Abstract
Calcitonin gene-related peptide (CGRP) is located with substance P in nerve varicosities in close apposition to principal neurons in airway parasympathetic ganglia. Substance P has multiple effects on airway parasympathetic neurons but the role of CGRP is unknown. Using intracellular current clamp recording of ganglionic neurons, stimulation of vagal afferent nerves in the presence of neurokinin receptor antagonists evoked hyperpolarization of the membrane potential which was blocked by the CGRP-1 receptor antagonist, CGRP8-37. Exogenous application of α-CGRP (0.001–0.1μM) hyperpolarized the membrane potential, which was either blocked or reversed to depolarization in the presence of CGRP8-37, whereas higher concentrations of α-CGRP (1.0–10.0μM) caused depolarization. Action potential accommodation in phasic-type neurons decreased in the presence of α-CGRP (0.1–10μm). The co-localization of substance P- and CGRP-immunoreactivity was observed in nerve varicosities within ganglia; prolonged exposure to capsaicin in vitro depleted substance P and CGRP immunostaining in nerve varicosities. These results demonstrate that CGRP has multiple effects on the excitability of airway parasympathetic neurons and may alter their activity, ultimately affecting parasympathetic tone in the lower airways.
1. INTRODUCTION
Calcitonin gene-related peptide (CGRP) belongs to a family of neuropeptides that includes adrenomedullin, amylin, and calcitonin. CGRP is a 37 amino acid peptide produced by alternative processing of the mRNA transcript encoded by the calcitonin-CGRP gene (reviewed in Wimalawansa, 1997). There are two known isoforms of CGRP, α-CGRP and β-CGRP, which differ by one amino acid in rats, and three in mouse and humans; based on mRNA expression levels, α-CGRP is the most abundant form in the nervous system (Morara et al., 1995). Two CGRP receptors have been pharmacologically identified based on their relative affinities for the peptide antagonist, CGRP8-37, which is selective for CGRP-1 receptors (Poyner et al., 2002). CGRP-2 receptors are activated by the α-CGRP analogs [Cys (ACM) 2,7]-CGRP and [Cys (Et) 2,7]-CGRP, but not in all species (Poyner et al., 2002).
CGRP is expressed in nerve fibers located in many visceral organs where, in most species, it is co-localized in sensory nerves with the neurokinin, substance P (Martling et al, 1988; van Rossum et al., 1997). Such neuropeptides associated with sensory nerve fibers are widely distributed in the airway mucosa, near the airway smooth muscle and around vasculature in most species. In addition to these areas, CGRP is also located in nerve fiber varicosities in close apposition to principal neurons in lower airway parasympathetic ganglia (Kummer, 1992). As CGRP is co-localized with substance P, CGRP may regulate substance P release or activity in the lower airways (Martling et al., 1988). Although it is known that substance P released from capsaicin-sensitive nerve terminals depolarizes airway parasympathetic ganglionic neurons (Myers and Undem, 1993) and enhances synaptic transmission in bronchial parasympathetic ganglia (Canning et al., 2002), the effect of co-released CGRP on these neurons is not known. In the present study, in vitro techniques were used to address the hypothesis that CGRP receptor activation alters the excitability of cholinergic neurons in airway parasympathetic ganglia. We also determined whether substance P and CGRP are contained within the same capsaicin-sensitive nerve terminals in bronchial ganglia.
2. METHODS
The methods for animal euthanasia and tissue collection were approved by the Johns Hopkins Animal Care and Use Committee, The Johns Hopkins University, Baltimore, Maryland, USA.
2.1 Tissue preparation for neuronal cell recordings
Male albino guinea pigs (Dunkin-Hartley) weighing 200–300g were killed by pentobarbital overdose (150mg/kg, i.p.) and exsanguinated. The thorax was opened, and the lungs, bronchi, and trachea were removed and placed in room temperature (20–21°C) Krebs buffer (composition in mM: NaCl, 118; KCl, 5.4; MgSO4, 1.0; CaCl2, 1.9; NaH2PO4, 1.0; NaHCO3, 25; dextrose, 11.1), saturated with 95% O2/5% CO2, pH 7.4.
The methods for tissue preparation and ganglia location have been described previously (Myers, 2000). Briefly, the left or right bronchus with attached vagus nerve was isolated from the trachea and lung parenchyma; the bronchus was cut longitudinally along the ventral surface and opened as a sheet. Using transmitted light, ganglia were located without the aid of staining on the serosal surface of the primary bronchus along peribronchial nerves (Myers, 2000). The bronchus was transferred and pinned, serosal side up, to the Sylgard-coated floor of a recording chamber (0.2 ml volume). The vagus nerve was gently drawn into a suction electrode for nerve stimulation. Once in the recording chamber, the tissue was continuously superfused with Krebs buffer (36–37°C, 5–8 ml/min) and equilibrated for at least 30 min prior to further experimental manipulation.
2.2 Membrane Properties of Ganglionic Neurons
Intracellular microelectrodes were fabricated from thick-walled capillary stock, filled with 3M KCl (pH 7.4), and connected by a Ag-AgCl wire in an electrode holder to an electrometer (Axoclamp 2A, Axon Instruments, Union City, CA). Once a neuron was impaled with the microelectrode, baseline control membrane properties were noted after the establishment of a stable recording of passive membrane properties, i.e. <1 mV change in resting potential, no change in input resistance (Ri) over time (5 min). The Ri of the neuron was calculated from the steady-state amplitude of the voltage transient produced by a hyperpolarizing constant-current step (−100 pA; 1–5 s duration). Changes in Ri were also monitored continuously by noting changes in the amplitude of the voltage transients produced by hyperpolarizing current steps (100 pA, 100 ms, 1 Hz). All cells were current clamped to −50±1mV, their average control resting potential (Myers et al., 1990) prior to measurement of control membrane properties. The effect of α-CGRP application (1–2min) on action potential properties included accommodation and the duration and amplitude of the cumulative after-hyperpolarization (AHP) duration that followed four consecutive action potentials (elicited by 3.0 nA, 20–33 Hz, 2 ms steps; see Myers, 1998). The accommodation characteristics of all neurons were analyzed by noting the pattern of action potentials elicited during a series of incrementing depolarizing steps (500 ms, 0.5–2.0 nA). Using this procedure, neurons exhibit either continuous repetitive action potential discharge (‘tonic’ neurons) or an initial burst of action potentials that terminate within 100 ms of the onset of the depolarizing step (‘phasic’ neurons; Myers, 1998). Antidromic stimulation of vagal afferents (1.2 ms pulses, 40 Hz for 5 sec) via the suction electrode caused capsaicin-sensitive slow potential(s) as reported previously (Myers et al., 1996, Canning et al., 2002).
