Abstract
Withdrawal from an acute bolus injection of ethanol produces affective or emotional signs that include anxiogenic-like behavior (Gauvin et al., 1992) and conditioned place aversion (Morse et al., 2000). The current study assessed whether brain reward deficits that accompany withdrawal from chronic ethanol dependence (Schulteis et al., 1995) are also observed upon withdrawal from acute intoxication. Rats were implanted with stimulating electrodes aimed at the medial forebrain bundle in the lateral hypothalamus and trained on a discrete trial current-intensity brain stimulation reward threshold paradigm. Ethanol intoxication was produced by bolus intraperitoneal injections of ethanol (1.0, 1.5, or 2.0 g/kg). Brain reward thresholds were monitored periodically following the bolus injection (3, 6, 9, 12, 24, 48, 72, 96 hr post-ethanol). Blood samples taken at various intervals post-ethanol revealed that peak blood alcohol levels (BAL) at all doses tested were reached within 10 min of injection. Following doses of 1.0, 1.5 and 2.0 g/kg ethanol, BAL had declined to undetectable levels within 3–6 hr post-injection. Withdrawal from a single injection of ethanol resulted in a significant but transient increase in brain reward thresholds only with the highest ethanol dose tested (2.0 g/kg). When acute intoxication and withdrawal episodes were repeated two additional times at weekly intervals, the peak magnitude and duration of threshold elevation increased significantly at the 2.0 g/kg dose of ethanol. A significant but transient increase in thresholds was also seen in the group treated with 1.5 g/kg ethanol during the 3rd and final week of testing. Results indicate that withdrawal from a single exposure to an intoxicating dose of ethanol produces significant brain reward deficits in addition to other affective disturbances previously reported, and that repeated weekly intoxication and withdrawal results in a progressive increase in magnitude and duration of the reward deficit.
Keywords: Dependence, Withdrawal, Abstinence, Addiction, Reward
1. Introduction
A number of prominent theoretical models of drug addiction attribute motivational significance to “homeostatic” or “allostatic” neuronal adaptations in the maintenance of the addictive phenotype as a state of drug dependence develops (Di Chiara et al., 1999; Koob & Le Moal, 2001, 2005a,b; Nestler, 2001; Schulteis & Koob, 1996). Within an affective or hedonic domain, the positive affective (rewarding) effects of drugs of abuse may come to be offset by opposing negative affective responses, including feelings of anxiety, restlessness, and depression/dysphoria. It has been argued that such affective components of the withdrawal syndrome may be of greater motivational relevance than somatic signs in maintaining drug seeking-behavior and compulsive drug use (Haertzen & Hooks, 1969; Henningfield, 1987; Jasinski et al., 1985; Koob & Le Moal, 2001; Schulteis & Koob, 1995, 1996). The spectrum of physiological and somatic signs of withdrawal vary widely across different classes of abused drugs such as opioids, sedative-hypnotics (ethanol, barbiturates, benzodiazepines), and psychostimulants (cocaine, amphetamines, nicotine)(see Koob & Le Moal, 2005b). However, it is notable that spontaneous or precipitated abstinence following chronic exposure to opioids, alcohol, cocaine, amphetamines, and nicotine uniformly results in significant elevations in brain stimulation reward thresholds (Epping-Jordan et al., 1998; Leith & Barrett, 1976; Lin et al., 1999; Liu & Schulteis, 2004; Markou & Koob, 1991; Schulteis et al., 1994, 1995). Therefore, brain reward deficits may be a common element of the withdrawal syndrome across all classes of drugs that are capable of stimulating the reward system.
Recent work in our laboratory (Liu & Schulteis, 2004) has demonstrated that brain reward deficits can be measured during withdrawal from a single acute treatment with morphine, suggesting that neuroadaptation in brain reward systems may begin with the first exposure to a drug. A similar acute withdrawal phenomenon has been described during spontaneous withdrawal from acute intraperitoneal injection of moderate to high doses of ethanol (2–4 g/kg) using other behavioral measures. For example Gauvin, Holloway and colleagues (1992, 1993), using a pentylenetetrazol (PTZ) vs. saline drug discrimination paradigm, observed that PTZ-appropriate responding emerged as blood alcohol levels (BAL) dropped to zero. This generalization to the anxiogenic-like stimulus induced by PTZ suggested that withdrawal from acute ethanol intoxication is accompanied by increased anxiety-like behavior, comparable to what is seen during withdrawal from chronic ethanol (Baldwin et al., 1991; Lal & Emmett-Oglesby, 1983; Lal et al., 1988; Rassnick et al., 1993). More recent work has confirmed the anxiogenic-like nature of acute withdrawal from bolus doses of ethanol (2–3 g/kg) using the elevated plus-maze paradigm (Morse et al., 2001). Moreover, it has been shown that withdrawal from acute ethanol doses of 3–4 g/kg produces conditioned aversion in the place conditioning paradigm (Morse et al., 2000). The current study sought to determine whether elevated brain reward thresholds, a reliable index of affective withdrawal from chronic ethanol (Schulteis et al., 1995) also could be observed following acute bouts of ethanol intoxication. A further objective was to determine whether repeated occasional bouts of intoxication (weekly intervals) might lead to a progressive increase in withdrawal severity as measured by brain reward deficits.
