Abstract
Aim of this study was to prepare polyamine-conjugated PAMAM dendrimers and study their permeability across Caco-2 cell monolayers. Polyamines, namely, arginine and ornithine were conjugated to the amine terminals of the G4 PAMAM dendrimers by Fmoc synthesis. The apical-to-basolateral (AB) and basolateral-to-apical (BA) apparent permeability coefficients (Papp) for the PAMAM dendrimers increased by conjugating the dendrimers with both of the polyamines. The enhancement in permeability was dependent on the dendrimer concentration and duration of incubation. The correlation between monolayer permeability and the decrease in transepithelial electrical resistance (TEER) with both the PAMAM dendrimers and the polyamine-conjugated dendrimers suggests that paracellular transport is one of the mechanisms of transport across the epithelial cells. Cytotoxicity of the polyamine-conjugated dendrimers was evaluated in Caco-2 cells by MTT (methylthiazoletetrazolium) assay. Arginine-conjugated dendrimers were slightly more toxic than PAMAM dendrimer as well as ornithine-conjugated dendrimers. Though investigations on the possible involvement of other transport mechanisms are in progress, results of the present study suggest the potential of dendrimer-polyamine conjugates as drug carriers to increase the oral absorption of drugs.
1. Introduction
Polyamines (ornithine, putrescine, spermidine and spermine) are ubiquitous and essential for cell growth and differentiation (Pegg A.E., 1988; MacRae et al., 1998). Intracellular concentrations of the polyamines are maintained via their biosynthesis and transcellular uptake (Schiper et al., 2003). Ornithine decarboxylase (ODC) is a key enzyme that regulates the decarboxylation of ornithine to putrescine in polyamine biosynthesis (Pegg A.E., 1988; Auvinen et al., 1992; Harold et al., 1996; O’Brien 1997; Mafune et al., 1999; Liu et al., 2000; Schiper et al., 2003). Once putrescine is formed, the activity of the other polyamine synthetic enzymes (spermidine synthase and spermine synthase) provide for the sequential formation of spermidine and spermine. The gastrointestinal mucosa has rapid turnover rate which equates to high rates of cell growth and differentiation. It has been reported that polyamines are involved in the growth and differentiation of gastrointestinal cells (Heby O., 1981; Johnson L.R., 1988; Seiler et al., 1996). In the stomach luminal polyamines stimulate repair of gastric mucosal injury (Luk et al., 1988), for example, putrescine injection was shown to stimulate oxyntic mucosal growth (Majumdar et al., 1987; Wang et al., 1990). The polyamines from food sources are found to be absorbed by passive diffusion process through paracellular route (Devens et al., 2000).
Efforts to design novel formulations to increase the absorption of poorly soluble and poorly absorbable drugs have been increasing recently. Dr. Kimura’s research group has used sodium laurate (C12) with taurine or L-glutamine to enhance the absorption of poorly absorbable drug (e.g. rebamipide) from colon and rectum without much toxicity (Messing et al., 1999; Aungst B.J. 2000; Yata et al., 2001; Endo et al., 2002, Miyake et al., 2003). However the absorption enhancement was not efficient enough for oral administration. Subsequently, this research group has explored other absorption enhancers such as polyamines, spermidine and spermine (Miyake et al., 2003). Several absorption enhancers such as cationic amino acids, poly-L-arginine (Miyake et al., 2006), poly-L-lysine (Ohtake et al., 2002) cationic gelatin (Ahnet al., 2004), and cationic lipids and liposomes have also shown significant increase in absorption but with considerable toxicity (Li et al., 2005; Seki et al., 2005).
Polyamidoamine (PAMAM) dendrimers are macromolecular hydrophilic polymers. They are synthesized from an initiator core (ammonia or ethylene diamine) around which the branches of the dendrimer originate. The dendritic structure is characterized by ‘layers’ between each focal point (or cascade) called generations (Boas et al., 2004). The core is sometimes denoted generation zero (G0), as no cascade points are present. The number of surface groups of PAMAM dendrimers increases exponentially from 4 to 64 as the generation size increases from G0–G4. Because of their highly branched structure with easy tailorability of surface groups with targeting moieties, and possibility of entrapment of guest molecule in the dendrimer core (dendritic box), they have been widely investigated as carriers for drug delivery and targeting (Jansen et al., 1994; Roy et al., 1997; Sakthivel et al., 1999; Wiwawattanapatapee et al., 2000; Ma et al., 2005). The permeability of different generations of the dendrimers across the epithelial cell monolayers was reported to be in the order of G4 ≫G0 ≈ G1 > G3 > G2, and the rate of permeability was concentration dependent (Florence et al., 2000; El-Sayed et al., 2001). In addition to size, concentration and charge also influence the dendrimer transport presumably by modulating the intercellular tight junctions. It is suggested that the possible mechanism of dendrimer transport across epithelial cells is adsorptive-mediated endocytosis, in addition to other active transport mechanisms including receptor-mediated, fluid-phase endocytosis, as well as carrier mediated processes (Florence A.T., 1997). Recent studies by D’Emanuele et al. (2005) demonstrate that conjugation of small molecular weight drugs (that are known p-glycoprotein substrates) to PAMAM dendrimers increases their transport indicating that dendrimers are not substrates for P-glycoprotein. Use of palmitoyl carnitine, which was found to decrease the lipid order of intestinal brush border membrane vesicles, along with G2-NH2 dendrimers was also shown to increase the Caco-2 cell monolayer permeability (D’Emanuele et al., 2004).
Polyamines are assumed to be transported into the cell by polyamine transporter protein (PAT) system (Cullis et al., 1999; Gardner et al., 2004). Based on their intestinal permeation enhancement effect, and their absorption by carrier mediated transport, we hypothesized that conjugation of the polyamines to the PAMAM dendrimers increases their permeability presumably because of dendrimer uptake by a combination of paracellular pathway as well as by the PAT system. As a first step to realize this objective, we have prepared ornithine and arginine-conjugated PAMAM (G4) dendrimers and their permeabilities across Caco-2 cell monolayer and cytotoxicity were studied.
2. Materials and Methods
2.1. Materials
PAMAM dendrimers (both G4-NH2 and 3.5 G-COOH) were obtained from Dendritic Nanotechnologies Inc. MI, U.S.A. Fluoresceine isothiocyanate dye (FITC), 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT), piperidine, N,N-dimethylformamide (DMF), N,N-diisopropylethylamine (DIPEA), diethyl ether, D2O and dihydrobenzoic acid (DHB), tetramethyl silane (TMS) and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) were purchased from Sigma-Aldrich (Saint-Louis, MO, U.S.A.). Trifluoroacetic acid (TFA), phosphate buffered saline (PBS, pH 7.4), acetone and acetonitrile (both HPLC grade) were purchased from Fisher Scientitifc (Pittsburgh, PA, U.S.A.). N-hydroxybenzotriazole (HOBt), and 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium (HBTU) were purchased from Anaspec (San Jose, CA, U.S.A.). Fmoc-L-Arg(pbf)-OH was from Novabiochem (San Diego, CA, U.S.A.). For permeability studies, 24 well Transwells® were purchased from Corning costar Inc. (NY, U.S.A.). Cell culture materials were purchased from Fisher Scientific Ltd. (Chicago, IL, U.S.A). Hank’s balanced salt solution (HBSS) with calcium and magnesium (without phenol red) was purchased from Cellgro (Herndon, VA).
