Abstract
Exposure of mice to single walled carbon nanotubes (SWCNTs) induces an unusually robust pulmonary inflammatory response with an early onset of fibrosis, which is accompanied by oxidative stress and antioxidant depletion. The role of specific components of the antioxidant protective system, specifically vitamin E, the major lipid-soluble antioxidant, in the SWCNT-induced reactions has not been characterized. We used C57BL/6 mice, maintained on vitamin E-sufficient or vitamin E-deficient diets, to explore and compare the pulmonary inflammatory reactions to aspired SWCNTs. The vitamin E-deficient diet caused a 90-fold depletion of α-tocopherol in the lung tissue and resulted in a significant decline of other antioxidants (GSH, ascorbate) as well as accumulation of lipid peroxidation products. A greater decrease of pulmonary antioxidants was detected in SWCNT-treated vitamin E-deficient mice as compared to controls. Lowered levels of antioxidants in vitamin E-deficient mice were associated with a higher sensitivity to SWCNT-induced acute inflammation (total number of inflammatory cells, number of polymorphonuclear leukocytes, released LDH, total protein content, and levels of pro-inflammatory cytokines, TNF-α and IL-6) and enhanced pro-fibrotic responses (elevation of TGF-β and collagen deposition). Exposure to SWCNTs markedly shifted the ratio of cleaved to full length extracellular superoxide dismutase (EC-SOD). Given that pulmonary levels of vitamin E can be manipulated through diet, its effects on SWCNT induced inflammation may be of practical importance in optimizing protective strategies.
Keywords: Carbon Nanotubes, Vitamin E Deficiency, Pulmonary Inflammatory Response, Oxidative Stress, Antioxidants, Inflammatory cells
INTRODUCTION
Normal uninterrupted functioning of the lung in oxygen-rich environment demands for a well-coordinated operation of fine-tuned antioxidant protective systems, including both enzymatic mechanisms and low molecular weight water- and lipid-soluble radical scavengers (Packer, 1992; Kagan et al., 2002a). This antioxidant demand becomes particularly important under conditions of elevated oxygen radical production induced by environmental/occupational factors or immune responses (Turner et al., 2002; McCunney, 2005; Jain and DalNogare, 2006). Nanoparticles - a new emerging class of technologically advanced materials – are of significant interest due to their ability to induce robust pulmonary inflammatory response often accompanied and exacerbated by massive production of reactive oxygen species, oxidative stress and damage (Muller et al., 2005; Shvedova et al., 2005). One of the most rapidly developing fields of nanotechnology is based on the use of single-walled carbon nanotubes (SWCNTs), and their applications range from the semiconductor industry to drug delivery and super-light and strong composite materials (de Jonge and Bonard, 2004). These broad modes of SWCNTs applications due to on their unique physico-chemical properties and vast surface area (Sinha and Yeow, 2005), the two factors that may be potentially critical for their interactions with cells and tissues, particularly in the lung. Indeed, we have recently demonstrated that exposure of mice to SWCNTs caused an unusually robust inflammatory response with a very early onset of fibrosis (Shvedova et al., 2005). The inflammatory response to SWCNTs invoked significant oxidative stress and antioxidant depletion (Kagan et al., 2006).
However, the role of specific components of the antioxidant protective system in the SWCNT-induced reactions needs further investigation. More specifically, vitamin E, the major lipid-soluble antioxidant of membranes and lipoproteins, has long been known to affect inflammatory responses in different tissue, including the lung, not only via direct quenching of oxidative stress (Rocksen et al., 2003; Singh et al., 2005) but also through modulation of oxidative eicosanoid pathways and prostaglandin synthesis (Toivanen, 1987; Sabat et al., 2001). Given that pulmonary levels of vitamin E can be manipulated through diet, its effects on SWCNT responses may be of practical importance in optimizing protective anti-inflammatory strategies. With this in mind, in the current study, we used two groups of C57BL/6 mice - maintained on either a vitamin E-sufficient diet (basal diet) or a vitamin E-deficient diet - to explore and compare pulmonary inflammatory reactions to aspired SWCNTs. We demonstrate that lowered levels of antioxidants in vitamin E-deficient mice are associated with a higher sensitivity to SWCNT-induced acute inflammation as well as subsequent pro-fibrotic responses.
MATERIALS AND METHODS
Chemicals and Reagents
Diffquick stain kit, picric acid, Mayer’s hematoxylin, 10% buffered formalin and thiobarbituric acid were obtained from Fisher Scientific (Pittsburgh, PA). Ca2+ + Mg2+-free PBS was purchased from Invitrogen Corporation (Grand Island, NY). BD™ Cytometric Bead Array Mouse Inflammation kit was obtained from BD Bioscience (San Diego, CA). TGF -β ELISA kit was purchased from Biosource International Inc. (Camarillo, CA). BioRad assay kit was purchased from BioRad Laboratories Inc. (Hercules, CA). Lactate dehydrogenase (LDH) activity kit was obtained from Pointe Scientific, Inc. (Lincoln Park, MI). Sircol Collagen Assay kit and pepsin were purchased from Accurate Chemical and Scientific Corporation (Westbury, NY). Sodium dodecyl sulfate (SDS), glutathione (GSH), phosphoric acid, eosin Y, phloxine B, chloramine T, methyl cellusolve, anti-β-actin antibody and acetic acid, were purchased from Sigma Chemicals Co. (St. Louis, MO). Methanol, ethanol, chloroform, hexane (HPLC grade), and water (HPLC grade) were purchased from Aldrich Chemical Co. (Milwaukee, WI). ThioGlo-1™ was obtained from Covalent Associates Inc. (Woburn, MA). Enhanced chemiluminescence detection reagents were purchased from Amersham Biosciences (Buckinghamshire, UK).
Animals
Specific pathogen-free adult female C57BL/6 mice (7–8 wk) were supplied by Jackson Lab (Bar Harbor, MN) and weighed 21.60 ± 0.26 g at time of use. Mice were given a vitamin E-deficient diet (contained less than 10 IU/kg vitamin E) or basal diet (50 IU/kg of diet) for a total of 24 weeks. The basal diet (Basal Diet™ 5755, Test Diet, Purina Mill, Richmond, IN) is a purified, synthetic diet that provides all the essential nutrients to support maintenance, growth, gestation and lactation in laboratory rodents. Vitamin E deficient diet (Purina Mill, Richmond, IN) is based on the Basal Diet™ 5755 from which a-tocopherol was removed (Table 1). Animals were daily supplied with fresh diets refrigerated at 4 °C. Mice were housed one mouse per cage in AAALAC-approved NIOSH animal facilities one week prior to use. Beta Chips (Northeastern Products Corp., Warrensburg, NY) were used for bedding and changed weekly. Animals were supplied with water and the respective diets ad libitum, in accordance with guidelines and policy set forth by the Institute of Laboratory Animals Resources, National Research Council.
Table 1.
