Abstract
Background
Maltreatment represents a major stressor in the lives of many youth. Given the known effects of stress exposure on subsequent biological stress response systems, researchers have been interested in the effects of maltreatment on the functioning of these systems. Experimental studies reveal that previous exposure to stress affects the symmetry between components of the physiological stress response to subsequent stress. The present study examined asymmetry between salivary alpha amylase (sAA), a sympathetic indicator, and cortisol reactivity to a social stressor among maltreated and comparison youth age 9 to 14 years. Consistent with earlier studies suggesting that stress leads to asymmetry between hypothalamic-pituitary-adrenal axis and sympathetic nervous system activity, we expected that maltreated youth would exhibit greater sAA-cortisol asymmetry than would comparison youth.
Methods
Forty-seven maltreated and 37 comparison youth visited the lab and engaged in a social stress protocol. We collected 2 saliva samples before the stressor and 4 after, at 0 minutes post stress and every 10 minutes for 30 minutes.
Results
Maltreatment status moderated the relation between sAA and cortisol activity in response to the stressor. Comparison youth showed significant links between the sAA and cortisol responses; maltreated youth had no significant associations between responses in the two biomarkers.
Conclusion
The data were consistent with sAA-cortisol asymmetry among maltreated youth. Further research should seek to replicate this finding and investigate its implication for developmental trajectories.
The present study examined the relation between child maltreatment and asymmetry between salivary alpha-amylase (sAA) and cortisol responses to stress. Given connections between the HPA axis and the SNS, one would anticipate symmetry between these systems, meaning that relatively strong responses in one system should correspond with relatively strong responses in the other. Recently researchers have suggested that the degree of asymmetry between biological stress systems may have implications for developmental outcomes (Bauer, Quas, and Boyce, 2002). Factors affecting degree of asymmetry between these biological stress systems remain poorly understood.
The biological stress response consists primarily of two systems: The locus cereleus-norepinephrine/sympathetic nervous system (LC-NE/SNS) and the hypothalamic-pituitary-adrenal (HPA) axis (Chrousos and Gold, 1992). The LC-NE/SNS is responsible for effects often referred to as the “fight or flight” response, which include increased cardiovascular tone, faster breathing rate, and increased blood flow to muscles (Cannon, 1914). The HPA axis involves multiple steps, including the release of corticotropin releasing hormone (CRH) from the hypothalamus, triggering the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary, causing the release of the steroid hormone cortisol from the adrenal glands.
The negative impact of maltreatment on psychological/behavioral development may be at least partially due to an altered stress response. Childhood trauma in general and maltreatment in particular are linked with alterations in both the HPA axis and in the LC-NE/SNS. Maltreated youth have demonstrated reduced stress reactivity in the HPA axis (Hart, Gunnar, and Cicchetti, 1995). One theory is that chronic stress leads to attenuation of the stress response (Goldstein & McEwen, 2002; Susman, 2006). Though few studies have examined the effect of maltreatment on the functioning of the LC-NE/SNS among children, some researchers report elevated urinary catecholamine levels among maltreated and traumatized children (De Bellis, Baum, Birmaher, et al. 1999; De Bellis, Lefter, Trickett, and Putnam, 1994). Thus, some research links maltreatment to alterations of both the HPA axis and the LC-NE/SNS, though the specific nature of the impact of maltreatment on stress reactivity remains unclear.
The HPA axis and the SNS are connected at multiple neural levels, and thus activity in these two systems should demonstrate some degree of symmetry (Chrousos and Gold, 1992; Lovallo and Thomas, 2000; Young, Abelson, and Cameron, 2005). Factors leading to individual differences in the degree of symmetry between these systems remain unclear. One possible factor may be different habituation rates of these systems given previous exposure to stress. Some evidence suggests faster habituation rates in the HPA axis (Gerra, Zaimovic, and Mascetti, 2001; Schommer, Hellhammer, and Kirschbaum, 2003). Schommer et al. (2003) and Gerra et al. (2001) both found decreased ACTH and cortisol responses over successive repetitions of the Trier Social Stress Test (TSST; Kirschbaum, Pirke, and Helhammer, 1993), but unchanged responses in level of epinephrine and NE. Animal research also suggests that prolonged stress may lead to asymmetry between these systems (Britton, Segal, Kuczenski, and Hauger, 1992).
Whereas experimental manipulations suggest that repeated stressors may lead to asymmetry between the HPA axis and the SNS, we know little about the effects of real life stressors on the symmetry between activity in these two systems in response to subsequent stress. If previous exposure to stress leads to asymmetry between the systems, we might expect individuals with a history of significant stress to show more asymmetry. This asymmetry may be important for understanding the impact of stress on atypical development. Recently, researchers have argued that examination of each stress system alone limits what we know about the role of the stress response in atypical development (Bauer et al., 2002). Each system independently has been linked to developmental trajectories in psychological and behavioral characteristics. Activity in the HPA axis covaries with aggression, disruptive behavior, depression, and inhibited behavior (e.g., De Souza, 1995; Duval et al., 2006; Shirtcliff et al., 2005; Shoal et al., 2003). Autonomic arousal has been associated with aggression, delinquency, and antisocial behavior (Lorber, 2004; Ortiz and Raine, 2004). Bauer et al. suggest that beyond relations with each system independently, we will learn more about the role of the biological stress response in mediating developmental outcomes if we consider the functioning of these systems together and examine the relations between them.
