Abstract
Objective:
To define auditory nerve and cochlear functions in two families with autosomal dominant axonal Charcot-Marie-Tooth (CMT).
Methods:
Affected members in two families with different point mutations of NF-L gene were screened with auditory brainstem responses (ABRs). Those with abnormal ABRs were further investigated with clinical, neurophysiological and audiological procedures. The point mutations of NF-L gene involved was Glu397Lys in 8 affected members of the family with AN, and Pro22Ser in 9 affected members of the family without AN.
Results:
ABRs and stapedial muscle reflexes were absent or abnormal in affected members of only one family consistent with auditory neuropathy (AN). In them, audiograms, otoacoustic emissions, and speech comprehension were normal. Absent or abnormal ABRs were, consistent with slowing of conduction along auditory nerve and/or brainstem auditory pathway. Wave I when present was of normal latency.
Conclusion:
Auditory nerve involvement in the presence of normal cochlear outer hair cell activity is asymptomatic in one of two families with CMT disorder with different point mutations of the NF-L gene. The nerve disorder is consistent with altered synchrony and slowed conduction.
Significance:
The absence of “deafness” may reflect the ability of central mechanisms to compensate for the slowly developing auditory nerve abnormalities.
Keywords: hereditary neuropathy, asymptomatic auditory neuropathy, NF-L gene, auditory brainstem responses, pathophysiological mechanisms
1. Introduction
Hearing loss and deafness is an uncommon phenotypic variant in Charcot-Marie-Tooth (CMT) (Satya-Murti et al., 1980; Raglan et al., 1987). Recent studies have focused attention on auditory function in CMT by identifying hearing disorder as a prominent feature in several distinct genetic variants including primary demyelinating forms (CMT1) due to mutation of PMP22 gene duplication (Boerkoel et al., 2002; Kovach et al., 2002; Hattori et al., 2003; Joo et al., 2004), primary axonal forms (CMT2) due to mutations of MPZ gene (Chapon et al., 1999; De Jonghe et al., 1999; Misu et al., 2000; Hattori et al., 2003; Starr et al., 2003), connexin 31 (GJB3) gene (Lopez-Bigas et al., 2001), connexin 32 (Cx32) gene (Boerkoel et al., 2002; Hattori et al., 2003), and in a mixed demyelinating/axonal autosomal recessive motor-sensory neuropathy, HMSN-Lom, particular to Roma populations due to a mutation of the NDRG1 gene (Kalaydjieva et al., 1998; Butinar et al., 1999; Kalaydjieva et al., 2000).
At least three of these studies (Butinar et al., 1999; Kovach et al., 2002; Starr et al., 2003) demonstrated that affected individuals showed normal physiological measures of cochlear outer hair cell activity (otoacoustic emissions, OAEs) but abnormal auditory nerve activity (auditory brain stem responses, ABRs), localizing the hearing loss to dysfunction of the auditory nerve. Pathological examination of the cochlea in one MPZ patient with deafness showed marked loss of both auditory ganglion cells and nerve fibers with normal numbers of inner hair cells and near normal numbers of outer hair cells (Starr et al., 2003). The vestibular nerve was also abnormal. Thus, deafness in CMT can be attributed to an accompanying neuropathy of the auditory nerve sparing the inner hair cells. A similar conclusion was drawn by both Spoendlin (Spoendlin, 1974) and Hallpike et al. (Hallpike et al., 1980) based on cochlear histopathology in subjects with CMT and deafness without the availability of physiological tests now used to define auditory neuropathy (AN).
Criteria for identifying a hearing disorder as due to auditory nerve dysfunction or AN include: 1) absence or profound abnormalities of neural components of the ABRs beyond those encountered for the same degree of hearing loss due to a cochlear sensory disorder; 2) normal outer hair cell activities reflected by OAEs and/or cochlear microphonics; 3) absent acoustic middle ear muscle reflexes; 4) temporal processing disorders affecting speech perception out of proportion to the pure tone hearing loss reflecting altered auditory temporal processes (Starr et al., 1996; Zeng et al., 2005). These same criteria can be found in disorders of auditory nerve fibers accompanying CMT (Butinar et al., 1999) as well as in disorders of inner hair cells and their synapses with auditory nerve accompanying mutations of Otoferlin (Roux et al., 2006; Varga et al., 2006). The analyses of animal models of auditory neuropathy due to particular disorders of inner hair cells, synapses, or auditory nerve may clarify whether there are objective measures that are specific for these different sites of dysfunction (Petit, 2006; Moser et al., 2006). While the benefits of cochlear implantation in auditory neuropathy is clear, there is as yet little evidence these benefits vary according to site of lesion (Shallop et al., 2004; Rouillon et al., 2006).