Composition of diets
| Composition of Diets | Minerals | Vitamins | |||
|---|---|---|---|---|---|
| Protein, % | 19.3 | Calcium, % | 0.6 | Thiamin Hydrochloride, ppm | 20.0 |
| Fat, % | 10.0 | Phosphorus, % | 0.4 | Riboflavin, ppm | 20.0 |
| Fiber1, % | 4.3 | Potassium, % | 0.4 | Nicotinic Acid, ppm | 90.0 |
| Carbohydrate, % | 60.6 | Magnesium, % | 0.065 | Pyridoxine Hydrochloride, ppm | 20.0 |
| Sodium, % | 0.2 | d-Calcium Pantothenate, ppm | 60.0 | ||
| Gross Energy, kcal/gm | 4.1 | Chlorine, % | 0.2 | Folic Acid, ppm | 4.0 |
| Fluorine, ppm | 5.0 | Biotin, ppm | 0.4 | ||
| Iron, ppm | 60.0 | i-Inositol, ppm | 200.0 | ||
| Ingredients | Zinc, ppm | 20.0 | Vitamin, B12, mcg/kg | 20.0 | |
| Manganese, ppm | 65.0 | Menadione Dimethylpyrimidinol | |||
| Casein-vitamin free, % | 21.00 | Copper, ppm | 15.0 | Bisulfite, ppm | 20.0 |
| Sucrose, % | 5.00 | Cobalt, ppm | 3.2 | Vitamin A Acetate, IU/gm | 22.0 |
| Non-nutritive fiber | Iodine, ppm | 0.6 | Vitamin D, IU/gm | 2.2 | |
| (Solka-floc), % | 3.00 | Chromium, ppm | 3.0 | dl-alpha-Tocopheryl Acetate, IU/kg | |
| Corn oil, % | 5.00 | Molybdenum, ppm | 0.8 | (sufficient diet) | 50.0 |
| Lard, % | 5.00 | Selenium, ppm | 0.2 | (deficient diet) | <10.0 |
| Dextrin, % | 43.65 | ||||
| DL-methionine, % | 0.15 | ||||
| RP vitamin mixture2, % | 2.00 | ||||
| Choline Chloride, % | 0.20 | ||||
| RP mineral mixture #103, % | 5.00 | ||||
| Total, % | 100.00 |
Based on the latest ingredient analysis information. Since nutrient composition of natural ingredients varies, analysis will differ accordingly.
RP vitamin mixture contains 1.94% sucrose
RP mineral mixture #10 contains 1.29% fiber
Particles
SWCNTs (CNI Inc., Houston, TX) were produced by the high pressure CO (HiPco) disproportionation technique, employing CO in a continuous-flow gas-phase as the carbon feedstock and Fe(CO)5 as the iron-containing catalyst precursor (non-purified SWCNTs) (Bronikowski et al., 2001). Purified SWCNTs were prepared by acid treatment to remove metal contaminates (Gorelik et al., 2000). Specific surface area was measured at −196 °C by the nitrogen absorption–desorption technique (Brunauer Emmet Teller method, BET) a using SA3100 Surface Area and Pore Size Analyzer, (Beckman Coulter Inc, Fullerton, CA), while diameter was measured by transmission electron microscopy (TEM). The mean diameters of purified SWCNTs were 1–4 nm. Surface area of purified SWCNTs was 1040 m2/g. Chemical analysis of total elemental carbon and metal (iron) in SWCNTs was performed at the Chemical Exposure and Monitoring Branch (DART/NIOSH, Cincinnati, OH). Elemental carbon in SWCNTs was assessed according to NIOSH Manual of Analytical Methods (NMAN) (Bronikowski et al., 2001), while metal content (iron) was analyzed using nitric acid dissolution and inductively coupled plasma-atomic emission spectrometry (ICP-AES) performed according to NMAM method 7300 for trace metals. Analysis performed by NMAN 5040 revealed that purified SWCNTs were comprised of 99.7 wt % elemental carbon. For purity assessment of HiPco SWCNTs, we used several standard analytical techniques including thermo gravimetric analysis with differential scanning colorimetry (TGA–DSC), thermo-programming oxidation (TPO), and Raman and near-infrared (NIR) spectroscopy (Birch, 2003; Arepalli et al., 2004; Dresselhaus et al., 2004). Comparative analytical data obtained by TGA–DSC, TPO, NIR and Raman spectroscopy revealed that more than 99% of carbon content in the SWCNT HiPco product was accountable in a carbon nanotube morphology.
Particulate Instillation
Mouse pharyngeal aspiration was used for particulate administration (Rao et al., 2003). Briefly, after anesthesia with a mixture of ketamine and xylazine (62.5 and 2.5 mg/kg subcutaneous in the abdominal area), the mouse was placed on a board in a near vertical position, and the animal’s tongue extended with lined forceps. A suspension (approximately 50 μl) of particulates prepared in PBS (SWCNTs at a dose of 0.0 and 40 μg/mouse) was placed posterior in the throat and the tongue held until the suspension was aspirated into the lungs. Control mice were administered sterile Ca2+ + Mg2+-free phosphate-buffered saline (PBS) as a vehicle. All mice in SWCNT and PBS groups survived this exposure procedure. This technique provided good distribution of particles widely disseminated in a peri-bronchiolar pattern within the alveolar region as was detected by histological evaluation. The mice revived unassisted after approximately 30–40 min. Animals treated with the particulates and PBS recovered easily after anesthesia with no behavioral or negative health outcomes. Mice were sacrificed on days 1, 7 and 28 days following exposure.
Bronchoalveolar Lavage (BAL)
Mice were weighed and sacrificed with intraperitoneal injection of sodium pentobarbital (>100 mg/kg) and exsanguinated. The trachea was cannulated with a blunted 22 gauge needle, and BAL was performed using cold sterile PBS at a volume of 0.9 ml for first lavage (kept separate) and 1.0 ml for subsequent lavages. Approximately 5 ml of BAL fluid per mouse was pooled and collected in sterile centrifuge tubes. Pooled BAL cells were washed in PBS by alternate centrifugation (800 × g for 10 min at 4 °C) and resuspension. Cell-free first fraction BAL aliquots were frozen or kept until processed.
BAL Cell Counting and Differentials
The degree of inflammatory response induced by the pharyngeal aspirated particulates was estimated by quantitating total cells, macrophages, polymorphonuclear leukocytes (PMNs) and lymphocytes recovered by BAL. Cell counts were performed using an electronic cell counter equipped with a cell sizing attachment (Coulter model Multisizer II with a 256C channelizer, Coulter Electronics, Hialeah, FL). Alveolar macrophages (AM), PMNs, and lymphocytes (LM) were identified by their characteristic cell shape in cytospin preparations stained with Diffquick (Fisher Scientific, Pittsburgh, PA), and differential counts of BAL cells was carried out. Three hundred cells per slide were counted.
Lung Lavage Fluid Cytokine Analysis
Levels of cytokines were assayed in the acellular BAL fluid following SWCNT exposures in mice given vitamin E-deficient/sufficient diets. The concentrations of TNF-α and IL-6 (sensitivity of assay is 5–7.3 pg/ml) were determined using the BD™ Cytometric Bead Array, Mouse Inflammation kit (BD Biosciences, San Diego, CA). The concentrations of TGF-β1, (sensitivity of assay is < 15.6 pg/ml) was determined using an ELISA kit (Biosource International Inc., Camarillo, CA).
Total Protein and Lactate Dehydrogenase (LDH) Activity in the BAL Fluids
Measurement of total protein in the BAL fluid was performed by a modified Bradford assay according to the manufacturer’s instructions (BioRad, Hercules, CA) with bovine serum albumin as a standard. The activity of LDH was assayed spectrophotometrically by monitoring the reduction of NAD+ at 340 nm in the presence of lactate (Pointe Scientific, Inc., Lincoln Park, MI).
Preparation of Lung Homogenates
The whole mouse lungs were separated from other tissues and weighed before being homogenized with a tissue tearer (model 985–370, Biospec Products Inc., Racine, WI) in PBS (pH, 7.4) for 2 min. The homogenate suspension was frozen at −80°C until processed.
Lung Fixation and Histopathology
All lung tissue prepared for histological analysis was done under standard conditions. Animals were deeply anesthetized with an overdose of sodium pentobarbital. The trachea was exposed, cannulated, and secured with a suture. Prior to instillation of fixative, the diaphragm was ruptured to allow collapse of the lungs. The lungs were subsequently fixed with 10% neutral buffered formalin. Thereafter, the trachea was ligated, and the lungs were excised and submerged in fixative for 5 days before embedding. Lung tissue slices were prepared from both right and left lung lobes and embedded in glycomethacrylate. Sections (1.5-μm) were prepared using a HM 355 rotary microtome (Carl Zeiss, Thornwood, NY). Lung sections were stained with hematoxylin and eosin (H&E). Airways, terminal bronchioles, and the lung parenchyma were examined microscopically for the presence of cellular changes and inflammation.