2. Materials and Methods
2.1 Animals
Male Wistar rats (n = 65, Harlan Labs, Indianapolis, IN) weighing 350–450 g at the time of testing were used. All rats were pair-housed in a temperature- and humidity-controlled room with a 12 hour light/12 hour dark cycle (lights ON at 6:00 AM). Rats had ad libitum access to food and water at all times. All experimental procedures were approved by the Subcommittee on Animal Studies of the VA San Diego Healthcare System, an AAALAC-accredited facility, and are in strict accordance with the “Guide for the Care and Use of Laboratory Animals” (revised 1996).
2.2 Ethanol treatments
Ethanol (15% w/v) was prepared by diluting a 95% stock solution of ethanol with sterile physiological saline. As described previously (Morse et al., 2000, 2001) all ethanol doses (1.0, 1.5, 2.0, and 3.0 g/kg) were injected intraperitoneally at the fixed concentration of 15% w/v (to avoid discomfort due to intraperitoneal injection of higher concentrations). Therefore instead of adjusting concentration, total volume of injection was adjusted to achieve desired dose.
2.3 Brain stimulation reward apparatus and procedure
The surgery, procedure, and apparatus have been described previously (Liu & Schulteis, 2004; Markou & Koob, 1991; Schulteis et al., 1995). Briefly, rats were anesthetized with halothane and a stainless-steel bipolar electrode (Plastics One, Roanoke, VA) was implanted in the lateral hypothalamus unilaterally (AP −0.5 mm from bregma, L 1.7 mm, 8.3 mm ventral from dura, incisor bar 5.0 mm above interaural line). To counterbalance any possible brain asymmetries, half the rats received implants on the right side of the brain, the other half on the left side.
Brain stimulation reward testing took place in 4 Plexiglas chambers (25 × 31 × 24 cm) enclosed in sound-attenuated cubicles (Med Associates, St. Albans, VT). The floors consisted of parallel aluminum rods spaced 1.25 cm apart. One wall of each chamber contained a metal wheel manipulandum requiring 0.2 N force to rotate ¼ turn. Rats were connected to the stimulation system through flexible bipolar leads covered with spring mesh (Plastics One, Roanoke, VA); the lead was attached to gold-contact swivel commutators (SL2C, Plastics One) mounted above the chamber.
Using a discrete-trial current-intensity threshold procedure (Bain & Kornetsky, 1989; Kornetsky et al., 1988), stimulation was delivered by constant current stimulators (Stimtech model 1200, San Diego Instruments, San Diego, CA) in the form of 60 Hz sinusoidal waves with a train duration set at 200 ms. To start each trial, a rat received a non-contingent electrical stimulus. A correct response was recorded if a rat rotated the wheel manipulandum at least ¼ turn within 7.5 s of the non-contingent electrical stimulus; each correct response produced a contingent stimulus identical in all parameters to the non-contingent stimulus. After each correct response, there was an inter-trial interval averaging 10 s (7.5–12.5 s). If no response occurred within 7.5 s of the non-contingent stimulus, the inter-trial interval followed and that trial ended. Any responding during the inter-trial interval resulted in a 10 s delay before the start of the next trial.
Stimulus intensities varied according to the method of limits and were presented in alternating ascending and descending series (two of each) with a step size of 5 μA; a given stimulus intensity was presented three times within each series. Threshold was defined for each series as the midpoint between the current intensity level at which at least two correct responses occurred and that level at which fewer than two correct responses occurred; the mean of the four series thresholds served as the estimated threshold for a given session. The duration of each session was approximately 30–40 min, and rats received two sessions per day, separated by 4 hr (end of first session to start of second).
2.4 Blood alcohol level (BAL) determination
Separate groups of rats (n=6/dose) were injected intraperitoneally with 1.0, 1.5, 2.0, or 3.0 g/kg ethanol. Doses were selected to produce peak BAL covering the range of 100–300 mg% based upon previously published BAL time-course data; a 4 g/kg dose utilized in the earlier studies was excluded because peak BAL at that dose were reported to be in excess of 400 mg% (Gauvin et al., 1992,1993; Morse et al., 2000), a BAL approaching anesthetic levels that may not be typical of any but the most extreme of alcohol drinkers. Because those earlier reports were conducted with rats of differing strain (Sprague-Dawley, Gauvin et al., 1992, 1993) and/or source (Wistar from Beckman Laboratory, The Scripps Research Institute, Morse et al., 2000), we felt it critical to re-examine the BAL time-course in our chosen subjects (Wistar strain from Harlan Labs). Within each dose group, rats had tail blood samples (< 0.3 ml/sample) collected into heparinized 1.5 ml Eppendorf tubes under one of three collection schedules: a) 5, 10, and 20 min post-injection, b) 40 min, 1 and 2 hr post-injection, or c) 3, 6 and 9 hr post-injection. Rats were rotated in semi-random fashion through the three collection schedules (dose remained constant for a given subject) at two week intervals. For each of the 3 sampling days, total volume collected was less than 5% of total blood volume for each rat, well within the guidelines established by the VA San Diego Healthcare System IACUC guidelines (maximum 10% of total blood volume every 2 weeks). Samples were immediately centrifuged, and plasma was removed and analyzed for ethanol content with an Analox AM1 alcohol analyzer (Analox Instruments, Lunenburg, MA). The analyzer was calibrated to a reference standard of 100 mg% ethanol.
