Abstract
Study Objectives:
To determine the combined effects of sleep restriction and low-dose alcohol on driving simulator performance, EEG, and subjective levels of sleepiness and performance in the mid-afternoon.
Design:
Repeated measures with 4 experimental conditions. Normal sleep without alcohol, sleep restriction alone (4 hours) and sleep restriction in combination with 2 different low blood alcohol concentrations (0.025 g/dL and 0.035 g/dL).
Setting:
Sleep Laboratory, Adelaide Institute for Sleep Health.
Participants:
Twenty-one healthy young men, aged 18–30 years, mean (±SD) = 22.5(±3.7) years, BMI = 25(±6.7) kg/m2; all had normal sleep patterns and were free of sleep disorders.
Measurements:
Participants completed a 70-minute simulated driving session, commencing at 14:00. Driving parameters included steering deviation, braking reaction time, and number of collisions. Alpha and theta EEG activity and subjective driving performance and sleepiness were also measured throughout the driving task.
Results:
All measures were significantly affected by time. Steering deviation increased significantly when sleep restriction was combined with the higher dose alcohol. This combination also resulted in a significant increase in alpha/theta EEG activity throughout the drive, as well as greater subjective sleepiness and negative driving performance ratings compared to control or sleep restriction alone.
Discussion:
These data indicate that combining low-dose alcohol with moderate sleep restriction results in significant decrements to subjective alertness and performance as well as to some driving performance and EEG parameters. This highlights the potential risks of driving after consumption of low and legal doses of alcohol when also sleep restricted.
Citation:
Vakulin A; Baulk SD; Catcheside PG; Anderson R; van den Heuvel CJ; Banks S; McEvoy RD. Effects of moderate sleep deprivation and low-dose alcohol on driving simulator performance and perception in young men. SLEEP 2007;30(10):1327-1333.
Keywords: Sleep, driving, alcohol, performance, perception
INTRODUCTION
SLEEP DEPRIVATION IS COMMON IN MODERN SOCIETY DUE TO BOTH PROFESSIONAL AND SOCIAL FACTORS.1 DRIVER SLEEPINESS IS A SIGNIFICANT CONTRIBUTOR to many motor vehicle accidents.2–5 Partial sleep deprivation and natural circadian variations in alertness are important factors that increase sleep propensity at certain times of day.6–9 When unssderlying sleepiness is mixed with a sedative substance such as alcohol, the impairments to neurobehavioral function, attention, and vigilance may be exacerbated.6–8 Cosnsumption of low (and legal) doses of alcohol, in addition to sleep deprivation is also common, especially amongst younger drivers—who are also in the highest risk group for sleep related vehicle accidents (SRVAs).5 Further investigations of the combined effects of alcohol and sleep restriction on both psychological and physiological aspects in young drivers are therefore warranted.
The neurobehavioral impact of sleep deprivation is affected both by the amount of prior sleep, as well as the period of continuous wakefulness. Impairment to vigilance and psychomotor activity accumulates throughout the day in a process governed by homeostatic and circadian interactions.10–14 Epidemiological studies show that the majority of SRVAs occur during the mid-afternoon and early morning, corresponding closely to the natural low points in alertness.2–5 Although sleep propensity during early morning is around 3 times higher compared to mid-afternoon, the increased volume of traffic during the day means the chance of an accident due to a combination of alcohol and sleepiness in the mid-afternoon is probably greater.5
A number of studies have shown that the combination of sleep restriction and low doses of alcohol result in a greater decrement in driving performance than alcohol or sleep deprivation alone.6–9 However, these studies examined a single blood-alcohol concentration (BAC). Thus, it is not clear at which point within current legally acceptable limits, that driving becomes impaired. In addition, few studies in this area have directly examined drivers' abilities to recognize their own sleepiness or appreciate the resulting impairment to driving performance.15–17 A recent laboratory study showed a reasonable correlation between subjective sleepiness, EEG, and driving performance measures17 although other studies have pointed to discrepancies between subjective and objective measures of driving performance, which may be gender specific.6–9 It is not known how and why drivers decide to continue driving when sleepy or how aware they are of their own performance deterioration. Moreover, there are very few data on how the interaction of alcohol and sleepiness affects these issues.
The aim of the current study was to investigate the effects of a realistic level of sleep restriction (4 hours in bed) in combination with 2 low-alcohol concentrations on simulated driving performance, EEG, and self-perceptions of driving performance and sleepiness in healthy young males. A mid-afternoon driving scenario was used to replicate a common situation—corresponding to the mid-afternoon circadian low, during which sleepiness is naturally augmented.
