Summary
Tuberculosis is a chronic disease requiring the constant expression of cellular immunity to limit bacterial growth. The constant expression of immunity also results in chronic inflammation, which requires regulation. While IFN-γ-producing CD4+ T helper cells (Th1) are required for control of bacterial growth they also initiate and maintain a mononuclear inflammatory response. Other T cell subsets are induced by Mycobacterium tuberculosis (Mtb) infection including those able to produce IL-17 (Th17). IL-17 is a potent inflammatory cytokine capable of inducing chemokine expression and recruitment of cells to parenchymal tissue. Both the IL-17 and the Th17 response to Mtb are largely dependent upon IL-23. Although both Th17 and Th1 cells are induced following primary infection with Mtb, the protective response is significantly altered in the absence of Th1 cells but not in the absence of Th17. In contrast, in vaccinated animals the absence of memory Th17 cells results in loss of both the accelerated memory Th1 response and protection.. Th1 and Th17 responses cross-regulate each other during mycobacterial infection and this may be important for immunopathologic consequences not only in tuberculosis but also other mycobacterial infections.
Keywords: Tuberculosis, cytokines, inflammation
1. Introduction
The recent explosion of data regarding the cytokines IL-17 and IL-23 has been informative for those studying protective and damaging immune responses. In tuberculosis, as in other persistent infections, these cytokines play an important role. In this review we will cover the role of IL-23 in the induction of IL-17-producing antigen-specific CD4+ T cells (Th17) and in the control of tuberculosis. We will also outline the role of both IL-23 and IL-17 in expression of vaccine-induce protection against tuberculosis. Finally, we will discuss the ability of mycobacteria to induce both Th1 and Th17 cells and how these cells contribute to inflammation.
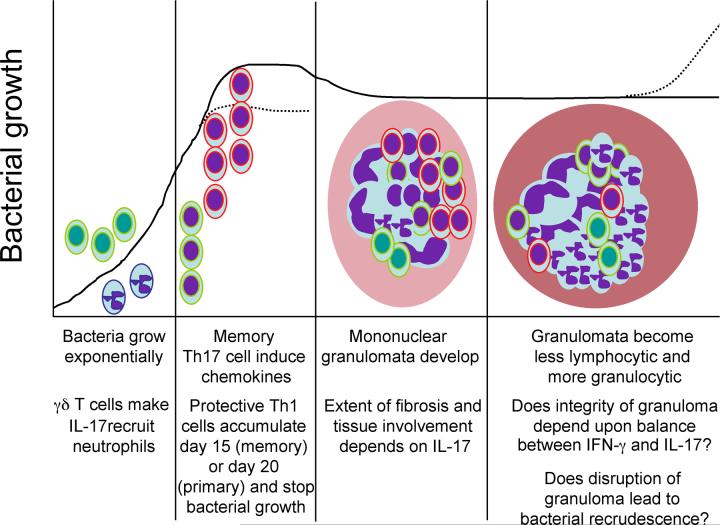
Mtb is delivered to the lung via an aerosol cloud that deposits 3-5 micron particles containing bacteria into the lower lung resulting in a granulomatous response within the alveolar parenchyma. It takes time for bacteria to grow and to initiate acquired immunity and inflammation in the lung tissue. Indeed in the mouse model of low dose aerosol infection, it takes 9-10 days for T cells within the draining lymph node to become activated [1]. Thereupon it takes 20 days for sufficient antigen-specific IFN-γ-producing T cells to accumulate in the lung and stop bacterial growth [2]. Bacterial growth occurs in an unrestrained manner in the absence of either an acquired immune response [3], IFN-γ [4], TNF-α [5] or IL-12p40 [6] and is restrained for only a short period in the absence of IL-12p35 [6], inducible nitric oxide synthase [7] or the ability to make a diverse antigen-specific response [8]. While the bacterial burden remains constant for a prolonged period of time in immunocompetent mice immunopathologic consequences continue to progress with the mononuclear granuloma becoming lymphopenic and granulocytic just prior to bacterial recrudescence [9]. The acquired immune response remains active throughout this period [10] and bacteria will begin to grow and disease recrudesce if the acquired response is limited [11]. IL-23 and IL-17 have roles throughout mycobacterial infection (Figure 1).
Figure 1. Tuberculosis represents a chronic interaction between host and pathogen with IL-23 and IL-17 playing roles throughout.