2.3 Pharmacological responses
The effect of bath applied α-CGRP on active, passive, and synaptic membrane properties of neurons were studied. α-CGRP, at concentrations (1–100 nM) previously shown to cause stimulation of tracheal and bronchial afferent nerve terminals (Gamse and Saria, 1985) and membrane depolarization of enteric ganglionic neurons (Johnson and Bornstein, 2004), were perfused directly over the parasympathetic ganglion preparation for 1–3 min, a period of time that allowed for a peak response to drug application on the resting potential to be observed. Once the resting potential returned to baseline (or current clamped to −50mV), the effect of α-CRGP on action potential or synaptic properties was determined. No more than two increasing concentrations of α-CGRP were tested on a neuron and a washout period of at least 10 min was used between successive α-CGRP applications. Once synaptic transmission was verified (presence of fast potentials) and prior to evoking slow potentials, atropine (0.1μM) and hexamethonium (100μM) were added to all Krebs buffer to inhibit muscarinic (Myers and Undem, 1996) and nicotinic (Myers et al., 1990) receptors associated with these neurons. For slow potentials, a control response was compared to a second response in the same cell to the same stimulus (10–30 minute interval) in the presence of α-CGRP. Slow potentials and the effects of α-CGRP were also studied in the presence of the CGRP receptor antagonist, CGRP8-37 (1.0μM), and/or neurokinin-1 and -3 receptor antagonists, SR140333 (0.1μM) and SR142801 (0.1μM), respectively (Canning et al., 2002). For antagonist studies, the control α-CGRP response was determined and the tissue was then exposed to antagonist for at least 10 min prior to the addition of α-CGRP in the presence of antagonist.
2.4 Immunofluorescent staining for CGRP and substance P in airway ganglia
CGRP release from nerve terminals in the airway ganglia was determined using the C-fiber activator, capsaicin. Ganglia were incubated (37°C) for 60min in oxygenated Krebs buffer with vehicle (control) or with capsaicin (30 μM) in an attempt to acutely deplete capsaicin-sensitive nerve endings of neuropeptides (Myers et al., 1996). The tissue was then fixed with 4% formaldehyde in phosphate buffered saline (PBS, 2h, 4°C), rinsed with PBS, cryoprotected overnight in PBS containing 18% sucrose, frozen and sectioned (12μm). The sections were blocked with donkey serum (10% in PBS with 1% Tween 20, 2 h), incubated overnight (4°C) in a mixture of goat polyclonal antiserum to choline acetyltransferase (ChAT, Chemicon, Temecula, CA; diluted 1:50) to facilitate identification of cholinergic bronchial ganglionic neurons, rabbit polyclonal antibody to CGRP (Peninsula Laboratories, Belmont, CA, diluted 1:200), and rat monoclonal to substance P (Chemicon; diluted 1:200). Sections were rinsed with PBS and incubated with a mixture of UV fluorochrome-labeled anti-goat antibody raised in donkey (Alexa 350, Molecular Probes, Inc., Eugene, OR, USA), rhodamine-labeled anti-rat antibody raised in donkey (Alexa 594, Molecular Probes, Inc.) and a fluorescein-labeled anti-rabbit antibody raised in donkey (Alexa 488, Molecular Probes, Inc) for 2 hours at room temperature. Separate sections were processed similarly but the primary antibody was excluded to evaluate non-specific staining. Washed slides were coverslipped with glycerol (pH 8.6). The tissues were photographed (Q-Imaging Retiga EXi camera, BioVision, Exton, PA), with an epifluorescence microscope (Olympus BX60, Olympus America, Inc., Melville, NY, USA) equipped with appropriate filter sets to allow separate visualization and overlay of Alexa 350, 488, and 594, using IPLab software (BioVision). The number of substance P- and/or CGRP-containing nerve profiles were counted in each micrograph as were the number of nucleated ChAT-positive neuronal profiles (Myers et al., 1996).
2.5 Materials
Reagents used to prepare the Krebs solution were purchased from J.T. Baker Chem. Co. (Phillipsburg, NJ, USA). SR142801 and SR142806 were gifts from GlaxoSmithkline (King of Prussia, PA). All remaining reagents, including α-CGRP (rat, synthetic), were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Stock solutions of atropine (10 mM) and hexamethonium (1 M), were dissolved in distilled water. SR142801 and SR142806 (1 mM each) were dissolved in dimethyl sulfoxide (DMSO), and indomethacin (0.3 M) was dissolved in ethanol. At the dilutions used in these studies (≥1:10,000), ethanol, DMSO, or distilled water had no effect on the active or passive properties of parasympathetic ganglionic neurons. Final dilutions of all drugs were made in Krebs buffer solution.
2.6 Data Analysis
All data are summarized as the mean ± the standard error of the mean of n experiments, where n is the number of responses with or without the specific concentration of CGRP (or CGRP with antagonist). Control values for resting membrane potential, membrane Ri, cumulative AHP duration, and amplitude were noted prior to α-CGRP application. These values were compared with peak changes evoked by α-CGRP in the same cell using paired Student’s t-test; similarly, peak changes caused by α-CGRP were compared in a paired fashion with α-CGRP in the presence of CGRP8-37. Statistical tests were performed using Statview statistics program (Abacus Concepts, Lafayette, CA). Statistical significance was accepted at the 0.05 level of probability (P).
3. RESULTS
3.1 Vagus nerve stimulation
Using intracellular current clamp recordings of bronchial ganglionic neurons on the guinea pig bronchus, antidromic stimulation of afferent (sensory) nerves evoked a depolarization (Fig. 1A) that can be blocked or reversed by tachykinin neurokinin-1 and –3 receptor (NK1-R, NK3-R) antagonists (Myers et al., 1996; Canning et al., 2002). In order to demonstrate that CGRP is released by antidromic stimulation of afferent nerves and has an effect on postganglionic neurons, the vagus nerve was stimulated in a similar manner in the presence of NK1-R, NK3-R antagonists, SR 142801 (1.0μM) and SR 142806 (0.1μM) to block depolarization associated with activation of these receptors. This evoked a hyperpolarization (−2±0.5mV) in 6 neurons (Fig. 1B). The hyperpolarization was blocked by the CGRP-1 receptor (CGRP-1R) (1.0 μM; n=6; Fig, 1C). Capsaicin (30μM, 30 min) pretreatment antagonist, CGRP8-37 abolished all vagus nerve stimulated potentials (n=4; Fig. 1D). These data are summarized in Fig. 1E.
