Abstract
Understanding the relationship between animal community dynamics and landscape structure has become a priority for biodiversity conservation. In particular, predicting the effects of habitat destruction that confine species to networks of small patches is an important prerequisite to conservation plan development. Theoretical models that predict the occurrence of species in fragmented landscapes, and relationships between stability and diversity do exist. However, reliable empirical investigations of the dynamics of biodiversity have been prevented by differences in species detection probabilities among landscapes. Using long-term data sampled at a large spatial scale in conjunction with a capture-recapture approach, we developed estimates of parameters of community changes over a 22-year period for forest breeding birds in selected areas of the eastern United States. We show that forest fragmentation was associated not only with a reduced number of forest bird species, but also with increased temporal variability in the number of species. This higher temporal variability was associated with higher local extinction and turnover rates. These results have major conservation implications. Moreover, the approach used provides a practical tool for the study of the dynamics of biodiversity.
With the destruction and reduction of forest habitat around the world, biological diversity of many areas is threatened and showing potentially dramatic declines (1–3). The reduction of suitable habitat area and the fragmentation of habitats into smaller patches are likely to increase the probability of local extinction of some species and to reduce species richness (4, 5). Once a landscape has been altered in this manner, the stability of the remaining communities also may be affected by the degree of habitat fragmentation. As habitats undergo fragmentation, it is important not only to investigate associated changes in species diversity, but also to understand the temporal variability of biodiversity in the remaining fragmented landscapes.
The relationship between stability of ecological communities and factors associated with decreases of community diversity has long been an important and much-debated theoretical and empirical issue (6–13). Relationships between environmental complexity and temporal changes in communities may differ depending on the spatio-temporal scales and the taxa considered. In the context of increasing concerns about potential effects of habitat changes at the landscape scale, several studies have investigated the relationship between local species richness and habitat patch size (4, 5), but few studies have considered temporal variability in species richness (14), and none has asked whether such variability could be attributed to landscape structure. Habitat fragmentation may affect bird populations and communities through different, although not necessarily mutually exclusive, mechanisms: through reduction in the amount of suitable habitat (15–17), through isolation of habitat patches, resulting in reduction in immigration/emigration that can lead to higher probabilities of local extinction (4, 5, 18–21), and through increased exposure to negative biotic factors associated with small patch size such as predation and brood parasitism (22, 23). The influence of these effects will depend on the species. Previous workers have shown that forest breeding bird species in the eastern U.S. states can be classified as area-sensitive and non-area-sensitive, based on the importance of forest patch size to their presence and persistence (24, 25).
In the present study, we merged data from two independent large-scale monitoring efforts, the North American Breeding Bird Survey (BBS) and the Land Use and Land Cover Classification of the U.S. Geological Survey, to test the prediction that higher temporal variability in species richness of area-sensitive species should be found in more fragmented landscapes. This prediction emerges from the expectation that fragmentation will cause decreases in species richness and increases in local extinction probabilities. Although species local colonization probabilities will tend to be smaller in fragmented habitats, these probabilities should be applied to larger numbers of species (there are more potential colonists in unsaturated habitats), frequently leading to more colonizing species. Equilibrium and nonequilibrium models incorporating these relationships lead to the prediction of higher temporal variability in species richness in more fragmented landscapes.
One important methodological problem faced when working on temporal changes of animal communities is that the probability of detecting a species may vary among species, time periods, and areas (26). If this is the case, and if total counts of individuals or species observed are used to estimate temporal variation (as has been done in most published studies, cf. refs. 27–29), then the results of such analyses may be misleading. For example, the number of species observed at a given time in an area associated with a given level of forest fragmentation is determined by the presence of individuals of the species and also by the probability of their detection by the observer. In most surveys involving the sampling of an extensive number of locations, the probability of detecting the species present is very likely to be smaller than one. The probability of species detection on routes of the BBS varies among species and among states (26). One way of estimating the number of species present in an area is to use a capture-recapture approach relying on the pattern of detection/nondetection of species in a series of temporal or spatial sampling replicates (26, 30–33). We used this approach to compute point estimates of number of area-sensitive and non-area-sensitive forest breeding bird species on all BBS routes of three mid-Atlantic U.S. states for all years during the period 1975–1996.