2.5 Experimental design for repeated intoxication and withdrawal
Following the establishment of stable baseline thresholds (± 15% on five consecutive days), rats received an intraperitoneal injection of saline vehicle on Monday of the first experimental week, with brain reward thresholds determined at 3, 6, 9, and 12 hr post-injection. Additional threshold determinations occurred once daily from Tuesday thru Friday of that same week, at 24, 48, 72 and 96 hr post-injection. Data from this initial week of testing served as the baseline for each rat, against which any changes in threshold during ethanol treatment weeks would be compared. After a two day rest period, separate groups of rats received their first injection of ethanol followed by threshold determinations at 3, 6, 9, 12, 24, 48, 72 and 96 hr post-ethanol. This procedure was repeated two additional times at weekly intervals, for a total of 4 weeks of testing, one baseline week and then three weeks of testing post-ethanol, with each rat receiving the same dose of ethanol on the 2nd and 3rd injection as they had received initially. A control group received vehicle instead of ethanol each week, to permit assessment of any non-ethanol induced alterations in thresholds across the testing period.
2.6 Statistical analysis
Data from all ethanol treatment weeks were expressed as the percentage of the baseline threshold from the corresponding test interval during the baseline week (e.g. 3 hr post-ethanol was expressed as a function of the 3 hr time-point during vehicle baseline determination, 12 hr to 12 hr, etc.). Subsequently, these data for each dose of ethanol (1.0, 1.5, 2.0 g/kg) were compared to the vehicle control group in three-factor mixed-design ANOVAs, with treatment (ethanol vs. vehicle) as a between-subjects factor, and both time post-injection (3–96 hr) and test week (1–3) as within-subjects factors. Follow-up comparisons consisted of interaction contrasts or simple main effects as dictated by the outcome of the overall ANOVA (with interactions of treatment with week and/or time factors the primary outcomes of interest), followed by individual means comparisons of ethanol to vehicle groups at specific time-points (Newman-Keuls, significance set at p < 0.05 two-tailed).
3. Results
3.1 Blood alcohol levels (BAL)
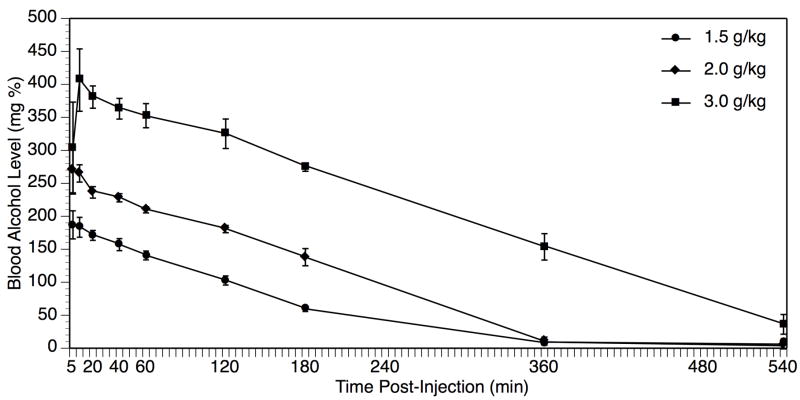
As shown in Figure 1, BAL increased in proportion to dose of ethanol injected, with peak BAL achieved either 5 or 10 min post-ethanol at all doses (peak BAL = 108.8 ± 16.3 mg%, 187 ± 21.3 mg%, 270 ± 34.5 mg%, and 406 ± 47.3 mg% following 1.0, 1.5, 2.0, and 3.0 g/kg respectively). Based upon the peak BAL data, doses of 1.0, 1.5, and 2.0 g/kg were selected for further study in the brain stimulation reward experiments, as they covered our desired target range of 100–300 mg% BAL; in contrast, the 3.0 g/kg dose resulted in higher BAL in our Wistar strain than had been reported previously in Sprague-Dawley rats (Gauvin et al., 1992, 1993). At 3 hr post-ethanol, the first point at which reward thresholds were determined, animals treated with 1.0 g/kg had undetectable BAL, whereas animals treated with 1.5 g/kg and 2.0 g/kg had residual BAL of 59 ± 3.4 mg% and 130 ± 13.1 mg% respectively. By 6 hr post-ethanol, rats treated with the 1.5 and 2.0 g/kg doses of ethanol also had undetectable BAL < 10 mg%.
Fig. 1.
Blood alcohol level (BAL) peaks within 5–10 min of bolus intraperitoneal injection of ethanol in doses of 1.0 g/kg (circles), 1.5 g/kg (diamonds), 2.0 g/kg (squares), or 3.0 g/kg (triangles). Data represent mean ± SEM BAL in mg% units (mg/100 dL). N = 4–6 per dose group.
3.2 Brain stimulation reward thresholds
Average baseline threshold levels across ethanol dose groups varied from 190.0 ± 13 to 217.91 ± 16.41 μA, and there was no significant difference across groups in baseline threshold (F[3,33] = 1.77, p > 0.05). A two-factor ANOVA within the Vehicle-treated group revealed no significant main effects of time post-ethanol or week, or interaction of the week and time factors (all F’s < 0.83, p’s > 0.60), indicating relatively stable thresholds that did not change across weeks of testing in this control group.