2.2. Synthesis of the arginine- and ornithine-conjugated dendrimers
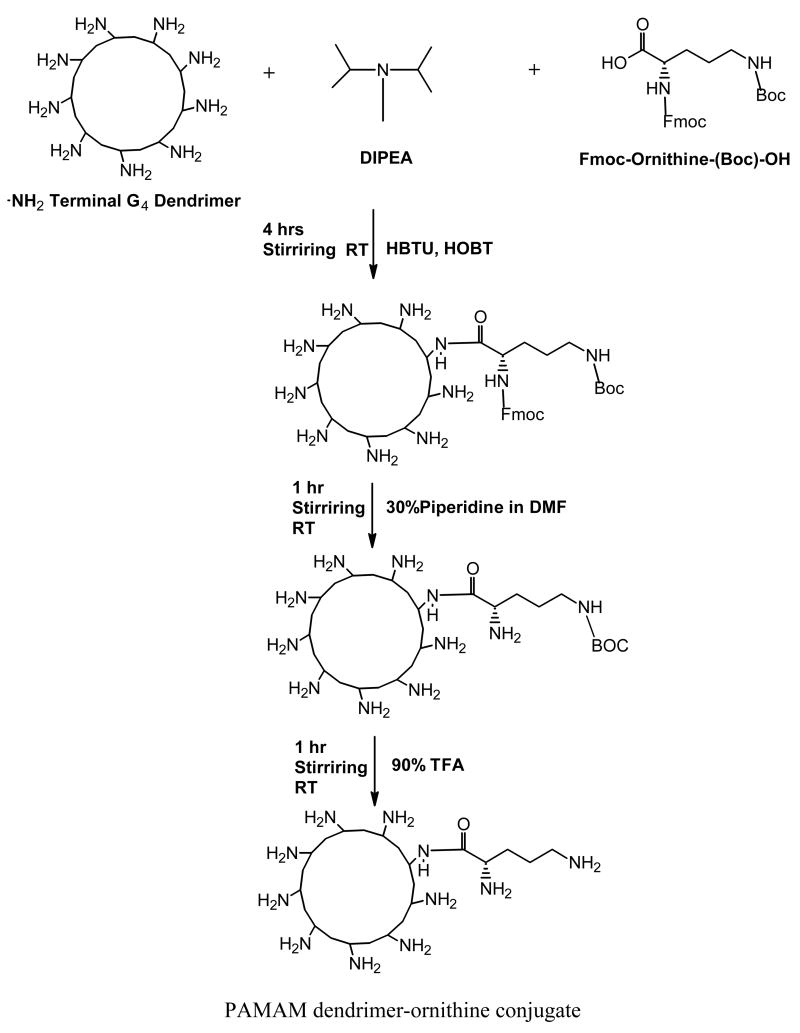
Arginine- and ornithine-conjugated PAMAM dendrimers were prepared using Fmoc synthesis (a schematic of the synthesis is shown in figure 1). Briefly, to 1.0 mmol of G4-NH2 dendrimer in 3.0 mL of DMF, 70 mmol each of HOBt, HBTU, Fmoc-L-Arg(pbf)-OH and 70 mmol of DIPEA were added. The reaction mixture was allowed to stir for 4.0 hrs at room temperature (Choi et al., 2004). The product was precipitated in about 5 mL of ethyl ether and washed with excess ether. The Fmoc groups of Fmoc-Arg(pbf)-coupled dendrimer were removed by adding 2.0 mL of 30% piperidine in DMF (v/v). After 1.0 hr of deprotection reaction, the mixture was precipitated in ethyl ether and washed with excess ethyl ether. A 3.0 mL of the reagent, trifluoroacetic acid/triisopropylsilane/deionized H2O (95:2.5:2.5, v/v) was used for deprotection of pbf groups of arginine (6 hrs at room temperature) and the final product was precipitated in ethyl ether and washed with excess ethyl ether. The product was then solubilized in deionized water and dialyzed against deionized water at 4°C overnight and freeze-dried to obtain a white powder of dendrimer-arginine conjugate (PAMAM-ARG). Dendrimer-ornithine conjugate (PAMAM-ORN) was synthesized the same way as above using Fmoc-ornithine(Boc)-OH and the deprotection of the BOC group was achieved by 90% TFA for 1 hr at room temperature. The yield for both dendrimer-ornithine and dendrimer-arginine was always more than 95%.
Figure 1.
A schematic of the conjugation of ornithine to the amine terminals of the G4 PAMAM dendrimers. Similar protocol was followed for the synthesis of arginine-conjugated PAMAM dendrimers using Fmoc-L-arginine-(pbf)-OH (Note: Though G4 dendrimer has 64 surface amine groups, only a few amine groups were depicted in this figure for convenience).
2.3. NMR and Mass spectral analysis
1H NMR spectra (chemical shift in ppm with respect to TMS set at zero) of arginine- and ornithine-conjugated PAMAM dendrimers were recorded on a Bruker AMX-400 (400 MHz) Spectrometer using D2O as the solvent (with 0.03% v/v TMS). To confirm the molecular weight of surface modified dendrimers, mass spectral analysis of the dendrimers was performed on a Bruker MALDI TOF (matrix assisted laser desorption/ionization-time of flight) using DHB as the matrix.
2.4. Florescence labeling of dendrimers
Fluorescently (FITC) labeled PAMAM dendrimers were prepared according to the methodology reported in the literature (El-Sayed et al., 2001; Jevaprasesphant et al., 2003). PAMAM dendrimer solution was diluted in PBS, pH 7.4. A 5 mg/mL solution of FITC in acetone was prepared and added to the dendrimer solution at a molar ratio of 1:1. The mixture was stirred overnight at room temperature. Fluorescently labeled dendrimer solution was purified by Sephacryl S-300 column chromatography with acetonitrile:Tris buffer (70:30 ) as the elution buffer. The elution fractions corresponding to the dendrimer size were collected, dialyzed against deionized water at 4°C, lyophilized and stored at 4°C for further studies. Stability of the FITC-labeled dendrimers was studied at 4°C and 37°C (for 5 days) by determining the free FITC in the samples using Agilent 1100 high pressure liquid chromatography with fluorescent detector (Agilent Technologies, Santa Clara, CA, U.S.A.). Phosphate buffered saline (PBS pH 7.4): acetonitrile (80:20) was used as the mobile phase. No second peak representing free FITC (retention time of 5.5 min) was found in the samples indicating that the labeled dendrimers were stable for 5 days.
2.5. Cell Culture
Caco-2 cells (passage number 37) were a kind gift from Dr. Radhey Kaushik, Department of Biology & Microbiology, South Dakota State University. The cells were grown at 37°C in T-75 flasks in an atmosphere of 5% CO2 and 95% relative humidity using RPMI 1640 Medium (pH 7.4) supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin solution. The cells were passaged by using 0.25% trypsin containing 0.20% ethylenediamine tetraacetic acid (EDTA).
2.6. Effect on monolayer integrity by measuring transepithelial electrical resistance (TEER)
Caco-2 cells were seeded onto the polystyrene 24-well Transwell® filters at a density of 1× 105 cells/cm2. The cells were grown in an atmosphere of 5% CO2 and 95% relative humidity using RPMI 1640 Medium (pH 7.4) supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin solution. The growth medium was changed every two days. Following incubation for 21 days, mean TEER value across the Caco-2 cell monolayers was found to be 1000±58 Ω.cm2. The effect of PAMAM dendrimers and surface modified dendrimers on Caco-2 cell monolayers was determined by measuring of the transepithelial electrical resistance across Caco-2 cell monolayers in presence of the dendrimers at donor concentrations of 1μg/mL and 10 μg/mL upon both apical and basolateral incubation. A EVOMX™ epithelial tissue voltammeter (World Precision Instruments, Sarasota, FL) was used to measure TEER values (Kitchens et al., 2006).