Another important and related methodological problem typically overlooked in most studies of temporal stability of populations or communities is the importance of considering sampling variance (34). The variance of a temporal sequence of point estimates of species richness can be written as the sum of two variance components, one of which is relevant to ecological hypotheses (true temporal variance in species richness) and one of which (sampling variance associated with the estimation process) is not. Here, we used a method designed to separate these components and estimate the true temporal variance in species richness for the purpose of investigating the potential association between this quantity and the level of forest fragmentation (34–36).
MATERIALS AND METHODS
The BBS provides information on the presence of bird species in North America at a landscape scale (37, 38). The survey started in 1966 and consists of >4,000 roadside routes located on secondary roads throughout the United States and southern Canada. Each route is 39.4 km long and is surveyed annually in June. A competent observer conducts 50 3-min point counts at 0.8-km intervals on the roadside, recording all birds heard and seen during the counts. Data are summarized for each route in lists of species detected on each of five groups of 10 point counts. Within the avian species pool, species are categorized in different groups (39), and we focused here on the forest breeding bird species. We partitioned this group into two subcategories, area-sensitive and non-area-sensitive species, according to studies carried out in the mid-Atlantic United States (24, 25). Land Use and Land Cover Classification data from the U.S. Geological Survey were used to quantify landscape structure within a circular scene radius of 19.7 km centered on each BBS route (area ≈1,200 km2) for the year 1974. A radius of half the length of a BBS route was chosen to guarantee that each landscape scene would contain the whole route. High-altitude photographs, usually at scales smaller than 1:60,000, were used to digitize and transfer land use and land cover data to 1:250,000 base maps in grid format (U.S. Department of Interior, Geological Survey 1987). Several variables were measured and computed to characterize the landscape associated with each survey route (38). For the BBS routes of the three U.S. states considered (Maryland, New York, and Pennsylvania), the high correlation among habitat fragmentation metrics measured for 1974 in the Land Use and Land Cover Classification prevented us from attempting to disentangle the effects of different components of landscape fragmentation (4). We thus focused on the global effect of forest fragmentation on the temporal dynamics of the bird communities, and we used the average size of forest patches as the summary statistic reflecting fragmentation: landscapes of BBS routes with a large number of forest patches contained smaller forest patches and a lower proportion of their total area was forested (unpublished data). As this variable was computed at the landscape scale, it incorporates all habitats potentially sampled by counts on the route, including both the area immediately surrounding the survey route and areas more distant to the route.
For each survey route for each year considered, species richness of the two groups of forest birds (area-sensitive and non-area-sensitive species) was estimated by using the jackknife estimator (30), which allows for heterogeneity among species in their probability of detection. The use of that estimator is justified in ref. 26. The estimates were computed by using software comdyn (40), which was specifically designed for the study of animal communities and which permits interactive analyses of BBS data through the internet (http://www.mbr-pwrc.usgs.gov/comdyn.html).
Temporal variation in species richness for each BBS route was estimated by using the approach proposed by Link and Nichols (ref. 34, see also refs. 35 and 36). The average sampling variance associated with annual estimates of species richness was subtracted from the overall total variance (estimated over time using the point estimates of species richness) to provide an estimate of the true temporal variance in species richness over that period. To express relative year-to-year variability in species number we used the coefficient of variation (CV) of species richness (41, 42). The CV of species richness was computed as the SD expressed as the proportion of the mean. It was thus the ratio of the square root of the estimated true temporal variance over the mean of the species richness estimates. Over the 22 years considered (1975–1996), only survey routes for which more than 15 annual estimates of species richness were available were used. Negative estimates of temporal variance in species richness were set to 0.