Figure 1.
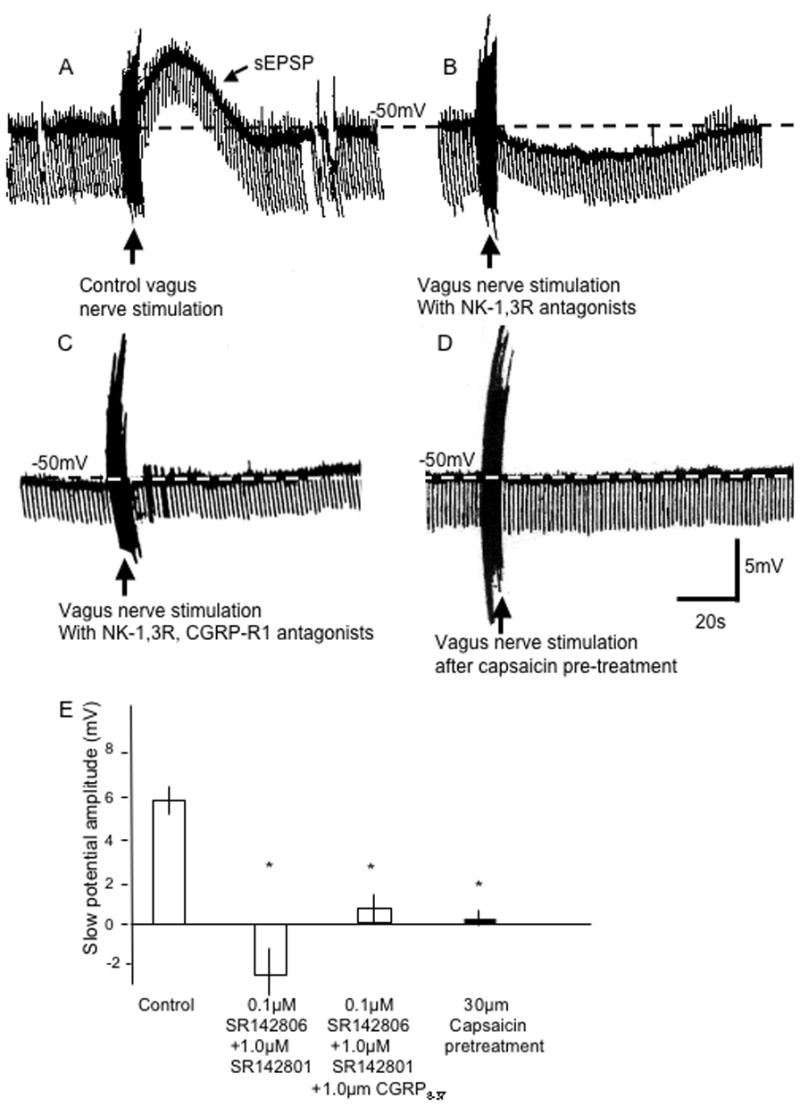
Effect of α-CGRP on sensory nerve-evoked slow excitatory post-synaptic potential (sEPSP). In A, vagus nerve stimulation (40V, 1.2ms, 40Hz, 5sec, at vertical arrow) elicits a control prolonged depolarization (sEPSP) that, in B, is changed to hyperpolarization in the presence of tachykinin neurokinin (NK)1- and 3-receptor antagonists, SR 142801 (1.0μM) and SR 142806 (0.1μM), respectively. In C, the vagus nerve stimulated hyperpolarization is no longer evident when the CGRP-R1 antagonist, CGRP8-37 (1μM), is added to the NK receptor antagonists. In D, 30μm capsaicin pretreatment (see Methods) blocks all potentials in another control neuron. Calibration bar in D is for all traces. E, summary of the effects of neurokinin receptor antagonists with and without CGRP8-37, and 30μM capsaicin pretreatment on the sEPSP. *indicates P<0.05 compared to control response.
3.2 Effects of bath-applied α-CGRP on the resting potential
We next attempted to mimic the effect of sensory nerve stimulation with bath application of α-CGRP. The lowest concentrations of α-CGRP (0.001, 0.01 μM; n = 8) caused a hyperpolarization of the resting membrane potential (Fig. 2A), which was associated with a slight increase in Ri (0.01μM α-CGRP, Fig. 2A; Table 1). Higher concentrations of α-CGRP (1.0, 10 μM; n = 6) consistently depolarized the resting membrane potential (Fig. 2B; Table 1) of bronchial neurons and had no effect on Ri (Fig. 2B; Table 1). The response to 0.1 μM α-CGRP was inconsistent with a depolarization of the membrane potential observed in four neurons, no change in membrane potential in three other neurons, and a hyperpolarization in one cell. The CGRP-1 receptor antagonist, CGRP8-37 (1.0 μM) inhibited the hyperpolarizing response to α-CGRP (0.001, 0.01 μM; n=4). Interestingly, 0.01 μM α-CGRP, a concentration that consistently hyperpolarized neurons (Fig. 2A) resulted in a depolarization of the resting membrane potential in 5 of 6 neurons when studied in the presence of CGRP8-37 (1.0 μM; Fig. 2C). α-CGRP8-37 had no effect on the depolarization response evoked by higher concentrations of α-CGRP (1.0, 10μM n=5); these data are summarized in Fig. 2D. In order to determine if depolarizations by higher concentrations of α-CGRP (1.0 and 10.0 μM) were causing release of substance P, we used NK-1 and NK-3 neurokinin receptor antagonists, SR 142801 (1.0 μM) and SR 142806 (0.1 μM), to try to block the depolarizing response; the depolarization was not significantly changed (n=4; Fig. 2D; P>0.05). Furthermore, because α-CGRP may cause prostaglandin D2 release, which was previously reported to cause hyperpolarization (Kajekar et al., 2003), we used the non-specific cyclooxygenase inhibitor, indomethacin (3.0 μM) which did not block the effects α-CGRP (0.001 μM, 1.0 μM) on the resting membrane potential (n=6 for each concentration; data not shown).
Figure 2.