We have examined auditory and vestibular nerve function in two families with CMT2E disorder due to NF-L point mutations without complaints of hearing impairment. For objective measures of auditory nerve and brainstem functions we used ABRs; for cochlear outer hair cell functions we used OAEs; and for vestibular function we used ENGs. Our study was motivated by a recent account of “deafness” occurring in a single member of a family with an NF-L mutation (Zuchner et al., 2004) but did not define whether the deafness were related to a particular variation in the NF-L gene affecting auditory nerve, or a nonspecific sensorineural hearing loss. The second rationale for the study was based on findings of asymptomatic abnormalities of peripheral nerve function in several forms of CMT (Dubourg et al., 2001; Infante et al., 2001) and cranial neuropathies (Ceranic and Luxon, 2004). We hypothesized that subclinical forms of auditory neuropathy might also be present in other hereditary neuropathies. For instance, abnormalities of auditory brainstem responses without subjective complaints of hearing problems have been described in individuals with Friedreich's ataxia (Satya-Murti et al., 1980), multiple sclerosis (Starr and Achor, 1975; Furst et al., 2000), and various degenerative disorders (Cassandro et al., 1986; Raglan et al., 1987). Asymptomatic vestibular nerve dysfunction can also occur in subjects with auditory neuropathy (Fujikawa and Starr, 2000). And finally, the clinical expression of auditory neuropathy may also occur when individuals are febrile, as in a special variant known as “temperature sensitive auditory neuropathy” (Starr et al., 1988).
We will report on two families with Charcot-Marie-Tooth disease having Type 2 axonal neuropathy without symptoms of hearing impairment. Both families were identified as having dominantly inherited disease genetically localized to 8p21 locus (Georgiou et al., 2002). We found that the disease in one family is associated with the neurofilament light (NF-L) Pro22Ser (Georgiou et al., 2002) mutation and in the other family with the (NF-L) Glu397Lys (Zuchner et al., 2004) mutation. Both families have comparable peripheral nerve involvement but only one family had an asymptomatic involvement of auditory nerve.
Subjects
The family with (NF-L) Glu397Lys with auditory nerve involvement will be designated as “NFL+AN” and the family with (NF-L) Pro22Ser will be designated as “NFL”. All subjects signed informed consent that was approved by the institutional review board for human research studies of the University of Ljubljana.
NFL family has 52 members extending over five generations with 18 having a peripheral neuropathy CMT2 phenotype; nine affected members were tested (Tables 1,2). None of the unaffected members were examined. The disease onset was most common in the first decade. The presenting sign was steppage gait in almost all patients. Symmetrical, slowly progressive distal weakness and wasting of lower limbs were found in all patients; upper limbs were involved in 6 patients. Pes cavus (an exaggeration of the normal arch of the foot) and hammer toes were present in the majority of patients and absent in only 2 patients. Six of the nine affected patients had claw hands. All had mildly reduced sensation that involved touch, pain, vibration, and position sense.
Table 1.
Patients' personal data and neurological findings.