Bacteria are deposited at low numbers within the lung and γδ T cells (green nuclei) make IL-17 (green lined cells) to recruit neutrophils (purple nuclei, purple lines). If mice have been vaccinated, memory Th17 cells (purple nuclei, green lines) make chemokines and accelerate the accumulation of memory Th1 cells (purple nuclei, red lines). In either the memory or the primary response it is the arrival of IFN-γ-producing cells (purple nuclei, red lines) at sufficient numbers that correlates with cessation of bacterial growth. Initially a mononuclear granuloma develops in the lung parenchyma (pink), the extent and content of which depends upon IL-17. As infection progresses, lymphocyte numbers decrease while polymorphonuclear cells increase. We propose here that the balance between the IL-12/IFN-γ and IL-23/IL-17 pathways define the late consequences of mycobacterial infection with disruption of the lung parenchyma (dark pink) and bacterial recrudescence being the consequences of imbalance.
2. Role of IL-23 and IL-17 in the primary protective response
Upon exposure of dendritic cells (DCs) to Mtb IL-12p70 and IL-23 are induced [12-14]. When CD4+ T cells are primed with Mtb-infected DCs and their cognate antigen and then restimulated, the efficient generation of Th17 cells is dependent upon the presence of IL-23 during the initial priming [12, 13], this is also true when M. bovis BCG is used [15]. Furthermore upon aerosol infection, the absence of IL-23 leads to ablation of the Th17 response and significant loss of IL-17mRNA expression in the lung [12]. These data demonstrate that IL-23 is essential to the expression of both the Th17 population and the IL-17 response to mycobacterial infection. This fits with other observations wherein the continued function of Th17 cells is associated with IL-23 [16]. These data do not define IL-23 as the initiator of the Th17 response to mycobacteria, indeed it is likely that this response is in mice dependent upon TGF-β [17, 18]. In contrast, a critical role for IL-23 in the initiation of Th17 cells in humans has recently been reported [19, 20]. Regardless of the specific role of IL-23, it is likely that this cytokine will be required for optimal Th17 responses to mycobacterial infection in humans.
IL-23 is also able to induce IFN-γ-producing Th1 cells in the absence of IL-12p70 as the residual Th1 response to Mtb seen in IL-12p35 gene-deficient mice, is lost when IL-23 is absent [12]. Neither the Th1 response nor protection is however lost in the absence of IL-23 [12, 21]. Conversely, when IL-23 is delivered as an adenoviral construct prior to infection, it increases the IFN-γ and IL-17 response in the lung and mediates improved protection compared to adenovirus alone [22]. In addition, when co-delivered with the Mtb antigen, Ag85, encoded as DNA in a plasmid vaccine, IL-23 was able to induce a strong Ag85-specific IFN-γ response in IL-12p40 gene-deficient mice [23]. These data suggest that while IL-23 plays a secondary role to IL-12 in the induction of IFN-γ-mediated protective response, it may be able to augment these responses.
T cells associated with innate response can also make IL-17. In particular, the γδ T cell population is a primary source of Mtb-induced IL-17 in the mycobacterial infection model [13, 24]. Following intratracheal delivery of BCG, IL-17 mRNA can be detected 1 day post-infection and in the absence of this cytokine the induction of chemokines and early neutrophil accumulation were reduced [24]. The IL-17 being produced in this model was from γδ T cells and in its absence there was also reduced mononuclear granulomatous inflammation later in infection [24] similar to the altered granulomatous response seen in the absence of γδ T cells [25]. Mtb-infected DCs can induce IL-17 in T cells from uninfected mice and this is largely a result of IL-23-dependent induction of IL-17 in the γδ T cell population [13]. Following a low dose aerosol infection with Mtb, γδ T cells are major producers of IL-17 [13] and it is likely that these cells are dependent upon IL-23 as well as there is no IL-17mRNA in IL-23p19 gene deficient mice [12]. There is also an invariant natural killer T (iNKT) cell population in the lung that recognizes lipopolysaccharide and pathogen glycolipids. These cells produce IL-17 upon stimulation and are required for the airway neutrophilia induced by these products [26]. It is not yet known if these cells are involved in the initial response to Mtb infection.