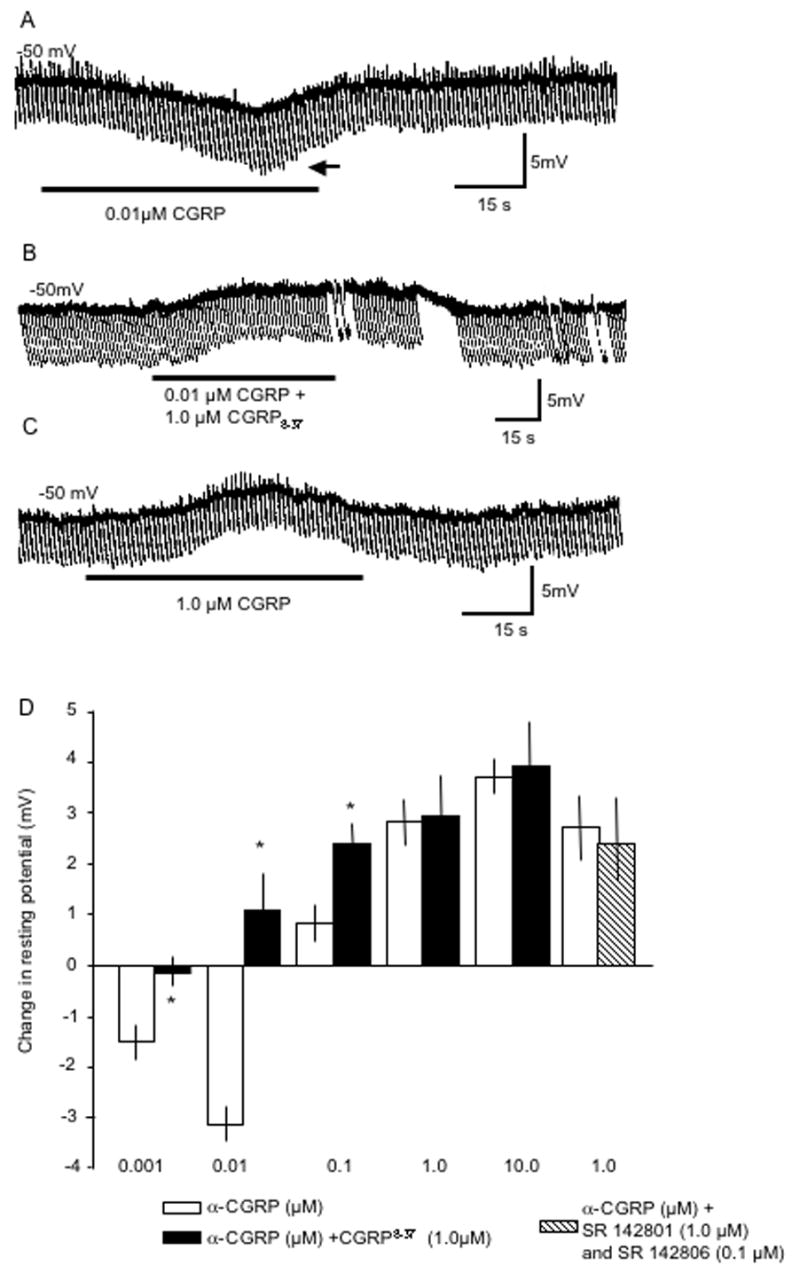
Bath-applied α-CGRP has multiple effects on the resting membrane potential and input resistance. In A, α-CGRP (0.01 μM, 1 min, at bar) hyperpolarized the resting membrane potential with an increase in membrane resistance (indicated at horizontal arrow). In B, pretreatment with the CGRP-1 receptor antagonist, CGRP8-37 (1.0 μM), reversed the hyperpolarization response induced by 0.01 μM α-CGRP. In C, in a different neuron than A and B, a higher concentration of α-CGRP (1.0 μM, 1 min, at bar) depolarized the resting membrane potential. In D, a summary of the concentration-dependent responses to α-CGRP on the resting potential in the absence (open bars) and presence of CGRP8-37 (1.0 μM, closed bars) or neurokinin receptor antagonists (hatched bar, see Results); n = 4–6 for each. *indicates P<0.05 when control α-CGRP responses were compared to the response in the presence of CGRP8-37.
Table 1.
Effect of α-CGRP on Neuronal Membrane Properties
| α-CGRP concentration (μM)
|
||||||
|---|---|---|---|---|---|---|
| Control | 0.001μM | 0.01 μM | 0.1 μM | 1.0 μM | 10.0 μM | |
| Resting Potential (mV) | −50±0.4 | −51.6±0.5* | −53.5±0.6* | −48.8±0.5 | −47.3±0.5* | −46.3±0.4* |
| Input Resistance (MΩ) | 36±5 | 41±7 | 48±9* | 34±7 | 27±13 | 29±12 |
| Single action potential | ||||||
| Duration (msec) | 3.9±0.7 | 4.1 0.5 | 4.0±0.5 | 3.9±0.5 | 3.9±0.5 | 3.8±0.5 |
| Amplitude (mV) | 56±11 | 56±12 | 54±6 | 50±9 | 50±11 | 48±9 |
| AHP amplitude (mV) | 18±5 | 19±6 | 18±4 | 17±7 | 17±6 | 15±3 |
| AHP duration (msec) | 58±11 | 62±19 | 56±5 | 53±7 | 54±11 | 51±9 |
| Four action potentials | ||||||
| AHP duration (msec) | 167±29 | 179±31 | 169±11 | 159±18 | 157±11 | 154±19 |
| Accommodation (Action potentials/500msec) | ||||||
| Phasic neurons | 3.8±1.1 | 6±3 | 10±3* | 18±5* | 19±3* | 18±3* |
| Tonic neurons | 19±2 | 18±5 | 18±4 | 19±5 | 19±2 | 21±3 |
Values are means ± SEM of 6–8 neurons for control or each concentration (in μM) of α-CGRP; see Methods for determination of membrane properties. Accommodation (number of action potentials per stimulus) determination was made with a 1.0nA stimulus. α-CGRP had no effect on single action potential properties, cumulative AHP amplitude or duration following four action potentials, or accommodation by tonic neurons.
indicates difference (P<0.05) from control values.