| motor | sensory | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt. No. |
Age | Gen der |
Onset | Initial sympto ms steppage gait |
UL | LL | Reflex | Claw hands |
Pes cavus/ varus |
pain / Touch |
Deep sense |
| NFL family NF-L Pro22Ser | |||||||||||
| III-6 | 70 | F | 0 - 10 | + | + | + | Absent Ankle |
+ | + | + | + |
| III-16 | 63 | F | 10 - 20 | + | + | + | A | + | + | − | + |
| III-18 | 57 | F | 0 - 10 | + | + | + | A | + | + | + | + |
| IV-1 | 48 | F | 0 - 10 | + | + | + | Absent Ankle |
+ | + | + | + |
| IV-4 | 51 | M | 0 - 10 | + | + | + | A | + | + | + | + |
| IV-11 | 42 | F | 0 - 10 | + | − | + | Absent Ankle |
− | − | + | + |
| IV-13 | 33 | F | 0 - 10 | + | + | + | Absent Ankle |
+ | − | + | + |
| V-1 | 23 | M | not done | not done | − | + | facilitation | − | + | − | − |
| V-2 | 25 | F | 0 - 10 | + | − | + | facilitation | − | + | − | + |
| NFL+AN family NF-L Glu397Lys | |||||||||||
| III-1 | 51 | M | 10 - 20 | + | + | + | A | + | + | + | + |
| III-4 | 56 | M | 10 - 20 | + | − | + | A | − | + | + | + |
| IV-1 | 19 | M | 10 - 20 | + | + | + | A | − | + | + | + |
| IV-2 | 26 | M | 10 - 20 | + | + | + | A | − | + | + | + |
| IV-3 | 37 | F | 10 - 20 | + | + | + | A | − | − | + | − |
| IV-4 | 29 | F | 20 - 30 | pain in the legs |
− | + | Absent Ankle |
− | + | − | + |
| IV-5 | 25 | M | 10 - 20 | + | − | + | A | − | + | + | + |
| V-1 | 17 | F | 10 - 20 | + | − | + | Absent Ankle |
− | + | − | + |
F = female; M = male; A = absent all reflexes; + = present; − = absent
Table 2.
Patients' personal data and investigations
| Pt. No. |
Age | Gen der |
PTA R/L |
Speech | Stap edial refle x R/L |
DPOAE R&L |
ABR | CV motor media n nerve m/sec |
CV sensor y median nerve |
MEP | Gap r/l |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NFL family NF-L Pro22Ser | |||||||||||
| III-6 | 70 | F | 25/30 | + | N | 36 | − | 7/5 | |||
| III-16 | 63 | F | 45/40 | − | N | − | − | 3/2 | |||
| III-18 | 57 | F | 20/20 | + | N | 38 | − | 5/9 | |||
| IV-1 | 48 | F | 30/40 | −/+ | N | 41 | − | 3/5 | |||
| IV-4 | 51 | M | 20/20 | − | N | 41 | − | 3/6 | |||
| IV-11 | 42 | F | 12/10 | N | +/+ | + | N | 39 | − | 3/4 | |
| IV-13 | 33 | F | 15/15 | + | N | 54 | − | 5/2 | |||
| V-1 | 23 | M | 10/10 | + | N | 34 | − | 3/5 | |||
| V-2 | 25 | F | + | N | 32 | − | 5/6 | ||||
| NFL+AN family NF-L Glu397Lys | |||||||||||
| III-1 | 51 | M | 13/3 | N | −/− | + | − | 46 | − | A | 6/5 |
| III-4 | 56 | M | 18/31 | A | −/− | + | − | 42 | − | 184/17 6 |
|
| IV-1 | 19 | M | 10/11 | N | −/− | + | − | 39 | − | A | 2/3 |
| IV-2 | 26 | M | 10/10 | N | +/− | + | A* | 46 | − | A | 3/3 |
| IV-3 | 37 | F | 11/11 | A | −/− | + | A* | 37 | − | 5/4 | |
| IV-4 | 29 | F | 10/10 | N | −/− | + | A | 46 | − | 5/6 | |
| IV-5 | 25 | M | 10/10 | N | +/+ | + | A* | 51 | − | 5/3 | |
| V-1 | 17 | F | 10/10 | N | +/+ | + | A | 34 | − | 3/5 | |
F = female; M = male; A = abnormal; + = present; − = absent; blank = not done; A*= abnormal and central auditory pathway lesion; PTA= pure tone audiogram; DPOAE=distortion product otoacoustic emissions; ABR= auditory brainstem response; CV= conduction velocity; MEP= motor evoked potentials
NFL+AN family had 17 members extending over five generations with 12 affected with the CMT2 phenotype. Eight affected members were examined in detail (Tables 1, 2). None of the unaffected members were tested. The disease onset was mainly in the second decade of life. The presenting symptom was steppage gait in almost all patients; an exception was one patient with painful feet (IV-4). Symmetrical, slowly progressive distal weakness and wasting of lower limbs were found in all patients; upper limbs were involved in 4 patients. Pes cavus and hammer toes were present in the majority of patients and absent in only one patient. Claw hands were found in only 1 member. All reflexes were absent in six members and only ankle reflexes were absent in two members of this family. Five had mildly impaired sensation that involved touch, pain, vibration, and position sense, two had diminished sensation for vibration and position senses and one had only touch and pain sensation diminished. Palpably enlarged nerves (ulnar at the elbow or peroneal at the ankle) were not noted. Also absent were scoliosis, limb ataxia, vocal cord paralysis, and respiratory affection. There were signs of upper motor neuron impairment in three affected members (III-1, IV-1, IV-2); only one (III-1) had clinical signs of corticospinal tract involvement (increased muscle tone, brisk patellar jerks and spastic gait). The other two patients were found abnormal on transcranial magnetic stimulation (TMS) testing. Other family members refused the TMS test.