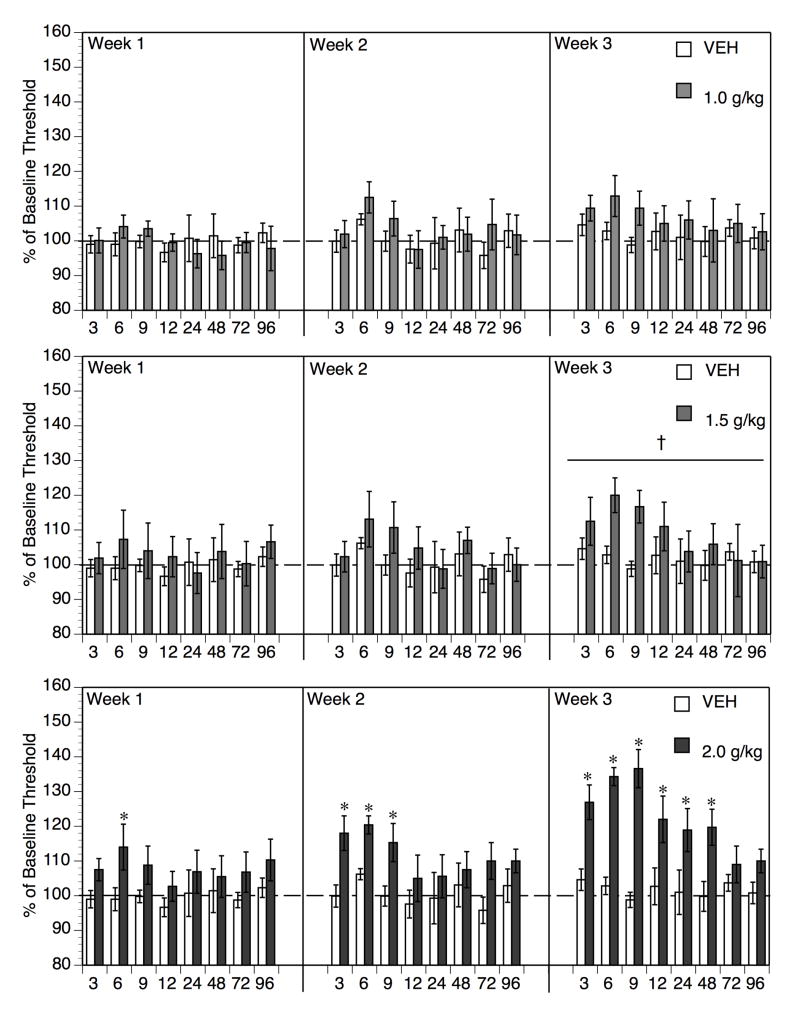
As shown in Figure 2, a single acute treatment with ethanol appeared to transiently increase brain reward thresholds at the highest dose (2.0 g/kg), with peak threshold elevation occurring at 6 hr post-ethanol when BAL had declined to virtually undetectable levels. Two additional ethanol treatments appeared to extend the duration of threshold elevation at this dose of ethanol. Lower doses of ethanol (1.0 and 1.5 g/kg) appeared to produce modest elevations at best, and only after repeated bouts of intoxication/withdrawal.
Fig. 2.
Withdrawal from a single bout of acute ethanol intoxication (Week 1) resulted in a significant but transient increase in brain reward threshold only with the highest dose of ethanol tested (2.0 g/kg, bottom panel, *p < 0.05 vs. Vehicle controls at given time-point post-injection). The effect was significant at 6 hr, a time when BAL had declined to virtually undetectable levels following this dose of ethanol (see Figure 1). Repeat treatment with this dose for 2 additional weeks resulted in a progressive broadening of the duration of significant threshold elevations. In contrast, treatment with 1.5 g/kg ethanol resulted in significant but transient elevations only after 3 repeated bouts of intoxication/withdrawal, no statistically reliable changes were seen after 1 or 2 treatments (center panel, †p < 0.05, Main Effect of Week, Week 1 vs Week 3 in ETOH 1.5 g/kg group). Finally, treatment with 1.0 g/kg did not produce any statistically reliable threshold changes regardless of treatment week (upper panel). Data represent mean ± SEM % of baseline threshold. N = 8–10 per dose group.
To evaluate the statistical significance of these observations, planned comparisons (Keppel, 1991) of each ethanol-treated dose group to vehicle control were conducted as described above (see 2.6 Statistical analysis). Comparison of the 1.0 g/kg dose to vehicle (Figure 2, upper panel) revealed no significant main effect of ethanol or interaction of ethanol with either time or week (all F’s < 1.40, p’s > 0.05). The only significant effect emerging from the overall analysis with the 1.0 g/kg dose was the main effect of time post-injection (F[7,126] = 2.10, p < 0.05), most likely attributable to the fact the slightly higher thresholds in the ethanol-treated group at the early time-points post-ethanol; although this effect appeared more pronounced during Weeks 2 and 3 than Week 1, the lack of main effect of week, or week/time and week/ethanol interaction did not justify further analysis with this dose of ethanol.
Comparison of the 1.5 g/kg-treated group to the vehicle control (Figure 2, center panel) revealed a significant main effect of week (F[2, 36] = 6.72, p < 0.005) and a significant week/ethanol interaction (F[2,36] = 3.45, p < 0.05), no other main effects or interactions were significant (all p’s > 0.10). Further analysis of the simple main effect of week in the 1.5 g/kg dose group revealed a significant difference between Week 1 and 3 (F[1,17] = 17.16, p < 0.001), but not Week 1 and Week 2 (F[1,17] = 1.35, p > 0.25) or between Week 2 and Week 3 (F[1,17] = 3.07, p > 0.05). Therefore, repeated treatment with ethanol at 1.5 g/kg induced a gradual elevation in reward thresholds, whereas there was no significant change in thresholds in the Vehicle group across weeks of testing. An inspection of Figure 2 (center panel) indicates that elevation of reward thresholds in the 1.5 g/kg-treated group in Week 3 appeared maximal in the first 12 hr post-ethanol.
