Abstract
Study Objectives:
Women with severe premenstrual syndrome (PMS) or premenstrual dysphoric disorder (PMDD) commonly report sleep disturbances, but the few studies using conventional polysomnographic measures have produced conflicting results. We investigated sleep quality and sleep composition using conventional and quantitative electroencephalographic analyses in women with severe PMS, as compared with that of controls.
Design and Participants:
Women (aged 18–40 years) were screened to ensure that their PMS symptoms were severe and that they had ovulatory menstrual cycles. Nine women with PMS or PMDD and 12 asymptomatic control subjects then had laboratory-based polysomnographic recordings at 2 phases of the menstrual cycle: follicular phase and late luteal (premenstrual) phase.
Results:
Women with severe PMS reported a significantly poorer subjective sleep quality during the late luteal phase (P = 0.02), but there was no evidence of disturbed sleep based on the polysomnogram specific to premenstrual symptom expression: both groups of women had increased wakefulness after sleep onset (P = 0.02) and increased sigma power (P < 0.01), especially in the 14-to 15-Hz band during non-rapid eye movement sleep, in the late luteal phase compared with the follicular phase. There were, however, some group differences in electroencephalographic measures regardless of menstrual phase, including decreased delta incidence (P = 0.02) and increased theta incidence and amplitude (P < 0.05) in women with PMS, suggesting the possibility of sleep electroencephalogram trait markers in women with PMS.
Conclusion:
Perceived poor quality sleep is a characteristic of severe PMS, but sleep composition based on polysomnographic measures and quantitative electroencephalographic analysis does not differ in association with premenstrual symptom expression in the late luteal phase.
Citation:
Baker FC; Kahan TL; Trinder J; Colrain IM. Sleep quality and the sleep electroencephalogram in women with severe premenstrual syndrome. SLEEP 2007;30(10):1283-1291.
Keywords: Menstrual cycle, sleep spindles, progesterone, premenstrual dysphoric disorder, women
BETWEEN 50% AND 80% OF WOMEN OF REPRODUCTIVE AGE EXPERIENCE AT LEAST A FEW PREMENSTRUAL SYMPTOMS THAT VARY FROM MILD TO SEVERE.1 UP to 18% of women have severe premenstrual syndrome (PMS) and 3% to 8% qualify for a diagnosis of premenstrual dysphoric disorder (PMDD).1, 2 PMDD is classified as a “depressive disorder not otherwise specified” in the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV). Severe PMS and PMDD are characterized by significant mood, behavioral, and somatic symptoms that occur exclusively during the luteal phase of the menstrual cycle. Symptoms occur after ovulation, any time in the 2 weeks before the onset of menstrual flow, and disappear shortly thereafter. Both severe PMS and PMDD are associated with significant functional impairment and impact quality of life,3 but their etiology remain poorly understood.
Survey data indicate that women with severe PMS or PMDD report sleep-related complaints, such as difficulty sleeping, fatigue, lethargy, and poor concentration.4,5 Further, sleep disturbance (hypersomnia or insomnia) is listed as 1 of the defining criteria for a diagnosis of severe PMS in the DSM-IV. There has, however, been minimal research about the nature and severity of these premenstrual sleep disturbances. Based on retrospective reports, Mauri and colleagues6 found that women with severe PMS reported frequent awakenings, difficulty reinitiating sleep after an arousal, failure to wake up at an expected time, increased incidence of unpleasant dreams, and generally poor sleep with increased tossing and turning in the late luteal phase. However, Parry et al7 found no differences between the late luteal phase and follicular phase in sleep time or time spent awake, as recorded in daily sleep logs, in a group of 23 patients with PMDD. There are therefore sparse and conflicting data as to whether self-reported sleep disturbance and/or poor sleep quality in the late luteal phase is a symptom of severe PMS.
The few studies using objective polysomnographic recordings have shown little evidence of sleep disturbance in the late luteal phase compared to other phases of the menstrual cycle in women with severe PMS or PMDD.8–11 Sleep efficiency has been found to be similar in women with severe PMS or PMDD in the symptomatic late luteal phase, compared with the asymptomatic follicular phase.8–11 Further, classic markers of disturbed sleep found in patients with depression,12 such as shorter latency to rapid eye movement (REM) sleep or longer sleep-onset latency have not been found in women with PMDD. However, 2 studies found trait-like differences in sleep architecture in women with PMS; women with severe PMS9 or PMDD10 had a greater percentage of stage 2 non-REM (NREM) sleep along with either decreased REM sleep10 or slow-wave sleep,9 compared with controls, an effect that was present in both phases of the menstrual cycle.
Most polysomnographic studies have varied in their definition and assessment of PMS or PMDD and have used Rechtschaffen and Kales13 criteria for scoring sleep stages. In this system, arousals of 3 to 14 seconds can effectively be ignored, despite them having an impact on daytime mood and sleepiness.14 Criteria set by the American Academy of Sleep Medicine15 allow characterization of brief arousals (≥ 3 seconds) but these criteria have not previously been applied in women with PMS or PMDD. Additional quantification of sleep electroencephalography (EEG) microarchitecture is possible with computer-based analysis. Power spectral analysis (PSA)with fast-Fourier transformation and period amplitude analysis (PAA) with 0-cross and 0-derivative algorithms are the most common methods used for quantification; however, they have not been used to evaluate the sleep of women with PMS or PMDD.
The aim of this study was to further assess subjective and objective sleep quality in women with severe PMS or PMDD in their late luteal phase, compared with the asymptomatic follicular phase of the menstrual cycle. In addition to subjective sleep quality and standard polysomnographic measures, we evaluated brief arousals15 and performed quantitative analysis of the NREM sleep EEG, using both PAA and PSA algorithms. Since sleep EEG spectra are altered in the late luteal phase compared with other phases of the menstrual cycle in women without premenstrual complaints,16 we also included a group of women with normal menstrual cycles as controls.