3.3 Effect of bath-applied α-CGRP on action potentials
The action potential characteristics of neurons were analyzed in the presence and absence of α-CGRP. Using a series of incrementing depolarizing steps (500 ms, 0.5–2.0 nA) neurons exhibited either continuous repetitive action potential discharge (‘tonic’ neurons) or an initial burst of action potentials that terminated (accommodated) within 100 ms of the onset of the depolarizing step (‘phasic’ neurons; Myers, 1998). α-CGRP decreased the action potential accommodation in phasic-type neurons: In the absence of α-CGRP, a 1.0 nA depolarizing stimulus elicited 3.8 ± 1.1 action potentials in phasic neurons before accommodating (n = 8; Fig. 3A). In the presence of α-CGRP (1.0 μM), six neurons responded by evoking 18±5 action potentials (P < 0.05; Fig. 3B). Threshold for this shift in accommodation was 0.01 μM (n = 4) with no further dose-related effects observed at concentrations greater than 1.0 μM (Fig 3C). CGRP8-37 (1.0 μM) did not block the effect on the decrease in accommodation evoked by α-CGRP (0.01–1.0 μM; n=4 for each concentration). Indomethacin (3.0 μM) did not block the effects of α-CGRP (1.0 μM) on the action potential accommodation (n=6). α-CGRP (0.01–10.0 μM) had no effect on accommodation in tonic neurons, single action potential properties, or on cumulative (four consecutive) action potential AHP durations (Table 1).
Figure 3.
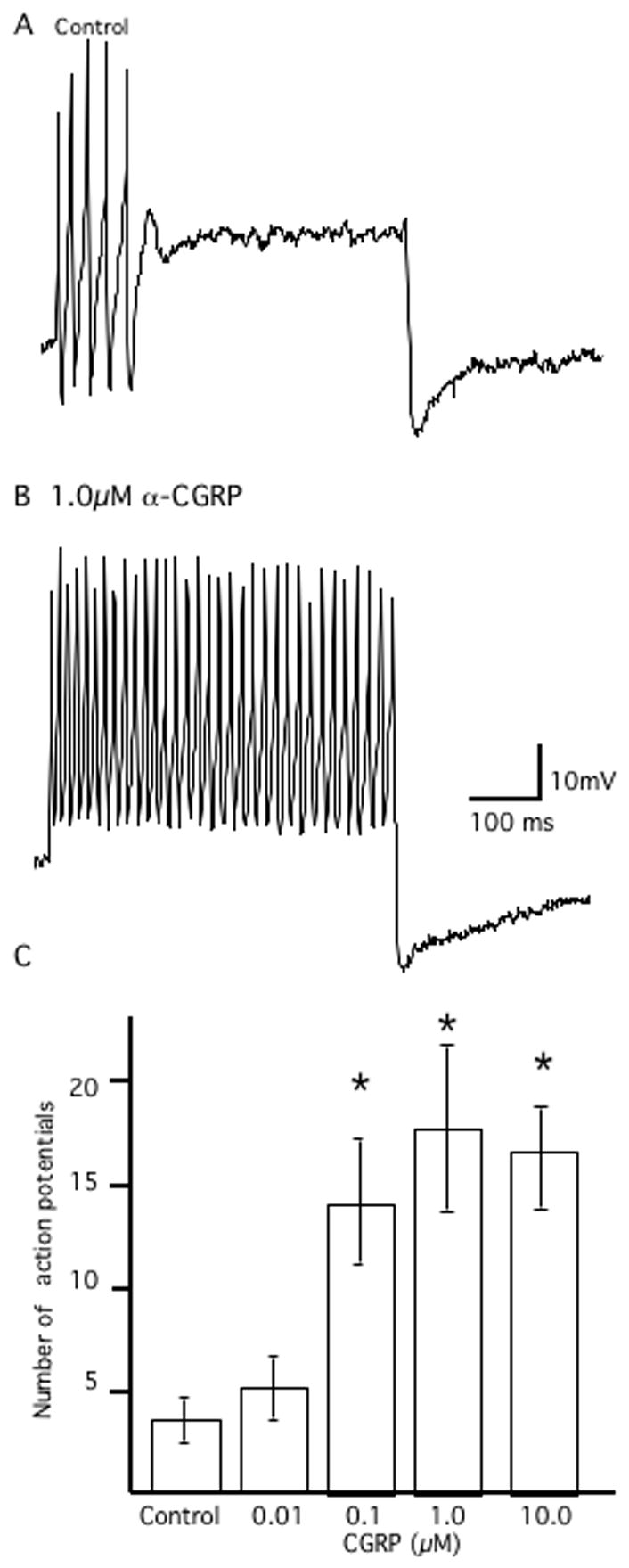
Effect of α-CGRP on action potential accommodation. In A, representative trace illustrating a control phasic neuron. A depolarizing step (500 ms, 1.0 nA) results in an initial burst of action potentials followed by accommodation (no action potentials) for the remainder of the stimulus. In B, in the presence of α-CGRP (0.1μM), the same neuron as A generates repetitive action potentials throughout the stimulus. Calibration bar in B is for both traces. C, summary of the effects of α-CGRP (0.01–10.0 μM) on the number of action potentials evoked during a sustained depolarizing stimulus (1 nA, 500msec). *indicates P<0.05 when control responses were compared to the response in the presence of α-CGRP.
3.4 Effect of capsaicin on CGRP in nerve terminals
In order to determine if CGRP and substance P are located in capsaicin-sensitive afferent nerves within intrinsic airway ganglia, we used immunofluorescent staining for these peptides with and without capsaicin pre-treatment. Bronchial ganglia contained ChAT-immunoreactive cell bodies and preganglionic nerve terminals (Fig. 4A) both of which did not have substance P- (Fig. 4B, arrow) nor CGRP-immunoreactivity (Fig. 4C, arrow). In vehicle control-treated tissue, substance P- (Fig. 4B) and CGRP-immunoreactivity (Fig. 4C) were co-localized in bead-like varicosities in bronchial ganglia. Substance P- and CGRP- immunoreactivity was co-localized in most varicosities in 11 control ganglia from four bronchi from four animals. When the tissue was exposed in vitro to 30μM capsaicin for 60 minutes, ChAT immunostaining was unaffected (Fig. 4D), and the number of substance P- (Fig. 4E) and CGRP-immunoreactive varicosities (Fig. 4F) was absent (Fig. 4E, F) or greatly reduced in 10 ganglia from four animals.
Figure 4.