2.7. Permeability of dendrimers across polarized Caco-2 cell monolayers
Caco-2 cells were seeded onto the polystyrene 24-well Transwell® filters of 3.0 μm mean pore size, 0.33 cm2 surface area (Corning Costar Inc., NY) at a density of 1 × 105 cells/cm2, and the growth medium was changed every two days. Polarization of the cells was monitored by measuring the TEER once every three days. The TEER of the blank Transwell® filters was found to be 178 ± 31 ohms. A TEER value of 1000 ± 58 ohms (including the background filter resistance) was observed after 21–28 days and these cell monolayers were used for the transport studies (El-Sayed et al., 2001, El-Sayed et al., 2002, Kitchens et al., 2006). The cells were washed with HBSS pH 7.4 buffer (with 10 mM HEPES) and incubated with HBSS pH 7.4 buffer for 1 hr prior to the experiment. For A-B transport study, 0.6 mL of the buffer was added to the basal side and a 0.1 mL of test solution was added to the apical side. For B-A transport study, 0.6 mL of the buffer was added to the apical side and a 0.1 mL of test solution was added to the basal side. Following addition of the test solution (dendrimers at 1 μg/mL and 10 μg/mL), the plates were incubated at 37°C, 5% CO2, 95% humidity, and shaking at 50 rpm while maintaining sink conditions. 200 μL samples were removed from the donor compartment at 30, 90, 150, and 210 min and analyzed for fluorescence using SpectraMax M2 (Molecular devices, Sunnyvale, CA). The calibration curves for both PAMAM-arginine (Y = 15.598X+224.69) and PAMAM-ornithine (Y = 85.935X-140.57) were linear between 0.1 and 5 μg/mL. Minimum detectable concentration was found to be 1 ng/mL. The volume of the receptor compartment was maintained constant at 0.6 mL by replacing it with fresh HBSS. The apparent permeability coefficient (Papp) was calculated using the equation:
Where ∂Q/∂t is the permeability rate, A is the surface area of the membrane filter, and C0 is the initial concentration in the donor compartment (Irvine et al., 1998).
2.8. In vitro cytotoxicity
In vitro cytotoxicity of the dendrimers was evaluated by MTT assay (Sgouras D. and Duncan R., 1990; Fischer et al., 2003). Caco-2 cells were seeded at a density of 4 × 104 cells/well in a 96 well plate. The cells were grown in an atmosphere of 5% CO2 and 95% relative humidity using RPMI 1640 Medium (pH 7.4) supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin solution. Following 24 hr incubation period, different concentrations (0.1 μg/mL, 1 μg/mL, 10 μg/mL, 50 μg/mL, 100 μg/mL and 200 μg/mL) of the native PAMAM dendrimers and surface modified dendrimers were added. After a 4 hr incubation period, 50 μL of 1:10 diluted MTT stock solution (5 mg/mL) was added and the cells were incubated for 4 hrs. The medium was removed and 150 μL of dimethyl sulfoxide (DMSO) was added to dissolve the MTT crystals and the optical density was read using a SpectraMax M2 microplate reader (Molecular devices, Sunnyvale, CA) with 590 nm as excitation wavelength and 650 nm as the background. The viability of cells exposed to dendrimers was expressed as a percentage of the viability of cells grown in the absence of dendrimers.
2.9. Statistical analysis
The statistical analysis was carried out using the student’s t test in GraphPad InStat 3.05 version software (San Diego, CA). p < 0.05 was considered significant.
3. Results
3.1. Polyamine-conjugation of the dendrimers
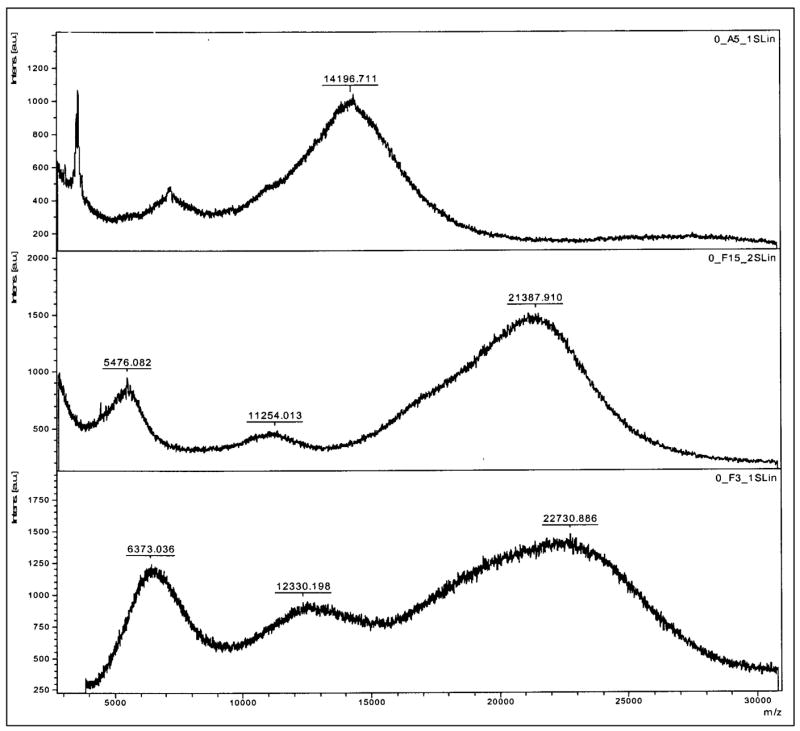
Initially, ornithine and arginine were coupled to the amine terminals of the PAMAM dendrimers by EDC (1-Ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride) coupling reaction (Malik et al., 2000). However, only a few ornithine and arginine molecules (7 molecules each) were found attached to the G4 PAMAM dendrimer. Increasing the molar ratio of dendrimer:arginine (and ornithine) did not yield any increase in the number of molecules attached to the dendrimer, instead dimer formation (such as dendrimer-ornithine-dendrimer, dendrimer-arginine-dendrimer) was observed. To avoid dimerization and to increase the degree of conjugation, F-moc synthesis protocol was used. To start with, different molar ratios of the dendrimers and the polyamines were used to optimize the conjugation reaction. When 1:35 ratio of dendrimer:Fmoc-polyamine (molar ratio) was used, approximately 28 molecules of ornithine and 26 molecules of the arginine were found coupled to the PAMAM dendrimers. Based on these preliminary results, a higher molar ratio of 1:70 was used to get all the 64 amine groups of the G4 PAMAM dendrimer conjugated with the polyamines. The surface modified dendrimers were characterized by 1H NMR and MALDI spectroscopic analysis (figure 2 a, b). The spectral analysis showed that out of 64 surface amine groups of G4 PAMAM dendrimer, 62 molecules of ornithine and 55 molecules of arginine were attached. The 1H NMR data of the dendrimers are as follows: PAMAM Dendrimer: 2.44 – NCH2 CH2 CO-, 2.63 –CONHCH2 CH2 N- & NCH2CH2N-, 2.72 – CONHCH2CH2N, 2.83 – NCH2CH2CO, 3.25 – ONHCH2CH2N. PAMAM-Ornithine: 1.73 - HCCH2CH2CH2NH (Orn), 1.91- H CCH2CH2CH2NH (Orn), 3.41- HCCH2CH2CH2NH (Orn), 3.92- HCCH2CH2CH2NH (Orn). PAMAM-Arginine: 1.68 -HCCH2CH2CH2NH (Arg), 1.93 - HCCH2CH2CH2NH (Arg) 3.25 -HCCH2CH2CH2NH (Arg), 4.01 - HCCH2CH2CH2NH (Arg).
Figure 2.