Methods
Subjects
Fourteen women with self-reported severe PMS and 15 women with mild or no symptoms of PMS (control subjects), aged 18 to 40 years, were recruited from the community and consented to participate in our study. The study was approved by the Institutional Review Board of SRI International. All women had a structured clinical interview for DSM-IV (SCID) to exclude any concurrent major psychiatric diagnosis. Women with severe PMS were still included if they had a past history (> 1 year) of psychiatric illness or alcohol or substance abuse; there is a high comorbidity of affective illness and PMDD.1 Women were questioned about their PMS symptoms in a customized module appended to the SCID that was based on the DSM-IV criteria for a PMDD diagnosis. In addition, all women completed the premenstrual symptoms screening tool (PSST), which is a simple rating scale, based on DSM-IV criteria for PMDD, designed to quickly determine whether a woman qualifies for a PMDD diagnosis.17 Based on the SCID and PSST assessments, women in the PMS group were given a provisional diagnosis of PMDD, and controls were confirmed as having no or mild PMS. Women were also questioned to ensure that they had regular sleep-wake schedules, regular menstrual cycles of 24 to 35 days, and good health and had not been taking any chronic medication, including hormonal contraceptives, for the previous 3 months (absence of psychoactive medication use was not validated by urinanalysis). Socioeconomic status, based on education and lifetime occupation of each participant, was also assessed using the Hollingshead socioeconomic scale.18
Screening Procedures
All women completed the Penn Daily Symptom Rating Form (DSR19) over at least 2 menstrual cycles. The DSR, which has been validated as a diagnostic tool for PMS,19 lists 17 common PMS symptoms, including the 11 symptoms listed in the DSM-IV as criteria for a PMDD diagnosis: depression, anxiety, mood swings, irritability, decreased interest, difficulty concentrating, fatigue, food cravings, hypersomnia or insomnia, feeling overwhelmed, and physical symptoms such as breast tenderness or headaches. Subjects rate each item on a 5-point scale (0 = none to 4 = extreme). Postmenstrual (follicular phase or FP) scores are calculated by adding the ratings of cycle days 5 through 10 (where day 1 is the first day of bleeding). Premenstrual (late luteal phase or LLP) scores are calculated by adding the ratings of the 6 days before menses. To qualify for severe PMS, women need to score 80 or greater on the DSR in their LLP and show an increase of at least 50% from the postmenstrual score for both screening months. To meet DSM-IV criteria for PMDD, women have to rate at least 5 PMDD symptoms as severe (3 or 4) on at least 2 premenstrual days, with the same symptoms being rated as absent or minimal (0 or 1) postmenstrually. According to these criteria, 5 women were confirmed as having PMDD. Of the remaining 9 women, 4 received a diagnosis of severe PMS according to the DSR criteria.19 Two of these subjects met DSM-IV criteria for PMDD for 1 month of screening but not the other, and another 2 subjects had 3 symptoms that strictly met the PMDD criteria. These 4 subjects were therefore included in the rest of the study. Four other women did not meet the criteria for severe PMS and were excluded. A final subject with PMS dropped out of the study before completion of the screening procedures. Based on their DSR scores, all the control subjects were confirmed as having no or mild PMS. Two of the control subjects dropped out before the study was completed, and one control subject was excluded because her menstrual cycle became irregular during the study. Nine women with severe PMS and 12 control subjects were included in the final analysis. Characteristics of the subjects are shown in Table 1.
Table 1.
Characteristics of Subjects Included in the Analysis.
| Women with severe PMS | Asymptomatic women (control subjects) | |
|---|---|---|
| Sample size | 9 | 12 |
| Age, y | 28 (6) | 31 (5) |
| Weight, kg | 65.6 (10.1) | 61.7 (8.2) |
| Height, m | 1.7 (0.05) | 1.7 (0.08) |
| Body mass index. kg/m2 | 23.2 (3.2) | 21.9 (2.1) |
| Menstrual cycle length, d | 30 (1) | 29 (2) |
| Age at menarche, y | 13 (2) | 13 (1) |
| Duration of PMS, y | 9 (7) | - |
| Premenstrual dysphoric disorder, no.a | 5 | 0 |
| Past history of major depression or alcohol/substance abuse/dependence, no. | 3 | 0 |
| Have children, no. | 0 | 0 |
| Socioeconomic statusb | 27 (9) | 23 (8) |
Values are means (SD) unless otherwise indicated. PMS refers to premenstrual syndrome.
According to DSM-IV diagnostic criteria.
See Ref18; no significant group difference.
During both the screening and recording phases, women used a commercially available self-testing kit that detects the presence of luteinizing hormone in urine (One Step LH Urine Ovulation Test, IND Diagnostic, Inc, CA) to confirm ovulation. All women were confirmed as having ovulatory menstrual cycles. The women also completed a modified version of the Pittsburgh Sleep Diary20 every day over 2 menstrual cycles, in which they reported bedtimes and wakeup times, time taken to fall asleep, number of awakenings, and duration spent awake during the night. They rated their sleep quality on a visual analogue scale with anchor points of “very bad” and “very good” and their morning alertness on a similar scale, with anchor points of “Not at all alert” and “Extremely alert.” Total sleep time was calculated from the diaries as time spent in bed excluding sleep-onset latency and wakefulness after sleep onset. Means were calculated for total sleep time, sleep quality, and morning alertness for midfollicular phase (Days 7–12) and LLP (6 days preceding menstruation) for each menstrual cycle and then averaged over both menstrual cycles. Sleep diary ratings were missing for 1 subject with PMS.
Study Design
Following the screening period, subjects were scheduled for a full night of clinical polysomnography in the controlled environment of our laboratory to confirm absence of any sleep disorder. This night also served as an adaptation to the laboratory. The women returned to the laboratory for recordings on 2 occasions during their menstrual cycle: once during the midfollicular phase (6–12 days after the onset of menstrual flow) and once during the LLP (9–13 days after the luteinizing hormone surge). Women entered the study at different phases of the menstrual cycle; 5 women with PMS and 8 control subjects had their follicular phase recording first. Women were requested to maintain their customary bedtime and wake-time schedules for at least 1 week before a study night. On study days, the women were requested to refrain from drinking caffeinated beverages after 15:00, not to drink any alcoholic beverages, and not to take naps. Women were screened for presence of alcohol with a breathalyzer on arrival at the sleep laboratory; all participants registered 0 on the breathalyzer. In the laboratory, each woman slept in a separate, sound-attenuated bedroom where the ambient temperature was maintained between 20°C and 22°C. Lights-out and lights-on times were based on the customary schedules for each individual.
Subjective Evaluations
Before going to bed, women completed the Beck Depression Inventory (BDI-II21). The BDI-II contains 21 questions about how a person has been feeling. Each item is scored on a scale of 0 to 3, and a total score indicates level of depression: 0 to 13, minimal depression; 14 to 19, mild depression; 20 to 28, moderate depression; and 29 to 63, severe depression. To better capture mood over the premenstrual week, women were asked to rate how they had been feeling for the past week instead of the usual past 2 weeks stipulated in instructions for administration of the BDI-II.21 Current mood was assessed using the Profile of Mood States, which consists of 65 adjectives to which participants respond on a 5-point scale, from 1 (not at all) to 5 (extremely), based on how they are feeling right then.22 A total mood score was calculated from the Profile of Mood States by summing the negative mood scores, subtracting the vigor score, and adding a constant of 100 to avoid negative scores. Within 1 hour of waking up in the morning, women completed the morning portion of the Pittsburgh Sleep Diary,20 which included ratings of sleep quality and alertness, as described above. Ratings of sleep quality and alertness were incomplete for 1 subject with PMS. The PMS group, therefore, has a sample size of 8 for the analyses of subjective sleep quality and alertness.