Examination of the HPA axis is long-established through salivary cortisol (Kirschbaum, Bartussek, and Strasburger, 1992). Recently, a marker of autonomic activity has been possible via salivary alpha-amylase (sAA), an enzyme released by the parotid gland (Granger, Kivlighan, El-Sheikh, Gordis, and Stroud, 2007). Protein release into the saliva is primarily controlled by sympathetic arousal, and in particular by beta-adrenergic activity (Bosch, de Geus, Veerman, Hoogstraten, and Amerongen, 2003). In addition, parasympathetic activity appears to augment release of salivary proteins, including sAA, due to the effect of parasympathetic activity on salivary flow rate (Bosch et al., 2003; Gjörstrup, 1979). Animal studies have demonstrated that stimulation of the sympathetic system leads to increased secretion of sAA in the saliva in the presence of parasympathetic activity (Gjörstrup, 1979). In humans, sAA increases in response to stress (Bosch et al., 2003; Nater, Rohleder, Gaab, et al., 2005; Rohleder, Nater, Wolf, Ehlert, and Kirschbaum, 2004). Some research shows that sAA correlates with plasma NE (Chatterton, Vogrldon, Ellman, and Hudgens, 1996), though this link has not been completely consistent (Nater, La Marca, Florin, et al., 2006). Stress-dependent release of sAA correlates with cardiovascular autonomic measures (Bosch et al., 2003). Beta adrenergic antagonist propranolol leads to lower levels of sAA at rest and in response to a stressor (van Stegeren, Rohleder, and Everaerd, 2006). Thus, saliva can provide indices of both SNS and HPA axis activity.
The present study examined the symmetry between HPA and SNS activity in maltreated and comparison adolescents. We expected that indices of HPA and SNS activity in response to stress would correlate positively among youth with no history of maltreatment. Consistent with the idea that stress may cause asymmetry between the systems, we expected that maltreated youth would demonstrate lower correlations between cortisol and sAA indices, and that maltreatment status would moderate the link between cortisol and sAA response to stress.
Materials and Methods
Participants
Eighty-four adolescents were selected from a larger, longitudinal study of the effects of maltreatment on adolescent development. Youth visited the lab with a parent or guardian for the first or second of three yearly interviews. Maltreated youth were recruited from select zip codes in a large urban area on the basis of recent (within the previous 2 months) referrals to the Department of Children and Family Services (DCFS) for child maltreatment (physical or sexual abuse or neglect). Comparison youth were recruited from the same or comparable census blocks. We excluded youth who used medications that would interfere with cortisol or sAA measures (e.g., synthetic corticosteroids or beta adrenergic agonists) or who reported smoking. In addition, based on parents’/guardians’ reports, no child had a history of cancer, diabetes, or thyroid problems. In the final sample of 84 adolescents, the mean age was 12.1 years (SD = 1.2, range = 9.1-14.5). The maltreated group included 47 youth (26 male), and the comparison group included 37 youth (18 male). Demographic information regarding the sample appears in Table 1. Ethnic composition did not differ by maltreatment status. Among the maltreated youth, 50% had been referred to DCFS for physical abuse, 34.2 for sexual abuse, 31.5 for emotional abuse, and 63.2 for neglect. Consistent with these percentages, most youth (78.9%) had more than one referral to DCFS (mean number of referrals = 3.8, SD = 2.7, range = 1-12, median = 3).
Table 1.
Demographic Information
| Comparison | Maltreated | |
|---|---|---|
| Yearly Family Income | ||
| Less than $30,000 | 17 | 34 |
| $30,000-60,000 | 13 | 8 |
| More than $60,000 | 7 | 5 |
| Parent’s Education | ||
| Less than high school | 4 | 22 |
| High School Diploma or GED | 11 | 9 |
| Some College | 9 | 12 |
| College degree or advanced degree | 13 | 4 |
| Ethnicity | ||
| Black | 45.9% | 38.3% |
| Caucasian | 8.1 | 12.8 |
| Latino/Latina | 37.8 | 40.4 |
| Other/Multiple ethnicities | 8.1 | 8.5 |
| Parent/Guardian | ||
| Biological or Adoptive Parent | 34 | 26 |
| Step-parent | 1 | 3 |
| Other family member | 2 | 9 |
| Foster parent | 0 | 9 |
Procedures
All procedures were approved by the university institutional review board and were carried out with the written understanding and assent of the adolescent participants and consent of their parents or guardians. Each adolescent and a parent or guardian attended a lab session lasting approximately 4 hours. As part of the procedures, adolescents engaged in a modified version of the TSST (Kirschbaum et al., 1993). During this procedure, the interviewer and a panel of 2 judges entered the room. The interviewer told the participant that he or she would read the beginning of a story, and the participant would have 5 minutes to develop the next part of the story, after which the participant would present the next part of the story to the panel of judges for 4 minutes. The interviewer and judges then left the room for 5 minutes. When they re-entered, the interviewer asked the adolescent to present the story. If the youth did not fill the 4 minutes, the interviewer used a standard set of prompts to encourage him or her to continue. After the 4 minutes, the interviewer asked the youth to perform a serial subtraction task before the judges. This task was designed to be challenging for the particular age of the adolescent (e.g., subtracting 7 serially beginning with 758).