The robust design was applied to estimate parameters of community change as proposed recently (43, 44). The robust design combines closed and open population capture-recapture models (45–47) to estimate changes in population size, survival rate and recruitment, and is especially useful when there is strong heterogeneity in the probability of detecting individuals. Applied to communities, this approach allowed us to estimate year-to-year local extinction and turnover rates on each BBS route over the study period (43).
RESULTS AND DISCUSSION
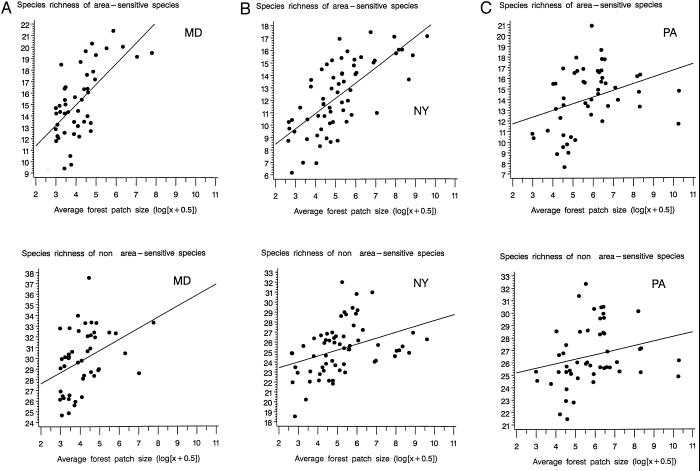
The average estimated number of area-sensitive and non-area-sensitive species on any BBS route in any year was, respectively, 15.2 (SE = 0.14) and 29.8 (0.16) in Maryland, 12.4 (0.10) and 25.2 (0.12) in New York, and 14.1 (0.13) and 26.6 (0.13) in Pennsylvania (all routes and years combined, n equals 1,038, 1,724, and 1,528 route-years, respectively). Twenty-six area-sensitive and 49 non-area-sensitive species that were detected at least on one of these BBS survey routes over the study period constituted the basic species pool available to all areas of the three Eastern states considered (Table 1). Average species richness over the study period was lower in survey routes in more fragmented landscapes than in less fragmented ones (Fig. 1). Analyses of covariance including state and average forest patch size as covariates showed that this relation was stronger for area-sensitive species than for non-area-sensitive ones (for area sensitive species F1.153 = 68.73, P = 0.0001, for non-area-sensitive species F1,153 = 18.79, P = 0.001). The final models showed main effects of state (F2,153 = 3.64, P = 0.0286) and a state by forest patch size interaction (F2,153 = 4.87, P = 0.0089) for area-sensitive species, but no significant state or interaction effects for non-area-sensitive species. Multivariate analysis of covariance documented that the relationship between patch size and species richness differed for area-sensitive and non-area-sensitive species (F1,153 = 17.06, P = 0.0001).
Table 1.
Non-area-sensitive and area-sensitive species used in the analyses
| Non-area-sensitive species | Non-area-sensitive species (continued) | ||
| Common name | Scientific name | Common name | Scientific name |
| Northern bobwhite | Colinus virginianus | American redstart | Setophaga ruticilla |
| Mourning dove | Zenaida macroura | Gray catbird | Dumetella carolinensis |
| Red-tailed hawk | Buteo jamaicensis | Carolina wren | Thryothorus ludovicianus |
| Barred owl | Strix varia | House wren | Troglodytes aedon |
| Eastern screech-owl | Otus asio | Brown creeper | Certhia americana |
| Great horned owl | Bubo virginianus | Brown-headed nuthatch | Sitta pusilla |
| Yellow-billed cuckoo | Coccyzus americanus | Black-capped chickadee | Poecile atricapillus |
| Black-billed cuckoo | Coccyzus erythropthalmus | Carolina chickadee | Poecile carolinensis |
| Downy woodpecker | Picoides pubescens | American robin | Turdus migratorius |