Comparison of the 2.0 g/kg dose to vehicle revealed a significant main effect of ethanol (F[1,16] = 20.32, p < 0.001) and week (F[2,32] = 8.21, p < 0.0013), as well as significant week/ethanol interaction (F[2,32] = 5.61, p < 0.01) and week/time (F[14,224] = 1.93, p < 0.025) interactions (all other terms p > 0.05). Two factor (week x time) repeated measures ANOVA in the 2.0 g/kg ethanol group revealed a significant main effect of week (F[2,14] = 8.58, p < 0.005) and week/time interaction (F[14,98] = 1.83, p < 0.05), whilst a similar analysis of the vehicle group revealed no main effects or interaction of the week and time factors as indicated above. Therefore, a time-dependent effect (i.e. post-injection time) that varied across treatment weeks was evident in the group treated with the 2.0 g/kg, but not the vehicle-treated group. Individual means comparisons of vehicle to 2.0 g/kg at each time-point (with Newman-Keuls correction) revealed only a transient effect of 2.0 g/kg ethanol at 6 hr post-ethanol in Week 1. In Week 2, thresholds were significantly elevated from 3–9 hr post-ethanol. Finally, in Week 3, thresholds were elevated from 3–48 hr post-ethanol.
4. Discussion
The current study extends the range of affective signs that accompany withdrawal from acute bouts of ethanol intoxication to include elevations in brain reward thresholds. Previous work had demonstrated that withdrawal from acute bolus doses of ethanol in the range of 2–4 g/kg elicited anxiogenic-like signs such as generalization to the pentylenetetrazol (PTZ) discriminative stimulus cue (Gauvin et al., 1992, 1993) and decreased exploration of the open arms in the elevated plus-maze (Morse et al., 2001). In addition, conditioned place aversion accompanied withdrawal from acute intraperitoneal ethanol at 3–4 g/kg doses (Morse et al., 2000). Significant effects in these studies typically were observed from 6–12 hr post-ethanol. Our data demonstrate that a single acute intraperitoneal injection of ethanol at the 2.0 g/kg dose elicits a transient elevation in brain reward thresholds (Figure 2A). Lower doses of ethanol (1.0–1.5 g/kg) did not produce a significant change in reward thresholds after a single administration. Therefore, withdrawal from a single bolus dose of ethanol produces a transient but significant elevation in reward thresholds, similar qualitatively but not in magnitude or duration to what we have previously reported during withdrawal from chronic ethanol exposure to a similar BAL (226 mg%; Schulteis et al., 1995).
Importantly, the elevated thresholds observed during withdrawal from acute and chronic ethanol are opposite in valence to the threshold-lowering effects produced by acute ethanol intoxication (Bain & Kornetsky, 1989; Lewis & June, 1990; Moolten & Kornetsky, 1990). It is noteworthy that Lewis and colleagues observed threshold reductions (i.e. “rewarding” effects) of ethanol only on the ascending limb of the BAL curve, and not on the descending limb, which is where our threshold determinations were made in the present study (Figure 1). Thus, threshold-lowering effects of acute ethanol (e.g. Lewis and June, 1990) give way to threshold elevations as BAL declined to undetectable levels (current study). The opposing nature of acute ethanol and ethanol “hangover” effects on brain reward thresholds is consistent with the broader literature on “hangover” effects following acute administration of ethanol (Gauvin et al., 1992,1993; Sanders, 1980; Staiger & White, 1988; Suzdak & Paul, 1987) wherein ethanol’s initial direct effects (e.g. anxiolytic, anticonvulsant, muscle relaxant) are followed by delayed drug-opposite effects (e.g anxiogenic, proconvulsant, muscle rigidity). These “hangover” signs accompanying withdrawal from a single bout of ethanol intoxication are similar to those observed during withdrawal from chronic ethanol exposure (Goldstein, 1974; Goldstein & Pal, 1971; Hunter et al., 1973, 1974; Majchrowicz, 1977, 1981; Schulteis et al., 1995) albeit in a milder form (lower magnitude and/or shorter duration) in the acute exposure situation.
The transient nature of the reward threshold elevation observed after a single ethanol treatment (2.0 g/kg) is consistent with earlier reports of anxiogenic-like effects reported in the PTZ discriminative stimulus (Gauvin et al., 1992) and elevated plus-maze paradigms (Morse et al., 2001) at this dose, where significant effects were noted at 6 and/or 8 hr post-withdrawal but not thereafter. Effects in the conditioned place aversion paradigm (Morse et al., 2000) were not significant at the 2.0 g/kg dose of ethanol, but this study examined only the 10 hr time-point, and it is possible that significant effects may have been observed at 6–8 hr. In all of these earlier studies, larger effects were noted at higher doses of ethanol (3–4 g/kg), typically peaking somewhat later at 10–12 hr post-ethanol. However, we chose not to examine these higher doses in the current study because the rather high peak BAL achieved at this dose (406 mg%), approaching anesthetic levels, exceeded the levels of intoxication that we wished to model. Indeed, pilot work with the 3.0 g/kg dose revealed a complete disruption of responding from 3–6 hr post-ethanol. Gauvin and colleagues (1992, 1993) had similarly reported disruption of responding at 3–4 g/kg doses of ethanol in their drug discrimination work, although their response schedules were considerably more demanding than those of our discrete trial paradigm.
Of greater interest to us than examination of effects at higher doses producing near-anesthetic BAL was an examination of the effects of repeated exposure to and withdrawal from ethanol at weekly intervals within the more moderate BAL ranges of 100–300 mg%. In this regard, it was noteworthy in the current study that the duration of threshold elevations increased significantly upon a 2nd and 3rd administration of 2.0 g/kg ethanol (Figure 2). In addition, a lower dose (1.5 g/kg) achieving peak BAL of 180 mg% elicited significant but transient elevations in reward thresholds after three weekly treatments.