Data Acquisition and Analysis
EEG, electrooculographic, and electromyographic recordings were made using E-series amplifiers and Profusion software (Compumedics, Abbotsford, Victoria, Australia) linked to appropriate transducers. Electrodes for EEG recordings were placed at F3, F4, Fz, C3, C4, Cz, O1, and O2 sites according to the international 10–20 system and were cross-referenced to A1 or A2. EEG signals were digitized at a sampling rate of 128 Hz, high-pass filtered at 0.3 Hz, and low-pass filtered at 30 Hz. For the purposes of this paper, only C3-A2 and C4-A1 EEG derivations were included in the analysis. Thirty-second epochs were scored according to standard criteria 13 by 2 scorers blind to menstrual cycle phase of the subjects. Interrater reliability of sleep scoring was set at 0.90, and discrepancies were resolved by a third scorer. Time in bed refers to the time from lights out to lights on. Total sleep time is the time spent asleep minus in-bed wakefulness during the time in bed. Sleep efficiency was calculated as a percentage of total sleep time during time in bed. Sleep-onset latency was taken as the time from lights out to the first 3 epochs of any stage of sleep. The time between sleep onset and the first epoch of REM sleep was defined as REM sleep-onset latency.
Quantitative EEG Analysis of NREM Sleep
Since most quantitative sleep EEG studies have focused on NREM sleep in patients with depressive disorders as well as in investigations about sleep EEG changes across the normal menstrual cycle, we focused on the NREM sleep EEG for our study. Power spectral and period amplitude analyses were performed on the C3-A2 EEG signal for NREM sleep (stages 2–4) using PASS PLUS EEG analysis software (Delta Software, St. Louis, Mo.). Spectral power reflects both the incidence and amplitude of waves in a particular EEG frequency band, whereas period amplitude analysis (PAA) can determine the separate contributions of wave amplitude and incidence.23 The methods, therefore, provide different and complementary information about the sleep EEG. Epochs containing movements or artifacts identified by visual inspection were excluded from analysis. The fast Fourier transform routine was performed on 30-second epochs of 4-second Welch-tapered windows with 2.0-second overlap, resulting in a frequency resolution of 0.25 Hz. Spectral power density (μV2/Hz) was calculated for the 0.3- to 1-Hz frequency bin and for subsequent 1-Hz frequency bins (identified by their lower boundary value) until 23 Hz. Power spectra were also averaged for the main frequency bands: delta (0.3 - ≤ 4 Hz), theta (4 - ≤ 8 Hz), alpha (8- ' 12 Hz), sigma (12 - ≤ 15 Hz), and beta1 (15 - ≤ 23 Hz). Both absolute and relative spectral power values are reported. Relative power was derived by dividing the power in each band by the sum of the power across all bands. Absolute power was normalized with a logarithmic transformation before analysis.
We also analyzed each main frequency band with PAA for NREM sleep. We used 0-cross measures for delta and theta frequencies and 0-derivative measures for all higher frequencies because these frequencies are often superimposed on slower waves and then do not cross 0 voltage. The specific PAA measures investigated were “time in band” or “derivative time in band” (sum of all half-wave or derivative half-wave durations = wave incidence, measured in seconds) per 30-second epoch and “average sample amplitude” or “derivative estimate of sample amplitude” (measured in μV).
Hormone Analysis
A blood sample was collected from subjects on each visit to the sleep laboratory. Samples, collected in tubes containing clot-activating factor, were centrifuged and serum was stored at −20°C until analysis. At the end of the study, all samples were analyzed for progesterone and estradiol using standard radioimmunoassay kits. The intraassay and interassay coefficients of variations were 8.8% and 9.7%, respectively, for the progesterone assay (Diagnostic Products Corporation), and 5.3% and 9.3%, respectively, for the estradiol assay (Diagnostic Systems Laboratories).
Statistical Analysis
Statistical procedures were performed using Statistica (StatSoft, Tulsa, OK). All results are reported as means (± SD) unless otherwise indicated. Visual analog scale measurements were normalized before statistical analysis through the arcsine square root transform. Initially, paired t tests were used to evaluate whether there were any significant effects for order of recording (follicular or luteal phase first) for polysomnographic and subjective sleep quality measures in study participants. There were no significant order effects. All subjective and objective measures were then analyzed with repeated-measures 2-way analyses of variance at a 95% confidence interval, with “menstrual phase” as the within factor and “subject group” as the between factor. When appropriate, the Tukey posthoc test was used to identify the origin of any differences.
Results
Hormone Concentrations
Analyses of variance did not show a significant effect of group or a group × phase interaction for progesterone levels. There was, however, the expected significant effect of menstrual phase (F1,18 = 18.6, P < 0.001, n = 11 controls), with values being higher in the LLP (controls: 7.6 ± 7.7 ng·mL−1; PMS: 7.5 ± 5.3 ng·mL−1) than in the FP (controls: 0.8 ± 0.5 ng·mL−1; PMS: 0.5 ± 0.2 ng·mL−1) in both groups of women. Estradiol levels did not show significant group, phase, or group × ng·mL−1 phase interaction effects. Controls had estradiol levels of 98.3 ± 43.2 ng·mL−1 and 110.4 ± 46 ng·mL−1 in their FP and LLP, respectively. Women with PMS had estradiol levels of 85.7 ± 19.5 pg.ml−1 and 102.4 ± 32.8 ng·mL−1 in their FP and LLP, respectively.
Subjective Assessments
As shown in Table 2, women with PMS had significantly higher scores on the BDI and the Profile of Mood States than controls during the LLP and compared with their own FP. Based on BDI scores, 4 women in the PMS group met criteria for moderate to severe depression during their LLP. In the morning following their overnight recordings, both groups of women rated their sleep quality as significantly worse during the LLP than the FP, which appeared to be a larger effect in the women with PMS (Table 2). Regardless of menstrual phase, women with PMS rated their morning alertness as being significantly lower than controls following their overnight recordings (Table 2). There were no significant group or menstrual-phase differences in subjective reports of sleep-onset latency, number of awakenings, or duration spent awake during the recording night.
Table 2.
Subjective Measures of Mood, Sleep Quality, and Morning Alertness in Women with PMS and Control Subjects During the Follicular and Late Luteal Phases of the Menstrual Cycle.
| Measure | Group | Menstrual Phase |
Analysis of variance | |
|---|---|---|---|---|
| Follicular | Late luteal | |||
| Beck depression inventorya | Control | 1 (2) | 1 (2) | Group effect: F1,18 = 28.6, P < 0.001 |
| PMS | 7 (5) | 20 (14)** | Phase effect: F1,18 = 8.6, P = 0.009 | |
| Interaction: F1,18 = 8.4, P = 0.01 | ||||
| Profile of mood states (total mood score) | Control | 89 (16) | 88 (14) | Group effect: F1,19 = 17.8, P < 0.001 |
| PMS | 105 (23) | 143 (46)*# | Phase effect: F1,19 = 5.4, P = 0.03 | |
| Interaction: F1,19 = 6.0, P = 0.02 | ||||
| Subjective sleep quality VAS. mmb | Control | 73 (14) | 63 (21)# | Group effect: F1,18 = 3.8, P = 0.07 |
| PMS | 64 (29) | 42 (28)# | Phase effect: F1,18 = 4.3, P = 0.05 | |
| Interaction: F1,18 = 0.9, NS | ||||
| Subjective morning alertness VAS, mmb | Control | 65 (12) | 67 (16) | Group effect: F1,18 = 8.6, P < 0.01 |
| PMS | 42 (26)* | 52 (28)* | Phase effect: F1,18 = 1.4, NS | |
| Interaction: F1,18 = 0.6, NS | ||||
Significantly different from controls (Tukey, P < 0.01)
Significantly different from follicular phase (Tukey, P < 0.05);
n = 11 control subjects.
n = 8 women with premenstrual syndrome (PMS).