3. Role of IL-23 and IL-17 in vaccination
While the role of IL-23 or IL-17 in the protective response to primary tuberculosis is dispensable, the fact that treatment with IL-23 can increase primary immunity [22] and that the IL-17 impacts the inflammatory response to mycobacteria [12, 24] suggests that the protective role of these cytokines may be improved by vaccination. In tuberculosis, the delivery of BCG as a live attenuated vaccine results in protection against disseminated disease but is less effective against pulmonary disease in adults [27]. The animal model of disease provides some explanation of this discrepancy as, mice with a population of Mtb-specific memory cells are better able to control bacteria following systemic rather than aerosol challenge [28]. The cessation of bacterial growth in the lung corresponds to the accumulation of IFN-γ-producing CD4 T cells and this accumulation, although accelerated in memory mice, takes 15 days to be effective [2, 28, 29]. This delay in expression of memory immunity needs to be addressed if improvements are to be made in the development of effective vaccines against this disease. In this regard we have demonstrated that the accelerated Th1 memory response seen in the lungs of vaccinated mice infected with Mtb is dependent upon IL-23 and IL-17 [2]. Specifically, upon vaccination a population of antigen-specific IL-17 producing memory T cells able to populate the lung is generated and these cells respond more quickly than memory Th1 cells to aerosol challenge; this population of cells is dependent upon IL-23. The IL-17 produced by these cells induces the chemokines CXCL9, CXCL10 and CXCL11 in the lung by day 12 post-challenge and in the absence of this response, the accelerated accumulation of Th1 memory cells is lost along with vaccine-induced protection [2]. These data demonstrate that vaccination results in the generation of a surveillance cell that can recognize an invading pathogen rapidly in the tissue and promote the recruitment of protective cells. It will be important to determine whether these surveillance cells can be established in high number in the lung without damaging consequences.
When used as an adjunct to DNA vaccination, IL-23 can augment the induction of protective Th1 and Th17 responses to a level similar to that seen for IL-12p70 [14, 23]. This is in contrast to an IL-27 containing plasmid, which does not augment protection upon DNA vaccination [14]; this is possibly due to the ability of IL-27 to down regulate Th17 responses [30, 31]. The BCG vaccine generates an early antigen-specific Th17 response following systemic infection but this is down regulated by IFN-γ [15]. When BCG is delivered intratracheally, a γδ T cell IL-17 response is induced [24] however upon subcutaneous delivery no IL-17 response is seen unless IL-12p40 is absent [23]. In this latter case, not only the potentially regulating IFN-γ is missing but also IL-23, suggesting that an IL-17-producing CD4+ T cell population can occur in response to mycobacteria in the absence of IL-23 [23].
3. The role of IL-23 and IL-17 in immunopathology of tuberculosis
IL-17 is recognized as an inflammatory cytokine capable of inducing chemokine gradients and initiating inflammation, particularly in the lung [32-34]. As IL-23 is responsible for the persistence and function of Th17 cells [16] it is also likely a key player in inflammation. It is surprising therefore that in the absence of IL-23 the inflammatory consequences of Mtb infection are modest. Specifically, while the extent of lung consolidation is similar between wild type and IL-23p19 gene-deficient mice, as disease progresses, the severity of the inflammation is increased and the extent of fibrin deposition decreased in the absence of IL-23 [12]. As discussed above, the absence of IL-17 also results in reduced mononuclear and polymorphonuclear infiltration early in the BCG model [24]. Further, despite the acknowledged role of IL-23 and IL-17 in neutrophil recruitment [34] and homeostasis [35] there are no differences in neutrophil numbers in the lungs of Mtb infected mice lacking IL-23 [12]. It is clear therefore that IL-23 and IL-17 are acting in a complex manner in the control of mycobacteria-induced inflammation. This is not so surprising as IL-17 can act as a mediator of macrophage accumulation [32] and we have shown that IL-17 can mediate induction of CXCL chemokines containing IL-17 associated promoter elements [2, 36]. These data suggest that during mycobacterial infection IL-17 can act to mediate accumulation of both polymorphic and mononuclear cells.