| Red-headed woodpecker | Melanerpes erythrocephalus | ||
| Northern flicker | Colaptes auratus | Area-sensitive species | |
| Chuck-will’s-widow | Caprimulgus carolinensis | ||
| Whip-poor-will | Caprimulgus vociferus | Common name | Scientific name |
| Ruby-throated hummingbird | Archilochus colubris | ||
| Eastern wood-pewee | Contopus virens | Red-shouldered hawk | Buteo lineatus |
| Blue jay | Cyanocitta cristata | Hairy woodpecker | Picoides villosus |
| Common raven | Corvus corax | Pileated woodpecker | Dryocopus pileatus |
| Fish crow | Corvus ossifragus | Red-bellied woodpecker | Melanerpes carolinus |
| European starling | Sturnus vulgaris | Great crested flycatcher | Myiarchus crinitus |
| Brown-headed cowbird | Molothrus ater | Acadian flycatcher | Empidonax virescens |
| Baltimore oriole | Icterus galbula | American crow | Corvus brachyrhynchos |
| Common grackle | Quiscalus quiscula | Rose-breasted grosbeak | Pheucticus ludovicianus |
| American goldfinch | Carduelis tristis | Scarlet tanager | Piranga olivacea |
| Eastern towhee | Pipilo erythrophthalmus | Summer tanager | Piranga rubra |
| Northern cardinal | Cardinalis cardinalis | Red-eyed vireo | Vireo olivaceus |
| Indigo bunting | Passerina cyanea | Black-&-white warbler | Mniotilta varia |
| Cedar waxwing | Bombycilla cedrorum | Worm-eating warbler | Helmitheros vermivorus |
| Yellow-throated vireo | Vireo flavifrons | Northern parula | Parula americana |
| Blue-headed vireo | Vireo solitarius | Black-throated blue warbler | Dendroica caerulescens |
| White-eyed vireo | Vireo griseus | Cerulean warbler | Dendroica cerulea |
| Prothonotary warbler | Protonotaria citrea | Ovenbird | Seiurus aurocapillus |
| Magnolia warbler | Dendroica magnolia | Northern waterthrush | Seiurus noveboracensis |
| Chestnut-sided warbler | Dendroica pensylvanica | Louisiana waterthrush | Seiurus motacilla |
| Yellow-throated warbler | Dendroica dominica | Kentucky warbler | Oporornis formosus |
| Black-throated green warbler | Dendroica virens | Canada warbler | Wilsonia canadensis |
| Pine warbler | Dendroica pinus | White-breasted nuthatch | Sitta carolinensis |
| Prairie warbler | Dendroica discolor | Tufted titmouse | Barolophus bicolor |
| Common yellowthroat | Geothlypis trichas | Blue-gray gnatcatcher | Polioptila caerulea |
| Yellow-breasted chat | Icteria virens | Wood thrush | Hylocichla mustelina |
| Hooded warbler | Wilsonia citrina | Veery | Catharus fuscescens |
Figure 1.
Relation between the mean species richness over the study period and the average forest patch size of a scene centered on each BBS route in 1974 (average forest patch size was log-transformed). Graphs were drawn for area-sensitive and non-area-sensitive species for the states of Maryland (A), New York (B), and Pennsylvania (C). Mean species richness was computed for survey routes for which more than 15 estimates of species richness were available over the 22-year period considered (1975–1996).