PMS refers to premenstrual syndrome; VAS, visual analogue scale.
Sleep Diaries
Since subjective evaluations of 1 night of sleep can be highly variable, we also investigated differences in ratings of total sleep time, sleep quality, and alertness in the FP and LLP, averaged over 2 menstrual cycles. Based on these averages taken from the sleep diaries, there were no significant group or menstrual-phase differences in total sleep time (control subjects: 453 ± 50 minutes [FP], 458 ± 45 minutes [LLP]; PMS: 461 ± 42 minutes [FP], 445 ± 58 minutes [LLP]), but there were differences in sleep quality and alertness. Women with PMS rated their sleep quality as being significantly poorer in the LLP compared with their FP (FP: 73 ± 12 mm, LLP: 56 ± 15 mm, Group × phase interaction: F1,18 = 7.28, P = 0.02, n = 8 women with PMS). Control subjects rated their sleep quality as similar in the FP and LLP (FP: 70 ± 12 mm, LLP: 71 ± 13 mm). Although tending in that direction, ratings of sleep quality did not differ significantly between control subjects and women with PMS in their LLP. There were no significant phase or interaction effects for morning alertness, but there was a significant group effect (I1,18 = 5.1, P = 0.04). Women with PMS rated their morning alertness (FP: 53 ± 23 mm, LLP: 49 ± 12 mm) significantly lower than did control subjects (FP: 66 ± 13 mm, LLP: 66 ± 17 mm), regardless of menstrual cycle phase.
Sleep Variables Derived From Laboratory-Based Polysomnographic Recordings
Time in bed (total recording time) was similar between groups of women and according to menstrual phase (Table 3). There were few significant differences in sleep variables attributable to either group or menstrual phase (Table 3). Women with PMS displayed a trend to have a poorer sleep efficiency than controls, regardless of menstrual phase, (F1,19 = 4.0, P = 0.06). Women with PMS had a significantly longer latency to REM sleep than controls, regardless of phase (F1,19 = 6.0, P = 0.03). Both groups of women had significantly more wakefulness after sleep onset (F1,19 = 6.7, P = 0.02) during the LLP phase than the FP. There was also a significant menstrual phase effect for the arousal index (F1,19 = 5.6, P = 0.03), with women having more microarousals per hour of sleep in the LLP than the FP.
Table 3.
Sleep Variables Derived From the Polysomnograms of 9 Women with Severe PMS and 12 Asymptomatic Women (Control Subjects) in the Midfollicular and Late Luteal Phases of Their Menstrual Cycles.
| Variable | Group | Menstrual Cycle Phase |
|
|---|---|---|---|
| Follicular | Late Luteal | ||
| Total recording time, min | Control | 492.3 (43.6) | 496.5 (50.8) |
| PMS | 482.4 (39.4) | 493.6 (32.7) | |
| Total sleep time, min | Control | 469.2 (52.2) | 464.7 (53.8) |
| PMS | 434.9 (44.1) | 442.0 (43.6) | |
| Sleep efficiency, % | Control | 95.1 (4.5) | 94.4 (2.8) |
| PMS | 90.3 (6.7) | 89.8 (9.0) | |
| Sleep onset latency, min | Control | 13 (18) | 9 (9) |
| PMS | 23 (24) | 17 (17) | |
| Wakefulness after sleep onset, min | Control | 10 (4.0) | 17 (9)* |
| PMS | 23 (27) | 33 (42)* | |
| Latency to REM sleep, min | Control | 79 (18) | 80 (26) |
| PMS | 108 (52)# | 103 (29)# | |
| Stage 1, % | Control | 5.6 (2.6) | 6.9 (2.6) |
| PMS | 7.7 (3) | 7.1 (2.9) | |
| Stage 2, % | Control | 52.4 (5.3) | 55.2 (4.5) |
| PMS | 51.6 (6.7) | 53.2 (5.5) | |
| Slow-wave sleep, % | Control | 16.8 (3.5) | 14.9 (3.3) |
| PMS | 18.4 (4.2) | 18.1 (5.2) | |
| REM sleep, % | Control | 25.4 (3.5) | 23.0 (3.7) |
| PMS | 22.4 (5.6) | 21.6 (5.6) | |
| Arousal index, No./h sleep | Control | 6.5 (2) | 7.7 (3)* |
| PMS | 6.6 (1) | 6.9 (2)* | |
Data are expressed as mean (SD). REM refers to rapid eye movement sleep; PMS, premenstrual syndrome.
Significantly different from follicular phase (P < 0.05).
Significantly different from controls (P = 0.03).
Quantitative NREM Sleep EEG
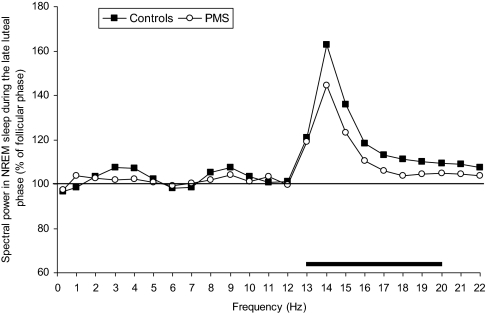
Figure 1 shows the average spectral power of NREM sleep, in the frequency range from 0.3 to 23 Hz, in women with PMS and control subjects during LLP relative to FP levels (set at 100%). Both groups of women showed a similar distribution of LLP-FP spectral power differences, with a prominent peak in the range of sleep spindles (12–15 Hz). The largest LLP increase was in the 14.25- to 15.0-Hz bin (F1,19 = 33.8, P < 0.001). Analysis of absolute spectral power in the broad frequency bands revealed significant menstrual phase effects for both the sigma (F1,19 = 12.1, P = 0.003) and beta1 (F1,19 = 15.6, P = 0.001) frequency bands. Similarly, relative power in the sigma (F1,19 = 13.7, P < 0.01) and beta1 (F1,19 = 9.5, P < 0.01) frequency bands was higher in the LLP compared with the FP, in both groups of women.
Figure 1.
Spectral electroencephalogram (EEG) power in non-rapid eye movement (NREM) sleep for women with severe premenstrual syndrome (PMS) and control subjects during the late luteal phase of the menstrual cycle, expressed relative to values in the follicular phase (horizontal line). Hz bins are identified by their lower boundary values. The horizontal bar at the bottom of the figure indicates significant menstrual-phase effects (analysis of variance, P < 0.05).