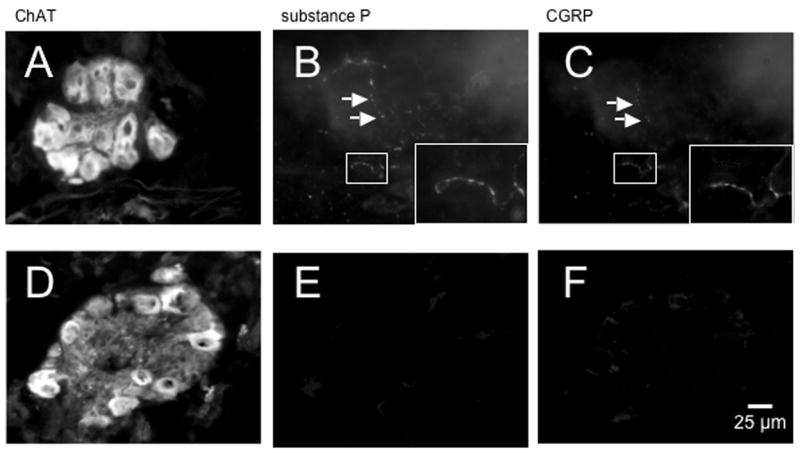
CGRP and substance P are co-localized in capsaicin-sensitive nerve terminals within bronchial parasympathetic ganglia. In A, immunofluorescent staining of a control ganglion for choline acetyltransferase (ChAT), showing neuronal cell bodies and pre-ganglionic nerve terminals. In B, the same ganglion as A stained for substance P and, in C, for CRGP (insets in lower right corner of B and C show small rectangle in images at higher magnification); substance P- and CGRP-immunoreactivity was co-localized around neurons in bead-like varicosities (rectangles, arrows in B and C). In D, ChAT-positive neurons were located in tissue pretreated in vitro with 30 μM capsaicin and, in E, the number of substance P and, in F, CGRP immunoreactive varicosities in the ganglion were absent. Similar results were seen in 11 control ganglia from four animals and 10 treated ganglia from four animals (see Results). Scale bar in F is for all micrographs.
4. DISCUSSION
Our results demonstrate that CGRP alters the electrophysiological membrane properties of airway parasympathetic ganglionic neurons and, possibly, their integrative function (reviewed by Myers, 2001). In the airway, CGRP is co-localized with substance P in nerve terminals near smooth muscle, glands, vasculature, mucosa (Martling et al., 1988), and, as demonstrated here, within intrinsic parasympathetic ganglia. The number and size of CGRP- (Kummer et al., 1992) and substance P-containing (Myers et al., 1996) varicosities in the ganglia are consistent with previous studies. Also, similar to previous studies (Kummer et al., 1992; Myers et al., 1996), the perikarya of intrinsic bronchial ganglionic neurons in guinea pigs do not contain substance P or CGRP, providing evidence for an extrinsic origin of the neuropeptide-containing nerves, unlike the intrinsic origin of substance P in the ferret tracheal plexus (Dey et al., 1991, 1996). Peripheral activation of afferent nerve endings within ganglia, either by antidromic electrical stimulation or capsaicin administration, induces depolarization of guinea pig (Myers et al., 1996; Myers and Undem, 1993) and human (Myers et al., 2005) airway cholinergic ganglionic neurons mediated predominantly by activation of NK3 receptors (Canning et al, 2002; Myers et al., 2005). The results from the present study demonstrate that CGRP is also released and also affects the function of parasympathetic neurons.
Antidromic stimulation of vagal sensory nerve fibers evokes a capsaicin-sensitive depolarization (Myers et al. 1996) that is mediated by activation of neurokinin receptors on parasympathetic ganglionic neurons (Canning et al., 2002). This response may be indicative of local reflex regulation of parasympathetic nerve activity (Mitchell et al., 1987). Although prejunctional (Belvisi et al., 1994; Murphy et al., 1998, Watson et al., 1993) and presynaptic (Canning et al., 2002) effects of neurokinin receptor activation on airway cholinergic nerves have been reported, those studies did not address the concomitant release of CGRP and its potential effect on parasympathetic ganglionic neurons. In this, and in our previous study (Myers et al., 1996), we observed vagus nerve-stimulated hyperpolarization in the presence of tachykinin neurokinin receptor antagonists possibly indicative of low concentrations of CGRP release that cause hyperpolarization. In the present study, it was expected that, if the hyperpolarization was due to CGRP release, the purported CGRP-1R antagonist, CGRP8-37, may inhibit this response. The inhibitory effect of CGRP8-37 provided evidence that the vagus nerve-stimulated hyperpolarization may be due to CGRP release and CGRP receptor activation.
We also tested whether exogenously applied α-CGRP could mimic the effect of nerve stimulation. α-CGRP had a biphasic, concentration-dependent effect on the resting membrane potential. Lower concentrations caused hyperpolarization of the resting membrane potential, mimicking nerve-stimulated hyperpolarizations, and, at higher concentrations, caused depolarization. Possible evidence for the presence of the CGRP-1R came from the ability of the CGRP-1R antagonist, CGRP8-37, to inhibit CGRP-induced hyperpolarization. Activation of the CGRP-1R has been reported to be associated with increased levels of cyclic adenosine monophosphate (Aiyar et al., 1999), and with the release of prostaglandins (Kress et al., 1999) and nitric oxide (Kubota et al., 1985; Wahl et al., 1994). We previously reported that prostaglandin D2 causes hyperpolarization of bronchial ganglionic neurons (Myers et al., 1991; Kajekar et al., 2003); however, the mechanism for the hyperpolarization response in the present study is not secondary to cyclooxygenase activation and consequent prostanoid synthesis (Springer et al., 2004), as indomethacin pretreatment failed to block the effects of α-CGRP on the resting membrane potential. Nitric oxide-releasing compounds have no effect on the resting membrane potential (unpublished observation). The mechanism for the depolarization evoked by higher concentrations of CGRP is more difficult to determine as there is not a selective and specific CGRP-2R receptor antagonist for this species (Hay et al., 2005) which may mediate this effect. However, we did determine that CGRP is not causing depolarization by evoking neurokinin release as the neurokinin receptor antagonists had not effect on this response.
CGRP also affected action potential properties of bronchial ganglionic neurons by decreasing accommodation in phasic neurons. It was previously reported that accommodation in guinea pig bronchial phasic neurons could be reduced either by activation of the potassium current, that has characteristics similar to A-current, or by inhibiting calcium-activated potassium current(s) (Myers, 1998; Kajekar et al., 2003). That α-CGRP had no effect on the AHP duration (an indicator of calcium-activated potassium current) following single or repetitive action potentials (Table 1) indicates that inhibition of calcium-activated potassium currents is not associated with the decrease in accommodation. This is also unlike our previously reported effects of prostaglandin D2 which decreased the AHP duration and decrease accommodation (Kajekar et al., 2003). The mechanism for the effect of CGRP on accommodation is not due to prostaglandin release (Springer et al., 2004), as indomethacin pretreatment failed to block this effect of α-CGRP. The effect of α-CGRP on action potential accommodation is unlikely related to voltage-dependent effects of α-CGRP as all tests on accommodation were performed after the cell returned to the resting potential and/or with the cell current clamped at −50mV. Based on our previous study (Myers, 1998), it is possible that the effect of α-CGRP on accommodation involves reduction of the A-current, which has no effect on single or multiple action potential characteristics, but decreases accommodation. That low concentrations of α-CGRP did not decrease accommodation and that the putative CGRP-1R antagonist, CGRP8-37, did not inhibit the effect of α-CGRP on accommodation may indicate that CGRP-1R are not involved in this response. The decrease in accommodation induced by CGRP would likely lead to a decrease in the filtering capacity of the ganglia (Myers et al., 1991; Kajekar et al., 2003) and, consequently, an overall increase in parasympathetic activity at effector tissue in the airway, such as smooth muscle (Myers and Undem, 1991) and glands.