METHODS
Study Design
The study used a repeated measures design with 4 conditions in a randomised and counterbalanced order: normal sleep without alcohol (CONTROL), sleep restriction to 4 hours in bed (02:00–06:00: SLP-RES), and sleep restriction combined with 1 of 2 legal blood-alcohol concentrations (SLP-RES+A1 [0.025 g/dL] and SLP-RES+A2 [0.035 g/dL]). Australia's legal alcohol limit is 0.05 g/dL.
Participants
Twenty-one healthy young males participated in the study. All participants were recruited from Adelaide University, the University of South Australia, or Flinders University after advertising in each University's employment websites; each subject was compensated for participating. They were medication free, had no previous or current sleeping problems, were nonsmokers, and had ≥2 years driving experience. All participants were screened using a preliminary telephone questionnaire before attending an introductory session in the laboratory. During this visit, participants completed general health/background and time of day preference18 questionnaires, as well as the Epworth Sleepiness Scale (ESS)19 and Pittsburgh Sleep Quality Index (PSQI).20 Subjects with ESS and PSQI scores above the normal range (i.e., ESS>10; PSQI>5)19–20 were excluded, as were extreme-morning or extreme-evening types.18 The study was approved by the University of South Australia and Repatriation General Hospital Human Research Ethics Committees.
Procedure
To check for compliance with the sleep restriction regime, participants were asked to wear activity monitors 7 days prior to each experimental session, and to call a time/date-stamped answering machine before going to bed the night before the experiment and in the morning upon waking. In addition, participants were asked to keep a diary of their sleep patterns for 7 days prior to each experiment and were excluded if they failed to comply with the sleeping protocol. Participants were excluded if they failed to comply with these instructions, and this was assessed on the basis of obvious inconsistencies in the actigraphy data, sleep diary, or answering machine records. Prolonged periods of inactivity in the actigraphy data before bedtime (02:00) and after wake time (06:00), as well as failure to call in at the designated times resulted in participant's exclusion from experiments for that particular week. Participants were asked to refrain from consuming alcohol or caffeinated drinks after 22:00 the night before and on the day of testing and to finish breakfast before 08:00 on the experimental day. Experimental sessions were randomized for each participant and conducted one week apart.
Upon arrival to the sleep laboratory at 12:00, participants' BACs were measured using a calibrated breathalyser (Dräger Alcotest 7410Plus), sleep diaries collected, activity monitor data downloaded, and answering machine checked for compliance with the sleeping regime. Participants then consumed a standard lunch, consisting of 2 toasted cheese rolls and a glass of water. At 12:30 participants had electrodes fitted for EEG/EOG (C3-A2, C4-A1, O1-A2, O2-A1). At 13:25 participants were given an alcoholic drink of low concentration (A1 target BAC 0.025 g/dL), higher concentration (A2 target BAC 0.035 g/dL), or a nonalcoholic mixer drink. The subjects were aware that alcohol was consumed to simulate real world situation, however they did not know the amount of alcohol consumed or the BAC reached. The alcohol used was 0.4 g/dL (40%) vodka mixed with a sugar-free, carbonated, noncaffeinated mixer. The driving began at 14:00 and continued nonstop for 70 minutes. Target BACs were achieved using dosages of alcohol derived from the mathematical formulae below,21 where total body water (TBW) is first estimated using age, height, and weight.
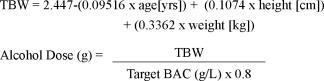 |
Driving Simulator
The AusEd driving simulator used in this study was developed at Sydney and Edinburgh Universities by Drs Engelman, Joffe, and Grunstein.6,22–26 The simulator is designed to assess driving impairment by measuring steering deviation, speed deviation, braking reaction time (in response to trucks), and the number and type of crashes. Steering deviation is defined as movement (in centimeters) of the car from the median position of the left hand side of the road. Speed deviation is defined as variation (in kilometers per hour) from the median speed within the 60–80 kph speed range. Five slow-moving trucks were presented at regular intervals throughout the driving task, with participants instructed to apply the brakes as soon as they saw one. Braking reaction time (BRT) was measured from each response. BRTs >3 sec were counted as lapses. Crash events were off road events, stopping events (>5 sec), and collisions with slow-moving trucks. The program was installed on a Windows 2000 workstation and displayed on a 19-inch BENQ FP937s monitor. A PC-compatible steering wheel and pedals (360 Modena Pro, Thrustmaster, Montreal, Canada) were used to control the simulation.
