Abstract
Objective
Previous studies in mice have detected quantitative trait loci (QTLs) on chromosome 7 that affect body composition. As a step toward identifying the responsible genes, we compared a chromosome 7 substitution strain C57BL/6J-Chr7129S1/SvImJ/Na (CSS-7) to its host (C57BL/6J) strain.
Methods and Procedures
Fourteen-week-old mice were measured for body size (weight, length), organ weight (brain, heart, liver, kidneys, and spleen), body and bone composition (fat and lean weight; bone area, mineral content, and density), and individual adipose depot weights (gonadal, retroperitoneal, mesenteric, inguinal, and subscapular). Differences between the CSS-7 strain and the host strain were interpreted as evidence for the presence of one or more QTLs on chromosome 7.
Results
Using this criterion, we detected QTLs for body weight, bone area, bone mineral content, brain, and heart weight, most adipose depot weights and some indices of fatness. A few strain differences were more pronounced in males (e.g., most adiposity measures) and others were more pronounced in females (e.g., bone area). QTLs for body length, lean weight, bone mineral density, and kidney, spleen, and liver weight were not detected.
Discussion
This study found several associations that suggest one or more QTLs specific to the weight of select tissues and organs exist on mouse chromosome 7. Because these loci are detectable on a fixed and uniform genetic background, they are reasonable targets for high-resolution mapping and gene identification using a congenic approach.
Body weight is the sum of the weight of all its component tissues and organs, with some tissues such as fat, bone, or muscle contributing more to the total weight than smaller organs, such as brain. One of the first questions raised among geneticists was whether the weight of organs and tissues was regulated by general genetic factors that influenced body size, or whether there were genes that acted to enlarge or reduce specific components (1). This question has been addressed in several ways, in earlier years by measuring the strength of correlation between the weight of different organs or tissues, e.g., Ref. (2), often comparing these relationships among animals of different genotype and sex. In later years this issue was addressed by examining the pattern of quantitative trait loci (QTLs) for organ or tissue weight, e.g., Ref. (3). This concept of distinguishing between general and specific factors is especially useful in understanding fatness since it is a trait in which a specific tissue (adipose) has expanded out of proportion to the other parts of the body.
But where are the general and specific genes that affect mouse body weight, and the weight of its components located in the genome? The first studies to address this question focused on the location of genetic markers for coat color, and how they predicted bone size (4) or adipose tissue weight (5). New molecular methods have moved these few initial linkages to the hundreds, with some chromosomes contributing more QTLs for mouse body composition and organ weight traits than others. One example of a chromosome with many obesity QTLs is mouse chromosome 7 (6,7). Numerous QTL linkages for the weight of tissues (bone, muscle and fat) as well as several organs map to chr7, and there are specific single genes that are known to contribute to these traits (based on data from spontaneous mutations or knockout strains). While there are many genes with a clearly defined effect on body weight or related traits, it is currently unknown whether the QTLs on chromosome 7 are due to alleles of these genes, or are due to alleles of other genes without previously described obesity effects. In humans, major single obesity genes contribute very little to normal variation in fatness (8). Similarly, we cannot assume that these known mouse single genes for body composition or the size of body components (on chromosome 7 or elsewhere) account for the natural variation among mice.
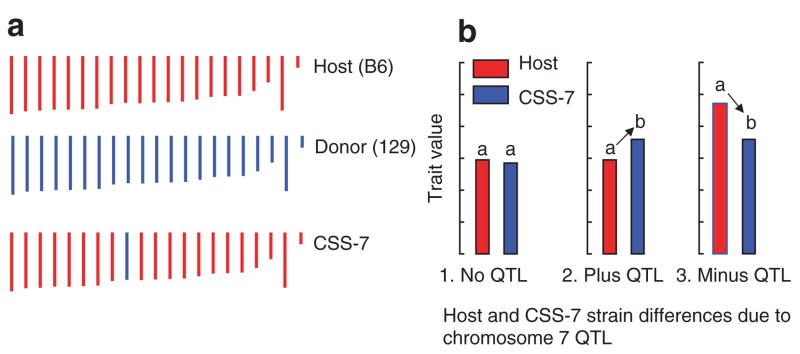
Many QTLs for organ and tissue weight aggregate on mouse chromosome 7, and one of these QTLs was detected in our laboratory when we studied the weight of adipose depots in an F2 intercross between the 129P3/J and C57BL/6ByJ strains of F2 mice (9). This QTL may be specific for adipose tissue weight because there were no related QTLs for body length or body weight found nearby (10). Our goal is to identify the gene that accounts for this linkage, and to determine whether the QTLs are specific to the weight of adipose tissue, or extends to other organs or tissues, such as bone, liver, brain, or lean mass. To begin the high-resolution mapping, we used a chromosome substitution strain (CSS; also known as a consomic strain). These strains are homozygous inbred strains that are identical to their host strain, except that a single chromosome is substituted with the corresponding chromosome from a donor strain (11–13). We used a CSS strain from a set developed in the laboratory of Joseph Nadeau that originates from the C57BL/6J (B6; host) and 129S1/SvImJ (129; donor) progenitor inbred strains. This strain, C57BL/6J-Chr7129S1/SvlmJ/Na (CSS-7), has a swapped chromosome 7(Figure 1a). When the CSS-7 and B6 strains are compared for specific traits, QTLs on the swapped chromosome can be identified (Figure 1b).