Methods
We identified members of NFL family who were affected with a peripheral neuropathy and identified by nerve conduction studies performed under standardized conditions (Stålberg and Falck, 1993). The measured results were compared with expected values and were considered normal when their Z-scores (the measured value according to the age and height minus the expected value divided by the standard deviation) were less than 2 (Stålberg and Falck, 1993). Motor studies of median, ulnar, peroneal and tibial nerves were measured and sensory studies of the median, ulnar, and sural nerves were accomplished antidromically. The subjects with peripheral neuropathy were then assessed by ABRs.
Auditory brainstem responses (ABRs)
Brainstem auditory evoked potentials were recorded from scalp electrodes (ipsilateral earlobe – vertex Cz) to alternating click stimuli presented from TDH-39P earphones at (10/sec) rate at 100 dB nHL. Masking of the opposite ear with white noise was used at the stimulus intensity 70 dB nHL. The grand average of three separate sets of 2,000 trials was made. Measurements were made from the grand average for each ear of the peak latency of the neural components and their inter-peak latency and compared with the values from 23 normal hearing subjects (12 females and 11 males) in our laboratory. Abnormalities of ABRs were defined by standard clinical procedures (Pratt et al., 1999). Those individuals with abnormal ABRs were subjected to additional tests as follows.
Clinical audiology
Hearing functions were measured monaurally in a sound attenuating booth using TDH-39P earphones. We tested: (1) pure tone audiometry (250–8,000 Hz) by air and bone conduction in eight members of NFL+AN, (2) speech comprehension to phonetically balanced words at threshold and at increasing intensities. Normal hearing subjects in our laboratory identify 100% of words by 30 dB HL, hearing level; (3) tympanograms; (4) ipsilateral acoustic middle ear muscle reflexes were tested to pure tones at 0.5, 1, 2, 4 kHz at intensities up to 100 dB HL; abnormality was defined by absence of reflex response; (5) distortion product otoacoustic emissions (DPOAEs) were measured with Grason-Stadler GSI 60; the stimulus levels used were 65 and 55 dB SPL; (6) psychoacoustic measure of temporal processes using the threshold for detecting brief silent periods in noise (gap detection) (Zeng et al., 1999). We used a web based protocol on a portable computer (www.bsos.umd.edu/hesp/zeng/gapdetection) with TDH-39P to test subjects binaurally in a quiet room in their homes. Three white noise stimuli of the same duration, one of which had a gap, were consecutively presented to the patient and were indicated by three filled circles on the computer screen. The patient had to identify which noise burst was “different”. If the correct noise stimulus were chosen, the gap became shortened till threshold was identified. Gap detection thresholds for subjects, naïve in performing psychoacoustic tests, are less than 7 ms.
Vestibular tests
Caloric stimulation with warm and cold water was used to obtain oculovestibular reflexes, while recording extra ocular movements.
Pyramidal tract conduction studies
Conduction studies were performed under standardized conditions in three affected members, one of whom had clinical signs of pyramidal tract involvement (Chang and Lien, 1991). Pyramidal tract central motor latencies were determined by subtracting latencies of the compound muscle action potentials (abductor digiti quinti or tibialis anterior muscles) to magnetic stimulation over the neck at the C7 level or over the back at the T12 level from latencies of the compound muscle potentials after transcranial magnetic stimulation of motor cortex (central motor latencies). Stimulus intensities were adjusted individually to obtain motor responses of either moderate (stimulation in the back) or maximal amplitudes (stimulation at the vertex).
