Abstract
Most anthropoid primates are slow to develop, their offspring are mostly single births, and the interbirth intervals are long. To maintain a stable population, parents must live long enough to sustain the serial production of a sufficient number of young to replace themselves while allowing for the death of offspring before they can reproduce. However, in many species there is a large differential between the sexes in the care provided to offspring. Therefore, we hypothesize that in slowly developing species with single births, the sex that bears the greater burden in the care of offspring will tend to survive longer. Males are incapable of gestating infants and lactating, but in several species fathers carry their offspring for long periods. We predict that females tend to live longer than males in the species where the mother does most or all of the care of offspring, that there is no difference in survival between the sexes in species in which both parents participate about equally in infant care, and that in the species where the father does a greater amount of care than the mother, males tend to live longer. The hypothesis is supported by survival data for males and females in anthropoid primate species.
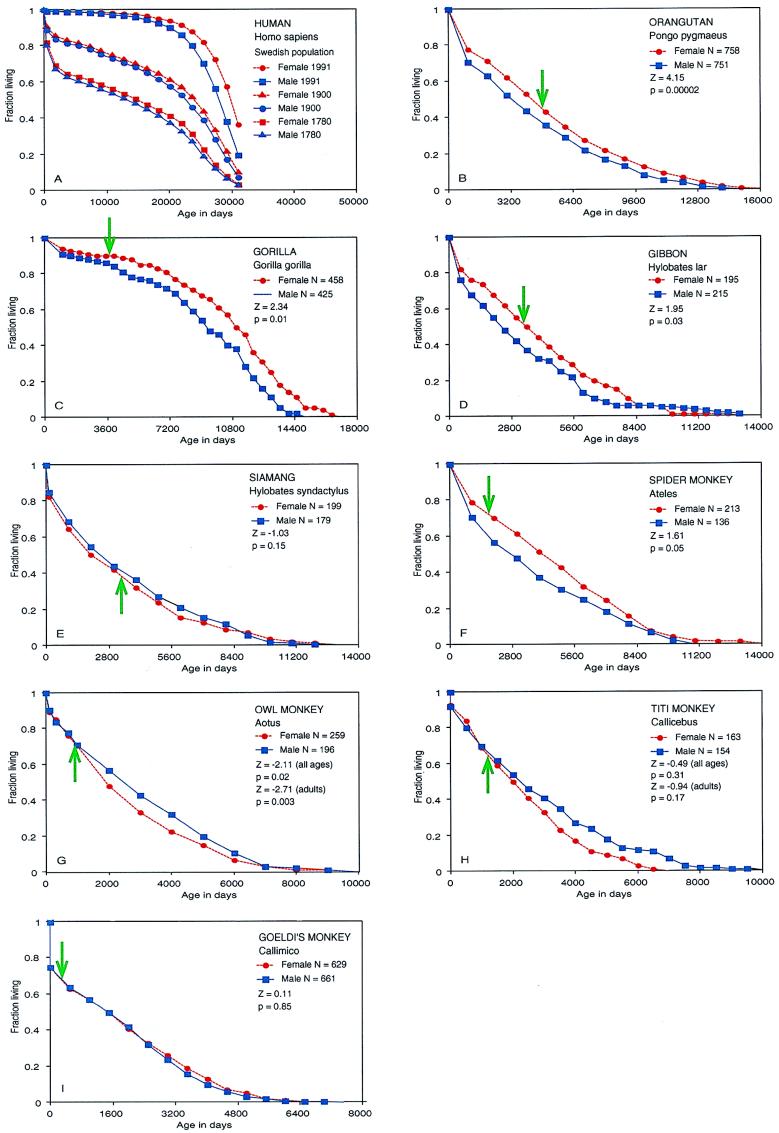
Most anthropoid primates are slow to develop, which is linked to large brain size (1, 2). We hypothesize that in slowly developing species with single births, the sex that bears the greater burden in the care of offspring will tend to survive longer. We have tested this hypothesis by reviewing the demographic literature and by constructing survival tables for anthropoid primates (see Fig. 1). The human graphs were plotted from published data (3, 4). The survival graphs and statistics below for nonhuman primates were calculated from raw birth and death records (5–12). Gehan’s generalized Wilcoxon test (13) was used to compare survival distributions for males and females of nine primate species. For orangutans, gorillas, gibbons, and spider monkeys, in which the female is primarily responsible for the care of offspring, we tested the null hypothesis against the hypothesis that females tend to outlive males; for siamangs and Goeldi’s monkeys, we tested the null hypothesis against the hypothesis that the sexes differed in survival; and in owl monkeys and titi monkeys, we tested the null hypothesis against the hypothesis that males tend to outlive females.