Figure 1.
(a) Chromosome composition of a chromosome substitution (consomic) strain (CSS-7). (b) Hypothetical patterns of trait values by strain and the interpretation of quantitative trait loci (QTLs) results: (1) The mean trait values of the host and CSS-7 strains do not differ (no QTLs are detected). (3) The CSS-7 has a lower trait value than the host strain (a minus QTL is detected).
We measured the body composition of mice from the CSS-7 as well as the host (B6) strain, and found QTLs for litter size, body weight, bone area, bone mineral content, brain weight, heart weight, and most indices of fatness including the five dissected adipose depots. Mice from the CSS-7 strain had litters with more offspring (7 ± 2) compared with the host (B6) strain mice (5 ± 2) [t(91) = 3.41, P < 0.001], which demonstrates presence of a QTL on chromosome 7 for litter size. Although the number of pups per mother is important, it does not account for the effects on body composition and organ weight found here because the effects of chromosome 7 substitution remained after litter size was used as a covariate (data not shown).
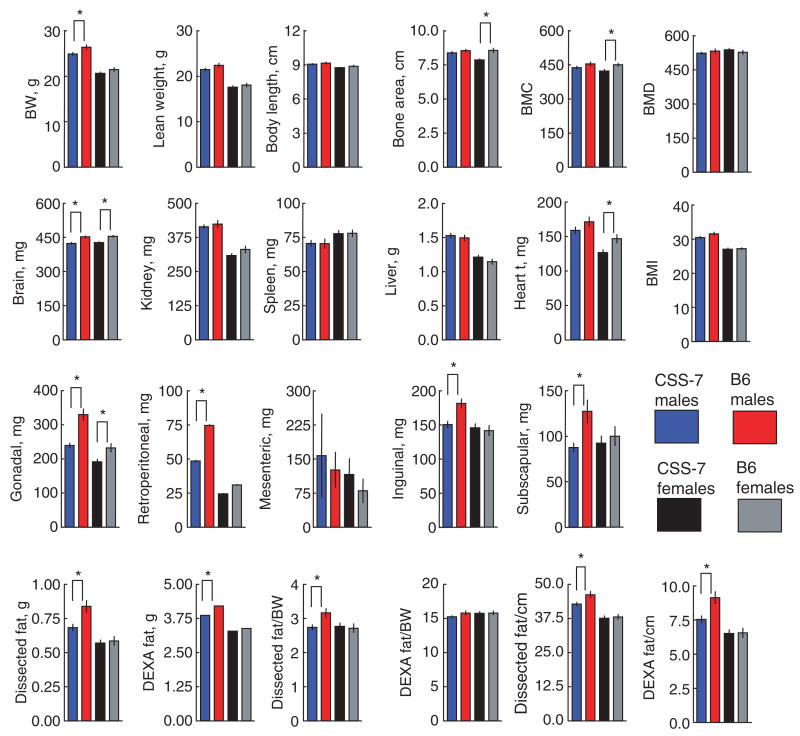
No significant linkages were detected for lean weight, liver, spleen or kidney weight, body length or bone mineral density. Some of the QTLs were male-specific (most indices of fatness, as well as retroperitoneal, inguinal, and sub-scapular depot weight), and one was female-specific (see Methods for the definition of sex-specific and sex-dependent effects) (bone area; Table 1; Figure 2). Body weight, heart weight, and bone mineral content were sex-dependent, with female CSS-7 and B6 groups differing by post hoc analysis. Sex-specific or sex-dependent linkage for body composition traits are widespread, and may be due to interactions between allelic variants in proteins with differing hormonal milieus. Three QTLs were common to male and female mice with roughly equal effect sizes in each sex: litter size, gonadal adipose depot weight, and brain weight. We also computed indexes of fatness in a variety of ways to match the methods of other investigators and earlier studies, and compared the indices by strain and sex. Most obesity indexes gave the same results: no QTL effects in female mice, but pronounced effects in male mice. Fat weight divided by body weight was one exception: although the direction of effect was the same, there were no significant effects of strain. For every trait studied except for litter size, when QTLs were detected, the effect of the introgressed chromosome 7 from the 129 strain was the same, i.e., it reduced the trait value (minus QTLs).