Electroencephalography
Objective sleepiness was assessed by combined alpha and theta EEG activity.24 Four EEG channels (C3-A2; C4-A1, O1-A2, O2-A1) were used, with 2 channels of EOG also recorded to assist in the identification of microsleeps (slow rolling eye movement, no eye movement, etc). EEG was digitally recorded using the PSG Online E-Series, and analyzed in 30-sec epochs using Profusion PSG 2 software (Compumedics, Melbourne, Australia). EEG activity in the alpha and theta frequency range (4–12Hz)24 was standardized by calculating the percentage of alpha and theta activity in each 30-second epoch and converting to a z-score to reduce the impact of substantial interindividual variation in EEG and to facilitate between-condition comparisons. This standardization was achieved by calculating the mean and standard deviation of all values recorded during the first 30 min (60 epochs) of the control condition drive (considered as the baseline), and expressing all other epochs from all conditions as a difference from baseline divided by baseline standard deviation (i.e., z-score) according to the formula25
 |
Microsleeps were analysed by manual scoring of the EEG trace,24 (conditions blinded) and defined as bursts of theta activity of ≥3 sec. The average duration of and cumulative number of microsleeps at 4.5 min intervals were determined for each subject during the 70-min driving task.
Subjective Measures
At 4.5-minute intervals during the simulated driving task, participants were prompted by an audio tone (58 dBA) to provide 2 subjective ratings according to scales visible at all times next to the monitor screen: Subjective Sleepiness: “How much sleepiness have you experienced since the last tone?” (1=none at all; 3=a little bit; 5=a moderate amount; 7=quite a lot; 9=an extreme amount), and Driving Performance: “Rate your driving performance since the last tone” (1=excellent; 3=good; 5=Okay; 7=very bad; 9=terrible).
Statistical Analysis
SPSS software was used to perform a repeated measures ANOVA (Greenhouse-Geisser adjusted) to examine the effects of time (4.5-min intervals) and experimental conditions (CONTROL, SLP-RES, SLP-RES+A1, and SLP-RES+A2) on driving simulator, EEG, and subjective measures. Significant ANOVA effects were further explored using pair-wise post hoc analysis corrected for multiple contrasts using Dunn-Sidak's procedure. The rate of change in objective and subjective measures over time was further evaluated by calculating the slope of each relationship over the entire time course of each drive within each subject. These were compared between conditions using ANOVA for repeated measures. Friedman's and chi-squared tests were used to evaluate difference in the number of crashes and reaction time lapses between conditions. All data are presented as means (±SEM).
RESULTS
All participants were aged 18–30 yrs (mean=22.5 [±3.7]), and were within normal limits for BMI (25 ±6.7 kg/m2) and ESS scores (5.8±2.2). On 2 occasions, participants were sent home before the start of the driving session because of a lack of actigraphy data indicating non-compliance with sleep restriction. They were asked to repeat the condition a week later. All other participants complied with the sleep restriction protocol. All participants had zero BAC upon arrival (12:00) to the laboratory during each condition (see Table 1). There was no evidence of participants napping or returning to bed after calling in at 06:00.
Table 1.