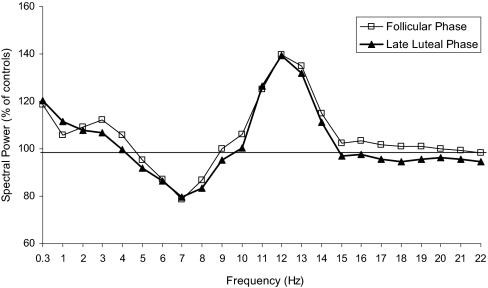
Figure 2 shows average spectral power of NREM sleep in women with PMS relative to controls (set at 100%). Spectral activity appears higher in the lower spindle frequency range in women with PMS than controls, regardless of menstrual phase, but there was no statistically significant difference between groups in any frequency band. However, there were trends for activity to be higher in PMS women for the 11- to 12-Hz (F1,19 = 2.3, P = 0.1) and 12- to 13-Hz (F1,19 = 3.8, P = 0.06) bins. There were no significant group differences in absolute or relative spectral power in the broad frequency bands.
Figure 2.
Spectral electroencephalogram power in non-rapid eye movement (NREM) sleep for women with severe premenstrual syndrome (PMS) relative to control subjects (horizontal line) during the follicular and late luteal phases of the menstrual cycle. Hz bins are identified by their lower boundary values.
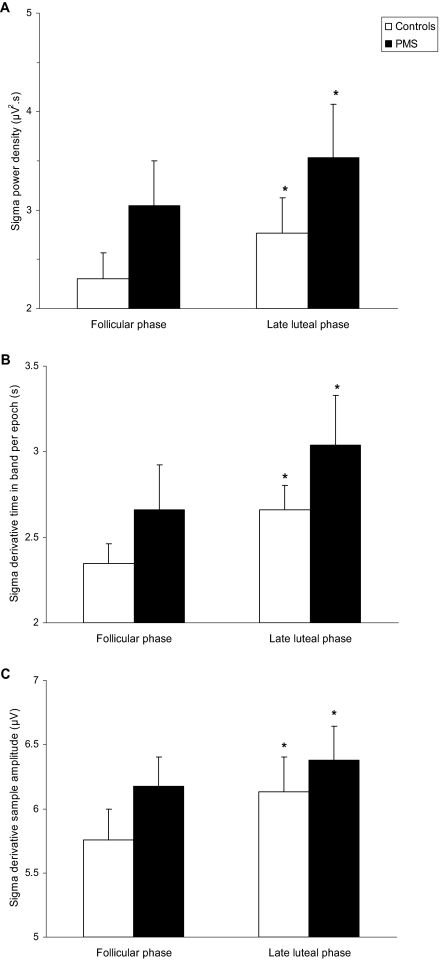
Period amplitude analysis of the NREM sleep EEG revealed significant menstrual phase differences in the sigma frequency band (Figure 3, Table 4). Both groups of women showed a significantly greater incidence (F1,19 = 18.6, P < 0.001) of waves per epoch, that were of a greater amplitude (F1,19 = 10.8, P < 0.01), in the sigma frequency band during the LLP compared with the FP. The incidence (F1,19 = 12.4, P < 0.01) and derivative sample amplitude (F1,19 = 11.0, P < 0.01) of beta1 waves were also significantly greater during the LLP in both groups of women. Amplitude was increased in the theta (F1,19 = 10.1, P < 0.01) and alpha (F1,19 = 7.2, P = 0.01) frequency bands during the LLP; however, theta and alpha incidence did not vary significantly according to menstrual phase (Table 4). Delta incidence was lower in the LLP (F1,19 = 4.6, P = 0.05) without any change in amplitude. There were no significant group differences in sigma or beta1 frequency bands, based on PAA, but there were some group differences in the delta and theta frequency bands (Table 4). Women with PMS had lower delta incidence (F1,19 = 7.2, P = 0.02) and higher theta incidence (F1,19 = 6.6, P = 0.02) and theta amplitude (F1,19 = 12.0, P < 0.01) than control subjects, regardless of menstrual cycle phase. There were no significant interaction effects for any of the variables.
Figure 3.
Results of quantitative analysis of the sigma frequency band (12–15 Hz) of the electroencephalogram (EEG) during non-rapid eye movement (NREM) sleep in women with severe premenstrual syndrome (PMS) and control subjects during the follicular and late luteal phases of the menstrual cycle. Power density (Panel A) was calculated based on a fast Fourier transform of the EEG signal; Time in band (Panel B) and Amplitude (Panel C) were calculated from period amplitude analysis. Values are shown as mean ± SEM. *Significantly different from follicular phase (P < 0.01).
Table 4.
Results Derived from Period Amplitude Analysis of the Electroencephalogram During Non-Rapid Eye Movement Sleep in 9 Women with Severe PMS and 12 Asymptomatic Women (Control Subjects) in the Mid-Follicular and Late Luteal Phases of Their Menstrual Cycles.
| Variable | Group | Menstrual Cycle Phase |
|
|---|---|---|---|
| Follicular | Late Luteal | ||
| Delta average sample amplitude, μV | Control | 15.6 (2.0) | 15.7 (2.1) |
| PMS | 17.3 (2.7) | 17.4 (2.9) | |
| Delta incidence, sec | Control | 25.0 (2.9) | 24.6 (3.2)* |
| PMS | 21.6 (2.6)# | 21.4 (2.3)*# | |
| Theta average sample amplitude, μV | Control | 6.9 (1.1) | 7.3 (1.4)* |
| PMS | 8.9 (1.1)# | 9.1 (1.3)*# | |
| Theta incidence, sec | Control | 2.0 (1.9) | 2.0 (1.9) |
| PMS | 4.0 (1.7)# | 4.0 (1.6)# | |
| Alpha derivative average sample amplitude, μV | Control | 6.7 (1.1) | 7.0 (1.3)* |
| PMS | 6.9 (0.5) | 7.1 (0.6)* | |
| Alpha incidence, sec | Control | 5.7 (1.0) | 5.6 (1.2) |
| PMS | 5.9 (0.9) | 5.6 (0.5) | |
| Sigma derivative average sample amplitude, μV | Control | 5.8 (0.8) | 6.1 (0.9)* |
| PMS | 6.2 (0.7) | 6.4 (0.8)* | |
| Sigma incidence, sec | Control | 2.3 (0.4) | 2.7 (0.5)* |
| PMS | 2.7 (0.8) | 3.0 (0.9)* | |
| Beta1 derivative average sample amplitude, μV | Control | 3.7 (0.4) | 4.0 (0.5)* |
| PMS | 3.9 (0.3) | 4.0 (0.5)* | |
| Beta1 incidence, sec | Control | 2.1 (0.4) | 2.5 (0.5)* |
| PMS | 2.3 (0.5) | 2.5 (0.6)* | |
Data are expressed as mean (SD). PMS refers to premenstrual syndrome.
Significantly different from follicular phase (P < 0.05)
Significantly different from controls (P < 0.05). See text for details.