We collected saliva samples 6 times, twice before and 4 times after the TSST. The first sample occurred 45 minutes before the stressor. The second sample was 10 minutes before the stressor, immediately after a 5 minute relaxation protocol involving listening to soft music while viewing a still slide of a beach scene. The third sample occurred immediately after the stressor, and the fourth, fifth, and sixth samples occurred 10, 20, and 30 minutes after the end of the stressor, respectively. We collected saliva samples via passive drool through a short straw into a vial. Data collection occurred primarily in the afternoon, with 96% of participants providing the baseline sample between 1200h and 1700h. The earliest baseline sample was collected at 1223h, and the latest was collected at 1735h. We control for time of day in regression analyses. Saliva samples were immediately frozen.
Samples were transported on ice to Penn State University and stored frozen at -80°C until assayed for cortisol and sAA. On the day of testing, all samples were centrifuged at 3000 rpm for 15 minutes to remove mucins.
α-Amylase
Samples were assayed for sAA using a commercially available kinetic reaction assay (Salimetrics, State College PA). The assay employs a chromagenic substrate, 2-chloro-p-nitrophenol, linked to maltotriose. The enzymatic action of sAA on this substrate yields 2-chloro-p-nitrophenol, which can be spectrophotometrically measured at 405 nm using a standard laboratory plate reader. The amount of α-amylase activity present in the sample is directly proportional to the increase (over a 2 minute period) in absorbance at 405 nm. Results are computed in U/mL of α-amylase using the formula: [Absorbance difference per minute × total assay volume (328 ml) × dilution factor (200)]/ [millimolar absorptivity of 2-chloro-p-nitrophenol (12.9) × sample volume (.008 ml) × light path (.97)]. Intrassay variation (CV) computed for the mean of 30 replicate tests was less than 7.5%. Inter-assay variation computed for the mean of average duplicates for 16 separate runs was less than 6%.
Cortisol
Samples were assayed for salivary cortisol using a highly-sensitive enzyme immunoassay US FDA (510k) cleared for use as an in vitro diagnostic measure of adrenal function (Salimetrics, State College, PA). The test used 25 μl of saliva (for singlet determinations), had a lower limit of sensitivity of .007 μg/dl (.19 nmol/L), range of sensitivity from .007 to 1.8 μg/dl (.19 to 49.7 nmol/L), and average intra-and inter-assay coefficients of variation of less than 5% and 10%, respectively .
Statistical Analyses
We conducted data analyses using SPSS statistical analysis software (version 15.0 for Windows). We first examined the effect of the TSST on cortisol and sAA levels. We conducted repeated measures ANCOVAs to examine whether level of cortisol or sAA varied by saliva sample. We examined the within subjects effect of saliva sample using Wilks’ lambda, as suggested by Stevens (1979). Because the data violated sphericity assumptions, we used multivariate tests, which do not require sphericity, unlike the univariate tests. Covariates included maltreatment status, time of day, sex, and pubertal stage, assessed via self- and parent/guardian-reported Tanner staging (Marshall and Tanner, 1969; 1970), which has been shown to demonstrate reasonable agreement with nurse practitioner ratings (Dorn, Susman, Nottelmann, Inoff-Germain, and Chrousos, 1990). In addition, we found higher sAA levels among African-American youth vs. Latino/Latina and Caucasian youth. Thus, we also controlled for ethnicity (African-American vs. Latino/Latina and White) in the regression analyses, though this did not affect the substantive findings.
Because sAA and cortisol show a time differential in response to stressors, we examined several indices of salivary cortisol and sAA. Thus, to examine whether overall HPA and autonomic activity and relative response to stress are correlated between the two systems, we calculated area under the curve scores with respect to ground (AUCG) and with respect to increase (AUCI) according to formulas provided by Pruessner, Kirschbaum, Meinlschmid, and Helhammer (2003). The AUCG and AUCI allow examination of summary scores over time, reflecting total output and total response to the stressor, respectively. These scores are based on trapezoid formulas. Specifically, AUCG is calculated by summing the areas of the trapezoids under the plot of the biomarker level at each time point for each case. AUCI is calculated by subtracting the rectangular area representing baseline from the AUCG score. We also examined baseline levels, peak values, and raw change from baseline to peak in both systems.
To examine whether the data are consistent with maltreatment status as a moderator of the symmetry between responses of cortisol and sAA, we conducted multiple regression analyses. Specifically, for each index of cortisol and sAA, we examined whether the interaction between maltreatment status and the sAA index accounted for variance in the cortisol index after including covariates and main effects of sAA and maltreatment status. Covariates included time of day, wave of visit, sex, pubertal stage, and ethnicity. We regressed each cortisol index onto the centered sAA index, maltreatment (-1 = comparison, +1 = maltreated), and the interaction between maltreatment and sAA, after controlling for covariates. To address positive skew in cortisol indexes (skew = 3.5, 2.0, and 1.6, respectively for baseline, peak and AUCG; SD = .27), we conducted log transformations of cortisol baseline, peak and AUCG. We conducted square root transformations of sAA baseline, peak, and AUCG as these indexes were moderately skewed (skew = 1.1, .8, and .8, respectively; SD = .27). In addition, to eliminate multivariate outliers, we eliminated cases with centered leverage values greater than 3k/n (where k = the number of predictors and n = the sample size) for each equation, as suggested by Cohen, Cohen, West, and Aiken (2003) for regression analyses with small samples. Elimination of multivariate outliers resulted in dropping one case in the equation predicting change in cortisol and two cases in predicting cortisol AUCI. To understand significant interaction effects, we compared correlation coefficients in the separate groups using Fisher’s R transformation to Z.