Mean (±SEM) Time-in-Bed and BAC Data for All Conditions (Approximate Times Shown for BAC Readings)
| Time In Bed (Min) | Blood-Alcohol Concentration (G/dl) |
||
|---|---|---|---|
| Pre-drive (14:00) | Post-drive (15:30) | ||
| Control | 511.5 (±19.09) | ZERO | ZERO |
| SLP-RES | 243.8 (±7.34) | ZERO | ZERO |
| SLP-RES + A1 | 243.1 (±6.57) | 0.025 (±0.002) | 0.013 (±0.002) |
| SLP-RES + A2 | 233.9 (±4.40) | 0.034 (±0.002) | 0.023 (±0.001) |
Driving Performance
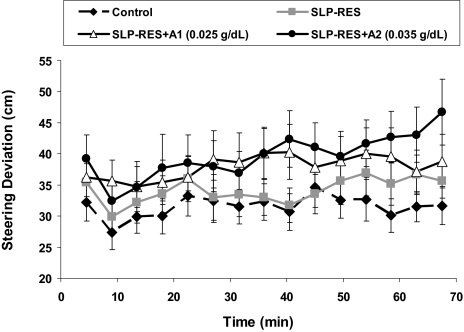
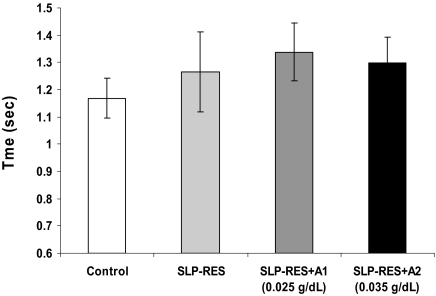
Steering deviation increased significantly over time (F5.5,110 = 3.5, P = 0.004), and was significantly affected by condition (F2,34 = 6.4, P = 0.006). Although there was no significant interaction effect, there was a trend for a steeper deterioration in steering with time on task for the SLP-RES+A2 condition compared with control (t20 = 1.8, P = 0.09, Figure 1). Post hoc analyses revealed significantly greater steering deviation when sleep restriction was combined with A1 or A2 alcohol doses, but no difference with sleep restriction alone (SLP-RES+A1: t20 = 2.2, P = 0.038 and SLP-RES+A2: t20 = 2.9, P = 0.009; see Figure 1). No significant effects were found for speed deviation. Braking reaction times were not significantly different between conditions (Figure 2). Chi-squared and Friedman's analyses revealed that significantly more participants crashed (χ'b23 = 15.4, P = 0.002), and there were more crashes overall (Friedman's χ'b23= 8.1, P = 0.045) during the driving task with the addition of sleep restriction and alcohol, but there were no significant differences in the number of participants who experienced a lapse or the total number of lapses between conditions (see Table 2).
Figure 1.
Mean (±SEM) steering deviation from the median position of the left-hand side of the road for the 70-min simulated driving task.
Figure 2.
Mean (±SEM) braking reaction times for the 4 conditions.
Table 2.
Percentage of Participants Crash and Lapse Data for All Conditions
| CONTROL | SLP-RES | SLP-RES + A1 | SLEP-RES + A2 | P-VALUE | |
|---|---|---|---|---|---|
| % participants crashed | 4.7 | 19 | 23.8 | 33.3 | 0.002 |
| % participants lapsed | 3 | 4 | 6 | 4 | 0.773 |
Electroencephalography
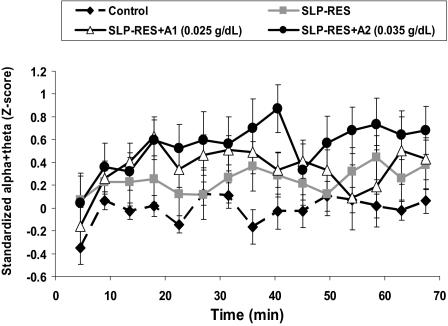
There were significant condition (F3,56 = 4.69, P = 0.006) and time (F6,119 = 3.70, P = 0.002) effects in the standardized percentage of EEG alpha and theta (see Figure 3). Post hoc analysis showed greater alpha and theta activity in the SLP-RES+A2 condition than control (t20 = 2.4, P = 0.026), but no other pair-wise differences. There was considerable intersubject variability in the total number of microsleeps during each condition, ranging from 0–22 throughout the control drive to between 0 and 92, 66, and 99 during SLP-RES, SLP-RES+A1, and SLP-RES+A2, respectively. While there were trends, there were no significant condition (F1.9,35.5 = 3.11, P = 0.06), time (F4.4.83.0 = 2.17, P = 0.074), or interaction effects in the number of microsleeps per 4.5-min period (overall mean±SD, 0.80±0.97), or the cumulative number of microsleeps over the entire drive (overall mean±SD 12±14). The overall mean (±SD) microsleep duration for every 4.5-min period was 3.94 (±0.28) sec and was relatively constant throughout the drive with no significant condition, time, or interactive effects.
Figure 3.
Mean (±SEM) of standardized alpha + theta EEG (4–12 Hz) activity for the 4 conditions over the entire 70-min simulated driving task.