That the absence of IL-23 and IL-17 in the lung leads to increased severity of inflammation with increased tissue damage and reduced fibrin deposition may be related to the recent demonstration that IL-17 alters the survival [37] and IL-17 and IL-23 alter the functional profile of neutrophils [38]. These data suggest that one possible function of these cytokines in the late stages of Mtb-induced inflammation may be to maintain the integrity of the granuloma by limiting neutrophil death, this should be investigated. It will be intriguing to determine the extent to which the Th1 and Th17 responses balance each other as disease progresses and whether loss of the IL-17 response results in early breakdown of the granuloma and earlier recrudescence. In this respect it is of interest that the absence of IL-27 leads to increased Th17 cells [39] and increased protection and mononuclear inflammation upon Mtb infection [40, 41], unfortunately it also leads to earlier death of animals [40] suggesting that while Th17 cells can balance Th1 mediated inflammation, Th1 cells are also required to balance Th17 mediated inflammation.
The relative levels of IL-12 and IL-23 induced by mycobacterially-infected cells will be crucial for the balance between Th1 and Th17 cells. In primary Mtb infection, Th17 and Th1 cells are induced with the same kinetics but there are 5-10 fold more Th1 than Th17 cells [2, 12]. In BCG infection the Th17 is rapidly suppressed by the Th1 response [15] and this may be related to differential induction of IL-12 and IL-23 by BCG compared to Mtb [14]. We have also shown that while IFN-γ dramatically increases IL-12p70 production by BCG-infected DCs, it also reduces IL-23; conversely IL-17 limits IL-12 production while augmenting IL-23 [15]. These data suggest that the cross-regulation of IL-12 and IL-23 by each other and by IFN-γ and IL-17 will be critical to the inflammatory outcome of any mycobacterial infection.
4. IL-23 and human tuberculosis
Genetic mutations resulting in altered IL-12-induced and IFN-γ-mediated responses have been reported since 1996, these mutations lead to increased susceptibility to mycobacterial disease [42-47]. However, while defects in the genes encoding IL-12Rβ1 and IL-12p40 have been associated with mycobacterial disease, there are no reported mutations in the genes for IL-12Rβ2 or IL-12p35 [48]. Importantly, the absence of IL-12p40 and IL-12Rβ1 will impair not only the IL-12p70 pathway but also the IL-23 pathway. Indeed in humans with IL-12Rβ1 deficiency, the ability of IL-23 to promote IFN-γ production is decreased [49]. As expected IL-23 also promotes IL-17 production however IL-12 reduces expression of this cytokine [50], suggesting a cross-regulatory role for these two cytokines in the human as well as the mouse model. This cross-regulation is intriguing in light of the demonstrated role for mutations in the gene for IL-23R in susceptibility to inappropriate inflammatory conditions, particularly of the gut [51]. Specifically, if there is an imbalance between IL-23 and IL-12 resulting in either excessive IFN-γ or IL-17-mediated inflammation in response to M. avium subsp. paratuberculosis this may explain the apparent association between this pathogen and Crohn’s disease [52]. Many questions remain to be addressed regarding the relative roles of IL-12 and IL-23 in mycobacterial disease in humans.
5. Conclusion
Tuberculosis is a disease that persists in the host. This persistence leads to a complex interaction between host and pathogen that must develop over time to balance protective and inflammatory roles. The role of IL-23 and IL-17 in this balance does not appear to be protective but rather as a regulator of inflammation. An important role for IL-23 and IL-17 has also been demonstrated for the expression of vaccine-induce protection with IL-23-dependent IL-17-producing memory T cells being required for chemokine expression and accelerated accumulation of protective IFN-γ-producing memory T cells. Finally, the balance between IL-12/IFN-γ and IL-23/IL-17 may be crucial to the regulation of inflammatory consequences not only of Mtb but also other mycobacterial infections. Understanding the relative roles of these cytokines in mediating protection and immunopathology will be important in light of the thrust towards inhibiting IL-23 as a treatment for autoimmune disease.
Acknowledgements
This work was supported by the Trudeau Institute, Inc.; a New York Community Trust-Heiser Fund Fellowship and a Career Development Award from AI057158 (North East Biodefense Center-Lipkin) to S.A.K.; NIH grants AI46530, AI067723, AG028878 to A.M.C.
Conflict of interest: There are no conflicting interests.