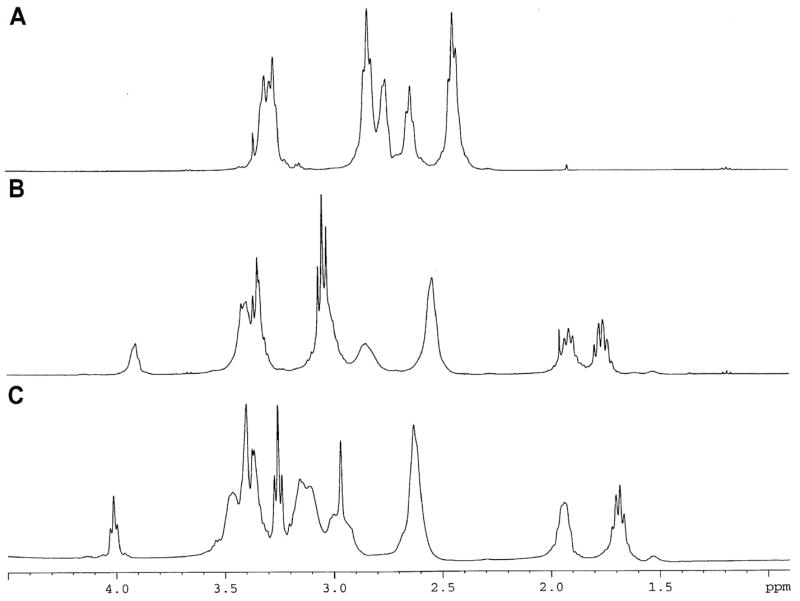
a. 1H NMR (400 MHz) spectra of (A) PAMAM, (B) PAMAM-ornithine, and (C) PAMAM-arginine.
b. MALDI-TOF spectra of (A) Standard PAMAM G4 dendrimer, (B) Ornithine coupled dendrimer showing 62 molecules coupled to PAMAM dendrimer, (C) Arginine coupled dendrimer showing 55 molecules coupled to PAMAM dendrimer.
3.2. Transepithelial electrical resistance (TEER)
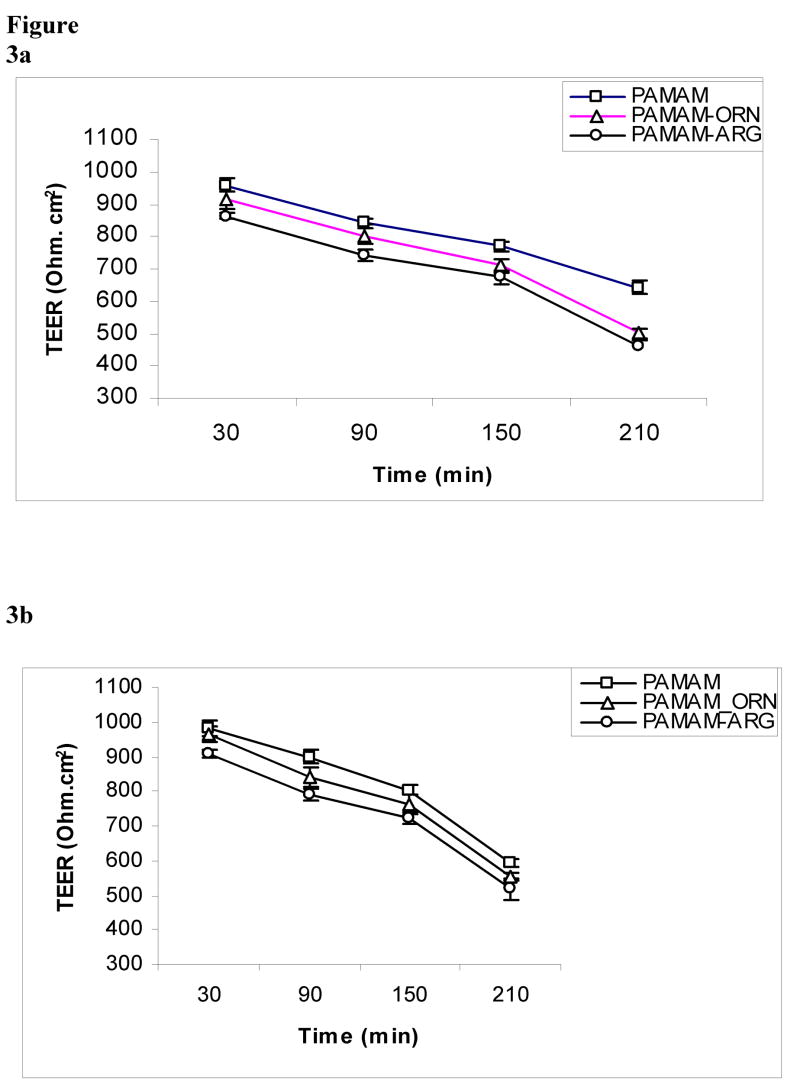
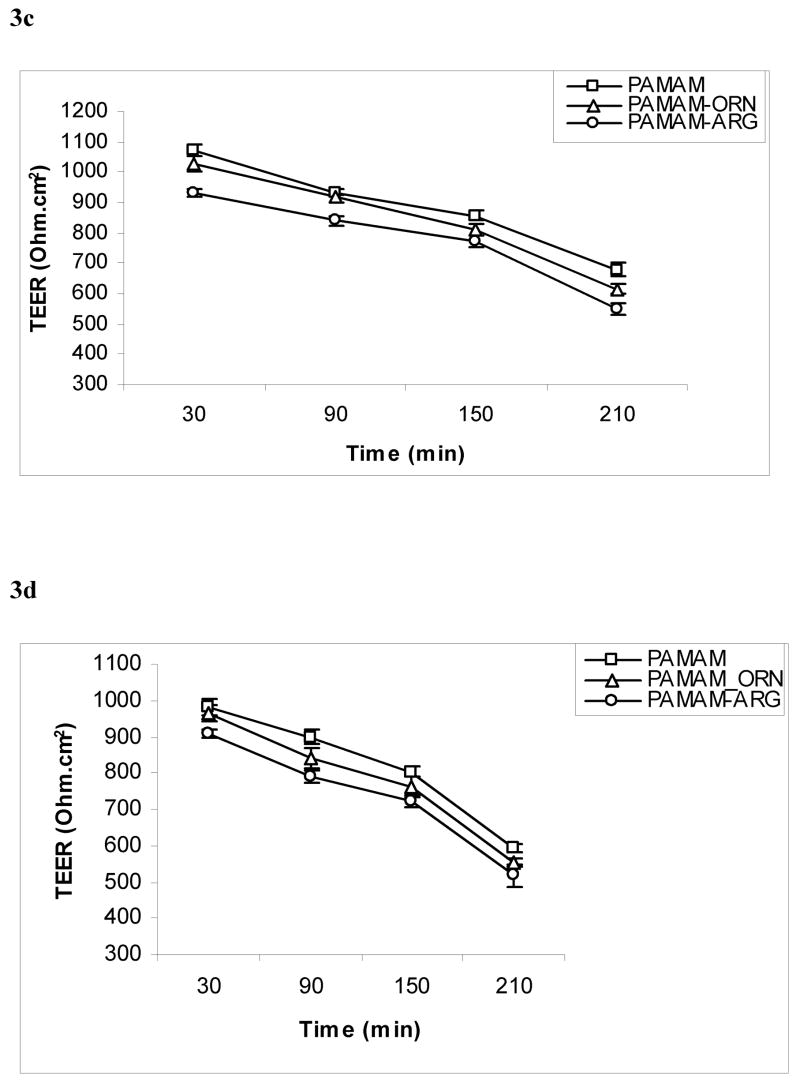
Transepithelial electrical resistance was measured at time intervals of 30, 90, 150, 210 min in presence of different concentrations (1 μg/mL and 10 μg/mL) of the dendrimers (PAMAM, PAMAM-arginine and PAMAM-ornithine) from both AB and BA directions. The TEER value with Caco-2 cell monolayer was 1000 ± 58 ohms (without background resistance subtracted). The decline in TEER for each type of the dendrimer was concentration and time dependent in both AB and BA directions as shown in figure 3a-d. Among the dendrimers tested, the decrease in TEER by arginine-conjugated dendrimer was most prominent and showed significant decrease as compared to PAMAM dendrimers (p<0.05). The basolateral incubation (BA) also showed faster and more pronounced decline in TEER as compared to apical incubation (AB) under similar conditions.
Figure 3.