Results
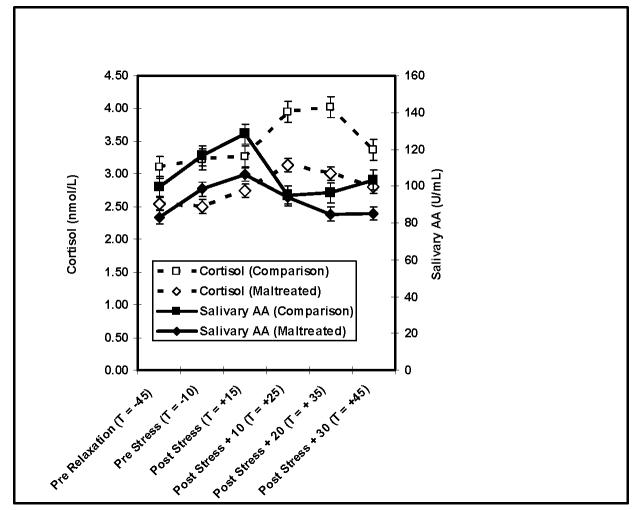
Response curves among maltreated and comparison youth appear in Figure 1. Cortisol and sAA have different response times, with cortisol responding to and recovering from stress more gradually, and sAA responding more immediately and recovering more quickly. For sAA, repeated measures analysis of variance revealed a significant within subjects main effect for saliva sample (Wilks’ λ = .83, Exact F (5,74) = 2.99, p < .05). Levels were lowest at pre-relaxation, increased after relaxation, peaked immediately post stressor, and returned to baseline levels by 10 minutes post stressor. A majority of the sample, 63.1%, had greater than 10% increases in sAA from baseline to post-stress peak.
Figure 1. Cortisol and sAA at Pre Relaxation (T = -45), Pre Stress (T = -10), and at 0 (T = +15), 10 (T = +25), 20 (T = +35), and 30 (T = +45) Min Post Stress in Maltreated and Comparison Youth.
Note: For sAA, repeated measures ANCOVA (within subjects factor saliva sample controlling for group, time of day, pubertal status, and ethnicity) Wilks’ λ = .83, Exact F (5,74) = 2.99, p < .05). Levels were lowest at pre-relaxation, increased after relaxation, peaked immediately post stressor, and returned to baseline levels by 10 minutes post stressor. For cortisol, repeated measures ANCOVA (within subjects factor saliva sample controlling for group, time of day, pubertal status, and ethnicity) Wilks’ λ = .88, Exact F (5,74) = 1.97, p < .10). Cortisol peaked at 10 minutes post-stressor and returned to baseline levels at 20 minutes post-stressor.
Repeated measures analysis of variance revealed an effect for time of sample on cortisol as well. Examination of the within subjects effect of saliva sample across all 6 samples revealed a marginally significant effect for saliva sample (Wilks’ λ = .88, Exact F (5,74) = 1.97, p < .10). A follow-up repeated measures ANCOVA examining change over time only during the linear rise during from pre-relaxation to 10 minutes post stressor, when responses peaked on average, revealed a significant effect for time (Wilks’ λ = .89, Exact F (3,76) = 3.17, p < .05). Cortisol peaked at 10 minutes post-stressor and returned to baseline levels at 20 minutes post-stressor. Similar to sAA, 64.5% of the sample had cortisol increases of great than 10% from baseline to post-stress peak.
Regression analyses reveal that in equations predicting cortisol AUCI, baseline, peak, and raw change scores, the maltreatment status by corresponding sAA index interaction was significant, consistent with the hypothesis that maltreatment status moderates the relation between cortisol and sAA activity. In the equation predicting cortisol AUCG, the interaction term was marginally significant. Correlations among biomarker indices across the whole sample of youth appear in Table 2. Several corresponding cortisol and sAA indices are correlated across the sample, including AUCI, baseline, and change. For each biomarker, the different indices are correlated with each other. Regression analyses appear in Table 3. Table 4 presents correlations between corresponding cortisol and sAA indexes among maltreated and comparison youth. Consistent with the hypothesis, among the comparison youth, the indexes are significantly correlated, whereas among the maltreated youth the correlations are not significant. Comparison of these correlation coefficients reveals that they are significantly different between the two groups. To illustrate the interaction, scatterplots of the relation between peak cortisol and peak sAA among maltreated and comparison youth appear in Figure 2. In these plots, peak cortisol is log transformed and peak sAA is square root transformed.
Table 2.