Subjective Measures
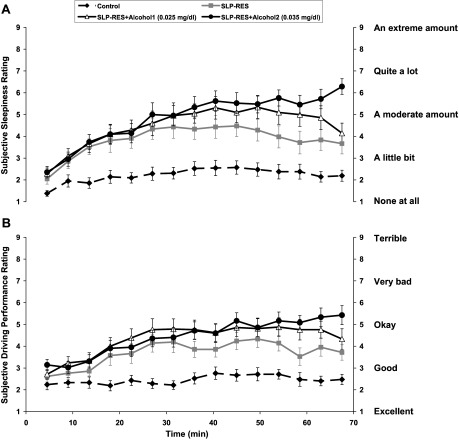
Subjective sleepiness ratings increased significantly over time (F3,54 = 26, P <0.001, see Figure 4a), showed significant differences between conditions (F3.6,72 = 22, P <0.001), and a significant condition-by-time interaction effect (F8,166 = 3.6, P <0.001). Post hoc tests showed significant differences between CONTROL and all other conditions (SLP-RES t20 = 4.2, P = 0.001, SLP-RES+A1 t20 = 5.2, P <0.001, SLP-RES+A2 t20 = 7.4, P <0.001) and between SLP-RES vs. SLP-RES+A2 (t20 = 2.3, P = 0.03). However, no significant differences were found between SLP-RES and SLP-RES+A1, or SLP-RES+A1 and SLP-RES+A2 conditions (see Figure 4a). The overall rate of change in sleepiness ratings over time was significantly greater during SLP-RES+A2 compared to all other conditions (Control t20 = 5.2, P <0.001, SLP-RES t20 = 2.6, P = 0.016, SLP-RES+A1 t20 = 2.5, P = 0.02).
Figure 4.
(A) Mean (±SEM) sleepiness perception scores for the 4 conditions for every 4.5-min period during the 70-min simulated driving task. (B) Mean (±SEM) driving performance perception scores for the 4 conditions for every 4.5-min period during the 70-min simulated driving task.
Subjective ratings of driving performance deteriorated significantly over time (F3.8,76 = 12.5, P <0.001, see Figure 4b), and showed significant differences between conditions (F2,46 = 23, P <0.001). There was a significant condition-by-time interaction (F10,199 = 2.7, P = 0.003). Post hoc analysis revealed significant differences between CONTROL and all other conditions (SLP-RES t20 = 3.8, P = 0.001, SLP-RES+A1 t20=4.6, P <0.001, SLP-RES+A2 t20 = 6.2, P <0.001) and significantly poorer driving performance ratings in the SLP-RES+A2 condition, compared with SLP-RES alone (t20 = 2.5, P = 0.022). However, there was no difference between SLP-RES and SLP-RES+A1, and no significant difference between the 2 alcohol conditions (see Figure 4b). The rate of change in driving performance ratings over time was significantly greater in SLP-RES+A2 versus control (t20 = 3.4, P = 0.003) but was not different between any other conditions (see Figure 4b).
DISCUSSION
This study shows that mixing alcohol (BAC below the legal limit in Australia of 0.05 g/dL) with sleep restriction results in a significant decline in some, but not all, objective and subjective driving performance measures during a mid-afternoon driving simulator task. Time-on-task contributed significantly to the changes observed in subjective measures of sleepiness and self-rated driving performance. Time-on-task also seemed to exaggerate the differences in steering deviation between the sleep restriction plus BAC 0.035 g/dL and control conditions (see Figure 1), although this difference was not statistically significant. Alcohol dose had a significant effect on subjective sleepiness but not subjective driving performance. While alcohol in the presence of sleep restriction clearly had an effect on driving performance (steering deviation and crashes), no discernible alcohol dose effect was observed.
The ability to keep the car in the middle of the lane is critical to safe driving, and is one of the more sensitive measures of driving impairment. Although steering deviation was not significantly affected by sleep restriction alone, alcohol at a BAC as low as 0.025 g/dL (half the Australian legal limit) in combination with sleep restriction was sufficient to significantly impair steering stability. This is important, as a single standard drink is enough to produce these levels of BAC in most individuals. Drinking one or 2 alcoholic beverages in the mid-afternoon is also common in young adult males and is legal for driving, regardless of prior sleep. This combination, however, may considerably reduce the threshold for safe driving, as suggested by the steering deviation data and an increase in off-road collisions following sleep restriction and alcohol ingestion in this study.
We are unable to say how the decrements in performance we found in this study might translate into real world crash risk. Validation studies comparing simulator performance with on-road performance and with crash statistics in very large cohorts of drivers would be needed to answer this question. However, there was about a 33% increase in steering deviation with SLP-RES+A2 and a 6-fold increase in the proportion of subjects showing a complete performance failure (“crash”) some time during the 70-min drive. These are large effects and we suspect, but cannot prove, they would be deleterious to real driving.
Studies have shown that EEG alpha and theta activity are a good indicator of sleepiness, but there is considerable variation between individuals.25 In our study, there was evidence for greater alpha and theta activity with higher alcohol concentrations, consistent with increased sleepiness levels during that condition. It is difficult to interpret the significance of EEG results because of its high variability. This variability may have obscured real differences between the other conditions, and a larger sample size may have been required.