Data analyses
We compared quantified data of the ABRs from 8 NFL+AN members (16 ears) and 9 NFL members (18 ears). Statistical measures of significant differences in ABRs as a function of family (NFL+AN vs. NFL) for the incidence of detecting components, their absolute latencies, and the intercomponent intervals were made using the test of proportions and Mann-Whitney U test. The values were compared to values obtained in the same way from normal subjects. Also Spearman's correlation was used in order to compare the I–II and III–V intercomponent interval with conduction velocities of median, ulnar, peroneal and tibial nerve (Table 3). There were insufficient numbers of family members tested for central motor latencies to examine for significant correlations with the I–III or III–V intercomponent interval.
Table 3.
Auditory Brainstem Potentials (ABRs): Incidence of detection, absolute latencies, and intercomponent times with statistical difference between the groups.
| n | Waves | ||||
|---|---|---|---|---|---|
| Incidence | I | II | III | IV-V | |
| NFL | 18 | 17 | 15 | 17 | 18 |
| NFL+AN | 16 | 6 | 5 | 10 | 12 |
| Difference between two | <.001 | <.001 | <.001 | <.001 | |
| proportions (p) | |||||
| Normal values | 23 | 23 | 23 | 23 | 23 |
| Latencies (ms) | |||||
| NFL | 18 | 1.65±.15 | 2.8±.16 | 3.84±.20 | 5.66±.21 |
| NFL+AN | 16 | 1.83±.16 | 3.3±.08 | 4.41±.17 | 6.67±.30 |
| Mann Whitney U test (p) | ns | <.001 | <.001 | <.001 | |
| Normal values – female | 12 | 1.61±.12 | 2.72±.12 | 3.72±.12 | 5.60±.20 |
| - male | 11 | 1.64±.13 | 2.84±.15 | 3.84±.17 | 5.79±.25 |
| Intercomponent intervals (ms) | I-II | II-III | III-V | I-V | |
| NFL | 14 | 1.15±.14 | 1.04±.13 | 1.82±.16 | 4.01±.12 |
| NFL+AN | 6 | 1.51±.14 | 1.1±.20 | 2.24±.25 | 4.76±.21 |
| Mann Whitney U test (p) | <.001 | ns | <.001 | <.001 | |
| Normal values – female | 12 | 1.11±.11 | 1.00±.08 | 1.88±.12 | 3.99±.15 |
| - male | 11 | 1.20±14 | 1.00±.09 | 1.15±.15 | 4.12±.25 |
n = Number of ABR averages; ns = non significant
3. Results
Symptoms of auditory and vestibular dysfunctions
There were two members of the NFL+AN family with symptoms of auditory or vestibular disorders: one (IV-2) noted difficulties understanding speech using the telephone and in noise, the other (IV-3) had occasional dizziness.
Auditory brain stem responses: (ABRs)
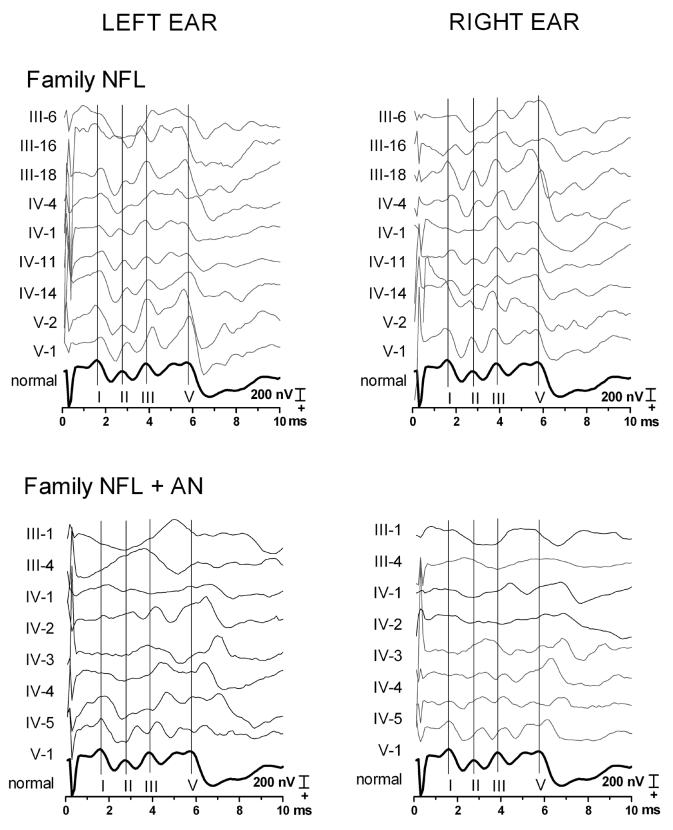
Individual ABRs waveforms from both families to monaural stimulation of each ear are shown in figure 1 along with the averaged ABRs from a group of normal hearing young adults (dark black trace). ABR components were easily identified and of normal latencies in all members of NFL family. In contrast, ABR components in NFL+AN family were either undetectable (Table 3 for details) or significantly delayed in latency compared to both normals and NFL family (p < .001). Importantly, when wave I was present, its latency did not differ significantly from both the controls and the NFL family (Table 3) consistent with a selective preservation of function of distal portions of auditory nerve early in the disorder.