Sirius Red Staining
Histological changes of the lungs were evaluated in H&E and Sirius red stained sections. The distributions of type I to type III collagen in the lung tissue were determined by morphometric evaluation of the specimens by a Sirius red-polarizing microscopy. Sirius red F3BA was dissolved in saturated picric acid at a concentration of 1g/L. Paraffin lung sections (five micrometer thick) were deparaffinized and dehydrated with xylene-alcohol series to distilled water, immersed in alcohol-saturated picric acid for 20 min, then washed with tap water until the yellow stain was cleared from the slides. To identify collagen fibers under the microscope, the sections were stained with F3BA/picric acid for 1–2 h, then washed with 0.01N HCL for 1 min, and counterstained with Mayer’s hematoxylin for 2 min. The slides were then dehydrated and mounted with cover slip (Junqueira et al., 1979). Type 1 and III collagen stained by red/orange color was visualized, and 6 randomly selected areas were scored under polarized microscopy using image analysis. With this morphometric method the average thickness of Sirius red positive connective tissues in the alveolar wall were quantitatively measured. Volume and surface density was measured using standard morphometric analyses of points and intercept counting (Underwood, 1970). Volume density was determined from counting the number of points over the Sirius red positive connective tissues in the alveolar regions. Surface density of the alveolar wall was determined from intercepts between a line overlay and the air to alveolar wall. These point and intercept counts were made using a 121-point/11-line overlay graticule (12.5 mm square with 100 divisions) at 60 × magnification taken at six random fields equally spaced across each section (one section per animal). This process was repeated twice for each animal. Areas containing airways or blood vessels greater than 25 mm in diameter were excluded from the analysis. Average thickness of the Sirius red positive connective tissues of the alveolar wall was computed from two times the ratio of volume density of point to the surface density of the alveolar wall.
Lung Collagen Measurements
Total lung collagen content was determined by quantifying total soluble collagen using the Sircol Collagen Assay kit (Accurate Chemical and Scientific Corporation, Westbury, NY). Briefly, lungs were homogenized in 0.7 ml of 0.5 M acetic acid containing pepsin (Accurate Chemical and Scientific Corporation, Westbury, NY) with 1:10 ratio of pepsin: tissue wet weight. Each sample was stirred vigorously for 24 h at 4°C, centrifuged, and 200 μl of supernatant was assayed according to the manufacturer’s instructions.
HPLC Assay of α-Tocopherol
Extracts of α-tocopherol from lung homogenates were prepared using a procedure described by Lang et al., (1986). A Waters HPLC system (Waters Assosiates, Milford, MA) with a 717 auto sampler, a Hewlett Packard ODS Hypersil column (5 mm, 200 × 4.6 mm), a Waters 600 controller pump, and a 474 fluorescence detector was used to measure vitamin E in samples. The wavelengths employed in the assay were 292 nm (excitation) and 324 nm (emission). Eluent was CH3OH, and the flow rate was 1 ml/min. Under these conditions, the retention time for α-tocopherol was 8 min. The data acquired was exported from the Waters 474 detector and analyzed using Millennium 2000 software.
HPLC Assay of Ascorbate
Lung homogenates were treated with 5% meta-phosphoric acid. Supernatant obtained after precipitation of proteins and sedimentation (2000 g × 10 min) was used for HPLC measurements. A ZORBAX Eclipse XDB-C18 column (5-μm particle size, 4.6 × 150 mm Agilent Technologies, Palo Alto, CA, U.S.A.) and a mobile phase of 1:24 methanol-water adjusted to pH 3.0 by acetic acid at a flow rate of 1.0 ml/min were used. A Shimadzu LC-10A HPLC system was used with an LC-600 pump and SPD-10A UV detector (Shimadzu, Kyoto, Japan) at 264 nm. Under these conditions, the retention time for ascorbate was 2.0 min. The ascorbate peak was completely eliminated by the addition of ascorbate oxidase to lung homogenates.
HPLC Assay of Malondialdehyde (MDA)
Accumulation of lipid peroxidation products was assessed by accumulation of malondialdehyde (MDA). MDA was analyzed by HPLC using a procedure described by Young and Trimble (Young and Trimble, 1991). Briefly, MDA was analyzed in lung homogenates following a reaction with phosphoric acid and thiobarbituric acid. After mixing, the reaction was incubated in boiling water for 1h in sealed glass tubes and then cooled to 4°C on ice. Samples were neutralized and proteins precipitated before injection into the column. A Waters HPLC system with a 717 auto sampler, a Waters Nova-Pak column (C18; 5 μm, 150 × 3.9 mm), a Waters 600 controller pump, and a 474 fluorescence detector was used to measure MDA in samples. The wavelengths employed in the assay were 532 nm (excitation) and 553 nm (emission). Eluent was 25 mM phosphate buffer:CH3OH, (1:1 v/v) at pH 6.5, and the flow rate was 0.8 ml/min. Under these conditions, the retention time for MDA was 6.5 min. The data acquired was exported from the Waters 474 detector and analyzed using Millennium 2000 software (Waters Assosiates, Milford, MA).
Fluorescence Assay for Low Molecular Weight Thiols
Low molecular weight thiol concentration in lung homogenates was determined using ThioGlo™-1, a maleimide reagent, which produces a highly fluorescent adduct upon its reaction with SH- groups (Shvedova et al., 2004). Low molecular weight thiol content was estimated by an immediate fluorescence response registered upon addition of ThioGlo™-1 to the lung homogenate. A standard curve was established by addition of GSH (0.04 – 4 μM) to 100 mM disodium phosphate buffer (pH 7.4) containing 10 μM ThioGlo™-1 (DMSO solution). A CytoFluor multiwell plate reader Series 4000 (Applied BioSystems, Foster City, CA) was employed for the assay of fluorescence using excitation at 360/40 nm and emission at 530/25 nm with a gain of 50. The data obtained were exported and analyzed using CytoFluor Software (Applied BioSystems, Foster City, CA).
Western Blot Analysis
Analysis of BAL fluid was performed as previously described (Fattman et al., 2001). Extracellular superoxide dismutase (EC-SOD) was detected with antibody (1:10,000) against mouse EC-SOD as previously described (Fattman et al., 2000; Fattman et al., 2001). After visualization by enhanced chemiluminescence, densitometry was performed and standardized to mouse β-actin using Kodak 1D software (Rochester, NY).
Statistics
Treatment related differences were evaluated using two-way ANOVA, followed by pair wise comparison using the Student-Newman-Keuls tests, as appropriate. Statistical significance was considered at p< 0.05.
RESULTS
Body weight
Body weight was assessed throughout the experimental schedule to monitor the general health of the animals given vitamin E-deficient/sufficient diets and exposed to the particles (Fig. 1). At 1 day post exposure, pharyngeal aspiration of SWCNTs (40μg/mouse) caused a significant weight loss compared to controls. No significant difference in the body weight was caused by vitamin E deficiency as compared to the control vitamin E-sufficient group 1 day after the exposure to PBS. Weight gains among groups were not significantly different at 7 days post exposure. The SWCNT depression of weight gain was significant again at 28 days post-exposure as compared to animals exposed to PBS. At this time point, no significant difference in body weight was caused by vitamin E deficiency.
Fig. 1. Differential weight gain of C57BL/6 mice given basal or vitamin E-deficient diets in response to pharyngeal aspiration with SWCNTs (40 μg/mouse).