Different individuals appear to react differently to sleep restriction, and while sleep restriction did not have a profound effect on all participants, some were very sleepy as assessed subjectively and by microsleeps. The lack of a significant change in the number of microsleeps between the experimental conditions is again likely to be due to large intersubject variability, and there may be some merit in future studies investigating individual differential vulnerability. However, any future investigations would need to be adequately powered to elucidate any significant difference.
Participants consistently reported feeling sleepier over time during all conditions. It was evident that they were also able to perceive the effect that sleep restriction had on their alertness and driving performance, with significantly higher reported sleepiness and worse driving performance compared with the control condition. Importantly, participants appeared able to differentiate between sleep restriction alone and the additional effects of alcohol, reporting highest levels of sleepiness and lowest performance during alcohol conditions (see Figure 4a). The ability to perceive sleepiness and to recognize performance decrements may however, independently depend on the level of sleepiness and alcohol.6,9 Significant effects of alcohol dose on subjective sleepiness but not subjective driving performance in this study are consistent with this view.6,9,5–17 For example, increased confidence and risk-taking behavior with alcohol could impair the perception of worsening driving performance despite the perception of increased sleepiness.
The differences in subjective ratings between sleep restriction alone and combined alcohol conditions became apparent after at least 30 min on task, suggesting a delayed interaction effect. A similar trend was also evident for steering performance. This suggests that short drives are less risky under these conditions. With longer drives, the delayed perception of alcohol effects may contribute to the decision to drive in the first place, and make the decision to stop driving more difficult later in the drive. In all conditions except the highest alcohol condition, there was some evidence for a decrease in sleepiness and improvements in driving performance in the last 15 min of the drive. Even though the BAC at the end of the drive in the highest alcohol condition was approximately 0.025 g/dL (similar to the lower alcohol condition at the beginning of the drive), ratings of sleepiness levels and performance impairments were highest. Others have also shown that the effects of alcohol on driving performance9 and sleep propensity30 may be delayed or at least persist well after BACs have returned to zero or near zero levels. Our results further support the idea that delayed effects of alcohol on performance and perception, and potential interaction effects with circadian, time of day, alertness and time-on-task factors, can cause significant sleepiness and performance decrements well after the decline in BAC. It would be interesting to explore (during a longer drive, e.g., 90–120 min) how long the effects of the higher alcohol dose lasts before improvements are evident. We believe that longer drives such as this are common and relevant to driving safety.
Methodological Limitations
Although simulator studies may not directly translate into a real driving situation, they do assess performance parameters fundamental to driving, such as steering deviation, reaction time, and vigilance. The AusEd simulator has previously been used to examine driving performance in a variety of experiments, and has demonstrated sensitivity to insults such as alcohol and/or sleep loss.6,22,23 We found no changes in braking reaction time following sleep restriction or sleep restriction combined with alcohol. Although generally considered to be important and sensitive to the effects of sleepiness, braking reaction time is a more complex parameter to measure and interpret. A limitation of this study, possibly accounting for the absence of experimental effects, was the small number of BRT responses, assessed during the drive. In future studies, we will employ a greater number of BRTs, but it is important to consider the effect this may have on alertness. In addition, the gold standard for measuring simple RT is the 10-minute PVT, with good RTs in the range 150–300 msec, becoming elongated with sleepiness (>300 msec, or >500 msec considered as lapses). Clearly, BRT as measured in this study (in the range 800–1400 msec) is much greater than PVT RT and therefore more difficult to interpret without a body of normative data. Few studies have investigated RT in this way, and more data are therefore needed—particularly in order to make conclusions about accident risk.
The absence of significant time-dependent effects in the objective driving and EEG measures may be due to the sample size. Based on the study design, sample size, and within-subject standard deviation of steering deviation in the order of 6 cm, we estimate that we could detect ~2 cm differences between conditions, ~4 cm differences over time, and ~7 cm condition- and time-dependent differences with 80% power and a two-tailed significance of 0.05. Similarly, based on a within-subject standard deviation in EEG z-scores in the order of 0.5, we estimate that we could detect ~0.15, ~0.3 and ~0.5 z-score differences between conditions, times, and condition × time respectively. These represent relatively small between-condition effects, but quite large time and condition-by-time effects. The lack of significant differences between sleep restriction and control and between alcohol dose conditions could reflect Type II error, although the risk is relatively small. However, the apparent lack of some condition by time-on-task effects could well reflect Type II error.