Table 1.
Strain, sex, and interaction effects on body composition and organ weights
| Tissue/organ or trait | Strain effect | Sex effect | Sex by strain |
|---|---|---|---|
| Body weight | F(1, 89) = 8.49, P = 0.01 | F(1, 89) = 135.39, P < 0.01 | F(1, 89) = 0.72, P = 0.40 |
| Body length | F(1, 89) = 3.14, P = 0.08 | F(1, 89) = 18.01, P < 0.01 | F(1, 89) = 0.10, P = 0.75 |
| Lean weight | F(1, 86) = 3.78, P = 0.06 | F(1, 86) = 137.45, P < 0.01 | F(1, 86) = 0.40, P = 0.53 |
| Bone area | F(1, 88) = 12.20, P < 0.01 | F(1, 88) = 3.96, P = 0.05 | F(1, 88) = 4.54, P = 0.04 |
| Bone mineral content | F(1, 88) = 9.42, P < 0.01 | F(1, 88) = 1.63, P = 0.21 | F(1, 88) = 0.65, P = 0.42 |
| Bone mineral density | F(1, 88) = 0.02, P = 0.90 | F(1, 88) = 0.42, P = 0.52 | F(1, 88) = 2.12, P = 0.15 |
| BMI | F(1, 89) = 2.49, P = 0.01 | F(1, 89) = 98.81, P < 0.01 | F(1, 89) = 1.47, P = 0.23 |
| Organs | |||
| Brain | F(1, 89) = 40.30, P < 0.01 | F(1, 89) = 0.35, P = 0.56 | F(1, 89) = 0.01, P = 0.93 |
| Kidneys | F(1, 89) = 2.45, P = 0.12 | F(1, 89) = 97.98, P < 0.01 | F(1, 89) = 0.35, P = 0.55 |
| Spleen | F(1, 89) = 0.00, P = 0.99 | F(1, 89) = 7.53, P = 0.01 | F(1, 89) = 0.01, P = 0.94 |
| Liver | F(1, 89) = 1.82, P = 0.18 | F(1, 89) = 97.98, P < 0.01 | F(1, 89) = 0.35, P = 0.55 |
| Heart | F(1, 89) = 8.50, P = 0.01 | F(1, 89) = 26.14, P < 0.01 | F(1, 89) = 0.49, P = 0.49 |
| Adipose depot | |||
| Gonadal | F(1, 89) = 34.33, P < 0.01 | F(1, 89) = 42.30, P < 0.01 | F(1, 89) = 4.83, P = 0.03 |
| Retroperitoneal | F(1, 89) = 12.09, P < 0.01 | F(1, 89) = 51.91, P < 0.01 | F(1, 89) = 4.32, P = 0.04 |
| Mesenteric | F(1, 89) = 6.65, P = 0.01 | F(1, 89) = 11.41, P < 0.01 | F(1, 89) = 0.03, P = 0.87 |
| Inguinal | F(1, 89) = 4.81, P = 0.03 | F(1, 89) = 13.12, P < 0.01 | F(1, 89) = 8.12, P = 0.01 |
| Subscapular | F(1, 89) = 7.08, P = 0.01 | F(1, 89) = 1.61, P = 0.21 | F(1, 89) = 3.20, P = 0.08 |
| Fatness indices | |||
| Dissected fat weight | F(1, 89) = 7.40, P = 0.01 | F(1, 89) = 34.26, P < 0.01 | F(1, 89) = 4.95, P = 0.03 |
| DEXA fat weight | F(1, 86) = 5.74, P = 0.02 | F(1, 86) = 57.58, P < 0.01 | F(1, 86) = 1.70, P = 0.20 |
| Dissected fat (g)/body weight | F(1, 89) = 3.13, P = 0.08 | F(1, 89) = 3.88, P = 0.05 | F(1, 89) = 5.10, P = 0.03 |
| DEXA fat (g)/body weight | F(1, 86) = 1.50, P = 0.22 | F(1, 86) = 1.22, P = 0.27 | F(1, 86) = 1.13, P = 0.29 |
| Dissected fat (g)/body length | F(1, 89) = 6.31, P = 0.01 | F(1, 89) = 30.47, P < 0.01 | F(1, 89) = 5.63, P = 0.02 |
| DEXA fat (g)/body length | F(1, 86) = 4.37, P = 0.04 | F(1, 86) = 51.44, P < 0.01 | F(1, 86) = 2.58, P = 0.11 |
Significant P values are underlined. Means ± s.e.m. are shown in Figure 2.