Figure 3a, b. Effect of polyamine-conjugated dendrimers (1 μg/mL) on transepithelial electrical resistance (TEER) across Caco-2 cell monolayers upon incubation on a: apical side b. basolateral side (n = 4 and p<0.05). (PAMAM-ORN: ornithine-conjugated PAMAM; PAMAM-ARG: arginine-conjugated PAMAM).
Figure 3c, d. Effect of polyamine-conjugated dendrimers (10 μg/mL) on transepithelial electrical resistance (TEER) across Caco-2 cell monolayers upon incubation on c: apical side d. basolateral side (n = 4 and p<0.05). (PAMAM-ORN: ornithine-conjugated PAMAM; PAMAM-ARG: arginine-conjugated PAMAM).
3.3. Permeability of dendrimers through Caco-2 cell monolayers
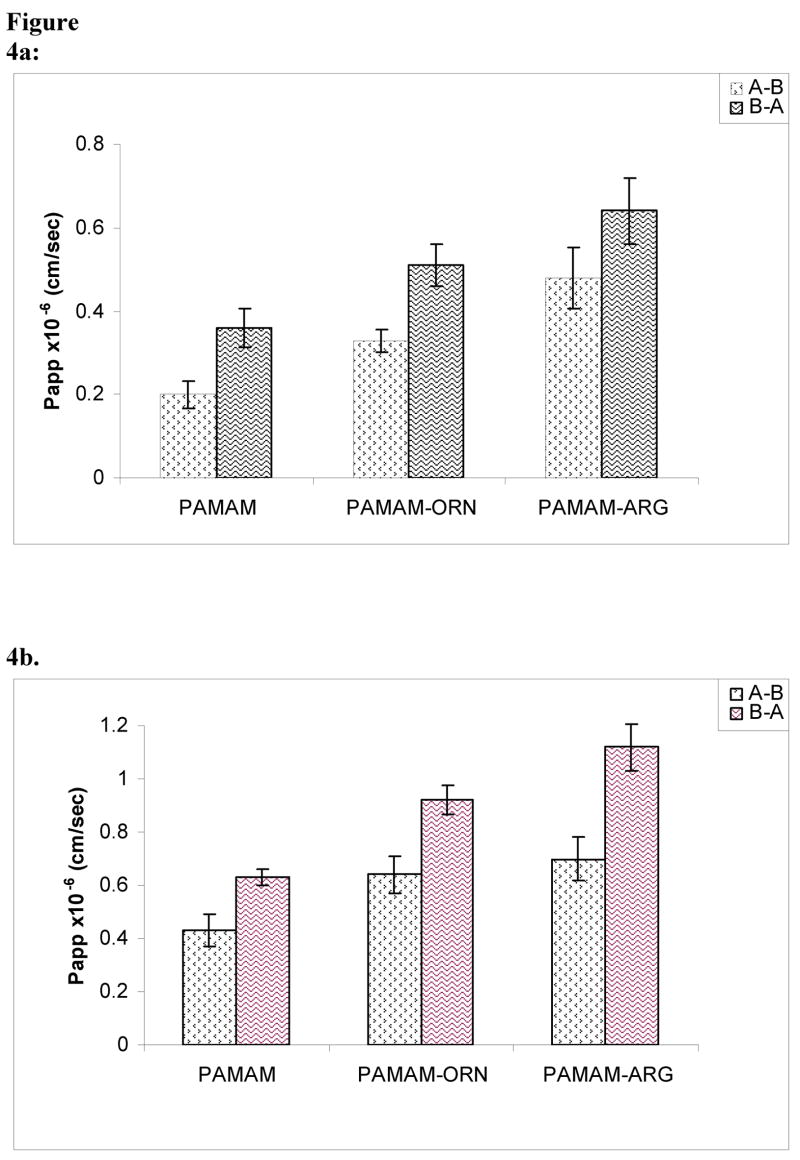
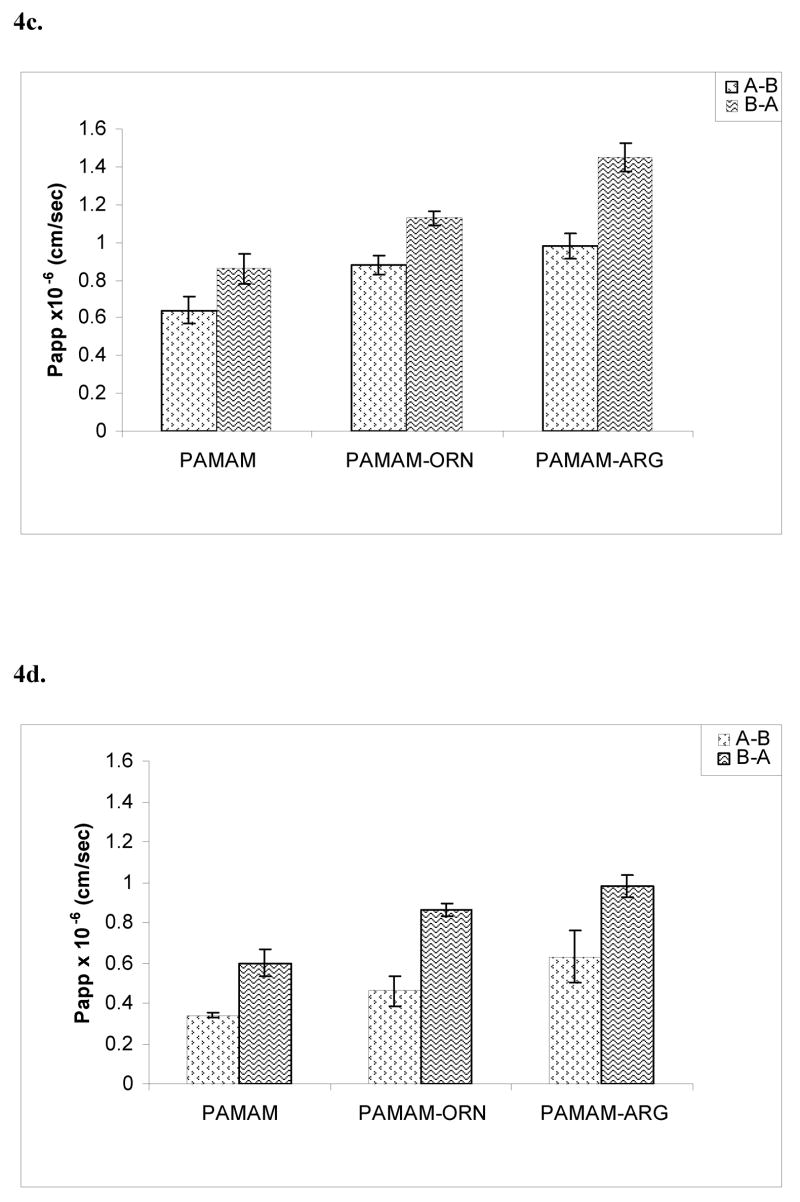
PAMAM dendrimers, ornithine- and arginine-conjugated dendrimers were labeled with FITC. Attachment of FITC to the dendrimers was verified by 1H-NMR and spectrofluorimetry. An average molar ratio of 1:0.5 dendrimer:FITC was obtained. In vitro stability tests were performed to ensure that FITC-labeled dendrimer was stable during the period of experimentation. HPLC analysis revealed that no free FITC was found in the dendrimer-FITC samples incubated at 4°C and 37°C (PBS pH 7.4) over a period of 5 days. At 1 μg/mL as well as 10 μg/mL, dendrimers showed incubation time and concentration dependent permeability profile as shown in the figures 4a–4f. Both ornithine- and arginine-conjugated dendrimers showed significantly higher AB permeability than the native PAMAM dendrimers (p<0.05). For instance, mean apparent permeability coefficient (Papp) of PAMAM dendrimers was 0.34 ± 0.01 × 10−6 cm/s for A-B direction and 0.61± 0.07 × 10−6 cm/s for the B-A direction after incubation for 90 min with 10μg/mL solution. Under the similar conditions, mean Papp of PAMAM-ornithine and PAMAM-arginine was 0.46 ± 0.07 × 10−6 and 0.63 ± 0.13 × 10−6 cm/s for AB direction, and 0.86 ± 0.03 × 10−6 and 0.98± 0.05 × 10−6 cm/s for BA direction respectively. Because all the dendrimers have shown some degree of toxicity when incubated over 4 hrs, permeabilities were not determined after 210 min. At both 1μg/mL and 10 μg/mL concentrations, permeability coefficients of the surface modified dendrimers were significantly higher than the simple PAMAM dendrimers (p<0.05).