It is noteworthy that during the second and third withdrawal episodes from the 2.0 g/kg dose, thresholds were significantly elevated by 3 hr post-ethanol, before ethanol levels had fully declined, and this is consistent with earlier reports of threshold elevations seen during withdrawal from chronic ethanol vapor exposure (Schulteis et al., 1995). Moreover, during the final withdrawal episode, thresholds in the 2.0 g/kg group remained significantly elevated for 48 hr post-ethanol, comparable in both peak magnitude and duration to the elevations observed after 14–21 days of chronic exposure in our earlier study. These findings suggest that even occasional bouts of ethanol intoxication can lead to rapid escalation in reward deficits to levels that approach those observed with a state of chronic dependence.
However, it must be appreciated that while subjects in the 2.0 g/kg group experienced three bouts of intoxication, each bout was followed by a period of withdrawal and prolonged abstinence, whereas subjects withdrawing from chronic ethanol in our prior work (Schulteis et al., 1995) experienced withdrawal only once at the cessation of chronic vapor exposure. In this regard, one must consider the “kindling” hypothesis of alcohol withdrawal, first formalized by Ballenger and Post (1978), which proposes that repeated episodes of abstinence interspersed between heavy episodes of drinking can result in “kindling” of alcohol withdrawal, in which the severity of withdrawal is potentiated as a direct result of repeated withdrawal experience. Several retrospective analyses of patient history in alcohol treatment programs have found that the patients with multiple detoxification histories show more pronounced withdrawal syndromes, involving both increased severity of individual signs and greater diversity in withdrawal signs noted (Ballenger & Post, 1978; Booth & Blow, 1993; Brown et al., 1988; Schuckit et al., 1995). Considerable work with animal models also supports the ability of repeated bouts of withdrawal to “kindle” withdrawal severity as measure by seizure susceptibility and/or electrophysiological markers of CNS hyperexcitability (e.g. Becker, 1996; Becker et al., 1997; Ulrichsen et al., 1992, 1998; Veatch & Gonzalez, 1996). A number of these studies indicate that withdrawal “kindling” is evident with relatively limited duration of exposure to ethanol. For example, Becker and colleagues (Becker, 1996; Becker et al., 1997, 1998) have shown that convulsions in mice are more severe if mice are exposed to repeated intermittent bouts (3 or more) of ethanol exposure interspersed with brief periods of abstinence (e.g. 16 hr exposure, 8 hr abstinence) than mice exposed to the same amount and duration of ethanol in a single continuous bout.
Therefore, it is quite possible that the magnitude and duration of brain reward threshold elevation seen in the current study after three weekly bouts of ethanol intoxication and withdrawal is due at least in part to repeated withdrawal “kindling”, and not merely to adaptive responses to repeated doses of ethanol alone. Recent work by Overstreet, Breese and colleagues suggests that affective signs of withdrawal (anxiety-like behavior in their studies) are subject to potentiation by repeated bouts of ethanol withdrawal (Breese et al., 2004, 2005; Knapp et al., 2005; Overstreet et al., 2002, 2004). However, withdrawal in these studies was from extended bouts of continuous access to ethanol liquid diet (5 days access interspersed with 2 days abstinence), conditions considerably different from the transient bouts of intoxication produced in our study by weekly intraperitoneal bolus injections of ethanol. Ultimate confirmation of the putative contribution of repeated bouts of withdrawal from acute intoxication to the present findings will require comparison to a control group that receives a single continuous exposure to the total dose of ethanol as that received (in separate weekly bouts) by the intermittent exposure group. The intraperitoneal bolus administration regimen employed herein is not ideally suited to provide such continuous exposure control conditions, but we have initiated studies using ethanol vapor exposure as the method of acute ethanol exposure, and in this model it should be possible to compare repeated brief bouts of exposure (e.g. 4–8 hr duration) to single continuous bouts (12–24 hr), similar to the studies of Becker and colleagues (1996, 1997, 1998, 2004).
Regardless of whether the current findings are due to progressive adaptation within the reward system to an accumulating history of total ethanol exposure, to “kindling” of the reward deficit state by repeated withdrawal experience, or both, they are compelling in their own right on several grounds. First, the transient but significant increase in brain stimulation reward threshold following a single episode of ethanol intoxication in a BAL range that human “binge” drinkers would often experience (200–300 mg%) demonstrates clearly that withdrawal from acute ethanol is accompanied by a brain reward deficit in addition to anxiogenic-like and aversive signs reported previously (Gauvin et al., 1992,1993; Morse et al., 2000, 2001). Moreover, occasional weekly bouts of intoxication with full abstinence between each bout engender a progressive increase in the reward deficit experienced with each subsequent withdrawal. Several studies indicate that repeated intoxication and withdrawal from chronic (Roberts et al., 2000; Schulteis et al., 1996) or more acute (Becker & Lopez, 2004) regimens of ethanol exposure potentiated subsequent ethanol consumption in limited access bouts of bottle-drinking or operant self-administration, suggesting motivational relevance of the repeated intoxication and/or withdrawal experience.