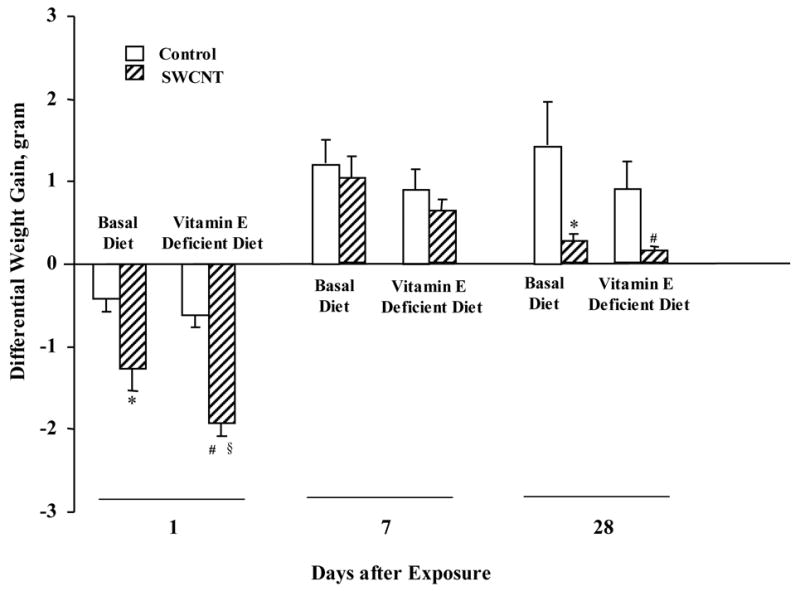
Mean ± SE (n = 12 mice/group). *p<0.05, vs mice given basal diet and aspirated with PBS, #p<0.05, vs mice given vitamin E-deficient diet and aspirated with PBS, §p<0.05, vs mice given basal diet and aspirated with SWCNTs
Depletion of vitamin E and oxidative stress response in the lung
Lung is a fast “vitamin E-reactive” tissue where dietary manipulations cause significant changes in vitamin E content both in terms of its depletion by deficient diets as well as its elevation after supplementation (Shvedova et al., 2002). In line with this, our results indicate that C57BL/6 mice kept for twenty weeks on a vitamin E-deficient diet responded by a remarkable depletion of vitamin E levels in the lung, i.e., a decrease of 90-fold compared to the control (kept on vitamin E sufficient diet) animals (Fig. 2A). The induced vitamin E deficiency resulted in depletion of other antioxidants (GSH and ascorbate) (Figs. 2B, 2D). SWCNT-induced accumulation of lipid peroxidation products was also enhanced by vitamin E deficiency (Fig. 2C). Thus generalized oxidative stress rather than depletion of vitamin E alone was induced by dietary manipulations.
Fig. 2. Depletion of vitamin E (A), GSH (B), ascorbate (D; 28 days post aspiration) and accumulation of lipid peroxidation products (C; 28 days post aspiration) in the lung of mice given basal or vitamin E-deficient diets in response to 40 μg/mouse SWCNTs.
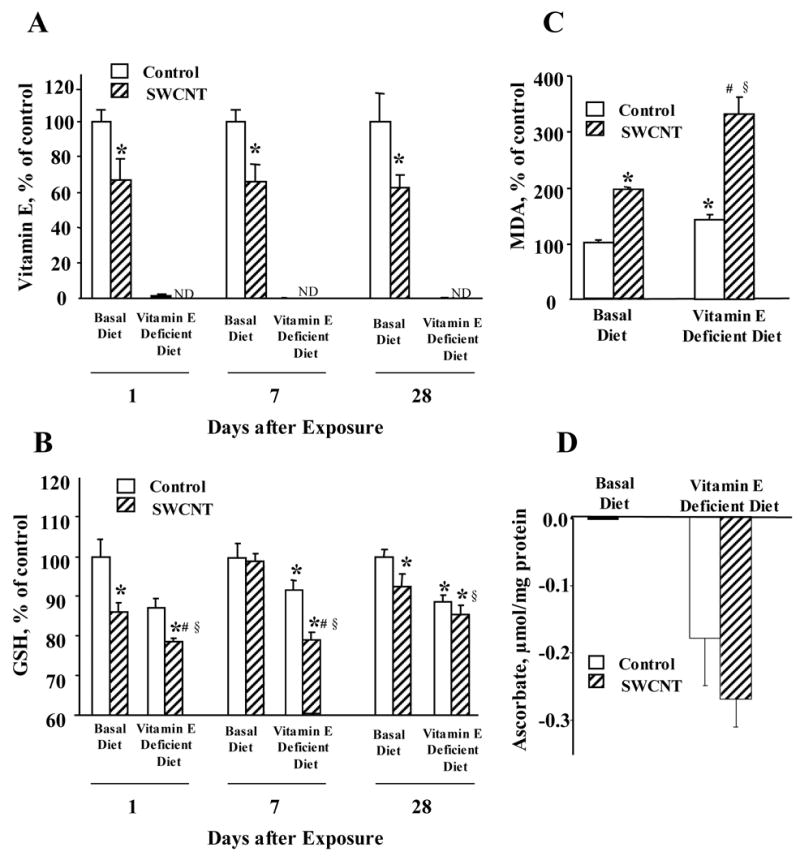
Mean ± SE (n = 6 mice/group). One hundred percent for vitamin E, GSH and MDA corresponds to 0.1 nmol/mg protein; 22 nmol/mg protein and 0.3 nmol/mg protein, respectively. *p<0.05, vs mice given basal diet and aspirated with PBS, #p<0.05, vs mice given vitamin E-deficient diet and aspirated with PBS, §p<0.05, vs mice given basal diet and aspirated with SWCNTs.
This is in line with a well accepted concept that individual low molecular weight radical scavengers are participants of a coordinated network rather than independent antioxidant players (Kagan and Packer, 1994). Next we assessed oxidative stress responses to SWCNTs in vitamin E-sufficient and deficient animals (Fig 2). A more severe depletion of pulmonary antioxidants was detected in SWCNT treated vitamin E-deficient mice as compared to controls.
We further evaluated effects of SWCNT and vitamin E-deficiency on extracellular superoxide dismutase (EC-SOD) - one of important enzymatic components of the pulmonary antioxidant protection. EC-SOD is a tetramer composed of either intact (Trp1–Ala222) or proteolytically cleaved (Trp1–Glu209) subunits. The latter form lacks the C-terminal extracellular matrix (ECM)-binding region (210RKKRRRESECKAA222–COOH) (Olsen et al., 2004). Previous studies have found that oxidative stress can alter the ratio between full-length and cleaved subunits in mouse models of pulmonary injury (Fattman et al., 2001; Oury et al., 2002; Tan et al., 2004). Although the mechanism of this alteration is unknown, inflammation appears to play an important role (Tan et al., 2006b). We found that exposure to SWCNTs lead to an 1.5–1.8 fold increase in the ratio of cleaved to full length EC-SOD as assayed in BAL fluid (Fig. 3). Notably, this change in EC-SOD occurred during the acute inflammatory phase (1 – 3 days post exposure), which further suggests that inflammatory cells are contributing to this response. No differences were seen between vitamin E-deficient and sufficient mice (data not shown).
Fig. 3. Changes in EC-SOD following SWCNT treatment.