Figure 1.
Survival curves for anthropoid primates. The green arrows indicate the average age of first reproduction in females (14).
RESULTS
In Fig. 1A, human data from the Swedish population from three historical periods indicate a female survival advantage going back to 1780, which are the earliest records available (3, 15). The female advantage is evident throughout more than two centuries in spite of large differences in mortality rates. Similar female advantages were recorded in the earliest data from England and France in the 19th century, and the female advantage has been present in most countries throughout the world in the 20th century (3, 4, 16). A female survival advantage has also been found among adults in the Ache, a well-studied hunter-gatherer population living in the forests of eastern Paraguay (17). These data strongly suggest that the survival advantage in human females has deep biological roots. Although human fathers have a significant role, human mothers generally bear the greater burden in caring for their offspring.
Female gorillas, orangutans, and chimpanzees have a proportionally larger survival advantage than human females in data obtained from captive populations [see Fig. 1 B and C and Gage et al. (18) for chimpanzees]. In chimpanzees (Pan troglodytes), there also are data from natural populations. In a 22-year study of 228 chimpanzees living in the Mahale Mountains, Nishida (19) found an equivalent number of male and female births but three times as many females as males in the adult population. This difference was not due to differential migration, and thus their observations indicate a strong female survival advantage. Goodall (20) also found a female survival advantage in her long-term study of chimpanzees at Gombe. Chimpanzee and orangutan mothers usually provide all the care for their offspring (20, 21), and females possess a very strong survival advantage. Gorilla mothers provide most of the care for their offspring, but the fathers protect and play with them (22). The female survival advantage in gorillas, although significant, is not as large as in chimpanzees or orangutans (see Table 1).
Table 1.
Survival ratios and male care in anthropoid primates
| Primate | Female/male survival ratio | Male care |
|---|---|---|
| Chimpanzee (18) | 1.418 | Rare or negligible (19, 20) |
| Spider monkey | 1.272 | Rare or negligible (23, 24) |
| Orangutan | 1.203 | None (21) |
| Gibbon | 1.199 | Pair-living, but little direct role (25) |
| Gorilla | 1.125 | Protects, plays with offspring (22) |
| Human (Sweden 1780–1991) | 1.052–1.082 | Supports economically, some care |
| Goeldi’s monkey | 0.974 | Both parents carry infant (26) |
| Siamang | 0.915 | Carries infant in second year (25) |
| Owl monkey | 0.869 | Carries infant from birth (27, 28) |
| Titi monkey | 0.828 | Carries infant from birth (27, 29) |
Fig. 1D shows that female white-handed gibbons (Hylobates lar) also enjoy a significant survival advantage. Gibbons live in pairs, but the females carry the infants without paternal assistance (25). Fig. 1E indicates that in contrast to the female advantage in the other apes, male siamangs (Hylobates syndactylus) have a slight survival advantage. Like gibbons, siamangs live in pairs; however, siamang fathers are the only male apes to carry their infants (25). Siamang mothers carry their infants during the first year, but the fathers largely take over this task during the second year until the offspring move independently (25).