Figure 4.
Figure 4a, b, c. Permeability of polyamine-conjugated dendrimers across polarized Caco-2 cell monolayer in AB (apical-to-basolateral) and BA (basolateral-to-apical) directions at a concentration of 1μg/mL after incubation for different time intervals, a. 90 min; b. 150 min; c. 210 min.. Results are reported as mean ± standard error (S.E.M.) (n = 4 and p<0.05). (PAMAM-ORN: ornithine-conjugated PAMAM; PAMAM-ARG: arginine-conjugated PAMAM).
Figure 4d, e, f. Permeability of polyamine-conjugated dendrimers across polarized Caco-2 cell monolayer in AB (apical-to-basolateral) and BA (basolateral-to-apical) directions at a concentration of 10 μg/mL after incubation for different time intervals, d. 90 min; e. 150 min; f. 210 min. Results are reported as mean ± standard error (S.E.M.) (n = 4 and p<0.05). (PAMAM-ORN: ornithine-conjugated PAMAM; PAMAM-ARG: arginine-conjugated PAMAM).
Permeabilities of the dendrimers across Caco-2 cell monolayers at each time duration (not the mean Papp) were calculated by determining the amount permeated only during that particular time period. Papp of all the dendrimers increased with time. For instance, Papp of PAMAM-ORN during 0–90 min was 0.46±0.076 × 10−6 cm/s, and it increased to 1.87 ±0.011 × 10−6 cm/s during 150–210 min (p<0.01). In case of PAMAM-ARG, Papp was 0.63±0.13 × 10−6 cm/s during 0–90 min, whereas, it increased to 2.28 ±0.0.09 × 10−6 cm/s during 150–210 min (p<0.01). The increase in the rate of permeability across the cell monolayers, as well as a decrease in TEER with time indicate that the increase in permeability with the surface modified dendrimers may be due to disruption of intercellular tight junctions as one of the mechanisms. However, effect of these dendrimers on the tight junctional proteins such as occludin and actin has to be investigated to confirm the mechanism.
3.4. In vitro cytotoxicity studies
The toxicity of the native PAMAM, PAMAM-arginine and PAMAM-ornithine to Caco-2 cells was studied by MTT assay. Figure 5 shows the concentration of different dendrimers and the percent cell viability after 4 hr incubation of dendrimers with Caco-2 cells at 37°C. No change in cell viability was observed with any of the dendrimers tested up to 2 hr of incubation (data not shown). At 0.1μg/mL concentration, cell viability with PAMAM, PAMAM-ORN and PAMAM-ARG were 87.4±1.7, 82.7±8.5 and 70.1±4.5% respectively. As the concentration increased to 200 μg/mL, percent cell viability with PAMAM, PAMAM-ORN and PAMAM-ARG were 61.2±4.2, 55.9±4.7 and 45.4±3.7% respectively. Interestingly, at all the concentrations tested, PAMAM-ARG was significantly more toxic than both PAMAM, PAMAM-ORN (P<0.01). Toxicity of the dendrimers increased with increasing in the duration of incubation (data not shown).
Figure 5.
Effect of polyamine-conjugated dendrimers (0.1μg/mL-200μg/mL) on Caco-2 cell viability after 4 hrs of incubation at 37°C. Results are reported as mean ± standard error (S.E.M.) (n = 4 and p<0.05).
4. Discussion
Dendrimers have been investigated as drug carriers for oral delivery as they have been shown to cross the epithelial layers at sufficient rates (Florence et al., 2000; Wiwattanapatapee et al., 2000). Anionic PAMAM dendrimers exhibited rapid transport rates across everted rat intestine as compared to positively charged dendrimers (El-Sayed et al., 2002; Kitchens et al., 2006). The difference in transepithelial transport between these dendrimers was attributed to the difference in interaction of the dendrimers with the negatively charged cell membrane. Positively charged dendrimers were shown to strongly associate with the tissue compared to the negatively charged dendrimers. In this study, PAMAM-NH2 dendrimers were conjugated with polyamines (ornithine and arginine) and their permeability across Caco-2 cell monolayers, and cytotoxicity were investigated. Though dendrimer-arginine conjugates were reported to enhance the gene transfection efficiency in 293 cells (Choi et al., 2004), their potential to increase permeability across Caco-2 cell monolayers has not been investigated.
The extent of dendrimer-polyamine conjugation may be controlled by adjusting the molar ratio of the Fmoc arginine/Fmoc ornithine and the PAMAM dendrimer. At a molar ratio of 70, most of the surface amino groups of the dendrimer were conjugated with the polyamine molecules (55 molecules of arginine/62 molecules of ornithine). The TEER studies indicate that the native PAMAM dendrimers and the surface modified dendrimers (polyamine-conjugated dendrimers) have significantly reduced the TEER values as compared to the control (p<0.05). The reduction in TEER was dependent on dendrimer concentration and duration of incubation. The reduction in TEER was more pronounced on the apical side as compared to the basal side.
At all the time intervals (90, 150, 210 min), and the concentrations (1 μg/mL, 10 μg/mL) tested, (papp of arginine-conjugated PAMAM dendrimers was significantly higher than the PAMAM-ornithine and native PAMAM dendrimers (p<0.05). The correlation between monolayer permeability (papp) and the decrease in TEER with both the PAMAM dendrimers and the surface modified dendrimers allows us to speculate that these dendrimers might have been transported across the epithelial cells via paracellular route. The higher BA permeabilities of the dendrimers over AB permeability may be attributed to differences in tight junctional structure, for example, Caco-2 cell monolayers possess tight junctional proteins at the apical but not the basolateral membrane (Noach et al., 1993; El-Sayed et al., 2002). The mechanism by which the polyamines improve the permeation of the dendrimers remain to be investigated, it is possible that the acidic molecules such as acidic mucopolysaccharides on the surface of the membrane and phosphatidyl serine, and/or polyamine transport system (PAT) are involved.
Many of the cell penetrating peptides such as TAT-derived peptide, protein transduction domains (PTD) or membrane translocalization signals (MTS) contain positively charged aminoacid residues arginine and lysine (Futaki S, 2002; Henry CM, 2003; Tung et al., 2003; Choi et al., 2004). It is not known if they enter the cell by endocytosis or other non-endocytic pathways. Arginine is a precursor for ornithine. Ornithine and other polyamines (such as putrescine) were shown to be transported into the cell by PAT system (Shao et al., 1996; Satriano et al., 2001). Though it is mere a speculation, ornithine and arginine-conjugated dendrimers uptake may also involve PAT system.
Dendrimers were reported to exhibit concentration and generation dependent toxicity in vitro. However, both acute and chronic toxicity evaluation in mice did not show any behavioral changes, weight loss or immunogenicity with G3 and G5 dendrimers (Roberts et al., 1996). In the present study, PAMAM-arginine was found to be more toxic than PAMAM-ORN and simple PAMAM dendrimer. It is reasonable to attribute the toxicity of PAMAM-arginine to its high permeability. Though the study was conducted only with G4 PAMAM dendrimers, it will be interesting to see the effect of generation of the polyamine-conjugated dendrimers on their toxicity and monolayer permeability.