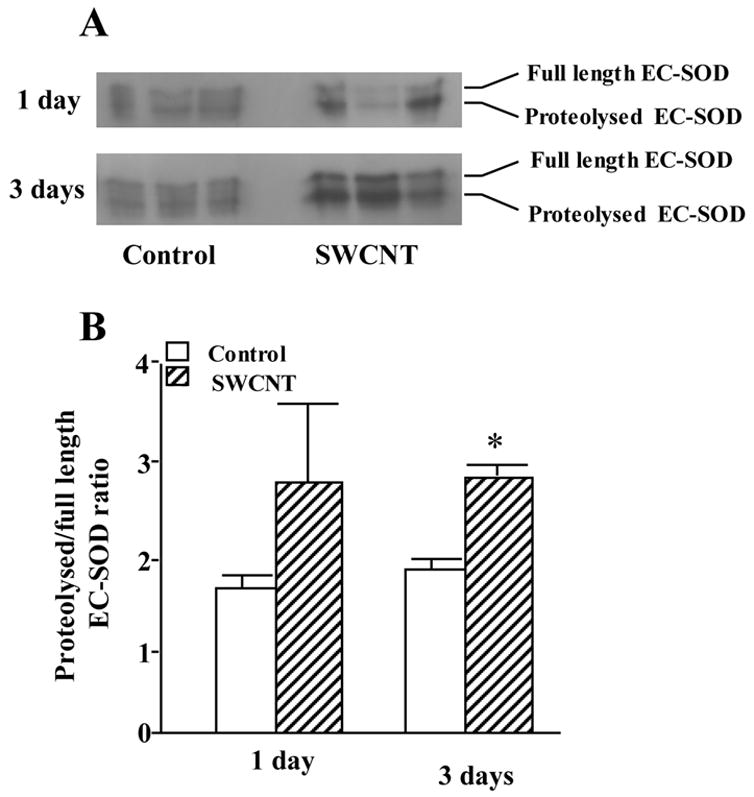
Bronchoalveolar lavage fluid EC-SOD levels are increased in SWCNT-treated mice compared to control mice by western blot analysis (A). Densitometry also shows that the ratio of proteolysed to full length EC-SOD is greatly increased 3 days after SWCNT treatment (B). Mean ± SE (n = 3 mice/group). * p<0.05 vs control group.
Inflammatory response
In agreement with our previous results, SWCNTs caused a robust, acute (1 day post exposure) inflammatory response in the lung of exposed C57BL/6 mice as evidenced by increased: 1) total number of inflammatory cells, 2) number of PMNs, 3) released LDH, 5) BAL fluid total protein content, and 6) BAL fluid levels of pro-inflammatory cytokines (TNF-α and IL-6) (Fig. 4). Morphologic findings were consistent with the rapid development of granulomatous bronchointerstitial pneumonia. Inflammatory reaction at one day after aspiration of SWCNTs (40 μg/mouse) was comprised of PMNs with a variable histiocytic response (Fig. 5A & 5B). These SWCNT-induced changes were significantly more pronounced in the lungs of vitamin E-deficient animals than in mice fed basal diet.
Fig. 4. Acute inflammatory response in lung of C57BL/6 mice exposed to SWCNT.
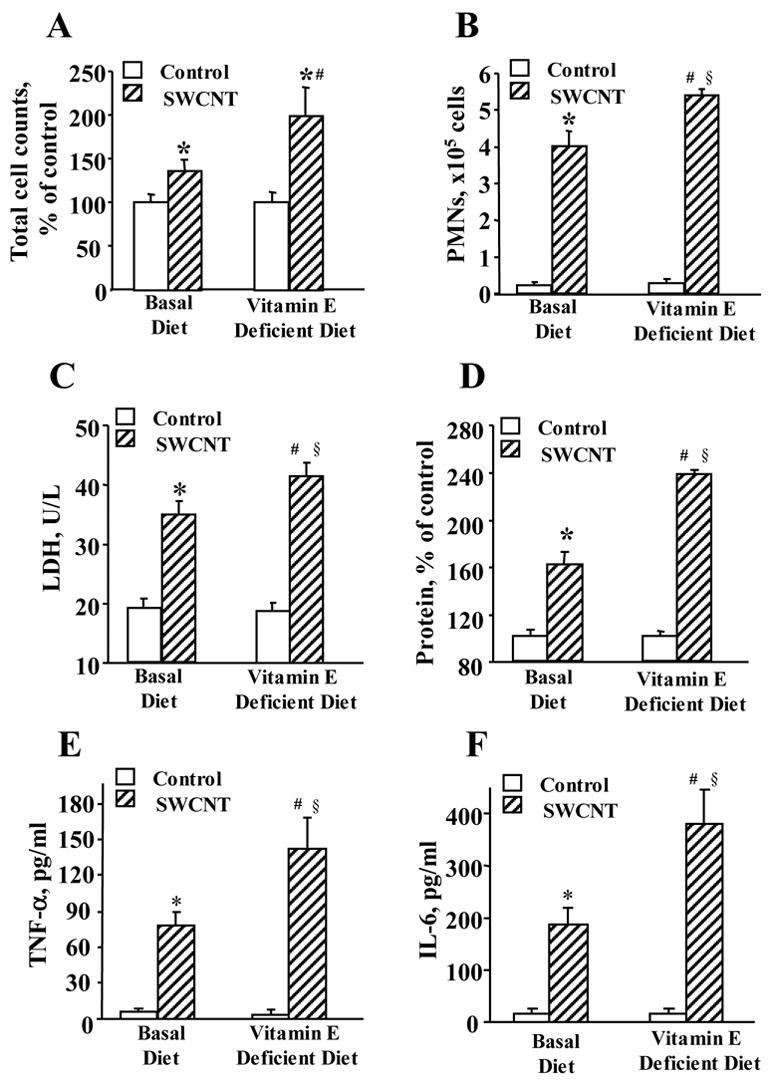
Inflammatory responses 24 h after aspiration of 40 μg/mouse SWCNT evaluated by the changes in the total cell counts (A), PMNs (B), LDH release (C), protein level (D) and accumulation of TNF-α (E) and IL-6 (F) in the BAL fluid of mice given basal or vitamin E-deficient diets. Mean ± SE (n = 12 mice/group). *p<0.05, vs given basal diet and aspirated with PBS, #p<0.05, vs mice given vitamin E-deficient diet and aspirated with PBS, §p<0.05, vs mice given basal diet and aspirated with SWCNTs.
Fig. 5. Light micrographs of H&E-stained sections from lung of mice given basal or vitamin E-deficient diets in response to SWCNT (40 μg/mouse).
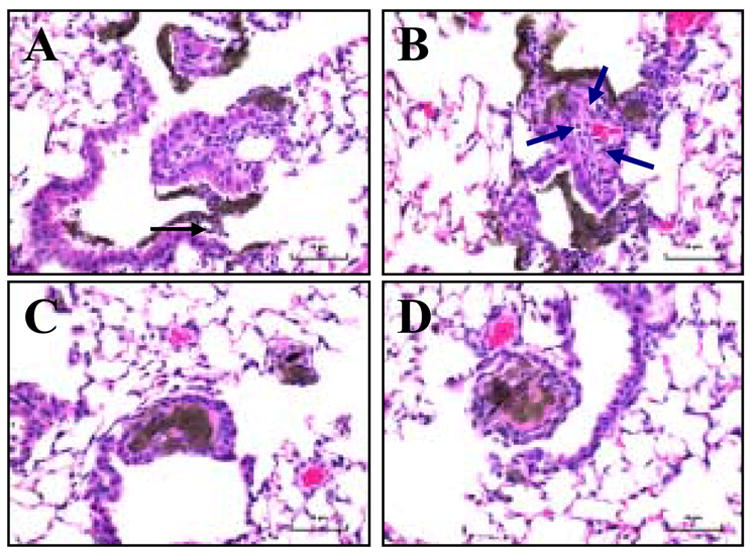
1 day post pharyngeal aspiration: A - basal diet, B - vitamin E-deficient diet; 28 days post pharyngeal aspiration: C - basal diet, D - vitamin E-deficient diet. Arrows indicated accumulation of PMNs. Magnification ×400.
Profibrotic response, assessed by the content of TGF-β 7 days after the exposure to SWCNTs, was also elevated to a greater extent in vitamin E-deficient animals as compared to control mice (Fig. 6). The nature of the inflammatory response evolved with time and at 28 days post-exposure, PMNs were no longer a major inflammatory component and the type of inflammation was leading to histiocytic granulomatous lesions (Fig. 5C & 5D). Evaluation of fibrosis at 28 days post exposure revealed a higher level of collagen deposition (Fig. 7A) and a greater extent of thickening of the alveolar septa (measured morphometrically) in SWCNT-treated, vitamin E-deficient vs control mice (Fig. 7B).