The administration of an audio tone every 4.5 minutes during the driving task, may have had an effect on subjects' overall alertness. This was not evident from subjective reports, which showed progressive sleepiness despite regular tone delivery. The EEG would usually indicate increased alertness during the responses themselves, but would in most cases slow down within 20–30 sec. The rationale for using 4.5 min for the subjective responses was that this period was adequate to obtain enough data points, but was also long enough to reduce subject's anticipation of the next tone. It is always difficult to assess and interpret subjective and objective parameters measured simultaneously. All subjects were subjected to the same auditory tone condition in a repeated measure design, thus allowing for comparison between the different conditions.
It was not possible to use EEG measurements to assess compliance with the sleep restriction protocol in this study. However the use of actigraphy, diaries, and answering machine gave a reasonable estimate of sleep and wake periods, although there is always a possibility of error between actigraphy data and subject's actual time in bed. All efforts were made to exclude subjects where there were doubts regarding compliance with the sleep restriction protocol.
We have investigated these issues using only young male drivers. Given known physiological gender differences relating to alcohol, general driving behavior, and numerous areas of sleep, further investigations in female drivers will also be important.28–34
In conclusion, these data suggest that young healthy men may be at a higher risk of SRVAs when sleep restriction and alcohol are combined. The legal alcohol limit in Australia and elsewhere of 0.05 g/dL is reasonable to minimize the risk of an accident in a fully rested individual, but where prior sleep opportunity is reduced by half, attempts to avoid alcohol should be made if intending to drive, to reduce the synergistic effects of alcohol and sleepiness. In addition, there is support for findings of other studies which suggest a persistent effect on performance and perception after BAC has returned to zero.9 Further research should also aim to examine the vulnerability of individuals to the synergistic effects of sleepiness and alcohol, in particular those already at risk of SRVAs such as patients suffering from obstructive sleep apnea.
ACKNOWLEDGMENTS
This study was supported by an Australian Brewer's Foundation Alcohol-Medical Research Grant.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have reported no financial conflicts of interest.
REFERENCES
- 1.Bonnet MH, Arand DL. We are chronically sleep deprived. Sleep. 1995;18:908–11. doi: 10.1093/sleep/18.10.908. [DOI] [PubMed] [Google Scholar]
- 2.Lyznicki JM, Doege TC, Davis RM, Williams MA. Sleepiness, driving, and motor vehicle crashes. JAMA. 1998;279:1908–13. doi: 10.1001/jama.279.23.1908. [DOI] [PubMed] [Google Scholar]
- 3.Philip P, Vervialle F, Le Breton P, Taillard J, Horne JA. Fatigue, alcohol, and serious road crashes in France: factorial study of national data. BMJ. 2001;322:829–30. doi: 10.1136/bmj.322.7290.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connor J, Norton R, Ameratunga S, et al. Driver sleepiness and risk of serious injury to car occupants: population based case-control study. BMJ. 2002;324:1125. doi: 10.1136/bmj.324.7346.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horne JA, Reyner LA. Sleep related vehicle accidents. BMJ. 1995;310:565–7. doi: 10.1136/bmj.310.6979.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banks S, Catcheside P, Lack L, Grunstein RR, McEvoy RD. Low levels of alcohol impair driving simulator performance and reduce perception of crash risk in partially sleep deprived subjects. Sleep. 2004;27:1063–7. doi: 10.1093/sleep/27.6.1063. [DOI] [PubMed] [Google Scholar]
- 7.Horne JA, Reyner LA, Barrett PR. Driving impairment due to sleepiness is exacerbated by low alcohol intake. Occup Environ Med. 2003;60:689–92. doi: 10.1136/oem.60.9.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett PR, Horne JA, Reyner LA. Sleepiness combined with low alcohol intake in women drivers: greater impairment but better perception than men? Sleep. 2004;27:1057–62. doi: 10.1093/sleep/27.6.1057. [DOI] [PubMed] [Google Scholar]