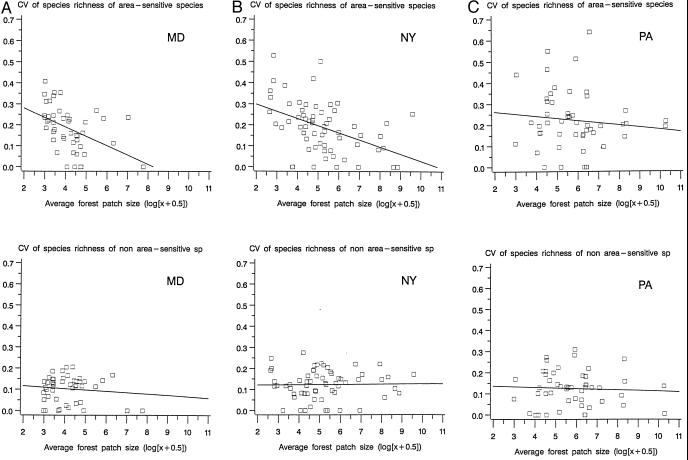
As predicted, we also observed an increase in the CV of species richness of area-sensitive species as average forest patch size decreased, especially for two of the three states (Fig. 2). There was no association between the CV of species richness and the average forest patch size for the group of non-area-sensitive species within any of the three states (Fig. 2). Analyses of covariance showed that there was indeed a negative association between the estimated CV of species richness and the average forest patch size for area-sensitive species but not for non-area-sensitive ones (for area-sensitive species F1,153 = 16.76, P = 0.0001, for non-area-sensitive species F1,153 = 0.41, P = 0.52). The final models showed no significant state or interaction effects for either species group. Multivariate analysis of covariance documented that the relationship between patch size and CV differed for area-sensitive and non-area-sensitive species (F1,153 = 14.55, P = 0.0002). The same global results were obtained when we used SD(log(N)) to express temporal variability in species number.
Figure 2.
Relation between the CV of species richness and the average forest patch size of a scene centered on each BBS route in 1974 (average forest patch size was log-transformed). Graphs were drawn for area-sensitive and non-area-sensitive species for the states of Maryland (A), New York (B), and Pennsylvania (C). CVs were computed for survey routes for which a minimum of 15 estimates of species richness was available over the 22-year period considered (1975–1996).
Thus, our analyses showed that temporal variations in species richness of forest bird species over a period of 22 years were associated with, and may have been affected by, landscape structure. As our approach allowed us to take into account both the detection probability of species and the estimated sampling error, such potentially important sources of bias are not likely to have affected our analyses. This is especially important for testing such hypotheses because sampling variance may differ depending on the landscape structure (sampling variance is linked to the probability of detection of species). For example, sampling variance of species richness tends to be greater when species probability of detection is lower, and could result in misleading inferences about the relationship between landscape structure and temporal variation in the number of species. The probabilistic approach does not provide the exact list of species that are responsible for change in total species richness, but permits more specific inference when applied to different groups of species (e.g., a priori distinction between species area-sensitive and non-area-sensitive).
As the landscape structure was characterized only at the beginning of the period over which the temporal variation of species richness was studied (in 1974), subsequent changes in the level of fragmentation of the landscapes may have affected our results. Temporal variation in species richness could have been caused, in particular, by systematic trends in species richness on the survey routes caused by changes in landscape structure. We nevertheless did not find any evidence of a trend in the number of species over time among survey routes. Moreover, when a linear trend in species richness through time was fitted within each survey route, and when this trend was further removed from the total variance of species richness, the results were not different (same final models selected; effect of patch size for area-sensitive species: P = 0.0001 and for non-area-sensitive ones: P = 0.41; the relationship between patch size and CV differs between the two groups: P = 0.001). Such an effect thus is not likely to have affected the analyses.
Temporal variations in species richness are the results of local extinctions and colonizations on the landscape. Estimation of local rates of extinction and colonization also must contend with missed species in count data and with heterogeneity in the probability of detecting the different species in a community. For this purpose, we applied the robust design to estimate the parameters of the bird communities (14, 43), and we found higher average annual rates of local extinction and local turnover (proportion of locally new species) in more fragmented landscapes (unpublished work). These analyses were carried out over the same period and set of BBS routes and thus further suggest that higher rates of both local extinction and local colonization have been occurring in the more fragmented landscapes at that spatial scale. Such dynamical aspects of species composition in highly fragmented landscapes may be relevant to the understanding of the relation between potential risk of regional extinction and deforestation (48). These results emphasize the need to investigate theoretically and empirically potential associations between changes in ecological integrity through time and changes in landscape structure (49).