Fig. 6. Level of TGF-β in the BAL fluids of mice given basal or vitamin E-deficient diets in response to SWCNTs (40 μg/mouse, 7 days post aspiration).
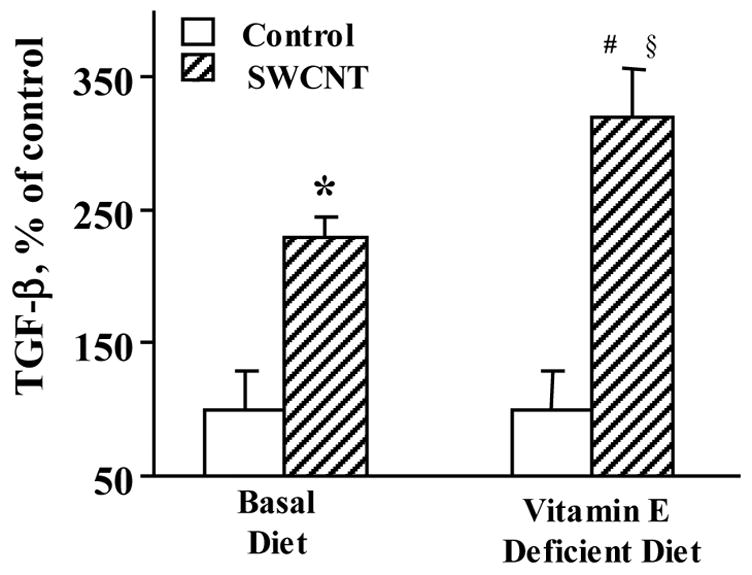
Mean ± SE (n = 8 mice/group). *p<0.05, vs given basal diet and aspirated with PBS, #p<0.05, vs mice given vitamin E-deficient diet and aspirated with PBS, §p<0.05, vs mice given basal diet and aspirated with SWCNTs.
Fig. 7. Evaluation of lung fibrosis in C57BL/6 mice exposed to SWCNT.
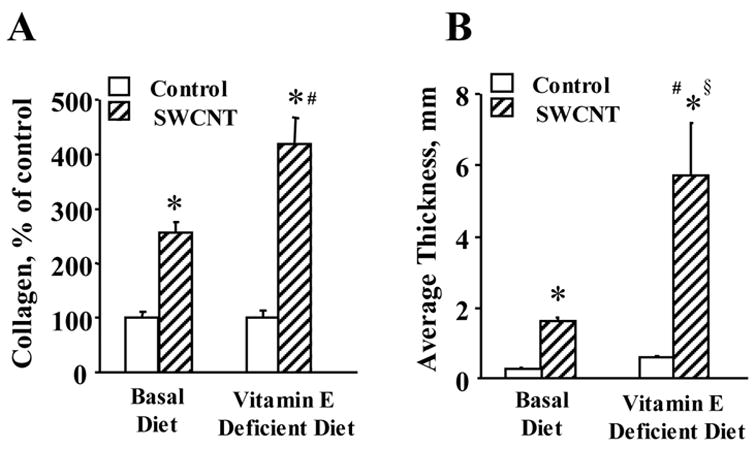
Collagen deposition (A) and morphometric determination of alveolar connective tissues (B) in the lung of mice given basal or vitamin E-deficient diets in response to SWCNTs (40 μg/mouse, 28 days post pharyngeal aspiration). Mean ± SEM (n = 8 mice/group). *p<0.05, vs mice given basal diet and aspirated with PBS, #p<0.05, vs mice given vitamin E-deficient diet and aspirated with PBS, §p<0.05, vs mice given basal diet and aspirated with SWCNTs.
DISCUSSION
Lung is the prime target for oxidative injury. Numerous studies have documented that inflammatory response to different chemicals and pathogens is intrinsically intertwined with simultaneously evolving oxidative stress (Nel et al., 2001). The importance of oxidative stress in pulmonary inflammation is strongly supported by a significant number of studies in which several antioxidants – mainly precursors of GSH, such as N-acetyl-cysteine (Sadowska et al., 2005), or SOD and SOD mimetics – have been successfully employed as protective anti-inflammatory agents (Salvemini et al., 2002; Kinnula and Crapo, 2003).
The antioxidant enzyme EC-SOD is a 135-kDa tetrameric enzyme that scavenges superoxide radicals in the extra-cellular space (Fattman et al., 2003). EC-SOD is highly expressed in mammalian lungs, where it is bound to the extra-cellular matrix through a positively charged heparin/matrix-binding domain. Proteolytic cleavage of the heparin/matrix-binding domain has been associated with loss of the enzyme from the extra-cellular matrix under experimental conditions that mimic human interstitial lung disease, such as bleomycin treatment or hyperoxia (Carlsson et al., 1995; Oury et al., 2002). Recently, it has been shown (Tan et al., 2006a) that exposure to asbestos resulted in decreased lung EC SOD protein levels and enzyme activity in mice. The loss of this enzyme may enhance oxidative stress and pulmonary injury induced by SWCNT exposure. Our results clearly demonstrate significant elevation of a neutrophil-associated form of EC-SOD lacking the heparin binding-domain early after the exposure to SWCNTs. This agrees well with a robust, acute accumulation of PMNs. However, vitamin E-deficient animals did not exert a greater response to SWCNTs in terms of accumulation of this form of EC-SOD, suggesting that low molecular weight antioxidants, rather than enzymatic antioxidant systems, such as EC-SOD, are more sensitive to the particle-induced inflammatory response. The vitamin E’s primary antioxidant action is confined to scavenging lipid radicals in the lipid phase of membranes (Burton et al., 1983) while non-protonated superoxide radicals are localized predominantly in the aqueous phase where they are catalytically dismutated by EC-SOD. Thus, it is not surprising that we found no differences in the EC-SOD responses to SWCNT induced oxidative stress between vitamin E-deficient and vitamin E-sufficient animals. The differential responsiveness of the EC-SOD activity vs vitamin E content to oxidative stress-inducing insults has been reported (Treitinger et al 2000).
Surprisingly, only very few investigations addressed the role of vitamin E in pulmonary inflammatory response (Singh et al., 2005). Using a model of vitamin E-deficiency Sabat et al., (2001) reported that dysregulation of immune cells in the lung was not directly related to antioxidant propensities of vitamin E but rather associated with its non-antioxidant features (interactions with protein kinase C and adhesion molecules) (Boscoboinik et al., 1991). Other studies reported that vitamin E supplementation decreased the number of PMNs during inflammatory response (Rocksen et al., 2003) but increased recruitment of macrophages (Belo et al., 2005). Notably, α-tocopherol transfer protein null (vitamin E-deficient) mice responded by an enhanced production of pro-inflammatory cytokines after LPS stimulation (Schock et al., 2004).
Effects of vitamin E on pulmonary responses to inhaled particles, to the best of our knowledge, have not been investigated. Our previous studies have established that SWCNT-induce a strong acute inflammatory response accompanied by accumulation of large numbers of ROS-producing PMNs and followed by the recruitment and activation of macrophages, as well as significant oxidative stress (Shvedova et al., 2005). Therefore, we were eager to assess the role of vitamin E in these processes. In our model vitamin E’s role seems to be primarily antioxidant protection as vitamin E-deficient animals exhibited a stronger inflammatory response and developed a more powerful oxidative stress response.
While several different non-antioxidant functions of vitamin E may be essential for the maintenance of cell integrity and functions, such as its role as anti-phospholipase A2 agent, stabilizer of the lipid bilayer of membranes against hydrolyzed and oxidized lipids (Kagan, 1989), its radical scavenging role seems to be dominating during SWCNTs induced responses. In a separate study, we used the EPR spin trapping technique to detect spin adducts of radicals generated by the SWCNT exposure. Using PBN as a spin trap, we were able to detect lipid-derived free radical adducts produced in the lung in response to SWCNT (Shvedova et al., unpublished data). In line with this, SWCNT enhanced lipid peroxidation induced in macrophages by stimulation with either zymosan or PMA (Kagan et al., 2006).