DEXA, dual-energy X-ray absorptiometry.
Figure 2.
Body composition, adipose depot and organ weights by strain and sex. Data are means ± s.e.m. The asterisk denotes sex-specific and sex-dependent quantitative trait loci (QTLs) effects. Fat weight was determined in two ways, by necropsy (dissection) and by DEXA. Fat weight was expressed as a ratio by dividing by body weight (BW) or body length (cm) × 100. BMC, bone mineral content (mg); BMD, bone mineral density (mg/cm2); BMI, body mass index (g/cm2) × 100; DEXA, dual-energy X-ray absorptiometry.
Female CSS-7 and B6 mice did not differ for body weight, length, or fat or lean weight (except for the weight of the gonadal depot), but did differ for bone area, bone mineral content, and heart and brain weight. In particular, no QTLs were detected for bone mineral density here, although a previous study using the identical host and donor strains did find one on chr7 (14). Our interpretation of these disparate results is that the bone QTLs are probably the same in both studies, but diet and age difference may change the specific bone-related trait detected.
In agreement with previous studies, the linkage for fatness on chromosome 7 was more apparent in male than female mice (9,15–18). The weight of fat tissue was measured here in two ways, through dissection of individual depots, and by whole-body dual-energy X-ray absorptiometry scanning; fat weight was then expressed relative to body size in several ways. The results for all indexes of fatness followed the same pattern, with the male CSS-7 strain having less fat than the B6 strain and little or no effect of strain on female mice. However the strain differences in percent fat (as measured by dual-energy X-ray absorptiometry) in male mice did not reach statistical significance. It may be that this method is a slightly less sensitive way to measure adiposity differences between strains.
Individual adipose depots were dissected because the location of fat (as well as its amount) is an important variable which is often used as a risk factor in clinical medicine (19). People with larger amounts of fat near the viscera, leading to an “apple” shape, are more likely to have diabetes and other metabolic complications compared with people equivalently fat but with a “pear” shape (i.e., having more fat in the arms and especially legs and buttocks) (19). There is no one-to-one correspondence between adipose depots in mice and in humans (20), but the gonadal, mesenteric, and retroperitoneal depots are inside the abdominal cavity in the mouse and might therefore be considered visceral fat, whereas the subscapular and inguinal depots are under the skin on the back and flank and might correspond to the depots that expand in the “pear” form of human obesity. In this study, the most striking differences were not between the visceral and subcutaneous depots but rather between one visceral depot (mesenteric) and all the others. Whereas most depots in CSS-7 mice were small relative to the other strains, the mesenteric depot tended to be larger. This finding is similar to the results of other studies measuring the effects of chromosome 7 on fat distribution in mice (16,17,21) and suggests these differences in fat distribution are reliable and biologically meaningful. The mesenteric depot traces the intestines and has more lymph nodes as well as other cell types associated with the immune system than other depots (22). Selective hypertrophy of the mesenteric depot is associated with chronic stress or inflammation (23,24).
There are disadvantages and advantages to using CSSs for QTL mapping. When QTLs are not detected, the result may be due to a lack of allelic loci which contribute to individual differences (i.e., no QTLs) or it could reflect a balance of “plus” and “minus” effects on the substituted chromosome. This possibility is one drawback to the use of these strains for QTL evaluation. However there is an important advantage to chromosome substitution studies: when QTLs are found in an F2 or backcross population, and the QTLs replicate in a CSS F2 strain, it suggests that the QTLs do not require a mixed genetic background for their expression. This step is an important one before investigators proceed to make and test congenic mouse strains, e.g., Ref. (25).