Abbreviations
- Mtb
Mycobacterium tuberculosis
- DCs
Dendritic cells
- Ag 85
Antigen 85
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chackerian A, Alt J, Perera T, Dascher C, SM B. Dissemination of Mycobacterium tuberculosis Is Influenced by Host Factors and Precedes the Initiation of T-Cell Immunity. Infect. Immun. 2002;70:4501–4509. doi: 10.1128/IAI.70.8.4501-4509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khader S, Bell G, Pearl J, Fountain J, Rangel-Moreno J, Cilley G, Shen F, Eaton S, Gaffen S, Swain S, Locksley R, Haynes L, Randall T, Cooper A. IL-23 and IL-17 in establishment of protective pulmonary CD4+ T cell responses upon vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 3.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 2001;193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 6.Cooper AM, Kipnis A, Turner J, Magram J, Ferrante J, Orme IM. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J. Immunol. 2002;168:1322–1327. doi: 10.4049/jimmunol.168.3.1322. [DOI] [PubMed] [Google Scholar]
- 7.Cooper AM, Pearl JE, Brooks JV, Ehlers S, Orme IM. Expression of nitric oxide synthase 2 gene is not essential for early control of Mycobacterium tuberculosis in the murine lung. Infect. Immun. 2000;68:6879–6882. doi: 10.1128/iai.68.12.6879-6882.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner J, Dobos K, Keen M, Frank A, Ehlers S, Orme I, Belisle J, Cooper A. A limited antigen-specific cellular response is sufficient for the early control of Mycobacterium tuberculosis in the lung but is insufficient for long-term survival. Infect. Immun. 2004;72:3759–3768. doi: 10.1128/IAI.72.7.3759-3768.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhoades ER, Frank AA, Orme IM. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tuberc. Lung Dis. 1997;78:57–66. doi: 10.1016/s0962-8479(97)90016-2. [DOI] [PubMed] [Google Scholar]
- 10.Winslow G, Roberts A, Blackman M, Woodland D. Persistence and turnover of antigen-specific CD4 T cells during chronic tuberculosis infection in the mouse. J. Immunol. 2003;170:2046–2052. doi: 10.4049/jimmunol.170.4.2046. [DOI] [PubMed] [Google Scholar]
- 11.Flynn J, Chan J. Tuberculosis: Latency and reactivation. Infect. Immun. 2001;69:4195–4201. doi: 10.1128/IAI.69.7.4195-4201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khader S, Pearl J, Sakamoto K, Gilmartin L, Bell G, Jelley-Gibbs D, Ghilardi N, deSauvage F, Cooper A. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J. Immunol. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 13.Lockhart E, Green A, Flynn J. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 14.Wozniak T, Ryan A, Triccas J, Britton W. Plasmid interleukin-23 (IL-23), but not plasmid IL-27, enhances the protective efficacy of a DNA vaccine against Mycobacterium tuberculosis infection. Infect. Immun. 2006;74:557–565. doi: 10.1128/IAI.74.1.557-565.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cruz A, Khader S, Torrado E, Fraga A, Pearl J, Pedrosa J, Cooper A, Castro A. CE:IFN-g regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection1. J. Immunol. 2006;177:1416–1420. doi: 10.4049/jimmunol.177.3.1416. [DOI] [PubMed] [Google Scholar]
- 16.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr. Op. Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Mangan P, Harrington L, O’Quinn D, Helms W, Bullard D, Elson C, Hatton R, Wahl S, Schoeb T, Weaver C. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 18.Veldhoen M, Hocking R, Atkins C, Locksley R, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, Tato C, Muul L, Laurence A, O’Shea J. Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum. 2007;56:2936–2946. doi: 10.1002/art.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson N, Boniface K, Chan J, McKenzie B, Blumenschein W, Mattson J, Basham B, Smith K, Chen T, Morel F, Lecron J, Kastelein R, Cua D, McClanahan T, Bowman E, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 21.Chackerian A, Chen S, Brodie S, Mattson J, McClanahan T, Kastelein R, Bowman E. Neutralization or absence of the interleukin-23 pathway does not compromise immunity to mycobacterial infection. Infect. Immun. 