In summary, as argued above affective components of withdrawal from alcohol as well as most other classes of abused drugs may be of significant motivational relevance to maintaining drug seeking-behavior and compulsive drug use in chronic users (Haertzen & Hooks, 1969; Henningfield, 1987; Jasinski et al., 1985; Koob & Le Moal, 2001, 2005a,b; Schulteis & Koob, 1995, 1996). The present findings indicate that these affective signs of withdrawal may increase progressively under conditions of limited intermittent ethanol intoxication and withdrawal, comparable to what has been seen with acute opioid dependence (Liu and Schulteis, 2004), and may consequently play a significant role in the transition from casual use to compulsive use of alcohol (Koob and Le Moal, 2005a, Schulteis et al., 1996).
Acknowledgments
This work was supported by PHS grant R01-AA12800 (GS). The authors wish to recognize the technical assistance of Susan Zyphur and Christina B. Trost in the collection of some of the data presented herein. Portions of these data were presented in initial form as a poster at the Research Society on Alcoholism (RSA) annual meeting in June, 2005, and were previously published in abstract-form only in the Society’s journal Alcoholism: Clinical and Experimental Research (Schulteis et al., 2005).
References
- Bain GT, Kornetsky C. Ethanol oral self-administration and rewarding brain stimulation. Alcohol. 1989;6:499–503. doi: 10.1016/0741-8329(89)90058-x. [DOI] [PubMed] [Google Scholar]
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 1991;103:227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Becker HC. The alcohol withdrawal “kindling” phenomenon: clinical and experimental findings. Alcohol Clin Exp Res. 1996;20:121A–124A. doi: 10.1111/j.1530-0277.1996.tb01760.x. [DOI] [PubMed] [Google Scholar]
- Becker HC, Diaz-Granados JL, Weathersby RT. Repeated ethanol withdrawal experience increases the severity and duration of subsequent withdrawal seizures in mice. Alcohol. 1997;14:319–326. doi: 10.1016/s0741-8329(97)87949-9. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Becker HC, Veatch LM, Diaz-Granados JL. Repeated ethanol withdrawal experience selectively alters sensitivity to different chemoconvulsant drugs in mice. Psychopharmacology (Berl) 1998;139:145–153. doi: 10.1007/s002130050699. [DOI] [PubMed] [Google Scholar]
- Booth BM, Blow FC. The kindling hypothesis: further evidence from a U.S. national study of alcoholic men. Alcohol Alcoholism. 1993;28:593–598. [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology. 2005;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ME, Anton RF, Malcolm R, Ballenger JC. Alcohol detoxification and withdrawal seizures: clinical support for a kindling hypothesis. Biol Psychiatry. 1988;23:507–514. doi: 10.1016/0006-3223(88)90023-6. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Cadoni C, Carboni E. Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Ann N Y Acad Sci. 1999;877:461–485. doi: 10.1111/j.1749-6632.1999.tb09283.x. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Goulden KL, Holloway FA. State-dependent stimulus control: cueing attributes of ethanol “hangover” in rats. Alcohol Clin Exp Res. 1993;17:1210–1214. doi: 10.1111/j.1530-0277.1993.tb05231.x. [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Youngblood BD, Holloway FA. The discriminative stimulus properties of acute ethanol withdrawal (hangover) in rats. Alcohol Clin Exp Res. 1992;16:336–341. doi: 10.1111/j.1530-0277.1992.tb01387.x. [DOI] [PubMed] [Google Scholar]
- Goldstein DB. Rates of onset and decay of alcohol physical dependence in mice. J Pharmacol Exp Ther. 1974;190:377–383. [PubMed] [Google Scholar]
- Goldstein DB, Pal N. Alcohol dependence produced in mice by inhalation of ethanol: grading the withdrawal reaction. Science. 1971;172:288–290. doi: 10.1126/science.172.3980.288. [DOI] [PubMed] [Google Scholar]
- Haertzen CA, Hooks NT., Jr Changes in personality and subjective experience associated with the chronic administration and withdrawal of opiates. J Nerv Ment Dis. 1969;148:606–614. doi: 10.1097/00005053-196906000-00004. [DOI] [PubMed] [Google Scholar]
- Henningfield J, Johnson RE, Jasinski DR. Clinical procedures for the assessment of abuse potential. In: Bozarth M, editor. Methods of Assessing the Reinforcing Properties of Abused Drugs. New York: Springer-Verlag; 1987. pp. 573–590. [Google Scholar]
- Hunter BE, Boast CA, Walker DW, Zornetzer SF. Alcohol withdrawal syndrome in rats: neural and behavioral correlates. Pharmacol Biochem Behav. 1973;1:719–725. doi: 10.1016/0091-3057(73)90036-1. [DOI] [PubMed] [Google Scholar]
- Hunter BE, Walker DW, Riley JN. Dissociation between physical dependence and volitional ethanol consumption: role of multiple withdrawal episodes. Pharmacol Biochem Behav. 1974;2:523–529. doi: 10.1016/0091-3057(74)90013-6. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Johnson RE, Kocher TR. Clonidine in morphine withdrawal. Differential effects on signs and symptoms. Arch Gen Psychiatry. 1985;42:1063–1066. doi: 10.1001/archpsyc.1985.01790340041006. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. Modulation of ethanol withdrawal-induced anxiety-like behavior during later withdrawals by treatment of early withdrawals with benzodiazepine/gamma-aminobutyric acid ligands. Alcohol Clin Exp Res. 2005;29:553–563. doi: 10.1097/01.alc.0000158840.07475.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005a;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiology of Addiction. San Diego: Academic Press; 2005b. [Google Scholar]