In Old World monkeys, females do most of the infant care. Female survival advantages have been reported in demographic studies of natural populations of two species. In toque macaques (Macaca sinica), females older than 4 years have a lower risk of dying than males of comparable age (30). In gelada baboons (Theropithecus gelada), females older than 8.5 years have a lower risk of dying than males of same age (31). In a demographic study of a large captive population of rhesus monkeys (Macaca mulatta), females were found to have a lower mortality rate than males up to age 7; the authors noted that the mortality rates for the older monkeys in this colony were much lower than expected and suggested that this was due to selective culling of the less robust older animals (32).
In New World monkeys, we found a significant female advantage in captive spider monkeys (see Fig. 1F and Table 1), and a substantial female survival advantage has been reported for a natural population of capuchin monkeys (Cebus olivaceus) (33). In both spider and capuchin monkeys, mothers do nearly all the infant care (23, 26). Past sexual maturity, owl monkey males (Aotus) have a strong survival advantage over females (Fig. 1G). Titi monkey males (Callicebus) also tend to live longer than females, but the effect is not statistically significant, possibly due to the small sample size (Fig. 1H). Both owl monkeys and titi monkeys live in monogamous pairs, and the fathers carry their offspring from shortly after birth except for brief nursing periods on the mother and occasional rides on older siblings (27). We have observed in captive owl monkeys that if the father dies, the mother will not carry the infant except during brief nursing periods. In Goeldi’s monkey (Callimico goeldi), we found nearly identical curves for males and females (see Fig. 1I). In Goeldi’s monkey, births are single; initially the mother carries the infant, but later the father carries it in cooperation with other family members (26). Goeldi’s monkeys also have accelerated maturation with first reproduction occurring at about 480 days, far earlier than any other monkey, and females have two birth seasons per year (26). Because of twinning, short interbirth intervals, and cooperative male care of infants (26, 34), our hypothesis does not apply to the callithricid group of anthropoids, the marmosets and tamarins.
DISCUSSION
In the majority of species, there is a female advantage throughout life, but in all anthropoids studied in which single births are typical and the male carries the infant, there is either no difference in survival between the sexes or there is a male survival advantage. These results run counter to the reasonable expectation that the increased energy expenditure and risk of falling associated with carrying an infant would result in increased rather than decreased mortality. In Table 1, the primates are ranked in descending order of female vs. male survival. As the female/male survival ratio decreases, paternal care for offspring tends to increase. In chimpanzees, spider monkeys, and orangutans, paternal care is rare or neglible, and the females have large survival advantages. In these three primates, females typically mate with multiple males and there may be substantial competition among males. This observation would suggest that higher mortality in males in these primates would be due in part to injuries suffered in competition for females. Unfortunately, the stud books used to construct these survival tables generally do not provide the causes of death so that we cannot directly evaluate the role of violence. However, aggressive interactions are rare among male spider monkeys, and females tend to be dominant (23, 24), which suggests that injuries in male competition are not responsible for the female survival advantage in these primates. Goodall (20) has suggested that dominance interactions might reduce reproductive success in male chimpanzees because they are incompatible with establishing consort relationships with females. By contrast, high-ranking female chimpanzees tend to live longer and their offspring are significantly more likely to survive (35). Female dominance interactions are generally more subtle and their hierarcharies more stable than in male chimpanzees (35). Finally, multimale mating systems do not necessarily lead to aggressive interactions among males as is evidenced by the low level of aggression and the high degree of male cooperation in tamarins (34).
A single breeding male per social group is the norm in gibbons, gorillas, siamangs, owl monkeys, and titi monkeys, but there is large variance in the female/male survival ratio in these primates, which is predicted by differences in the male parenting roles but not by male competition. This contrast is most striking in gibbons and siamangs, which are members of the same genus and are pair-living but covary in male care and female/male survival ratios. One factor that we have not taken into account because of the lack of comparable data across primates is the possibility that survival ratios might be influenced by the amount of cooperative interactions among care-givers of the same sex.
In Table 1 there is a tendency for body weight (14) to decrease with the female survival ratio; however, the correlation between these variables is not statistically significant (P = 0.11; R2 = 0.236). This tendency is not seen in the two most closely related primates in the table, gibbons and siamangs, where it is the heavier siamangs that exhibit male care and a lower female survival ratio.