- 9.Barrett PR, Horne JA, Reyner LA. Alcohol continues to affect sleepiness related driving impairment, when breath alcohol levels have fallen to near-zero. Hum Psychopharmacol Clin Exp. 2004;19:421–3. doi: 10.1002/hup.601. [DOI] [PubMed] [Google Scholar]
- 10.Vitaterna MH, Takahashi JS, Turek FW. Overview of circadian rhythms. Alco Res Health. 25:85–93. 200. [PMC free article] [PubMed] [Google Scholar]
- 11.Moore RY. Circadian rhythms: basic neurobiology and clinical applications. Annu Rev Med. 1997;48:253–66. doi: 10.1146/annurev.med.48.1.253. [DOI] [PubMed] [Google Scholar]
- 12.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–41. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 13.Broughton RJ. SCN controlled circadian arousal and the afternoon “nap zone”. Sleep Res Online. 1998;1:166–78. [PubMed] [Google Scholar]
- 14.Turek FW, Zee PC. New York: Marcel Dekker; 1999. Regulation of sleep and circadian rhythms: lung biology in health and disease. [Google Scholar]
- 15.Reyner LA, Horne JA. Falling asleep whilst driving: are drivers aware of prior sleepiness? Int J Legal Med. 1998;111:120–3. doi: 10.1007/s004140050131. [DOI] [PubMed] [Google Scholar]
- 16.Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 17.Horne JA, Baulk SD. Awareness of sleepiness when driving. Psychophysiology. 2004;41:161–5. doi: 10.1046/j.1469-8986.2003.00130.x. [DOI] [PubMed] [Google Scholar]
- 18.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 19.Johns MW. A new method for measuring daytime sleepiness. The Epworth Sleepiness Scale. Sleep. 1991;14:540–6. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 20.Buysse DJ, Reynolds CF, Monk TH. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiat Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 21.Crow KA, Batt RD. vol 1. Florida: CRC Press; 1989. Pharmocokinetics, medicolegal aspects, and general interest: human metabolism of alcohol. [Google Scholar]
- 22.Desai AV, Marks G, Jankelson D, Grunstein RR. Do sleep deprivation and time of day interact with mild obstructive sleep apnoea to worsen performance and neurobehavioural function. J Clin Sleep Med. 2006;2:63–70. [PubMed] [Google Scholar]
- 23.Desai AV, Wilsmore B, Bartlett DJ, et al. The utility of the AusEd™ driving simulator in the clinical assessment of driver fatigue. Behaviour Research Methods. doi: 10.3758/bf03193039. (In press, 2006) [DOI] [PubMed] [Google Scholar]
- 24.Rechtschaffen A, Kales A. Techniques, and Scoring System for Sleep Stages of Human Subjects: A Manual of Standardised Terminology. Los Angeles: UCLA Brain Information Service; 1968. [Google Scholar]
- 25.Horne JA, Reyner LA. Counteracting driver sleepiness: effects of napping, caffeine and placebo. Psychophysiology. 1996;33:306–9. doi: 10.1111/j.1469-8986.1996.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 26.Roehrs T, Burduvali E, Banahoom A, Drake C, Roth T. Ethanol and sleep loss: a “dose” comparison of impairing effects. Sleep. 2003;26:981–5. doi: 10.1093/sleep/26.8.981. [DOI] [PubMed] [Google Scholar]
- 27.Roehrs T, Claiborue D, Knox M, Roth T. Residual sedating effects of ethanol. Alcohol Clin Exp Res. 1994;18:831–4. doi: 10.1111/j.1530-0277.1994.tb00047.x. [DOI] [PubMed] [Google Scholar]
- 28.Mumenthaler MS, Taylor JL, O'Hara R, Fisch HU, Yesavage JA. Effects of menstrual cycle and female sex steriods on ethanol pharmacokinetics. Alcohol Clin Exp Res. 1999;23:250–5. [PubMed] [Google Scholar]
- 29.Lieber CS. Ethnic and gender differences in ethanol metabolism. Alcohol Clin Exp Res. 2000;24:417–8. [PubMed] [Google Scholar]
- 30.Thomasson H. Alcohol elimination: faster in women? Alcohol Clin Exp Res. 2000;24:419–20. [PubMed] [Google Scholar]
- 31.DeJoy DM. An examination of gender differences in traffic accident risk perception. Accid Anal Prev. 1992;24:237–46. doi: 10.1016/0001-4575(92)90003-2. [DOI] [PubMed] [Google Scholar]
- 32.Glendon AI, Dorn L, Davies DR, Matthews G, Taylor RG. Age and gender differences in perceived accident likelihood an driver competences. Risk Anal. 1996;16:755–62. doi: 10.1111/j.1539-6924.1996.tb00826.x. [DOI] [PubMed] [Google Scholar]
- 33.Manber R, Bootzin RR. Sleep and the menstrual cycle. Health Psychol. 1997;16:209–14. doi: 10.1037//0278-6133.16.3.209. [DOI] [PubMed] [Google Scholar]
- 34.Armitage R, Smith C, Thompson S, Hoffmann R. Sex differences in slow-wave activity in response to sleep deprivation. Sleep Res Online. 2001;4:33–41. [Google Scholar]