Different processes may be responsible for the association between variability in species richness and landscape fragmentation. In particular, different aspects of forest fragmentation may be responsible for changes over time in the number of species (4, 5, 50), and regional and local diversity may be linked by different mechanisms (51). Investigating the respective role of these effects of fragmentation would require using an array of landscapes with independent variations in the numbers of patches, average patch sizes, and proportions of forest cover. Ideally, such an approach would need to be carried out experimentally to be able to make strong inference about the causal nature of the relationships. The estimation approach we used represents a potentially useful tool to investigate such questions at the large spatial and temporal scales necessarily involved.
This study provides a test of an important prediction linking community stability and landscape structure. Habitat fragmentation not only reduces the numbers of some forest bird species, it also may be responsible for an increase in the subsequent temporal variability of the communities through increased rates of local extinction and turnover at the landscape scale. Our approach moreover provides a set of inference methods to investigate theoretical and applied questions dealing with the dynamics of communities, taking into account variation associated with sampling methods. This should permit investigation of important questions of both theoretical and applied interest to community ecology and conservation in both temperate and tropical ecosystems.
Acknowledgments
We thank the BBS volunteer observers, recorders, and coordinators.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: BBS, North American Breeding Bird Survey; CV, coefficient of variation.
References
- 1.Wilson E O. Biodiversity. Washington, DC: Natl. Acad. Press; 1988. [Google Scholar]
- 2.Ehrlich P R, Daily G C. Ambio. 1993;22:64–68. [Google Scholar]
- 3.Pimm S L, Russell G J, Gittleman J L, Brooks T M. Science. 1995;269:347–350. doi: 10.1126/science.269.5222.347. [DOI] [PubMed] [Google Scholar]
- 4.Andrén H. Oikos. 1994;71:355–366. [Google Scholar]
- 5.Opdam P. Land Ecol. 1991;5:93–106. [Google Scholar]
- 6.May R M. Stability and Complexity in Model Ecosystems. Princeton: Princeton Univ. Press; 1973. [PubMed] [Google Scholar]
- 7.Goodman D. Q Rev Biol. 1975;50:237–266. [Google Scholar]
- 8.Pimm S L. Nature (London) 1984;307:321–326. [Google Scholar]
- 9.Connell J H, Sousa W P. Am Nat. 1983;121:789–824. [Google Scholar]
- 10.Tilman D, Downing J A. Nature (London) 1994;367:363–365. [Google Scholar]
- 11.Givnish T J. Nature (London) 1994;371:113–114. [Google Scholar]
- 12.Tilman D. Ecology. 1996;77:350–363. [Google Scholar]
- 13.Kareiva P, Wennergren U. Nature (London) 1995;373:299–302. [Google Scholar]
- 14.Rosenzweig M L. Species Diversity in Space and Time. Cambridge, U.K.: Cambridge Univ. Press; 1995. [Google Scholar]
- 15.Andrén H. Oikos. 1996;76:235–242. [Google Scholar]
- 16.Connor E F, McCoy E D. Am Nat. 1979;113:791–833. [Google Scholar]
- 17.Haila Y. Oikos. 1983;41:334–349. [Google Scholar]
- 18.MacArthur R H, Wilson E O. The Theory of Island Biogeography. Princeton: Princeton Univ. Press; 1967. [Google Scholar]
- 19.Villard M-A, Merriam G, Maurer B A. Ecology. 1995;76:27–40. [Google Scholar]