Parital Correlations Among sAA and Cortisol Indices
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. sAA-AUCG | -- | ||||||||
| 2. sAA-AUCI | .31** | -- | |||||||
| 3. Baseline sAA | .74** | -.38** | -- | ||||||
| 4. Peak sAA | .96** | .38** | .64** | -- | |||||
| 5. sAA Change | .43** | .92** | -.23* | .58** | -- | ||||
| 6. Cort-AUCG | .15 | -.07 | .20+ | .15 | -.00 | -- | |||
| 7. Cort-AUCI | .07 | .30* | -.10 | .15 | .36** | .10 | -- | ||
| 8. Baseline Cort | .05 | -.26* | .23* | .01 | -.23* | .77** | -.46** | -- | |
| 9. Peak Cort | .11 | .01 | .13 | .14 | .08 | .86** | .34** | .46** | -- |
| 10. Cort Change | .05 | .19+ | -.06 | .13 | .27* | .28* | .89** | -.29** | .61** |
p < .05.
p < .01.
p < .10.
Note: df = 76 for all coefficients. Coefficients control for maltreatment status, time of day, ethnicity, pubertal status, sex, and wave of visit. sAA = Salivary alpha-amylase. Cort= Cortisol. AUCG = Area under the curve with respect to ground. AUCI = Area under the curve with respect to increase.
Table 3.
Regression Equations Predicting Cortisol from sAA, Maltreatment, and Maltreatment by sAA Interactions
| β | T | F | Adj R2 | |
|---|---|---|---|---|
| Cortisol AUCI | ||||
| sAA AUGI | .42 | 3.91** | ||
| Maltreatment status | -.14 | -1.25 | ||
| sAA AUCI by Maltreatment Status | -.42 | -3.88** | ||
| Equation | 8.49a** | .25 | ||
| Cortisol AUCG | ||||
| sAA AUGG | .18 | 1.59 | ||
| Maltreatment status | -.19 | -1.60 | ||
| Salivary A-A AUCG by Maltreatment Status | -.21 | -1.96+ | ||
| Equation | 3.04b* | .10 | ||
| Baseline Cortisol | ||||
| Baseline sAA | .33 | 2.79** | ||
| Maltreatment Status | .04 | .35 | ||
| Baseline sAA by Maltreatment Status | -.32 | -2.80** | ||
| Equation | 4.12b** | .14 | ||
| Peak Cortisol | ||||
| Peak sAA | .20 | 1.69 | ||
| Maltreatment Status | -.17 | -1.49 | ||
| Peak sAA by Maltreatment Status | -.24 | -2.26* | ||
| Equation | 3.41b* | .10 | ||
| Cortisol Change | ||||
| sAA Change | .29 | 2.70* | ||
| Maltreatment status | -.09 | -.78 | ||
| sAA Change by Maltreatment Status | -.33 | -3.14** | ||
| Equation | 6.43c** | .19 |
p < .05.
p < .01.
p < .10.
df = 3,73
df = 3,75
df = 3,74
Note: Equations control for pubertal status, sex, time of day, ethnicity (African-American vs. Latino/Latina or white) and wave of visit. To reduce positive skew, cortisol AUCG was subjected to log transformation, and sAA AUCG was subjected to square root transformation. Predictors were centered. Maltreatment status coded as -1 = comparison and +1 = maltreated. sAA and cortisol change are raw change from baseline to post-stress peak levels.
Table 4.
Correlations between corresponding cortisol and sAA indices
| Comparison (n = 37) | Maltreated (n = 47) | |
|---|---|---|
| AUCI | .48a** | .11a |
| AUCG | .37a* | -.04a |
| Baseline | .44a** | -.01a |
| Peak | .41a* | -.06a |
| Change (Baseline to Peak) | .51b** | .01b |
p < .05.
p < .01.
AUCI = Area under the curve with respect to increase.
AUCG = Area under the curve with respect to ground.
coefficients sharing this subscript differ at p < .05 (one-tailed).
coefficients sharing this subscript differ at p < .01 (one-tailed).
Figure 2. Scatterplots of the Relation between Peak Cortisol and Peak sAA among Maltreated and Comparison Youth.
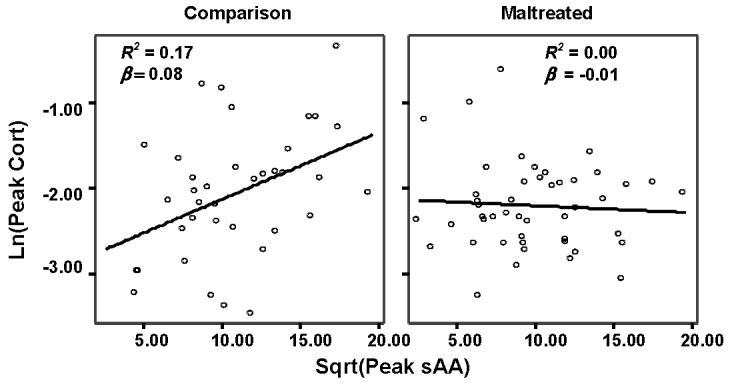
Note: Fitted lines reflect linear regression lines. Peak cortisol is log transformed. Peak sAA is square root transformed.