In the airway, CGRP can mediate multiple effects, some of which have potential implications in airway homeostasis and disease (Dakhama et al., 2004). The immunohistochemical and electrophysiological data presented in this study provide evidence that CGRP is also located in capsaicin-sensitive nerve terminals within cholinergic ganglia and affects the electrophysiological properties of these neurons. CGRP in the lower airway of guinea pigs is synthesized in the cell bodies of small and large diameter vagal sensory neurons, predominantly in the superior vagal (jugular) sensory nerve ganglia (Hunter and Undem, 1999). Our results provide additional evidence that sensory nerves located in lower airway parasympathetic ganglia function to regulate the excitability of the principal neurons within the ganglia. As the levels of substance P (Fischer et al., 1996) and CGRP (Myers et al., 2002; Kwong et al., 2001) are upregulated in airway sensory nerves by airway inflammation, the role of CGRP in the regulation of reflex responses may be enhanced in diseased or inflamed airway and, thus, possibly represents a novel therapeutic target (Brain and Cox, 2006).
Acknowledgments
The authors thank H.K. Rohde for expert technical assistance. This work was supported by a NIH-funded grant from the National Heart Lung and Blood Institutes (ACM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiyar N, Disa J, Stadel JM, Lysko PG. Calcitonin gene-related peptide receptor independently stimulates 3′,5′-cyclic adenosine monophosphate and Ca2+ signaling pathways. Mol Cell Biochem. 1999;197:179–185. doi: 10.1023/a:1006962221332. [DOI] [PubMed] [Google Scholar]
- Belvisi MG, Patacchini R, Barnes PJ, Maggi CA. Facilitatory effects of selective agonists for tachykinin receptors on cholinergic neurotransmission: Evidence for species differences. Br J Pharmacol. 1994;111:103–110. doi: 10.1111/j.1476-5381.1994.tb14030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain SD, Cox HM. Neuropeptides and their receptors: innovative science providing novel therapeutic targets. Br J Pharmacol. 2006;147(S1):S202–11. doi: 10.1038/sj.bjp.0706461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Reynolds SM, Anukwu LU, Kajekar R, Myers AC. Endogenous neurokinins facilitate synaptic transmission in guinea pig airway parasympathetic ganglia. Am J Physiol Regul Integr Comp Physiol. 2002;283(2):R320–330. doi: 10.1152/ajpregu.00001.2002. [DOI] [PubMed] [Google Scholar]
- Dakhama A, Larsen GL, Gelfand EW. Calcitonin gene-related peptide: role in airway homeostasis. Curr Opin Pharmacol. 2004;4:215–220. doi: 10.1016/j.coph.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Dey RD, Altemus JB, Michalkiewicz M. Distribution of vasoactive intestinal peptide- and substance P-containing nerves originating from neurons of airway ganglia in cat bronchi. J Comp Neurol. 1991;304:330–340. doi: 10.1002/cne.903040213. [DOI] [PubMed] [Google Scholar]
- Dey RD, Altemus JB, Rodd A, Mayer B, Said SI, Coburn RF. Neurochemical characterization of intrinsic neurons in ferret tracheal plexus. Am J Respir Cell Mol Biol. 1996;14:207–216. doi: 10.1165/ajrcmb.14.3.8845170. [DOI] [PubMed] [Google Scholar]
- Fischer A, McGregor GP, Saria A, Philippin B, Kummer W. Induction of tachykinin gene and peptide expression in guinea pig nodose primary afferent neurons by allergic airway inflammation. J Clin Invest. 1996;98:2284–2291. doi: 10.1172/JCI119039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamse R, Saria A. Potentiation of tachykinin-induced plasma protein extravasation by calcitonin gene-related peptide. Eur J Pharmacol. 1985;114:61–66. doi: 10.1016/0014-2999(85)90520-5. [DOI] [PubMed] [Google Scholar]
- Hay DL, Christopoulos G, Christopoulos A, Poyner DR, Sexton PM. Pharmacological discrimination of calcitonin receptor: receptor activity-modifying protein complexes. Mol Pharmacol. 2005;67:1655–1665. doi: 10.1124/mol.104.008615. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Undem BJ. Identification and substance P content of vagal afferent neurons innervating the epithelium of the guinea pig trachea. Am J Respir Crit Care Med. 1999;159:1943–1948. doi: 10.1164/ajrccm.159.6.9808078. [DOI] [PubMed] [Google Scholar]
- Johnson PJ, Bornstein JC. Neurokinin-1 and -3 receptor blockade inhibits slow excitatory synaptic transmission in myenteric neurons and reveals slow inhibitory input. Neuroscience. 2004;126:137–147. doi: 10.1016/j.neuroscience.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Kajekar R, Undem BJ, Myers AC. Role of cyclooxygenase activation and prostaglandins in antigen-induced excitability changes of bronchial parasympathetic ganglionic neurons. Am J Physiol Lung Cell Mol Physiol. 2003;284:L581–587. doi: 10.1152/ajplung.00332.2002. [DOI] [PubMed] [Google Scholar]
- Kress M, Guthmann C, Averbeck B, Reeh PW. Calcitonin gene-related peptide and prostaglandin E2 but not substance P release induced by antidromic nerve stimulation from rat skin in vitro. Neuroscience. 1999;89:303–310. doi: 10.1016/s0306-4522(98)00280-2. [DOI] [PubMed] [Google Scholar]
- Kubota M, Moseley JM, Butera L, Dusting GJ, MacDonald PS, Martin TJ. Calcitonin gene-related peptide stimulates cyclic AMP formation in rat aortic smooth muscle cells. Biochem Biophys Res Commun. 1985;132:88–94. doi: 10.1016/0006-291x(85)90992-1. [DOI] [PubMed] [Google Scholar]
- Kummer W. Ultrastructure of calcitonin gene-related peptide-immunoreactive nerve fibres in guinea-pig peribronchial ganglia. Reg Peptides. 1992;37:135–142. doi: 10.1016/0167-0115(92)90662-e. [DOI] [PubMed] [Google Scholar]