5. Conclusion
The concept of conjugation of polyamines to the dendrimers to increase the flux across Caco-2 cell monolayers is novel and has not been reported. PAMAM dendrimer permeability was enhanced by conjugating with polyamines such as ornithine and arginine. The enhanced permeability and concomitant reduction in TEER with these modified dendrimers indicate the paracellular pathway as one of the mechanisms of their transport. However, it is well known that the polyamines are transported by polyamine transporter system in a metabolism dependent pathway. Further studies are needed to understand the involvement of polyamine transporter protein (PAT) in the transport of polyamine-conjugated dendrimers across caco-2 cell monolayers..
Acknowledgments
This work was partly supported by a grant from National Institutes of Health (NIH R15 CA121980-01) to SP, and the Governor’s Individual Research Seed Grant, SD and SDSU Research Support Fund, South Dakota State University, SD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahnet CH, Chae SY, Bae YH, Kim SW. Synthesis of biodegradable multiblock copolymers of poly(L-lysine) and poly(ethylene glycol) as a non-viral gene carrier. J Control Rel. 2004;97:567–574. doi: 10.1016/j.jconrel.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Aungst BJ. Intestinal permeation enhancers. J Pharm Sci. 2000;89:429–442. doi: 10.1002/(SICI)1520-6017(200004)89:4<429::AID-JPS1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Auvinen M, Parsinen A, Anderson LC, Holtta E. Ornithine decarboxylation activity is critical for cell transformation. Nature. 1992;360:355–358. doi: 10.1038/360355a0. [DOI] [PubMed] [Google Scholar]
- Boas U, Heggard PMH. Dendrimers in drug research. Chem Soc Rev. 2004;33:43–63. doi: 10.1039/b309043b. [DOI] [PubMed] [Google Scholar]
- Choi JS, Nam K, Park KJY, Kim JB, Lee JK, Park JS. Enhanced transfection efficiency of PAMAM dendrimer by surface modification with L-arginine. J Control Rel. 2004;94:445–456. doi: 10.1016/j.jconrel.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Cullis PM, Green RE, Merson-Davies L, Travis N. Probing the mechanism of transport and compartmentalization of polyamines in mammalian cells. Chem Biol. 1999;6:717–729. doi: 10.1016/s1074-5521(00)80019-8. [DOI] [PubMed] [Google Scholar]
- D’Emanuele A, Jevapresesphant R, Penny J, Deadwood D. The use of dendrimer-propranalol prodrug to bypass efflux transporters and enhance oral bioavailability. J Control Rel. 2004;95:447–453. doi: 10.1016/j.jconrel.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Devens BH, Weeks RS, Burns MR, Carlson CL, Brawer MK. Polyamine depletion therapy in prostate cancer. Prostate Cancer Prost Dis. 2000;3:275–279. doi: 10.1038/sj.pcan.4500420. [DOI] [PubMed] [Google Scholar]
- Domanski DM, Klajnert B, Bryszewska M. Influence of PAMAM Dendrimers on human red blood cells. Bioelectrochemistry. 2004;63:189–191. doi: 10.1016/j.bioelechem.2003.09.023. [DOI] [PubMed] [Google Scholar]
- El-Sayed M, Kiani MF, Naimark MD, Hikal AH, Ghandehari H. Extravasation of poly (amidoamine)(PAMAM) dendrimers across microvascular network endothelium. Pharm Res. 2001;18:23–28. doi: 10.1023/a:1011066408283. [DOI] [PubMed] [Google Scholar]
- El-Sayed M, Ginski M, Rhodes C, Ghandehari H. Transepithelial transport of poly(amidoamine) dendrimers across Caco-2 cell monolayers. J Control Rel. 2002;81:355–365. doi: 10.1016/s0168-3659(02)00087-1. [DOI] [PubMed] [Google Scholar]
- Endo Y, Hanada K, Miyake M, Ogawara K, Higaki K, Kimura T. Mechanisms of cytoprotective effect of amino acids on local toxicity caused by sodium laurate, a drug absorption enhancer, in intestinal epithelium. J Pharm Sci. 2002;91:730–743. doi: 10.1002/jps.10049. [DOI] [PubMed] [Google Scholar]
- Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kassel T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24:1121–1131. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- Florence AT. The oral absorption of micro-and nanoparticles: neither exceptional nor unusual. Pharm Res. 1997;14:259–266. doi: 10.1023/a:1012029517394. [DOI] [PubMed] [Google Scholar]
- Florence AT, Sakthivel T, Toth I. Oral uptake and translocation of a polylysine dendrimer with a lipid surface. J Control Rel. 2000;65:253–259. doi: 10.1016/s0168-3659(99)00237-0. [DOI] [PubMed] [Google Scholar]
- Futaki S. Arginine-rich peptides: potential for intracellular delivery of macromolecules and the mystery of translocation mechanisms. Int J Pharm. 2002;245:1–7. doi: 10.1016/s0378-5173(02)00337-x. [DOI] [PubMed] [Google Scholar]
- Gardner RA, Delcros JG, Konate F, Breitbeil F, III, Martin B, Sigman M, Huang M, Phanstiel O., IV N1-Substituent effects in the selective delivery of polyamine conjugates into cells containing active polyamine transporters. J Med Chem. 2004;47:6055–6069. doi: 10.1021/jm0497040. [DOI] [PubMed] [Google Scholar]
- Harold BB, Margaret AP. Circular dichromism assay for decarboxylation of optically pure aminoacids: application to ornithine. Anal Biochem. 1996;238:191–194. doi: 10.1006/abio.1996.0274. [DOI] [PubMed] [Google Scholar]
- Heby O. Role of polyamines in the control of cell proliferation and differentiation. Differentiation. 1981;19:1–20. doi: 10.1111/j.1432-0436.1981.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Henry CM. Breaching barriers. Chem Eng News. 2003;81:35–43. [Google Scholar]
- Irvine JD, Takahashi L, Lockhart K, Cheong J, Tolan JW, Selick HE, Grove JR. MDCK (Madin-Darby Canine Kidney) cells: a tool for membrane permeability screening. J Pharm Sci. 1998;88:28–33. doi: 10.1021/js9803205. [DOI] [PubMed] [Google Scholar]
- Janasen JFGA, de Brabander-van den Berg EMM, Meijer EW. Encapsulation of guest molecules in to a dendritic box. Science. 1994;266:1226–1229. doi: 10.1126/science.266.5188.1226. [DOI] [PubMed] [Google Scholar]
- Jevaprasesphant R, Penny J, Attwood D, McKeown NB, D’Emanuele A. Engineering of dendrimer surfaces to enhance transepithelial transport and reduce cytotoxicity. Pharm Res. 2003;20(10):1543–1550. doi: 10.1023/a:1026166729873. [DOI] [PubMed] [Google Scholar]
- Johnson LR. Regulation of gastrointestinal mucosal growth. Physiol Rev. 1988;68:456–502. doi: 10.1152/physrev.1988.68.2.456. [DOI] [PubMed] [Google Scholar]
- Kitchens KM, Kolhatkar RB, Swaan PW, Eddington ND, Ghandehari H. Transport of poly(amidoamine) dendrimers across Caco-2 cell monolayers: Influence of size, charge and fluorescent labeling. Pharm Res. 2006;23(12):2818–2826. doi: 10.1007/s11095-006-9122-2. [DOI] [PubMed] [Google Scholar]