Discussion
The results of this study suggest that among comparison youth, sAA and cortisol responses to stress demonstrate symmetry, whereas among maltreated youth, they demonstrate asymmetry. Among comparison youth, sAA baseline and peak levels, AUC scores with respect to both ground and increase, and change from baseline to peak are correlated with corresponding indexes in cortisol. The correlations suggest that among comparison youth, the magnitude of the response to stress in the ANS covaries with the magnitude of response in the HPA axis. In contrast, among maltreated youth, responses across the two systems are uncorrelated, suggesting asymmetry. Regression analyses reveal significant or marginally significant sAA index by maltreatment status interactions in predicting the corresponding cortisol index, consistent with the hypothesis that asymmetry between cortisol and sAA response is moderated by maltreatment status. Whereas the overall activity of the two systems in response to the TSST appears to demonstrate some symmetry, as indicated by the correlations between these systems in the whole sample, the maltreatment by sAA interactions and follow-up correlations reveal that the cortisol and sAA indexes were correlated only in the comparison group. Among the maltreated adolescents, the systems demonstrate asymmetry. These data are consistent with the notion that maltreatment may cause asymmetry between these systems, possibly by interrupting the connections between them or by causing attenuation in one system but not the other (Ressler and Nemeroff, 2000).
Given the connections between the two systems, symmetry between their activity makes sense. For example, the lateral paraventricular nucleus (PVN) connects to areas in the hindbrain responsible for sympathetic activity via neurons that secrete CRH (Chrousos and Gold, 1992). Catecholaminergic pathways project from the LC to the PVN (Brown et al., 1982; Chrousos and Gold, 1992; Dunn and Berridge, 1990). Experimental manipulations support the interconnectedness of these systems. For example, administration of CRH increases catecholaminergic activity and norepinephrine (NE) levels (Brown, Fisher, Speiss, Rivier, and Vale, 1982; Dunn and Berrdige, 1990). CRH antagonists cause reduced responsiveness in the LC-NE system (Dunn and Berridge, 1990). NE stimulates the release of CRH in the PVN (Brown et al., 1982; Dunn and Berridge, 1990), and β-adrenergic blockers reduce the behavioral consequences of CRH (Chrousos and Gold, 1992; Dunn and Berridge, 1990).
Despite these connections, the two systems may be activated by different kinds of stressors and may respond to stress differently over time. Some research suggests that CRH pathways function differently in responding to metabolic versus psychological stressors, and thus that CRH may affect the autonomic nervous system (ANS) under conditions of metabolic but not psychological stress (Malarkey, Lipkus, and Cacioppo, 1995). Moreover, the HPA axis may be particularly sensitive to fear and frustration, whereas the ANS may be more generally responsive (Frankenhaeuser, 1982; Lovallo and Thomas, 2000).
Chronic stress may have different implications for the HPA axis and ANS and may thus affect the degree of symmetry between them. Chronically elevated levels of cortisol and CRH may promote long term changes in the functioning of CRH in response to stress (Tarullo and Gunnar, 2006). Given connections between CRH and the ANS, these changes may have implications for the relation between the HPA axis and the ANS. Experimental manipulations have revealed that repeated stress leads to asymmetry between the two systems. Some researchers report attenuated responses in the HPA axis to experimental manipulations but not in the LC-NE/SNS (Gerra et al., 2001; Schommer et al., 2003). In our sample, peak cortisol levels in the maltreated group were marginally lower than peak cortisol levels in the comparison group (F(1,78) = 3.40, p = .07), whereas no such difference emerged in sAA. In contrast, some researchers have found a decreased response over time in the SNS, with sustained HPA axis responding (Britton et al., 1992). Beyond effects of lab-based stressed exposure on these stress response systems, different types of psychopathology that are linked with stress and maltreatment may relate to different kinds of alterations in the stress response systems (Gunnar and Quevedo, 2007). Understanding the role that alterations in both stress response systems and their degree of symmetry play in the link between maltreatment and different forms of psychopathology is an important future direction.
Though our data do not clarify the cause of asymmetry between the systems or the role of CRH in each of these systems, the asymmetry of these systems among the maltreated youth may be due to a reduced response in the HPA axis but not in the LC-NE/SNS. A reduced responsiveness to stress among maltreated youth would be consistent with the hypothesis that stress systems might attenuate in responsivity due to allostatic load (Goldstein and McEwen, 2002; Susman, 2006). As Susman notes, a downregulation of the stress response would protect the individual from chronic energy expenditure and arousal in the face of repeated stressors. Given the potential harmful effects of chronically high levels of cortisol, an attenuation in the responsiveness of the HPA axis in the face of chronic stress may be protective (Fries, Hesse, Hellhammer and Hellhammer, 2005; Goldstein & McEwen, 2002; Sapolsky, 2004). Consistent with lab based studies (Gerra et al. 2001; Schommer et al., 2003), data consistent with such attenuation occurred in the HPA axis but not the LC-NE/SNS, which may be more sensitive to certain types of arousal and more resistant to attenuation.
Replication of these findings will be important before we can understand the implications of the asymmetry for maltreated youth. Different profiles of symmetry or asymmetry in HPA axis/SNS activity may have different implications for behavioral outcomes (Bauer et al., 2002). For example low or high activity in both systems may put youth at risk for aggression or anxiety, respectively. Further studies should examine whether asymmetry is a risk factor for negative outcomes. Conversely, asymmetry may protect youth who have extremely high or low reactivity in one system. A moderate response in one system may serve a protective function among youth with extremely high or low reactivity in the other stress response system.