Discussion
A detailed analysis of the sleep EEG, objective polysomnography, and subjective sleep quality was conducted in women with severe PMS and asymptomatic control subjects at 2 phases of the menstrual cycle. The women with severe PMS had significantly higher depression scores when symptomatic during the LLP, compared with the FP and compared with controls, but sleep architecture was altered to a similar extent in both groups in the LLP. Women with PMS and control subjects had more wakefulness after sleep onset and showed a prominent increase in spindle frequency activity in the LLP. Expression of premenstrual symptoms, therefore, is not associated with specific changes in the sleep EEG. However, we found some intriguing differences in sleep architecture between women with PMS and control subjects that were not limited to the symptomatic LLP, suggesting that the sleep EEG may be altered in women with PMS in a trait-like manner, independent of symptom expression.
The finding that objectively measured sleep macrostructure is apparently unrelated to the presence of severe premenstrual symptoms supports those of previous studies.8–11 The present quantitative EEG data and assessment of microarousals add to the previous work in indicating that there is also no change in sleep microstructure specific to premenstrual symptom expression. Further, the sleep diary data indicate a dissociation between objective and subjective sleep-quality measures in that women with severe PMS perceived their sleep quality as poorer in the LLP in association with their premenstrual symptoms. A similar tendency was found based on the subjective ratings of sleep quality after the overnight polysomnographic study. Santo and colleagues also reported, in conference proceedings 24, that women with PMDD had a poorer sleep quality in their LLP. Sleep quality has been assumed to be related mainly to objective sleep efficiency,25 which did not vary according to menstrual phase in our subjects. Perceived sleep quality is also influenced by frequency and duration of wakefulness during the night.26 Both groups of women had increased polysomnographically defined wake after sleep onset and a marginal increase in the arousal index in LLP recordings; possibly, women with PMS may be more sensitive to the increased wake after sleep onset and sleep fragmentation when rating their sleep quality. Alternatively, the poor ratings of sleep quality in the women with PMS may be a consequence of a negative reporting bias in association with their negative premenstrual symptoms. A similar tendency for negative evaluations of sleep quality has been reported for depressed patients27 and patients with irritable bowel syndrome.28 Another interesting finding based on the self-report data in our study is that the women with PMS rated their alertness as significantly lower, at both menstrual phases, compared with controls. Possibly, the lower levels of alertness may be attributed to a tendency for poorer sleep efficiency in the women with PMS.
Although there were no symptom-related menstrual-phase differences in sleep architecture specific to women with PMS, we found evidence of group differences in sleep structure irrespective of menstrual phase. Women with severe PMS had a longer REM-onset latency than control subjects. Possibly, the data reflect a tendency for a delayed circadian rhythm, given that the timing of REM sleep is under circadian regulation.29 There is evidence that women with PMDD have altered timing of several hormones, including delayed onset of melatonin in the luteal phase compared with the FP,30 suggesting the possibility of an underlying circadian abnormality in these women compared with control subjects. Previous studies have not found any differences in REM-onset latency between women with PMS or PMDD and control subjects, but 2 studies have found other trait-like alterations in sleep in women with severe PMS or PMDD, including decreased REM sleep or slow-wave sleep, in association with increased stage 2 sleep, compared with controls.9,10 Two further studies, however, have found no differences in sleep architecture between women with PMDD and control subjects.8,11 These variable results between studies may be a consequence of different definitions of PMS and PMDD, different experimental conditions, variable sample sizes, and variations in symptoms experienced by the women.
The link between depressed mood and sleep composition in patients with depressive disorders is complex. Although several studies have shown substantial differences in sleep macrostructure, such as reduced REM-onset latency and slow-wave sleep, in patients with major depressive disorder (MDD) compared with controls, a meta-analysis of published work showed that no single sleep variable was specific for MDD.31 Also, not all patients with MDD show substantial sleep disturbances.12 Even investigations of subtle changes in sleep, based on quantitative EEG analysis, have not shown consistent differences between patients with MDD and control subjects,32–35 which may partly be due to variation in disease severity. There also is wide variation in the type and severity of symptoms experienced by women with severe PMS or PMDD, with some women reporting irritability and anxiety, and others reporting depressed mood and emotional lability, as their major symptoms.3 This variability in symptom expression may lead to variability in effects on sleep composition between women.
In our study, detailed analysis of the sleep EEG revealed subtle differences between women with severe PMS and control subjects, regardless of menstrual phase. These differences, therefore, were evident independent of premenstrual symptom expression in women with PMS. Compared with controls, women with PMS had decreased incidence of delta and increased incidence and amplitude of theta waveforms in NREM sleep. Decreased delta power or delta wave incidence is hypothesized to reflect a deficiency in sleep-regulatory processes.33 Although not a consistent finding in all patients, several studies have reported decreased slow-wave activity or delta wave incidence in NREM sleep33,34 in patients with depression. Further, a correlational analysis of the association between sleep EEG measures and depressive symptoms showed that delta wave counts (incidence) was significantly related to depressive symptoms in a large group of patients with MDD.36 Since depressed mood is a common symptom of PMS and PMDD,1 it is possible that delta sleep EEG characteristics may be similarly altered in these women, as in patients with MDD. Interestingly, slow-wave sleep abnormalities in patients with mood disorders may persist even when patients are in remission, thus appearing as a trait marker.12
We also found a trend for group differences in the lower sigma frequency band, based on power spectral analysis. Women with PMS tended to have higher power, specifically in the 12- to 13-Hz band compared with control subjects, in both menstrual phases. This frequency band is in the range of sleep spindle activity, which can change as a function of several variables, including homeostatic and circadian factors, menstrual cycle phase, pregnancy, and pharmacologic agents such as benzodiazepines.37 GABAA receptors in the thalamus play an essential role in the generation of sleep spindles,38 and altered activity in the spindle frequency range of the sleep EEG may reflect altered GABAA receptor function. Indeed, abnormal GABAergic function has been reported in women with PMDD, regardless of menstrual phase, and is hypothesized to be involved in the etiology of PMDD.39 For example, cortical GABA levels were found to be reduced in the FP in women with PMDD compared with control subjects.40 Future studies are needed to clarify possible differences in the sleep EEG and sleep regulation in women with severe PMS or PMDD. Challenge studies, such as with sleep deprivation, may also be worthwhile in revealing differences between women with severe PMS and asymptomatic women.
As an integral part of our study, we investigated differences in sleep composition and the NREM sleep EEG at 2 phases of the menstrual cycle. Women with normal menstrual cycles and women with PMS showed a substantial increase in EEG activity in the sigma frequency band in LLP compared with FP, confirming results from a previous study.16 Similar to Driver et al,16 we found that activity was maximally increased in the upper spindle frequency band (14–15 Hz). PAA of the EEG indicates that both amplitude and incidence of waves in the sigma frequency band increase during the LLP. The increased spectral power in the sigma frequency band, therefore, is due to a combination of increased amplitude and incidence of sigma waves. Ishizuka et al41 found only a menstrual cycle phase variation in spindle frequency, with no change in spindle amplitude; however, their analysis only included predefined sleep spindles with an amplitude greater than 5 μV rather than analysis of the sigma frequency band. The increased sigma power that we found in our study likely translates into increased spindle density; spectral power density in the frequency range of spindles correlates well with spindle density.42 Increased spindle frequency activity is reminiscent of the effects of the progesterone metabolite, allopregnanolone, on the EEG in rats.43 Allopregnanolone, which is increased in association with increased progesterone levels, enhances GABA-induced GABAA receptor currents, facilitating the occurrence of sleep spindles (Reviewed in 44). The increased spindle frequency activity in the LLP, therefore, probably represents an interaction between endogenous progesterone metabolites and GABAA receptors.