2006;74:6092–6099. doi: 10.1128/IAI.00621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Happel K, Lockhart E, Mason C, Porretta E, Keoshkerian E, Odden A, Nelson S, Ramsay A. Pulmonary interleukin-23 gene delivery increases local T-cell immunity and controls growth of Mycobacterium tuberculosis in the lungs. Infect. Immun. 2005;73:5782–5788. doi: 10.1128/IAI.73.9.5782-5788.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wozniak T, Ryan A, Britton W. Interleukin-23 restores immunity to Mycobacterium tuberculosis infection in IL-12p40-deficient mice and is not required for the development of IL-17-secreting T cell responses. J. Immunol. 2006;177:8684–8692. doi: 10.4049/jimmunol.177.12.8684. [DOI] [PubMed] [Google Scholar]
- 24.Umemura M, Yahagi A, Hamada S, Begum M, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, Matsuzaki G. IL-17-Mediated Regulation of Innate and Acquired Immune Response against Pulmonary Mycobacterium bovis Bacille Calmette-Guerin Infection. J. Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 25.D’Souza CD, Cooper AM, Frank AA, Mazzaccaro RJ, Bloom BR, Orme IM. An anti-inflammatory role for gd T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J. Immunol. 1997;158:1217–1221. [PubMed] [Google Scholar]
- 26.Michel M, Keller A, Paget C, Fujio M, Trottein F, Savage P, Wong C, Schneider E, Dy M, Leite-de-Moraes M. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J. Exp. Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colditz G, Brewer T, Berkey C, Wilson M, Burdick E, Fineberg H, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 28.Cooper AM, Callahan JE, Keen M, Belisle JT, Orme IM. Expression of memory immunity in the lung following re-exposure to Mycobacterium tuberculosis. Tuberc. Lung Dis. 1997;78:67–73. doi: 10.1016/s0962-8479(97)90017-4. [DOI] [PubMed] [Google Scholar]
- 29.Jung Y, Ryan L, Lacourse R, North R. Properties and protective value of the secondary versus primary T helper type 1 response to airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 2005;201:1915–1924. doi: 10.1084/jem.20050265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stumhofer J, Laurence A, Wilson E, Huang E, Tato C, Johnson L, Villarino A, Huang Q, Yoshimura A, Sehy D, Saris C, O’Shea J, Hennighausen L, Ernst M, Hunter C. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 31.Batten M, Li J, Yi S, Kljavin N, Danilenko D, Lucas S, Lee J, de Sauvage F, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat. Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 32.Sergejeva S, Ivanov S, Lotvall J, Linden A. IL-17 as a recruitment and survival factor for airway macrophages in allergic airway inflammation. Am. J. Resp. Cell Mol. Biol. 2005;33:248–253. doi: 10.1165/rcmb.2004-0213OC. [DOI] [PubMed] [Google Scholar]
- 33.Kolls J, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Miyamoto M, Prause O, Sjostrand M, Laan M, Lotvall J, Linden A. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J. Immunol. 2003;170:4665–4672. doi: 10.4049/jimmunol.170.9.4665. [DOI] [PubMed] [Google Scholar]
- 35.Stark M, Huo Y, Burcin T, Morris M, Olson T, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Shen F, Hu Z, Goswami J, Gaffen S. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J. Biol. Chem. 2006;281:24138–24148. doi: 10.1074/jbc.M604597200. [DOI] [PubMed] [Google Scholar]
- 37.Dragon S, Saffar A, Shan L, Gounni A. IL-17 attenuates the anti-apoptotic effects of GM-CSF in human neutrophils. Mol. Immunol. 2007 doi: 10.1016/j.molimm.2007.04.027. epub. [DOI] [PubMed] [Google Scholar]
- 38.Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, Belladonna M, Vacca C, Conte C, Mosci P, Bistoni F, Puccetti P, Kastelein R, Kopf M, Romani L. The IL-23/IL-17 pathway promotes inflammation and impairs antifungal immune resistance. Eur. J. Immunol. 2007 doi: 10.1002/eji.200737409. in press. [DOI] [PubMed] [Google Scholar]