The study has several limitations. First, the small sample size and cross-sectional design of the study limit power to test interactions and differences in effect sizes between groups. In addition, cortisol and sAA response curves to stress are timed differently. Thus, examination of their asymmetry must consider response over time. The AUC ground and change scores reflect ways to examine the relation between the sum totals of each system in response to stress. The small sample size limits our ability to examine asymmetry regarding other characteristics of the response curves over time in cortisol and sAA. The sample size also precludes our examining whether psychopathology plays a role in HPA and SNS patterns of responding to stressors. The non-experimental, cross-sectional design precludes clarification of the causal relationships between maltreatment and asymmetry between these two stress responses systems. We also did not conduct physical examinations of the youth to rule out potentially confounding medical conditions, although we did obtain some information regarding medical history and medication use.
Nonetheless, this study is new in examining the effect of maltreatment on asymmetry between the SNS and HPA axis in adolescents. Maltreatment is linked with alterations in both of these biological response systems and with a variety of negative outcomes. Researchers recently have suggested that the pattern of asymmetry between the HPA axis and SNS may be related to atypical development (Bauer et al., 2002). Thus, the role of maltreatment in asymmetry may have relevance for the links between maltreatment as a major life stressor and youth outcomes.
Acknowledgements
This research was supported in part by the Behavioral Endocrinology Laboratory and the Child Youth and Families Consortium at The Pennsylvania State University, by NIH grants K23 HD041428 and R01 HD039129, and by the University of Southern California Urban Initiative. Thanks are due to Becky Hamilton and Mary Curran for biotechnical support with immunoassays.
Footnotes
Financial Disclosures
Douglas A. Granger discloses that he is the founder and current president of (and correspondingly holds an equity position in) Salimetrics LLC. No other potential conflicts of interests exist for any of the authors regarding this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elana B. Gordis, Department of Psychology, University at Albany, SUNY, egordis@albany.edu
Douglas A. Granger, Department of Biobehavioral Health, Penn State University, dag11@psu.edu
Elizabeth J. Susman, Department of Biobehavioral Health, Penn State University, esusman@psu.edu
Penelope K. Trickett, School of Social Work, University of Southern California, pennyt@usc.edu
References
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. J. Dev. Behav. Pediatr. 2002;23:102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Bosch JA, de Geus EJ, Veerman ECI, Hoogstraten J, Amerongen AVN. Innate secretory immunity in response to laboratory stressors that evoke distinct patterns of cardiac autonomic activity. Psychosom. Med. 2003;65:245–258. doi: 10.1097/01.psy.0000058376.50240.2d. [DOI] [PubMed] [Google Scholar]
- Britton KT, Segal DS, Kuczenski R, Hauger R. Dissociation between in vivo hippocampal norepinephrine response and behavioral neuroendocrine responses to noise stress in rats. Brain Res. 1992;574:125–130. doi: 10.1016/0006-8993(92)90808-m. [DOI] [PubMed] [Google Scholar]
- Brown MR, Fisher LA, Spiess J, Rivier C, Rivier J, Vale W. Corticotropin-releasing factor: Actions on the sympathetic nervous system and metabolism. Endocrinology. 1982;111:928–931. doi: 10.1210/endo-111-3-928. [DOI] [PubMed] [Google Scholar]
- Cannon WB. The interrelations of emotions as suggested by recent physiological researches. Am. J. Psychol. 1914;25:256–282. [Google Scholar]
- Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, Reiss AL. Dirunal salivary cortisol in pediatric post traumatic stress disorder. Biol. Psychiatr. 2002;51:575–582. doi: 10.1016/s0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- Chatterton RT, Vogelsong KM, Lu Y, Ellman AB, Hudgens GA. Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clin. Physiol. 1996;16:433–448. doi: 10.1111/j.1475-097x.1996.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders: Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Devel. Psychopathol. 2001;13:783–804. [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. Third ed. LEA; Mahwah, NJ: 2003. [Google Scholar]
- De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, et al. Developmental traumatology part I: Biological stress systems. Biol. Psychiatr. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Lefter L, Trickett PK, Putnam FW. Urinary catecholamine excretion in sexually abused girls. J. Am. Acad. Child Adol. Psychiatr. 1994;33:320–237. doi: 10.1097/00004583-199403000-00004. [DOI] [PubMed] [Google Scholar]
- De Souza EB. Corticotropin releasing factor receptors: Physiology, pharmacology, biochemistry and role in central nervous system and immune disorders. Psychoneuroendocrinology. 1995;20:789–819. doi: 10.1016/0306-4530(95)00011-9. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Susman EJ, Nottelmann ED, Inoff-Germain G, Chrousos GP. Perceptions of puberty: Adolescent, parent, and health care personnel. Dev. Psychol. 1990;26:322–239. [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotrophin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res. Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Duval F, Mokrani M, Monreal-Ortiz J, Fattah S, Champeval C, Schulz P, Macher JP. Cortisol hypersecretion in unipolar major depression with melancholic and psychotic features: Dopaminergic, noradrenergic and thyroid correlates. Psychoneuroendocrinology. 2006;31:876–888. doi: 10.1016/j.psyneuen.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Frankenhaeuser M. Challenge-control interaction as reflected in sympathetic-adrenal and pituitary-adrenal activity: Comparison between the sexes. Scand J. Psychol. 1982;1(Supp):158–164. doi: 10.1111/j.1467-9450.1982.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Helhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gerra G, Zaimovic A, Mascetti GG, et al. Neuroendocrine responses to experimentally-induced psychological stress in healthy humans. Psychoneuroendocrinology. 2001;26:91–107. doi: 10.1016/s0306-4530(00)00046-9. [DOI] [PubMed] [Google Scholar]