One of the essential antioxidant functions of vitamin E may be its control of apoptotic lipid signaling. Phosphatisylserine (PS) externalization on the surface of apoptotic cells is known to act as an eat-me signal for macrophages (Kagan et al., 2000). Moreover, oxidation of PS is important for its externalization, hence may affect recognition and engulfment of apoptotic cells by macrophages (Kagan et al., 2002b). The significance of PS oxidation for the development of the inflammatory response is that PS-dependent signaling switches off production of ROS by macrophages and stimulates the production and release of anti-inflammatory, pro-fibrotic cytokines (TGF-β) (Inghilleri et al., 2005). It is tempting to speculate that vitamin E deficiency causes enhanced PS oxidation/externalization, stimulates engulfment and clearance of apoptotic cells by macrophages, and hence facilitates the creation of a pro-fibrotic environment. Interestingly, non-functionalized SWCNTs are poorly recognized by macrophages (Jia et al., 2005; Kagan et al., 2006). However, coating of SWCNTs with PS not only makes them highly recognizable but also affects macrophage functions in ways similar to those of apoptotic cells. This opens interesting new prospectives and applications for coated SWCNTs as regulators of immune response.
Acknowledgments
supported by NIOSH OH008282, NIH HL70755, NORA 92700Y, the Human Frontier Science Program.
Footnotes
DISCLAIMER: The findings and conclusions in this report are those of the authors and do not necessary represent the view of the National Institute for Occupational Safety and Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arepalli S, Nikolaev P, Gorelik O. Analytical characterization of single wall carbon nanotubes. Encyclopedia of Nanoscience and Nanotechnology. 2004;1:51–66. [Google Scholar]
- Belo MA, Schalch SH, Moraes FR, Soares VE, Otoboni AM, Moraes JE. Effect of dietary supplementation with vitamin E and stocking density on macrophage recruitment and giant cell formation in the teleost fish, Piaractus mesopotamicus. J Comp Pathol. 2005;133:146–154. doi: 10.1016/j.jcpa.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Birch ME. NIOSH Manual of Analytical Methods (NMAM 5040), Chapter Q. 4. NIOSH; Cincinnati, OH: 2003. Elemental Carbon. Monitoring of diesel exhaust particulate in the workplace. DHHS publication No 2003–2154. [Google Scholar]
- Boscoboinik D, Szewczyk A, Hensey C, Azzi A. Inhibition of cell proliferation by alpha-tocopherol. Role of protein kinase C. J Biol Chem. 1991;266:6188–6194. [PubMed] [Google Scholar]
- Bronikowski MJ, Willis PA, Colbert DT, Smith KA, Smalley RE. Gas-phase production of carbon single-walled nanotubes from carbon monoxide via the HiPco process: A parametric study. J Vac Sci Technol. 2001;19:1800. [Google Scholar]
- Burton GW, Joyce A, Ingold KU. Is vitamin E the only lipid-soluble, chain-breaking antioxidant in human blood plasma and erythrocyte membranes? Arch Biochem Biophys. 1983;221:281–290. doi: 10.1016/0003-9861(83)90145-5. [DOI] [PubMed] [Google Scholar]
- Carlsson LM, Jonsson J, Edlund T, Marklund SL. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc Natl Acad Sci USA. 1995;92:6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge N, Bonard JM. Carbon nanotube electron sources and applications. Philos Transact A Math Phys Eng Sci. 2004;362:2239–2266. doi: 10.1098/rsta.2004.1438. [DOI] [PubMed] [Google Scholar]
- Dresselhaus MS, Dresselhaus G, Charlier JC, Hernandez E. Electronic, thermal and mechanical properties of carbon nanotubes. Philos Transact Ser A Math Phys Eng Sci. 2004;362:2065–2098. doi: 10.1098/rsta.2004.1430. [DOI] [PubMed] [Google Scholar]
- Fattman CL, Chu CT, Kulich SM, Enghild JJ, Oury TD. Altered expression of extracellular superoxide dismutase in mouse lung after bleomycin treatment. Free Radic Biol Med. 2001;31:1198–1207. doi: 10.1016/s0891-5849(01)00699-2. [DOI] [PubMed] [Google Scholar]
- Fattman CL, Enghild JJ, Crapo JD, Schaefer LM, Valnickova Z, Oury TD. Purification and characterization of extracellular superoxide dismutase in mouse lung. Biochem Biophys Res Commun. 2000;275:542–548. doi: 10.1006/bbrc.2000.3327. [DOI] [PubMed] [Google Scholar]
- Fattman CL, Schaefer LM, Oury TD. Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med. 2003;35:236–256. doi: 10.1016/s0891-5849(03)00275-2. [DOI] [PubMed] [Google Scholar]
- Gorelik O, Nikolaev P, Arepalli S. Purification procedures for single-walled carbon nanotubes. NASA contractor report, NASA/CR-2000–208926 2000 [Google Scholar]
- Inghilleri S, Morbini P, Oggionni T, Barni S, Fenoglio C. In situ assessment of oxidant and nitrogenic stress in bleomycin pulmonary fibrosis. Histochem Cell Biol. 2005;24:1–9. doi: 10.1007/s00418-005-0116-7. [DOI] [PubMed] [Google Scholar]
- Jain R, DalNogare A. Pharmacological therapy for acute respiratory distress syndrome. Mayo Clin Proc. 2006;81:205–212. doi: 10.4065/81.2.205. [DOI] [PubMed] [Google Scholar]
- Jia G, Wang H, Yan L, Wang X, Pei R, Yan T, Zhao Y, Guo X. Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environ Sci Technol. 2005;39:1378–1383. doi: 10.1021/es048729l. [DOI] [PubMed] [Google Scholar]
- Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Kagan VE. Tocopherol stabilizes membrane against phospholipase A, free fatty acids, and lysophospholipids. Ann N Y Acad Sci. 1989;570:121–135. doi: 10.1111/j.1749-6632.1989.tb14913.x. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Fabisiak JP, Shvedova AA, Tyurina YY, Tyurin VA, Schor NF, Kawai K. Oxidative signaling pathway for externalization of plasma membrane phosphatidylserine during apoptosis. FEBS Lett. 2000;477:1–7. doi: 10.1016/s0014-5793(00)01707-5. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Kisin ER, Kawai K, Serinkan BF, Osipov AN, Serbinova EA, Wolinsky I, Shvedova AA. Toward mechanism-based antioxidant interventions: lessons from natural antioxidants. Toward mechanism-based antioxidant interventions: lessons from natural antioxidants. Ann NY Acad Sci. 2002a;959:188–198. doi: 10.1111/j.1749-6632.2002.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Packer L. Light-induced generation of vitamin E radicals: assessing vitamin E regeneration. Methods Enzymol. 1994;234:316–320. doi: 10.1016/0076-6879(94)34099-4. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Shvedova AA, Tyurina YY, Tyurin VA, Fabisiak JP, Schor NF. Peroxidation of phosphatidylserine in mechanisms of apoptotic signaling. Methods Enzymol. 2002b;352:159–174. doi: 10.1016/s0076-6879(02)52016-4. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Tyurina YY, Tyurin VA, Konduru N, Potapovich AI, Osipov AN, Kisin ER, Schwegler-Berry D, Mercer R, Castranova V, Shvedova AA. Direct and indirect effects of single walled carbon nanotubes on RAW 264.7 macrophages: role of iron. Toxicol Lett. 2006;165:88–100. doi: 10.1016/j.toxlet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med. 2003;167:1600–1619. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- Lang JK, Cohil L, Packer L. Simultaneous determination of tocopherols, ubiquinols and ubiquinones in blood, plasma, tissue homogenates and subcellular fractions. Anal Biochem. 1986;157:106–116. doi: 10.1016/0003-2697(86)90203-4. [DOI] [PubMed] [Google Scholar]