- Kitchens KM, El-Sayed MEH, Ghandehari H. Transepithelial and endothelial transport of poly(amidoamine) dendrimers. Adv Drug Del Rev. 2005;57:2163–2176. doi: 10.1016/j.addr.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Li W, Ishida T, Okada Y, Oku N, Kiwada H. Increasing gene expression by cationic liposomes (TFL-3) in lung metastases following intravenous injection. Biol Pharm Bull. 2005;28:701–706. doi: 10.1248/bpb.28.701. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang L, Lin Y, Teng Q, Zhao C, Hu H, Chi W. Ornithine decarboxylase activity and its gene expression are increased in benign hyperplastic prostate. Prostate. 2000;43:83–87. doi: 10.1002/(sici)1097-0045(20000501)43:2<83::aid-pros2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Luk GD, Marton LJ, Baylin SB. Ornithine decarboxylase is important in intestinal mucosal maturation and recovery from injury in rats. Science. 1988;210:195–198. doi: 10.1126/science.6774420. [DOI] [PubMed] [Google Scholar]
- Ma Z, Li J, He F, Wilson A, Pitt B, Li S. Cationic lipids enhance siRNA-mediated interferon response in mice. Biochem Biophys Res Commun. 2005;330:755–759. doi: 10.1016/j.bbrc.2005.03.041. [DOI] [PubMed] [Google Scholar]
- MacRae M, Kramer DL, Coffino P. Developmental effects of polyamine depletion in caenorhabditis elegans. Biochem J. 1998;333(Pt2):309–315. doi: 10.1042/bj3330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafune K, Tanaka Y, Mimori K, Mori M, Takubo K, Makuochi M. Increased expression of ornithine decarboxylase messenger RNA in human esophageal carcinoma. Clin Cancer Res. 1999;5:4073–4078. [PubMed] [Google Scholar]
- Majumdar AP, Johnson LR. Effect of putrescine on oxyntic gland and colonic mucosal growth in rats. Life Sci. 1987;41:961–966. doi: 10.1016/0024-3205(87)90683-7. [DOI] [PubMed] [Google Scholar]
- Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H, Weener JW, Meijer EW, Paulus W, Duncan R. Dendrimers: Relationship between structure and biocompatibility in vitro and preliminary studies on the biodistribution of 125I–labeled polyamidoamine dendrimers in vivo. J Controlled Rel. 2000;65:133–148. doi: 10.1016/s0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- Messing EM, Love RR, Tutsch KD, Verma AK, Douglas J, Pomplun M, Simsiman R, Wilding G. Low-dose difluoromethylornithine and polyamine levels in human prostate tissue. J Natl Cancer Inst. 1999;91:1416–1417. doi: 10.1093/jnci/91.16.1416. [DOI] [PubMed] [Google Scholar]
- Miyake M, Oka Y, Minami T, Toguchi H, Odomi M, Ogawara K, Higaki K, Kimura T. Combinatorial use of sodium laurate with taurine or L-glutamine enhances colonic absorption of rebamipide, poorly absorbable anti-ulcer drug without any serious histopathological mucosal damages. J Pharm Sci. 2003;92:911–921. doi: 10.1002/jps.10362. [DOI] [PubMed] [Google Scholar]
- Miyake M, Kamada N, Oka Y, Mukai T, Munami T, Toguchi H, Odomi M, Ogawara K, Higaki K, Kimura T. Development of suppository formulation safely improving rectal absorption of rebamipide, a poorly absorbable drug, by utilizing sodium laurate and taurine. J Control Rel. 2004;99:63–71. doi: 10.1016/j.jconrel.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Miyake M, Minami T, Hirota M, Toguchi H, Odomi M, Ogawara K, Higaki K, Kimura T. Novel oral formulation safely improving intestinal absorption of poorly absorbable drugs: Utilization of polyamines and bile acids. J Control Rel. 2006;111:27–34. doi: 10.1016/j.jconrel.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Noach ABJ, Kurosaki Y, Blom-Roosemalen MCM, Boer AGD, Breimer DD. Cell-polarity dependent effect of chelation on the paracellular permeability of confluent Caco-2 cell monolayers. Int J Pharm. 1993;90:229–237. [Google Scholar]
- O’Brien TG, Megosh LC, Gillard G, Soler AP. Ornithine decarboxylase over expression is a sufficient condition for tumor promotion in mouse skin. Cancer Res. 1997;57:2630–2637. [PubMed] [Google Scholar]
- Ohtake K, Natsume H, Ueda H, Morimoto Y. Analysis of transient and reversible effects of poly-L-arginine on the in vivo nasal absorption of FITC-dextrans in rats. J Control Rel. 2002;82:263–275. doi: 10.1016/s0168-3659(02)00128-1. [DOI] [PubMed] [Google Scholar]
- Pegg AE. Polyamine metabolism and its importance in neoplastic growth and as a target for chemotherapy. Cancer Res. 1988;48:759–774. [PubMed] [Google Scholar]
- Roberts JC, Bhalgat MK, Zera RT. Preliminary biological evaluation of polyamidoamine(PAMAM) starburst dendrimers. J Biomed Mater Res. 1996;30:53–65. doi: 10.1002/(SICI)1097-4636(199601)30:1<53::AID-JBM8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Roy R. Recent developments in the rational design of multivalent glycoconjugates. Topics in Current Chem. 1997;187:241–274. [Google Scholar]
- Sakthivel T, Toth I, Florence AT. Distribution of lipidic 2.5 nm diameter dendrimer carrier after oral administration. Int J Pharm. 1999;183:51–55. doi: 10.1016/s0378-5173(99)00043-5. [DOI] [PubMed] [Google Scholar]
- Satriano J, Isome M, Casero RA, Jr, Thomson SC, Blantz RC. Polyamine transport system mediates agmatine transport in mammalian cells. Am J Physiol Cell Physiol. 2001;281:329–334. doi: 10.1152/ajpcell.2001.281.1.C329. [DOI] [PubMed] [Google Scholar]
- Schiper RG, Romijn JC, Cuijpers VMJI, Verhofstad AAJ. Polyamines and prostate cancer. Biochem Soc Trans. 2003;31(2):375–380. doi: 10.1042/bst0310375. [DOI] [PubMed] [Google Scholar]
- Seiler N, Delcros JG, Moulinoux JP. Polyamine transport in mammalian cells: An Update. Int J Biochem Cell Biol. 1996;28:843–861. doi: 10.1016/1357-2725(96)00021-0. [DOI] [PubMed] [Google Scholar]
- Seki T, Kanabayashi H, Nagao T, Chono S, Tomita M, Hayashi M, Tabata Y, Morimoto K. Effect of aminated gelatin on the nasal absorption of insulin in rats. Biol Pharm Bull. 2005;28:510–514. doi: 10.1248/bpb.28.510. [DOI] [PubMed] [Google Scholar]
- Sgouras D, Duncan R. Methods for the evaluation of biocompatibility of soluble synthetic polymers which have potential for biomedical use:1. Use of the tetrazolium-based colorimetric assay (MTT) as a preliminary screen for the evaluation of in vitro cytotoxicity. J Mater Sci Med. 1990;1:67–78. [Google Scholar]
- Shao D, Xiao L, Ha HC, Casero RA., Jr Isolation of a polyamine transport deficient cell line from human non-small lung carcinoma line NCI H157. J Cell Physiol. 1996;166:43–48. doi: 10.1002/(SICI)1097-4652(199601)166:1<43::AID-JCP5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Tung CH, Weissleder R. Arginine containing peptides as delivery vectors. Adv Drug Deliv Rev. 2003;55:281–294. doi: 10.1016/s0169-409x(02)00183-7. [DOI] [PubMed] [Google Scholar]
- Wang JY, Johnson LR. Luminal polyamines stimulate repair of gastric mucosal stress ulcers. Am J Physiol. 1990;259:G584–G592. doi: 10.1152/ajpgi.1990.259.4.G584. [DOI] [PubMed] [Google Scholar]
- Wiwattanapatapee R, Carreno-Gomez B, Malik N, Duncan R. Anionic PAMAM dendrimers rapidly cross adult rat intestine in vivo: A potential oral delivery system? Pharm Res. 2000;17(8):991–998. doi: 10.1023/a:1007587523543. [DOI] [PubMed] [Google Scholar]
- Yata T, Endo Y, Sone M, Ogawara K, Higaki K, Kimura T. Amino acids protect epithelial cells from local toxicity by absorption enhancer, sodium laurate. J Pharm Sci. 2001;90:1456–1465. doi: 10.1002/jps.1097. [DOI] [PubMed] [Google Scholar]