- Gjörstrup P. Amylase secretion in the rabbit parotid gland when stimulating the sympathetic nerves during parasympathetic activity. J. Physiol. 1979;296:443–351. doi: 10.1113/jphysiol.1979.sp013015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, McEwen B. Allostasis, homeostats, and the nature of stress. Stress. 2002;5:55–58. doi: 10.1080/102538902900012345. [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, El-Sheikh M, Gordis EB, Stroud LR. Salivary α-amylase in biobehavioral research: Recent developments and applications. Ann. N.Y. Acad. Sci. 2007;1098:122–144. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annu. Rev. Psychol. 2007;58:145–73. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Hart J, Gunnar M, Cicchetti D. Salivary cortisol in maltreated children: evidence of relations between neuroendocrine activity and social competence. Dev. Psychopathol. 1995;7:11–26. [Google Scholar]
- Kirschbaum C, Bartussek D, Strasburger CJ. Cortisol responses to psychological stress and correlations with personality traits. Pers. Indiv. Differ. 1992;13:1353–1357. [Google Scholar]
- Kirschbaum C, Pirke K, Hellhammer DH. The “Trier Social Stress Test”: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Thomas TL. Stress hormones in psychophysiological research: Emotional, behavioral, and cognitive implications. In: Cacioppo TJ, Tassinary LG, Bernston GG, editors. Handbook of Psychophysiology. 2nd Ed. Cambridge University Press; New York: 2000. pp. 342–367. [Google Scholar]
- Malarkey WB, Lipkus IM, Cacioppo JT. The dissociation of catecholamine and hypothalamic-pituitary-adrenal responses to daily stressors using dexamethasone. J. Clin. Endocrin. Metab. 1995;80:2458–2463. doi: 10.1210/jcem.80.8.7629242. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in girls. Arch. Dis. Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 1969;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nater UM, Rohleder N, Gaab J, Berger S, Jud A, Kirschbaum C, et al. Human salivary alpha-amylase reactivity In a psychosocial stress paradigm. Int. J. Psychophysiol. 2005;55:333–342. doi: 10.1016/j.ijpsycho.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Nater UM, La Marca R, Florin L, Moses A, Langhans W, Koller MM, et al. Stress-induced changes in human salivary alpha-amylase activity-associations with adrenergic activity. Psychoneuroendocrinology. 2006;31:49–58. doi: 10.1016/j.psyneuen.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Raine A. Heart rate level and antisocial behavior in children and adolescents: a meta-analysis. J. Am. Acad. Child Adol.Psychiatr. 2004;43:154–162. doi: 10.1097/00004583-200402000-00010. [DOI] [PubMed] [Google Scholar]
- Pruessner J, Kirschbaum C, Meinlschmid G, Helhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent child. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Raine A. The interaction of biological and social measures in the explanation of antisocial and violent behavior. In: Stoff D, Susman E, editors. Developmental psychobiology of aggression. Cambridge University Press; New York: 2005. pp. 13–42. [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress. Anxiety. 2000;12(suppl 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Nater UM, Wolf JM, Ehlert U, Kirschbaum C. Psychosocial stress-induced activation of salivary alpha-amylase: An indicator of sympathetic activity? Ann. NY Acad. Sci. 2004;1032:258–263. doi: 10.1196/annals.1314.033. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Social status and health in humans and other animals. Annu. Rev. Anthropol. 2004;33:393–418. [Google Scholar]
- Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary systems to repeated psychosocial stress. Psychosom. Med. 2003;65:450–460. doi: 10.1097/01.psy.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Botth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Dev. Psychopathol. 2005;171:167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Shoal GD, Giancola PR, Kirillova G. Salivary cortisol, personality, and aggressive behavior in adolescent boys: A 5-year longitudinal study. J. Am. Acad. Child Adol. Psychiatr. 2003;42:1101–1107. doi: 10.1097/01.CHI.0000070246.24125.6D. [DOI] [PubMed] [Google Scholar]
- Stevens J. Comment on Olson: Choosing a test statistic in multivariate analysis of variance. Psychol. Bull. 1979;86:355–360. [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neuroscience and Biobehavioral Reviews. 2006;30:376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Horm. Behav. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- van Stegeren A, Rohleder N, Everaerd W. Salivary alpha amylase as marker for adrenergic activity during stress: Effect of betablockade. Psychoneuroendocrinology. 2006;31:137–141. doi: 10.1016/j.psyneuen.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Young EA, Abelson J, Cameron OG. Interaction of brain noradrenergic system and the hypothalamic-pituitary-adrenal (HPA) axis in man. Psychoneuroendocrinology. 2005;30:807–814. doi: 10.1016/j.psyneuen.2005.03.009. [DOI] [PubMed] [Google Scholar]