Although not to the same extent as in the sigma frequency band, activity in the beta1 frequency band was increased in the LLP, due to a combination of increased incidence and amplitude of beta1 waveforms. Elevated beta activity during sleep is thought to represent hyperarousal and characterizes the sleep of people with primary insomnia.45 Women, therefore, may be in a state of central nervous system hyperarousal during sleep in their LLP, which may impact the perception of their sleep. This menstrual phase difference, when combined with their depressed symptoms, may lead women with severe PMS to be more likely to complain of sleep disturbances during the LLP than at other times of the menstrual cycle.
Our study has several limitations, which need to be considered. Not all women in our symptomatic group had equally severe premenstrual symptoms. While all women with PMS met the provisional criteria for a diagnosis of PMDD based on their retrospective reports and clinical interview, only 5 of the women continued to meet the criteria after prospective evaluation. Although the other 4 women did not meet the criteria for PMDD, they were included in the study because their PMS was still severe and impacted their quality of life. Further, recent clinical and epidemiologic studies have shown there is a strong overlap between severe PMS and PMDD, with severe PMS still being highly correlated with functional impairment.3 Although the stringent criteria for PMDD may not be met, particularly the requirement of 5 specific PMDD symptoms, severe PMS is still considered clinically significant.1,3 We therefore believe that our results are applicable to women who have clinically significant PMS, including those meeting the DSM-IV criteria for PMDD. Another factor that needs to be considered is that PMS severity can vary from month to month within an individual. The women in our study continued to complete the daily symptom rating form throughout the recording period, which allowed us to confirm that they were experiencing severe premenstrual symptoms during the recording period. However, based on the BDI ratings, there was a range in the severity of depression that the women were experiencing during the LLP recording period. Indeed, there is large variability in the type, as well as timing, of symptoms experienced by women with severe PMS or PMDD.1 Given this variability, it would be worthwhile to investigate sleep EEG changes in relation to a range of severity of premenstrual mood symptoms in a larger sample of women. Finally, a limitation of our study is that we did not use actigraphy or polysomnography to confirm that the subjects kept their regular sleep-wake schedules on the night before laboratory recordings. Given these limitations and the small sample size, our results need to be interpreted with caution until replicated and extended in other studies.
In conclusion, we have shown that women with severe PMS perceive their sleep quality to be poorer in association with their symptoms in the LLP. However, there are no specific alterations in sleep macrostructure or microstructure associated with premenstrual symptoms. Detailed analysis of the sleep EEG revealed potential trait-like differences in women with severe PMS, which need to be investigated further.
ACKNOWLEDGMENTS
This study was supported by a developmental grant from SRI International (FC Baker). Blood sample analysis was supported by NICHD (SCCPRR) Grant U54-HD28934, “University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core.” We thank all research staff for their invaluable technical support and are especially grateful for the assistance of Laura deTar on the project. We also thank all volunteers for their dedicated participation.
Footnotes
Disclosure Statement
This was not an industry supported study. Drs. Baker, Kahan, Trinder, and Colrain have reported no financial conflicts of interest.
REFERENCES
- 1.Halbreich U. The etiology, biology, and evolving pathology of premenstrual syndromes. Psychoneuroendocrinology. 2003;28(Suppl 3):55–99. doi: 10.1016/s0306-4530(03)00097-0. [DOI] [PubMed] [Google Scholar]
- 2.Angst J, Sellaro R, Merikangas KR, Endicott J. The epidemiology of perimenstrual psychological symptoms. Acta Psychiatr Scand. 2001;104:110–6. doi: 10.1034/j.1600-0447.2001.00412.x. [DOI] [PubMed] [Google Scholar]
- 3.Freeman EW. Premenstrual syndrome and premenstrual dysphoric disorder: definitions and diagnosis. Psychoneuroendocrinology. 2003;28(Suppl 3):25–37. doi: 10.1016/s0306-4530(03)00099-4. [DOI] [PubMed] [Google Scholar]
- 4.Mortola JF, Girton L, Beck L, Yen SS. Diagnosis of premenstrual syndrome by a simple, prospective, and reliable instrument: the calendar of premenstrual experiences. Obstet Gynecol. 1990;76:302–7. [PubMed] [Google Scholar]
- 5.Strine TW, Chapman DP, Ahluwalia IB. Menstrual-related problems and psychological distress among women in the United States. J Womens Health (Larchmt) 2005;14:316–23. doi: 10.1089/jwh.2005.14.316. [DOI] [PubMed] [Google Scholar]
- 6.Mauri M, Reid RL, MacLean AW. Sleep in the premenstrual phase: a self-report study of PMS patients and normal controls. Acta Psychiatr Scand. 1988;78:82–6. doi: 10.1111/j.1600-0447.1988.tb06304.x. [DOI] [PubMed] [Google Scholar]
- 7.Parry BL, Cover H, Mostofi N, et al. Early versus late partial sleep deprivation in patients with premenstrual dysphoric disorder and normal comparison subjects. Am J Psychiatry. 1995;152:404–12. doi: 10.1176/ajp.152.3.404. [DOI] [PubMed] [Google Scholar]
- 8.Chuong CJ, Kim SR, Taskin O, Karacan I. Sleep pattern changes in menstrual cycles of women with premenstrual syndrome: a preliminary study. Am J Obstet Gynecol. 1997;177:554–8. doi: 10.1016/s0002-9378(97)70145-5. [DOI] [PubMed] [Google Scholar]
- 9.Lee KA, Shaver JF, Giblin EC, Woods NF. Sleep patterns related to menstrual cycle phase and premenstrual affective symptoms. Sleep. 1990;13:403–9. [PubMed] [Google Scholar]
- 10.Parry BL, Mendelson WB, Duncan WC, Sack DA, Wehr TA. Longitudinal sleep EEG, temperature, and activity measurements across the menstrual cycle in patients with premenstrual depression and in age-matched controls. Psychiatry Res. 1989;30:285–303. doi: 10.1016/0165-1781(89)90020-6. [DOI] [PubMed] [Google Scholar]
- 11.Parry BL, Mostofi N, LeVeau B, et al. Sleep EEG studies during early and late partial sleep deprivation in premenstrual dysphoric disorder and normal control subjects. Psychiatry Res. 1999;85:127–43. doi: 10.1016/s0165-1781(98)00128-0. [DOI] [PubMed] [Google Scholar]