- Kornetsky C, Bain GT, Unterwald EM, Lewis MJ. Brain stimulation reward: effects of ethanol. Alcohol Clin Exp Res. 1988;12:609–616. doi: 10.1111/j.1530-0277.1988.tb00250.x. [DOI] [PubMed] [Google Scholar]
- Lal H, Emmett-Oglesby MW. Behavioral analogues of anxiety. Animal models. Neuropharmacology. 1983;22:1423–1441. doi: 10.1016/0028-3908(83)90111-9. [DOI] [PubMed] [Google Scholar]
- Lal H, Harris CM, Benjamin D, Springfield AC, Bhadra S, Emmett-Oglesby MW. Characterization of a pentylenetetrazol-like interoceptive stimulus produced by ethanol withdrawal. J Pharmacol Exp Ther. 1988;247:508–518. [PubMed] [Google Scholar]
- Leith NJ, Barrett RJ. Amphetamine and the reward system: evidence for tolerance and post-drug depression. Psychopharmacologia. 1976;46:19–25. doi: 10.1007/BF00421544. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, June HL. Neurobehavioral studies of ethanol reward and activation. Alcohol. 1990;7:213–219. doi: 10.1016/0741-8329(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Lin D, Koob GF, Markou A. Differential effects of withdrawal from chronic amphetamine or fluoxetine administration on brain stimulation reward in the rat--interactions between the two drugs. Psychopharmacology (Berl) 1999;145:283–294. doi: 10.1007/s002130051060. [DOI] [PubMed] [Google Scholar]
- Liu J, Schulteis G. Brain reward deficits accompany naloxone-precipitated withdrawal from acute opioid dependence. Pharmacol Biochem Behav. 2004;79:101–108. doi: 10.1016/j.pbb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Comparison of ethanol withdrawal syndrome in humans and rats. Adv Exp Med Biol. 1977;85B:15–23. doi: 10.1007/978-1-4615-9038-5_2. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Reversal in central nervous system function during ethanol withdrawal in humans and experimental animals. Fed Proc. 1981;40:2065–2072. [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia: An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Moolten M, Kornetsky C. Oral self-administration of ethanol and not experimenter-administered ethanol facilitates rewarding electrical brain stimulation. Alcohol. 1990;7:221–225. doi: 10.1016/0741-8329(90)90008-z. [DOI] [PubMed] [Google Scholar]
- Morse AC, Schulteis G, Holloway FA, Koob GF. Conditioned place aversion to the “hangover” phase of acute ethanol administration in the rat. Alcohol. 2000;22:19–24. doi: 10.1016/s0741-8329(00)00099-9. [DOI] [PubMed] [Google Scholar]
- Morse AC, Urena VM, Schulteis G, Koob GF. 2002 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2001. Anxiety-like behavior in the elevated plus maze during hangover following acute and repeated ethanol administration in rats. CD-ROM, program no. 980.14. [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Sanders B. Withdrawal-like signs induced by a single administration of ethanol in mice that differ in ethanol sensitivity. Psychopharmacology (Berl) 1980;68:109–113. doi: 10.1007/BF00432126. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Reich T, Hesselbrock VM, Bucholz KK. The histories of withdrawal convulsions and delirium tremens in 1648 alcohol dependent subjects. Addiction. 1995;90:1335–1347. doi: 10.1046/j.1360-0443.1995.901013355.x. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Hyytia P, Heinrichs SC, Koob GF. Effects of chronic ethanol exposure on oral self-administration of ethanol or saccharin by Wistar rats. Alcohol Clin Exp Res. 1996;20:164–171. doi: 10.1111/j.1530-0277.1996.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Liu J, Zyphur S, Fuqua L. Brain reward deficits accompany withdrawal from acute bouts of ethanol intoxication. Alcoholism: Clinical and Experimental Research. 2005;29(Suppl):16A. [Google Scholar]
- Schulteis G, Koob GF. Reinforcement processes in opiate addiction: a homeostatic model. Neurochem Res. 1996;21:1437–1454. doi: 10.1007/BF02532385. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci U S A. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Gold LH, Stinus L, Koob GF. Relative sensitivity to naloxone of multiple indices of opiate withdrawal: a quantitative dose-response analysis. J Pharmacol Exp Ther. 1994;271:1391–1398. [PubMed] [Google Scholar]
- Staiger PK, White JM. Conditioned alcohol-like and alcohol-opposite responses in humans. Psychopharmacology (Berl) 1988;95:87–91. doi: 10.1007/BF00212773. [DOI] [PubMed] [Google Scholar]
- Suzdak PD, Paul SM. Ethanol stimulates GABA receptor-mediated Cl- ion flux in vitro: possible relationship to the anxiolytic and intoxicating actions of alcohol. Psychopharmacol Bull. 1987;23:445–451. [PubMed] [Google Scholar]
- Ulrichsen J, Clemmesen L, Hemmingsen R. Convulsive behaviour during alcohol dependence: discrimination between the role of intoxication and withdrawal. Psychopharmacology (Berl) 1992;107:97–102. doi: 10.1007/BF02244972. [DOI] [PubMed] [Google Scholar]
- Ulrichsen J, Haugbol S, Brandt CF, Allerup P, Hemmingsen R. Irreversibility of kindled alcohol-withdrawal behaviour in rats. Alcohol Alcoholism. 1998;33:230–243. doi: 10.1093/oxfordjournals.alcalc.a008387. [DOI] [PubMed] [Google Scholar]
- Veatch LM, Gonzalez LP. Repeated ethanol withdrawal produces site-dependent increases in EEG spiking. Alcohol Clin Exp Res. 1996;20:262–267. doi: 10.1111/j.1530-0277.1996.tb01638.x. [DOI] [PubMed] [Google Scholar]