- 39.Khader S, Solache A, Pearl J, Fountain J, Rangel-Moreno J, Martino C, Randall T, Cooper A. IL-27R on CD4 T cells limits the protective response to Mycobacterium tuberculosis but not as a result of limiting the generation of Th17 cells. Eur. J. Immunol. 2007 submitted. [Google Scholar]
- 40.Holscher C, Holscher A, Ruckerl D, Yoshimoto T, Yoshida H, Mak T, Saris C, Ehlers S. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J. Immunol. 2005;174:3534–3544. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- 41.Pearl JE, Shabaana AK, Solache A, Gilmartin L, Ghilardi N, deSauvage F, Cooper AM. IL-27 signaling compromises control of bacterial growth in mycobacteria-infected mice. J. Immunol. 2004;173:7490–7496. doi: 10.4049/jimmunol.173.12.7490. [DOI] [PubMed] [Google Scholar]
- 42.Akahoshi M, Nakashima H, Miyake K, Inoue Y, Shimizu S, Tanaka Y, Okada K, Otsuka T, Harada M. Influence of interleukin-12 receptor b1 polymorphisms on tuberculosis. Hum Genet. 2003;112:237–243. doi: 10.1007/s00439-002-0873-5. [DOI] [PubMed] [Google Scholar]
- 43.Tso HW, Lau YL, Tam CM, Wong HS, Chiang AK. Associations between IL12b polymorphisms and tuberculosis in the Honk Kong chinese population. J. Inf. Dis. 2004;190:913–919. doi: 10.1086/422693. [DOI] [PubMed] [Google Scholar]
- 44.Doffinger R, Dupius S, Picard C, Fieschi C, Feinberg J, Barcenas-Morales G, Casanova J-L. Inherited disorders of IL-12 and IFN-G mediated immunity: a molecular genetics update. Mol. Immunol. 2001;38:903–909. doi: 10.1016/s0161-5890(02)00017-2. [DOI] [PubMed] [Google Scholar]
- 45.Fieschi C, Bosticardo M, de Beaucoudrey L, Boisson-Dupuis S, Feinberg J, Santos OF, Bustamante J, Levy J, Candotti F, Casanova J. A novel form of complete IL-12/IL-23 receptor b1-deficiency with cell surface-expressed non-functional receptors. Blood. 2004;104:2095–2101. doi: 10.1182/blood-2004-02-0584. [DOI] [PubMed] [Google Scholar]
- 46.Sakai T, Matsuoka M, Aoki M, Nosaka K, Mitsuya H. Missense mutation of the interleukin-12 receptor B1 chain-encoding gene is associated with impaired immunity against Mycobacterium avium complex infection. Blood. 2001;97:2688–2694. doi: 10.1182/blood.v97.9.2688. [DOI] [PubMed] [Google Scholar]
- 47.Lichtenauer-Kaligis EGR, de Boer T, Verreck FAW, van Voorden S, Hoeve MA, van de Vosse E, Ersoy F, Tezcan I, van Dissel JT, Sanal O, Ottenhoff THM. Severe Mycobacterium bovis BCG infections in a large series of novel IL-12 receptor B1 deficient patients and evidence for the existence of partial IL-12 receptor B1 deficiency. Eur. J. Immunol. 2003;33:59–69. doi: 10.1002/immu.200390008. [DOI] [PubMed] [Google Scholar]
- 48.Filipe-Santos O, Bustamante J, Chapgier A, Vogt G, de Beaucoudrey L, Feinberg J, Jouanguy E, Boisson-Dupuis S, Fieschi C, Picard C, Casanova J. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin Immunol. 2006;18:347–361. doi: 10.1016/j.smim.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 49.Hoeve M, de Boer T, Langenberg D, Sanal O, Verreck F, Ottenhoff T. IL-12 receptor deficiency revisited: IL-23-mediated signaling is also impaired in human genetic IL-12 receptor beta1 deficiency. Eur. J. Immunol. 2003;33:3393–3397. doi: 10.1002/eji.200324343. [DOI] [PubMed] [Google Scholar]
- 50.Hoeve M, Savage N, de Boer T, Langenberg D, de Waal Malefyt R, Ottenhoff T, Verreck F. Divergent effects of IL-12 and IL-23 on the production of IL-17 by human T cells. Eur. J. Immunol. 2006;36:661–670. doi: 10.1002/eji.200535239. [DOI] [PubMed] [Google Scholar]
- 51.Duerr R, Taylor K, Brant S, Rioux J, Silverberg M, Daly M, Steinhart A, Abraham C, Regueiro M, Griffiths A, Dassopoulos T, Bitton A, Yang H, Targan S, Datta L, Kistner E, Schumm L, Lee A, Gregersen P, Barmada M, Rotter J, Nicolae D, Cho J. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feller M, Huwiler K, Stephan R, Altpeter E, Shang A, Furrer H, Pfyffer G, Jemmi T, Baumgartner A, Egger M. Mycobacterium avium subspecies paratuberculosis and Crohn’s disease: a systematic review and meta-analysis. Lancet Inf. Dis. 2007;7:607–613. doi: 10.1016/S1473-3099(07)70211-6. [DOI] [PubMed] [Google Scholar]