- 12.Benca R. Principles and Practice of Sleep Medicine 4th Ed. Philadelphia: Elsevier Saunders; 2005. Mood Disorders. In: Kryger M, Roth T, Dement W, eds; pp. 1311–26. [Google Scholar]
- 13.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects National Institutes of Health Publication No. 204. Washington, DC: U.S. Government Printing Office; 1968. [DOI] [PubMed] [Google Scholar]
- 14.Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: a meta-analysis. Sleep. 1996;19:318–26. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- 15.American Sleep Disorders Association ASDA EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 16.Driver HS, Dijk DJ, Werth E, Biedermann K, Borbely AA. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81:728–35. doi: 10.1210/jcem.81.2.8636295. [DOI] [PubMed] [Google Scholar]
- 17.Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203–9. doi: 10.1007/s00737-003-0018-4. [DOI] [PubMed] [Google Scholar]
- 18.Hollingshead AB. New Haven, CT: Yale University Press; 1965. Two-factor index of social position. [Google Scholar]
- 19.Freeman EW, DeRubeis RJ, Rickels K. Reliability and validity of a daily diary for premenstrual syndrome. Psychiatry Res. 1996;65:97–106. doi: 10.1016/s0165-1781(96)02929-0. [DOI] [PubMed] [Google Scholar]
- 20.Monk TH, Reynolds CF, 3rd, Kupfer DJ, et al. The Pittsburgh Sleep Diary. J Sleep Res. 1994;3:111–20. [PubMed] [Google Scholar]
- 21.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory, 2nd ed. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- 22.McNair DM, Lorr M, Droppleaman LF. Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1992. 1992. [Google Scholar]
- 23.Feinberg I, Maloney T, Campbell IG. Effects of hypnotics on the sleep EEG of healthy young adults: new data and psychopharmacologic implications. J Psychiatr Res. 2000;34:423–38. doi: 10.1016/s0022-3956(00)00038-8. [DOI] [PubMed] [Google Scholar]
- 24.Santo JB, Chevrier E, L'Esperance P, Boivin DB. Subjective assessment of sleep quality across the menstrual cycle in women with premenstrual dysphoric disorder. Sleep. 2002:A503. [Google Scholar]
- 25.Keklund G, Akerstedt T. Objective components of individual differences in subjective sleep quality. J Sleep Res. 1997;6:217–20. doi: 10.1111/j.1365-2869.1997.00217.x. [DOI] [PubMed] [Google Scholar]
- 26.Baker FC, Maloney S, Driver HS. A comparison of subjective estimates of sleep with objective polysomnographic data in healthy men and women. J Psychosom Res. 1999;47:335–41. doi: 10.1016/s0022-3999(99)00017-3. [DOI] [PubMed] [Google Scholar]
- 27.Armitage R, Trivedi M, Hoffmann R, Rush AJ. Relationship between objective and subjective sleep measures in depressed patients and healthy controls. Depression Anxiety. 1997;5:97–102. doi: 10.1002/(sici)1520-6394(1997)5:2<97::aid-da6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 28.Elsenbruch S, Thompson JJ, Hamish MJ, Exton MS, Orr WC. Behavioral and physiological sleep characteristics in women with irritable bowel syndrome. American J Gastroenterol. 2002;97:2306–14. doi: 10.1111/j.1572-0241.2002.05984.x. [DOI] [PubMed] [Google Scholar]
- 29.Czeisler CA, Zimmerman JC, Ronda JM, Moore-Ede MC, Weitzman ED. Timing of REM sleep is coupled to the circadian rhythm of body temperature in man. Sleep. 1980;2:329–46. [PubMed] [Google Scholar]
- 30.Parry BL, Berga SL, Mostofi N, Klauber MR, Resnick A. Plasma melatonin circadian rhythms during the menstrual cycle and after light therapy in premenstrual dysphoric disorder and normal control subjects. J Biol Rhythms. 1997;12:47–64. doi: 10.1177/074873049701200107. [DOI] [PubMed] [Google Scholar]
- 31.Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992;49:651–68. doi: 10.1001/archpsyc.1992.01820080059010. discussion 69–70. [DOI] [PubMed] [Google Scholar]
- 32.Armitage R, Hoffmann R, Trivedi M, Rush AJ. Slow-wave activity in NREM sleep: sex and age effects in depressed outpatients and healthy controls. Psychiatry Res. 2000;95:201–13. doi: 10.1016/s0165-1781(00)00178-5. [DOI] [PubMed] [Google Scholar]
- 33.Borbely AA, Tobler I, Loepfe M, et al. All-night spectral analysis of the sleep EEG in untreated depressives and normal controls. Psychiatry Res. 1984;12:27–33. doi: 10.1016/0165-1781(84)90135-5. [DOI] [PubMed] [Google Scholar]
- 34.Kupfer DJ, Ulrich RF, Coble PA, et al. Application of automated REM and slow wave sleep analysis: II. Testing the assumptions of the two-process model of sleep regulation in normal and depressed subjects. Psychiatry Res. 1984;13:335–43. doi: 10.1016/0165-1781(84)90081-7. [DOI] [PubMed] [Google Scholar]
- 35.Landolt HP, Gillin JC. Similar sleep EEG topography in middle-aged depressed patients and healthy controls. Sleep. 2005;28:239–47. doi: 10.1093/sleep/28.2.239. [DOI] [PubMed] [Google Scholar]
- 36.Perlis ML, Giles DE, Buysse DJ, Thase ME, Tu X, Kupfer DJ. Which depressive symptoms are related to which sleep electroencephalographic variables? Biol Psychiatry. 1997;42:904–13. doi: 10.1016/S0006-3223(96)00439-8. [DOI] [PubMed] [Google Scholar]
- 37.De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7:423–40. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- 38.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–85. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 39.Sundstrom Poromaa I, Smith S, Gulinello M. GABA receptors, progesterone and premenstrual dysphoric disorder. Arch Womens Ment Health. 2003;6:23–41. doi: 10.1007/s00737-002-0147-1. [DOI] [PubMed] [Google Scholar]
- 40.Epperson CN, Haga K, Mason GF, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59:851–8. doi: 10.1001/archpsyc.59.9.851. [DOI] [PubMed] [Google Scholar]
- 41.Ishizuka Y, Pollak CP, Shirakawa S, et al. Sleep spindle frequency changes during the menstrual cycle. J Sleep Res. 1994;3:26–9. doi: 10.1111/j.1365-2869.1994.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 42.Dijk DJ. EEG slow waves and sleep spindles: windows on the sleeping brain. Behav Brain Res. 1995;69:109–16. doi: 10.1016/0166-4328(95)00007-g. [DOI] [PubMed] [Google Scholar]
- 43.Lancel M, Faulhaber J, Schiffelholz T, et al. Allopregnanolone affects sleep in a benzodiazepine-like fashion. J Pharmacol Exp Ther. 1997;282:1213–8. [PubMed] [Google Scholar]
- 44.Lancel M. Role of GABAA receptors in the regulation of sleep: initial sleep responses to peripherally administered modulators and agonists. Sleep. 1999;22:33–42. doi: 10.1093/sleep/22.1.33. [DOI] [PubMed] [Google Scholar]
- 45.Perlis ML, Merica H, Smith MT, Giles DE. Beta EEG activity and insomnia. Sleep Med Rev. 2001;5:363–74. doi: 10.1053/smrv.2001.0151. [DOI] [PubMed] [Google Scholar]