METHODS AND PROCEDURES
Mice
Joseph Nadeau provided C57BL/6J-Chr7129S1/SvImJ/Na CSS N11F4 mice (CSS-7), which were used to breed N11F5 and N11F6 mice for the experiment reported here. Genetic composition of the CSS-7 strain was conformed by genotyping markers on chromosome 7 (A.A. Bachmanov, M. Avigdor, N. Bosak et al., unpublished data). Mice from the progenitor C57BL/6J (B6; stock no. 000664) strain were purchased from the Jackson Laboratory (Bar Harbor, ME) and used to produce inbred mice. The CSS-7 (18 female/33 male) and B6 (20 female/22 male) mice were bred and measured during the same period of time. All animals were kept in a temperature- and humidity-controlled vivarium at 23 ± 2 °C in a 12/12-h light/dark photoperiod with lights on at 7:00 am Mice were housed in polypropylene cages (33 × 20 × 20 cm) with stainless steel wire tops and provided with Harlan Teklad Rodent diet 8604 (24% protein, 4% fat) and water ad libitum. The pups in each litter were counted a few days after birth. Mice were weaned at 3 weeks of age and housed with their littermates of the same sex until age of ~8 weeks, when they were housed alone to conduct an ethanol preference test (26). The weight gain of mice when ethanol was available to drink was not affected by the 129 chromosome substitution. All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Monell Chemical Senses Center.
Body composition
Mice were euthanized between 84 and 127 days of age (mean ± s.d., 102 ± 10 days) and their body weight and body length were measured, as well as and body composition was determined by dual-energy X-ray absorptiometry (no. 51601; GE Medical Systems Lunar, Madison, WI). The mice were dissected and the five organs (brain, heart, liver, kidney, spleen) and five bilateral adipose depots (gonadal, retroperitoneal, mesenteric, inguinal, and subscapular) were removed and weighed. We also computed several common indices of mouse fatness for comparison with the results of earlier studies.
Data analysis
Data were analyzed using a two-way ANOVA using strain and sex as factors, followed by LSD post hoc tests. Other analyses were done which also examined age and litter size as covariates, but because the results were essentially the same as the ANOVA, they are not included for simplicity. When there was a main effect of strain, we considered that one or more QTLs were detected for that trait. When significant sex-by-strain interactions were detected, the QTL was considered sex-specific, and post hoc tests determined which sex was most affected. In cases where there was no significant strain-by-sex interaction, but same-sex strains differed by post hoc analysis, only for one sex did we refer to these QTLs as sex-dependent. We used a P value of 0.05 as the criterion for significance. All data analyses were conducted with STATISTICA 6.1 (StatSoft, Tulsa, OK).
Acknowledgments
Grants from the National Institutes of Health funded this research (R01DK 058797 to D.R.R. and R01AA011028 to A.A.B.). Joseph Nadeau kindly provided breeding pairs of the chromosome substitution mouse strain. The advice of David B. West and Craig H. Warden is gratefully acknowledged. Michael G. Tordoff, Gary K. Beauchamp, and Patricia Watson commented on drafts of the manuscript. Maureen P. Lawler conducted bioinformatics searches, and her assistance is appreciated. Preliminary work assessing chromosome 7 obesity QTLs was done in the laboratories of Maja Bucanories and R. Arlen Price.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Wright S. General, group and special size factors. Genetics. 1932;17:603–619. doi: 10.1093/genetics/17.5.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark FH. Correlation and body proportions in mature mice of the genus peromyscus. Genetics. 1941;26:283–300. doi: 10.1093/genetics/26.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenney-Hunt JP, Vaughn TT, Pletscher LS, et al. Quantitative trait loci for body size components in mice. Mamm Genome. 2006;17(6):526–37. doi: 10.1007/s00335-005-0160-6. [DOI] [PubMed] [Google Scholar]
- 4.Castle W, Gates WH, Reed SC, Law LW. Studies of a size cross in mice, II. Genetics. 1936;21:310–323. doi: 10.1093/genetics/21.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danforth C. Hereditary adiposity in mice. J Hered. 1927;18:153–162. [Google Scholar]