In humans, the female survival advantage begins shortly after conception and continues throughout life with the largest advantage, in terms of the differential between male and female age-specific death rates, occurring during the child-rearing years (36). In some human groups including the contemporary United States and Swedish populations, there is evidence for a second smaller peak in the female survival advantage around age 60 (4, 36). Although it is difficult to obtain precise sexual differentials for age-specific mortality rates from the small populations available for nonhuman primates, the data from gorillas and gibbons exhibit two peaks in female survival advantage at the same stages in the life cycle as in humans. By contrast, the male survival advantage in owl monkeys and titi monkeys emerges shortly after maturity during the peak period of paternal responsibility for the care of offspring.
Human females have lower risks than males of dying from the 13 most prevalent causes of death (37), which indicates the female survival advantage has an extremely broad base. The hormonal basis of this effect is evidenced by the finding that postmenopausal women who currently receive estrogen replacement have a lower risk of death as compared with postmenopausal women who have never received supplemental estrogen (38). Experimental findings from avian species in which males typically have a large parenting role indicate that testosterone administration reduces male parenting behavior, suppresses the immune system, and increases the frequency of injury from fighting (39). It would be interesting to determine whether these results would be obtained in male-caretaking primates.
Finally, a close dyadic bond develops between father and offspring in siamangs, owl monkeys, and titi monkeys, which is much stronger than the maternal bond in these primates (25, 28, 29). It is conceivable that the strength of these bonds and their underlying neurochemical and hormonal bases might enhance survival .
Acknowledgments
We thank the anonymous reviewers for their helpful comments. This research was supported by the Hixon Fund and by a grant from the Howard Hughes Medical Institute Undergraduate Biological Science Education Program.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Allman J, McLaughlin T, Hakeem A. Proc Natl Acad Sci USA. 1993;90:118–122. doi: 10.1073/pnas.90.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith H, Crummet T, Brandt K. Year Phys Anthropol. 1994;37:177–231. [Google Scholar]
- 3.Keyfitz N, Fleiger W. World Population. Chicago: Univ. of Chicago Press; 1968. [Google Scholar]
- 4.United Nations. Demographic and Social Statistics Database. New York: Computer disk published by the United Nations Department for Economic and Social Information and Policy Analysis; 1994. [Google Scholar]
- 5.Perkins L. International Studbook of the Orangutan (Pongo pygmaeus) Atlanta: Fulton County Zoo; 1996. [Google Scholar]
- 6.Kirchshofer R. International Studbook of the Gorilla. Frankfurt: The Frankfurt A. M. Zoological Garden; 1987–1993. [Google Scholar]
- 7.Brockett R C. 1994 North American Studbook Aotus. Atlanta: Fulton County Zoo; 1994. [Google Scholar]
- 8.Moore D, Hannon K. White-Handed Gibbon Regional Studbook. Syracuse, NY: Burnett Park Zoo; 1992. [Google Scholar]
- 9.Fiore W J. Siamang Studbook. Montgomery, AL: Montgomery Zoo; 1996. [Google Scholar]
- 10.Kaemmerer K, Stevens A. Titi Monkey Studbook. Dallas: Dallas Zoo; 1997. [Google Scholar]