- 20.Bellamy P E, Hinsley S A, Newton I. Oecologia. 1996;108:64–71. doi: 10.1007/BF00333215. [DOI] [PubMed] [Google Scholar]
- 21.Hanski I, Simberloff D. In: Metapopulation Biology. Hanski I A, Gilpin M E, editors. San Diego: Academic; 1997. pp. 5–26. [Google Scholar]
- 22.Martin T E. Ecology. 1988;69:74–84. [Google Scholar]
- 23.Paton P W C. Conserv Biol. 1994;8:17–26. [Google Scholar]
- 24.Whitcomb R F, Robbins C S, Lynch J F, Whitcomb B L, Klimkiewicz M K, Bystrak D. In: Forest Island Dynamics in Man-Dominated Landscapes. Burgess R L, Sharpe D M, editors. New York: Springer; 1981. pp. 125–205. [Google Scholar]
- 25.Robbins C S, Dawson D K, Dowell B A. Wildl Monogr. 1989;103:1–34. [Google Scholar]
- 26.Boulinier T, Nichols J D, Sauer J R, Hines J E, Pollock K H. Ecology. 1998;79:1018–1028. [Google Scholar]
- 27.Curnutt J L, Pimm S L, Maurer B A. Oikos. 1996;76:131–144. [Google Scholar]
- 28.Bengtsson J, Baillie S R, Lawton J. Oikos. 1997;78:249–256. [Google Scholar]
- 29.Cyr H. Oikos. 1997;79:549–558. [Google Scholar]
- 30.Burnham K P, Overton W S. Ecology. 1979;60:927–936. [Google Scholar]
- 31.Bunge J, Fitzpatrick M. J Am Stat Assoc. 1993;88:364–373. [Google Scholar]
- 32.Colwell R K, Coddington J A. Philos Trans R Soc London B. 1994;345:101–118. doi: 10.1098/rstb.1994.0091. [DOI] [PubMed] [Google Scholar]
- 33.Nichols J D, Conroy M J. In: Measuring and Monitoring Biological Diversity: Standard Methods for Mammals. Wilson D E, Cole F R, Nichols J D, Rudran R, Foster M, editors. Washington, DC: Smithsonian Institution Press; 1996. pp. 226–234. [Google Scholar]
- 34.Link W A, Nichols J D. Oikos. 1994;69:539–544. [Google Scholar]
- 35.Burnham K P, Anderson D R, White G C, Brownie C, Pollock K H. Am Fish Soc Monogr. 1987;5:1–437. [Google Scholar]
- 36.Skalski J R, Robson D S. Techniques for Wildlife Investigations: Design and Analysis of Capture Data. San Diego: Academic; 1992. [Google Scholar]
- 37.Robbins C S, Sauer J R, Greenberg R S, Droege S. Proc Natl Acad Sci USA. 1989;86:7658–7662. doi: 10.1073/pnas.86.19.7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flather C H, Sauer J R. Ecology. 1996;77:28–35. [Google Scholar]
- 39.Peterjohn B, Sauer J R. Bird Populations. 1993;1:1–15. [Google Scholar]
- 40.Hines, J. E., Boulinier, T., Nichols, J. D., Sauer, J. R. & Pollock, K. H. (1998) Bird Study, in press. [DOI] [PMC free article] [PubMed]
- 41.Lewontin R C. Syst Zool. 1966;15:141–142. [Google Scholar]
- 42.McArdle B H, Gaston K J. Oikos. 1995;74:165–171. [Google Scholar]
- 43.Nichols, J. D., Boulinier, T., Hines, J. E., Pollock, K. H. & Sauer, J. R. (1998) Ecol. Appl., in press. [DOI] [PMC free article] [PubMed]
- 44.Nichols, J. D., Boulinier, T., Hines, J. E., Pollock, K. H. & Sauer, J. R. (1998) Conserv. Biol., in press. [DOI] [PMC free article] [PubMed]
- 45.Pollock K H. J Wildl Manag. 1982;46:752–757. [Google Scholar]
- 46.Pollock K H, Nichols J D, Brownie C, Hines J E. Wildl Monogr. 1990;107:1–97. [Google Scholar]
- 47.Nichols J D. Bioscience. 1992;42:94–102. [Google Scholar]
- 48.Brooks T, Balmford A. Nature (London) 1996;380:115. [Google Scholar]
- 49.Fahrig L, Merriam G. Conserv Biol. 1994;8:50–59. [Google Scholar]
- 50.Faaborg J, Brittingham M, Donovan T, Blake J. In: Ecology and Management of Neotropical Migratory Birds. Marin T E, Finch D M, editors. New York: Oxford Univ. Press; 1996. pp. 357–380. [Google Scholar]
- 51.Ricklefs R E. Science. 1987;235:167–171. doi: 10.1126/science.235.4785.167. [DOI] [PubMed] [Google Scholar]