- McCunney RJ. Asthma, genes, and air pollution. J Occup Environ Med. 2005;47:1285–1291. doi: 10.1097/01.jom.0000188561.75578.bf. [DOI] [PubMed] [Google Scholar]
- Muller J, Huaux F, Moreau N, Misson P, Heilier JF, Delos M, Arras M, Fonseca A, Nagy JB, Lison D. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol. 2005;207:221–231. doi: 10.1016/j.taap.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Nel AE, Diaz-Sanchez D, Li N. The role of particulate pollutants in pulmonary inflammation and asthma: evidence for the involvement of organic chemicals and oxidative stress. Curr Opin Pulm Med. 2001;7:20–26. doi: 10.1097/00063198-200101000-00004. [DOI] [PubMed] [Google Scholar]
- Olsen DA, Petersen SV, Oury TD, Valnickova Z, Thogersen IB, Kristensen T, Bowler RP, Crapo JD, Enghild JJ. The intracellular proteolytic processing of extracellular superoxide dismutase (EC-SOD) is a two-step event. J Biol Chem. 2004;279:22152–22157. doi: 10.1074/jbc.M401180200. [DOI] [PubMed] [Google Scholar]
- Oury TD, Schaefer LM, Fattman CL, Choi A, Weck KE, Watkins SC. Depletion of pulmonary EC-SOD after exposure to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2002;283:L777–L784. doi: 10.1152/ajplung.00011.2002. [DOI] [PubMed] [Google Scholar]
- Packer L. Interactions among antioxidants in health and disease: vitamin E and its redox cycle. Proc Soc Exp Biol Med. 1992;200:271–276. doi: 10.3181/00379727-200-43433. [DOI] [PubMed] [Google Scholar]
- Rao GV, Tinkle S, Weissman DN, Antonini JM, Kashon ML, Salmen R, Battelli LA, Willard PA, Hoover MD, Hubbs AF. Efficacy of a technique for exposing the mouse lung to particles aspirated from the pharynx+ 2003;66:1441–1452. doi: 10.1080/15287390306417. [DOI] [PubMed] [Google Scholar]
- Rocksen D, Ekstrand-Hammarstrom B, Johansson L, Bucht A. Vitamin E reduces transendothelial migration of neutrophils and prevents lung injury in endotoxin-induced airway inflammation. Am J Respir Cell Mol Biol. 2003;28:199–207. doi: 10.1165/rcmb.4899. [DOI] [PubMed] [Google Scholar]
- Sabat R, Kolleck I, Witt W, Volk H, Sinha P, Rustow B. Immunological dysregulation of lung cells in response to vitamin E deficiency. Free Radic Biol Med. 2001;30:1145–1153. doi: 10.1016/s0891-5849(01)00523-8. [DOI] [PubMed] [Google Scholar]
- Sadowska AM, van Overveld FJ, Gorecka D, Zdral A, Filewska M, Demkow UA, Luyten C, Saenen E, Zielinski J, De Backer WA. The interrelationship between markers of inflammation and oxidative stress in chronic obstructive pulmonary disease: modulation by inhaled steroids and antioxidant. Respir Med. 2005;99:241–249. doi: 10.1016/j.rmed.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Salvemini D, Riley DP, Cuzzocrea S. SOD mimetics are coming of age. Nat Rev Drug Discov. 2002;1:367–374. doi: 10.1038/nrd796. [DOI] [PubMed] [Google Scholar]
- Schock BC, Van der Vliet A, Corbacho AM, Leonard SW, Finkelstein E, Valacchi G, Obermueller-Jevic U, Cross CE, Traber MG. Enhanced inflammatory responses in alpha-tocopherol transfer protein null mice. Arch Biochem Biophys. 2004;423:162–169. doi: 10.1016/j.abb.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Kisin E, Mercer R, Murray A, Johnson VJ, Potapovich A, Tyurina Y, Gorelik O, Arepalli S, Schwegler-Berry D, Antonini J, Evans DE, Ku BK, Ramsey D, Maynard A, Kagan VE, Castranova V, Baron P. Unusual inflammatory and fibrogenic pulmonary responses to single walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L698–L708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Kisin E, Murray A, Goldsmith T, Reynolds JS, Castranova V, Frazer DG, Komminen iC. Metal working fluids: sub-chronic effects on pulmonary functions in B6C3F1 mice given vitamin E deficient and sufficient diets. Toxicology. 2002;177:285–297. doi: 10.1016/s0300-483x(02)00188-9. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Murray AR, Schwegler-Berry D, Gandelsman VZ, Baron P, Maynard A, Gunther MR, Castranova V. Exposure of human bronchial epithelial cells to carbon nanotubes causes oxidative stress and cytotoxicit. Proc. Soc. Free Rad Research Meeting, European Section; June 26–29, 2003; 2004. pp. 91–103. [Google Scholar]
- Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress and inflammation. Annu Rev Nutr. 2005;25:151–174. doi: 10.1146/annurev.nutr.24.012003.132446. [DOI] [PubMed] [Google Scholar]
- Sinha N, Yeow JT. Carbon nanotubes for biomedical applications. IEEE Trans Nanobioscience. 2005;4:180–195. doi: 10.1109/tnb.2005.850478. [DOI] [PubMed] [Google Scholar]
- Tan RJ, Fattman CL, Niehouse LM, Tobolewski JM, Hanford LE, Li Q, Monzon FA, Parks WC, Oury TD. Matrix metalloproteinases promote inflammation and fibrosis in asbestos-induced lung injury in mice. Am J Respir Cell Mol Biol. 2006a;35:289–297. doi: 10.1165/rcmb.2005-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan RJ, Fattman CL, Watkins SC, Oury TD. Redistribution of pulmonary EC-SOD after exposure to asbestos. J Appl Physiol. 2004;97:2006–2013. doi: 10.1152/japplphysiol.00480.2004. [DOI] [PubMed] [Google Scholar]
- Tan RJ, Lee JS, Manni ML, Fattman CL, Tobolewski JM, Zheng M, Kolls JK, Martin TR, Oury TD. Inflammatory cells as a source of airspace extracellular superoxide dismutase after pulmonary injury. Am J Respir Cell Mol Biol. 2006b;34:226–232. doi: 10.1165/rcmb.2005-0212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toivanen JL. Effects of selenium, vitamin E and vitamin C on human prostacyclin and thromboxane synthesis in vitro. Prostaglandins Leukot Med. 1987;26:265–280. doi: 10.1016/0262-1746(87)90036-9. [DOI] [PubMed] [Google Scholar]
- Treitinger A, Spada C, Verdi JC, Miranda AF, Oliveira OV, Silveira MV, Moriel P, Abdalla DS. Decreased antioxidant defence in individuals infected by the human immunodeficiency virus. Eur J Clin Invest. 2000;30:454–459. doi: 10.1046/j.1365-2362.2000.00642.x. [DOI] [PubMed] [Google Scholar]
- Turner ND, Braby LA, Ford J, Lupton JR. Opportunities for nutritional amelioration of radiation-induced cellular damage. Nutrition. 2002;18:904–912. doi: 10.1016/s0899-9007(02)00945-0. [DOI] [PubMed] [Google Scholar]
- Underwood EE. Quantitative stereology. Reading, MA: Addison-Wesley Publishing Co; 1970. [Google Scholar]
- Young IS, Trimble ER. Measurement of malondialdehyde in plasma by high performance liquid chromatography with fluorimetric detection. Ann Clin Biochem. 1991;28:504–508. doi: 10.1177/000456329102800514. [DOI] [PubMed] [Google Scholar]