- 11.Newland K. North American Regional Studbook South American Spider Monkeys. Kansas City, KS: Sedgwick County Zoo; 1994. [Google Scholar]
- 12.Warneke M. Callimico goeldii. Chicago: Chicago Zoological Society; 1996. [Google Scholar]
- 13.Lee E T. Statistical Methods for Survival Data Analysis. New York: Wiley; 1992. [Google Scholar]
- 14.Rowe N. The Pictorial Guide to The Living Primates. East Hampton, NY: Pagonias; 1996. [Google Scholar]
- 15.Keyfitz N, Fleiger W. World Population Growth and Aging. Chicago: Univ. of Chicago Press; 1990. [Google Scholar]
- 16.United Nations. 1948 Demographic Yearbook. United Nations, New York: Statistical Office; 1949. [Google Scholar]
- 17.Hill K, Hurtado A M. Ache Life History. New York: Aldine de Gruyter; 1996. [Google Scholar]
- 18.Dyke B, Gage T B, Alford P L, Swenson B, Williams-Blangero S. Am J Primatol. 1995;37:25–55. doi: 10.1002/ajp.1350370104. [DOI] [PubMed] [Google Scholar]
- 19.Nishida T. The Chimpanzees of the Mahale Mountains. Tokyo: Univ. of Tokyo Press; 1990. [Google Scholar]
- 20.Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. Cambridge, MA: The Belknap Press of Harvard Univ. Press; 1986. [Google Scholar]
- 21.Rodman P, Itani J. In: Primate Societies. Smuts B, Cheney D, Seyfarth R, Wrangham R, Struhsaker T, editors. Chicago: Univ. of Chicago Press; 1986. pp. 146–154. [Google Scholar]
- 22.Stewart K, Harcourt A. In: Primate Societies. Smuts B, Cheney D, Seyfarth R, Wrangham R, Struhsaker T, editors. Chicago: Univ. of Chicago Press; 1986. pp. 155–164. [Google Scholar]
- 23.Robinson J, Janson C. Primate Societies. Chicago: Univ. of Chicago Press; 1986. pp. 69–82. [Google Scholar]
- 24.van Roosmalen M G M. Habitat Preferences, Diet, Feeding Strategy, and Social Organization of the Black Spider Monkey (Ateles paniscus paniscus) in Surinam. Leersum, The Netherlands: Rijksinstituut voor Natuurbeheer; 1980. [Google Scholar]
- 25.Chivers D J. The Siamang in Malaya. Basel: Karger; 1974. [PubMed] [Google Scholar]
- 26.Kinzey W G. New World Primates: Ecology, Evolution, and Behavior. New York: Aldine de Gruyter; 1997. [Google Scholar]
- 27.Robinson J, Wright P, Kinzey W. Primate Societies. Chicago: Univ. of Chicago Press; 1986. pp. 44–53. [Google Scholar]
- 28.Wright P. In: Aotus: the Owl Monkey. Baer J, Weller R, Kakoma I, editors. San Diego: Academic; 1994. pp. 97–112. [Google Scholar]
- 29.Hoffman K, Mendoza S, Hennessy M, Mason W. Dev Psychobiol. 1995;28:399–407. doi: 10.1002/dev.420280705. [DOI] [PubMed] [Google Scholar]
- 30.Dittus W. Behavior. 1977;63:281–322. [Google Scholar]
- 31.Dunbar R. J Anim Ecol. 1980;49:485–506. [Google Scholar]
- 32.Dyke B, Gage T B, Mamelka P M, Goy R W, Stone W H. Am J Primatol. 1986;10:257–269. doi: 10.1002/ajp.1350100306. [DOI] [PubMed] [Google Scholar]
- 33.Robinson J G. Behavior. 1988;104:202–231. [Google Scholar]
- 34.Garber P. Evol Anthropol. 1997;5:187–199. [Google Scholar]
- 35.Pusey A, Williams J, Goodall J. Science. 1997;277:828–831. doi: 10.1126/science.277.5327.828. [DOI] [PubMed] [Google Scholar]
- 36.Smith D. Human Longevity. London: Oxford Univ. Press; 1993. [Google Scholar]
- 37.Anderson R, Kochanek K, Murphy S. Mon Vital Stat Rep. 1997;45:1–80. [Google Scholar]
- 38.Grodstein F, Stampfer M, Colditz G, Willett W, Manson J, Joffe M, Rosner B, Fuchs C, Hankinson S, Hunter D, et al. N Engl J Med. 1997;336:1769–1775. doi: 10.1056/NEJM199706193362501. [DOI] [PubMed] [Google Scholar]
- 39.Wingfield J C, Jacobs J, Hillgarth N. Ann NY Acad Sci. 1997;807:22–41. doi: 10.1111/j.1749-6632.1997.tb51911.x. [DOI] [PubMed] [Google Scholar]