- 6.Eppig JT, Bult CJ, Kadin JA, et al. The Mouse Genome Database (MGD): from genes to mice—a community resource for mouse biology. Nucleic Acids Res. 2005;33:D471–D475. doi: 10.1093/nar/gki113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wuschke S, Dahm S, Schmidt C, Joost HG, Al-Hasani H. A meta-analysis of quantitative trait loci associated with body weight and adiposity in mice. Int J Obes (Lond) 2007;31(5):829–841. doi: 10.1038/sj.ijo.0803473. [DOI] [PubMed] [Google Scholar]
- 8.Reed D, Ding Y, Xu W, Cather C, Price RA. Human obesity does not segregate with the chromosomal regions of Prader-Willi, Bardet-Biedl, Borjeson or Wilson-Turner syndromes. Int J Obes Relat Metab Disord. 1995;19:599–603. [PubMed] [Google Scholar]
- 9.Reed DR, McDaniel AH, Li X, Tordoff MG, Bachmanov AA. Quantitative trait loci for individual adipose depot weights in C57BL/6ByJ x 129P3/J F(2) mice. Mamm Genome. 2006;17(11):1065–1077. doi: 10.1007/s00335-006-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reed DR, Li X, McDaniel AH, et al. Loci on chromosomes 2, 4, 9, and 16 for body weight, body length, and adiposity identified in a genome scan of an F2 intercross between the 129P3/J and C57BL/6ByJ mouse strains. Mamm Genome. 2003;14(5):302–313. doi: 10.1007/s00335-002-2170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abiola O, Angel JM, Avner P, et al. The nature and identification of quantitative trait loci: a community’s view. Nat Rev Genet. 2003;4(11):911–916. doi: 10.1038/nrg1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nadeau JH, Singer JB, Matin A, Lander ES. Analysing complex genetic traits with chromosome substitution strains. Nat Genet. 2000;24(3):221–225. doi: 10.1038/73427. [DOI] [PubMed] [Google Scholar]
- 13.Silver LM. Mouse Genetics: Concepts and Applications. Oxford University Press; New York: 1995. p. 376. [Google Scholar]
- 14.Ishimori N, Li R, Walsh KA, et al. Quantitative trait loci that determine BMD in C57BL/6J and 129S1/SvImJ inbred mice. J Bone Miner Res. 2006;21(1):105–112. doi: 10.1359/JBMR.050902. [DOI] [PubMed] [Google Scholar]
- 15.Dhar M, Webb LS, Smith L, et al. A novel ATPase on mouse chromosome 7 is a candidate gene for increased body fat. Physiol Genomics. 2000;4(1):93–100. doi: 10.1152/physiolgenomics.2000.4.1.93. [DOI] [PubMed] [Google Scholar]
- 16.Diament AL, Warden CH. Multiple linked mouse chromosome 7 loci influence body fat mass. Int J Obes Relat Metab Disord. 2004;28(2):199–210. doi: 10.1038/sj.ijo.0802516. [DOI] [PubMed] [Google Scholar]
- 17.York B, Truett AA, Monteiro MP, et al. Gene-environment interaction: a significant diet-dependent obesity locus demonstrated in a congenic segment on mouse chromosome 7. Mamm Genome. 1999;10(5):457–462. doi: 10.1007/s003359901023. [DOI] [PubMed] [Google Scholar]
- 18.Ishimori N, Li R, Kelmenson PM, et al. Quantitative trait loci that determine plasma lipids and obesity in C57BL/6J and 129S1/SvImJ inbred mice. J Lipid Res. 2004;45(9):1624–1632. doi: 10.1194/jlr.M400098-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Vague J. Importance of the measurement of fat distribution in pathology. Bull Mem Soc Med Hop Paris. 1950;66(31–32):1572–1574. [PubMed] [Google Scholar]
- 20.Pond CM. The fats of life. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- 21.Warden CH, Fisler JS, Shoemaker SM, et al. Identification of four chromosomal loci determining obesity in a multifactorial mouse model. J Clin Invest. 1995;95:1545–1552. doi: 10.1172/JCI117827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen P, McCarthy JC. The effects of selection for high and low body weight on the proportion and distribution of fat in mice. Anim Prod. 1980;31:1–11. [Google Scholar]
- 23.Pond CM. Paracrine relationships between adipose and lymphoid tissues: implications for the mechanism of HIV-associated adipose redistribution syndrome. Trends Immunol. 2003;24(1):13–18. doi: 10.1016/s1471-4906(02)00004-2. [DOI] [PubMed] [Google Scholar]
- 24.Sadler D, Mattacks CA, Pond CM. Changes in adipocytes and dendritic cells in lymph node containing adipose depots during and after many weeks of mild inflammation. J Anat. 2005;207(6):769–781. doi: 10.1111/j.1469-7580.2005.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bevova MR, Aulchenko YS, Aksu S, Renne U, Brockmann GA. Chromosome-wise dissection of the genome of the extremely big mouse line DU6i. Genetics. 2006;172(1):401–10. doi: 10.1534/genetics.104.040196. [DOI] [PMC free article] [PubMed] [Google Scholar]