- Kummer W, Fischer A, Kurkowski R, Heym C. The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double labeling immunohistochemistry. Neuroscience. 1992;49:715–737. doi: 10.1016/0306-4522(92)90239-x. [DOI] [PubMed] [Google Scholar]
- Kwong K, Wu ZX, Kashon ML, Krajnak KM, Wise PM, Lee LY. Chronic smoking enhances tachykinin synthesis and airway responsiveness in guinea pigs. Am J Respir Cell Mol Biol. 2001;25:299–305. doi: 10.1165/ajrcmb.25.3.4557. [DOI] [PubMed] [Google Scholar]
- Martling CR, Saria A, Fischer JA, Hokfelt T, Lundberg JM. Calcitonin gene-related peptide and the lung: neuronal coexistence with substance P, release by capsaicin and vasodilatory effect. Regul Pept. 1988;20:125–139. doi: 10.1016/0167-0115(88)90046-8. [DOI] [PubMed] [Google Scholar]
- Mitchell RA, Herbert DA, Baker DG, Basbaum CB. In vivo activity of tracheal parasympathetic ganglion cells innervating tracheal smooth muscle. Brain Res. 1987;437:157–160. doi: 10.1016/0006-8993(87)91537-x. [DOI] [PubMed] [Google Scholar]
- Morara S, Sternini C, Provini L, Rosina A. Developmentally regulated expression of alpha- and beta-calcitonin gene-related peptide mRNA and calcitonin gene-related peptide immunoreactivity in the rat. J Comp Neurol. 1995;354:27–38. doi: 10.1002/cne.903540104. [DOI] [PubMed] [Google Scholar]
- Murphy SM, Mathew SE, Rodgers HF, Lituri DT, Gibbins IL. Synaptic organization of neuropeptide-containing preganglionic boutons in lumbar sympathetic ganglia of guinea pigs. J Comp Neurol. 1998;398:551–567. doi: 10.1002/(sici)1096-9861(19980907)398:4<551::aid-cne7>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Myers AC. Ca2+ and K+ currents regulate accommodation and firing frequency in guinea pig bronchial parasympathetic ganglionic neurons. Am J Physiol Lung Cell Mol Physiol. 1998;275:L357–364. doi: 10.1152/ajplung.1998.275.2.L357. [DOI] [PubMed] [Google Scholar]
- Myers AC. Anatomical characteristics of tonic and phasic postganglionic neurons in guinea pig bronchial parasympathetic ganglia. J Comp Neurol. 2000;419:439–450. [PubMed] [Google Scholar]
- Myers AC. Transmission in autonomic ganglia. Respir Physiol. 2001;125:99–111. doi: 10.1016/s0034-5687(00)00207-3. [DOI] [PubMed] [Google Scholar]
- Myers AC, Goldie R, Hay WP. A novel role for tachykinin neurokinin-3 receptors in regulation of human bronchial ganglia neurons. Am J Resp Crit Care Med. 2005;171:212–216. doi: 10.1164/rccm.200405-600OC. [DOI] [PubMed] [Google Scholar]
- Myers AC, Kajekar R, Undem BJ. Allergic inflammation-induced neuropeptide production in rapidly adapting afferent nerves in guinea pig airways. Am J Physiol Lung Cell Mol Physiol. 2002;282(4):L775–781. doi: 10.1152/ajplung.00353.2001. [DOI] [PubMed] [Google Scholar]
- Myers AC, Undem BJ. Analysis of preganglionic nerve evoked cholinergic contractions of the guinea pig bronchus. J Auton Nerv Sys. 1991;35:175–184. doi: 10.1016/0165-1838(91)90095-k. [DOI] [PubMed] [Google Scholar]
- Myers AC, Undem BJ. Electrophysiological effects of tachykinins and capsaicin on guinea-pig bronchial parasympathetic ganglion neurones. J Physiol (London) 1993;470:665–679. doi: 10.1113/jphysiol.1993.sp019881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers AC, Undem BJ. Muscarinic receptor regulation of synaptic transmission in airway parasympathetic ganglia. Amer J Physiol Lung Cell Mol Physiol. 1996;270:L630–636. doi: 10.1152/ajplung.1996.270.4.L630. [DOI] [PubMed] [Google Scholar]
- Myers AC, Undem BJ, Kummer W. Anatomical and electrophysiological comparison of the sensory innervation of bronchial and tracheal parasympathetic ganglionic neurons. J Auton Nerv Sys. 1996;61:162–168. doi: 10.1016/s0165-1838(96)00081-1. [DOI] [PubMed] [Google Scholar]
- Myers AC, Undem BJ, Weinreich D. Electrophysiological properties of neurons in guinea pig bronchial parasympathetic ganglia. Am J Physiol Lung Cell Mol Physiol. 1990;259:L403–409. doi: 10.1152/ajplung.1990.259.6.L403. [DOI] [PubMed] [Google Scholar]
- Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, Muff R, Fischer JA, Foord SM. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- Springer J, Amadesi S, Trevisani M, Harrison S, Dinh QT, McGregor GP, Fischer A, Geppetti P, Groneberg DA. Effects of alpha calcitonin gene-related peptide in human bronchial smooth muscle and pulmonary artery. Regul Pept. 2004;118:127–34. doi: 10.1016/j.regpep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- van Rossum D, Hanisch UK, Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci Biobehav Rev. 1997;21:649–678. doi: 10.1016/s0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- Wahl M, Schilling L, Parsons AA, Kaumann A. Involvement of calcitonin gene-related peptide (CGRP) and nitric oxide (NO) in the pial artery dilatation elicited by cortical spreading depression. Brain Res. 1994;637:204–210. doi: 10.1016/0006-8993(94)91234-3. [DOI] [PubMed] [Google Scholar]
- Watson N, Maclagan J, Barnes PJ. Endogenous tachykinins facilitate transmission through parasympathetic ganglia in guinea-pig trachea. Br J Pharmacol. 1993;109:751–759. doi: 10.1111/j.1476-5381.1993.tb13638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimalawansa SJ. Amylin, calcitonin gene-related peptide, calcitonin, and adrenomedullin: a peptide superfamily. Crit Rev Neurobiol. 1997;11:167–239. doi: 10.1615/critrevneurobiol.v11.i2-3.40. [DOI] [PubMed] [Google Scholar]


