial neural crest cells. Development. 2001;128:2143–2152. doi: 10.1242/dev.128.11.2143. [DOI] [PubMed] [Google Scholar]
- Schilling TF, Kimmel CB. Segment and cell type lineage restrictions during pharyngeal arch development in the zebrafish embryo. Development. 1994;120:483–494. doi: 10.1242/dev.120.3.483. [DOI] [PubMed] [Google Scholar]
- Trumpp A, Depew MJ, Rubenstein JL, Bishop JM, Martin GR. Cre-mediated gene inactivation demonstrates that FGF8 is required for cell survival and patterning of the first branchial arch. Genes Dev. 1999;13:3136–3148. doi: 10.1101/gad.13.23.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veitch E, Begbie J, Schilling TF, Smith MM, Graham A. Pharyngeal arch patterning in the absence of neural crest. Curr Biol. 1999;9:1481–1484. doi: 10.1016/s0960-9822(00)80118-9. [DOI] [PubMed] [Google Scholar]
- Wada N, Javidan Y, Nelson S, Carney TJ, Kelsh RN, Schilling TF. Hedgehog signaling is required for cranial neural crest morphogenesis and chondrogenesis at the midline in the zebrafish skull. Development. 2005;132:3977–3988. doi: 10.1242/dev.01943. [DOI] [PubMed] [Google Scholar]
- Walshe J, Mason I. Expression of FGFR1, FGFR2 and FGFR3 during early neural development in the chick embryo. Mech Dev. 2000;90:103–110. doi: 10.1016/s0925-4773(99)00225-7. [DOI] [PubMed] [Google Scholar]
- Walshe J, Mason I. Fgf signalling is required for formation of cartilage in the head. Dev Biol. 2003;264:522–536. doi: 10.1016/j.ydbio.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book - a guide for the laboratory use of zebrafish (Danio rerio) Oregon: University of Oregon Press; 1995. [Google Scholar]
- Weston JA, Yoshida H, Robinson V, Nishikawa S, Fraser ST, Nishikawa S. Neural crest and the origin of ectomesenchyme: neural fold heterogeneity suggests an alternative hypothesis. Dev Dyn. 2004;229:118–130. doi: 10.1002/dvdy.10478. [DOI] [PubMed] [Google Scholar]
- Wilke TA, Gubbels S, Schwartz J, Richman JM. Expression of fibroblast growth factor receptors (FGFR1, FGFR2, FGFR3) in the developing head and face. Dev Dyn. 1997;210:41–52. doi: 10.1002/(SICI)1097-0177(199709)210:1<41::AID-AJA5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]