Figure 1.
Auditory brainstem responses (ABRs) recorded earlobe (active) to vertex (reference) for NFL family (upper panels) and NFL+AN (lower panels); to left ear stimulation (left); to right ear stimulation (right). Cochlear microphonics were cancelled by combining the averages to the condensation and rarefaction stimuli. The vertex positive components are plotted upwards. Vertical lines have been placed at the peaks of the normal ABR wave I, and V, plotted in bold below. The waves I, II, III, and IV/V were labeled with roman numerals. Measures of incidence, of latencies and of intercomponent intervals are in Table 3. NFL family members are numbered by Roman numerals (III-V) referring to their generation and by Arabic numbers referring to their position in that generation. The NFL family has normal latency of ABRs except for III-6 on the left side contaminated by muscle artifact. NFL+AN members in generation III and IV show absent ABRs or presence of only delayed wave V. In contrast members of the youngest generation (V) show normal latency wave I but delays of subsequent waves (II to V). Details of the measures can be found in Table 3.
Measures of intercomponent intervals (Table 3) were normal in NFL family members but abnormal in NFL+AN. We measured the III–V interval bilaterally in five subjects of the NFL+AN family with these components as a function of the I–II interval and compared the measures with those from the NFL family. Results of Mann Whitney test show that the NFLAN family had significant increases of both I–II (p=0.0026) and III–V (p<0.0001) intervals compared to the NFL alone family.
One of the two NFL+AN family members with a central slowing of conduction also had evidence of slowing of central pyramidal tracts (IV-1).
Clinical Auditory tests
Audiograms
Audiograms in NFL +AN were normal except in three subjects showing a mild 30 dB elevation of threshold at high frequencies (6 and 8 kHz). Otoacoustic emissions: In NFL+AN, DPOAEs were present from 0.5 to 5 kHz in the NFL-AN family except in the two oldest members whose DPOAEs were absent at 4 and 5 kHz
Speech identification scores
Scores were normal in all members of NFL+AN.
Gap detection
Thresholds were within normal limits (< 7 ms) in all. We could not obtain a threshold for one individual with right sided cerebral infarct and could not ascertain if the task demands or specifics of the acoustic percept were the cause Acoustic middle ear muscle reflexes: Middle ear muscle reflexes were present bilaterally in three (IV-4, IV-5, V-1), absent bilaterally in 3 (III-1, III-4, IV-3), and absent unilaterally in 2 (IV-1, IV-2). Tympanograms were normal in all.
Electronystagmography – ENG
Tests results were made in four family members and were normal.
Peripheral nerve and motor evoked potential studies
Nerve conduction studies demonstrated mixed axonal and demyelinating involvement. Mean values of NFL and NFL+AN for motor nerves were: 39/44 m/s for the median, 40/44 m/s for the ulnar, 32/28 m/s for the peroneal and 33/31 m/s for the tibial nerves. Of 36 motor conduction velocities measured in NFL family, 25 were abnormally slowed, 6 were unobtainable (no compound muscle action potentials – CMAPs), and only 5 were normal. The corresponding numbers for NFL+AN for 32 motor conduction velocities were 23, 9 and 1. The sensory action potentials of ulnar, median, and sural nerves were absent in all patients of both families. The Spearman correlation between III–V conduction times of ABRs with peripheral nerve conduction times of the median nerve was significant for the NFL+AN family r=0,69 and p=0,03 whereas the correlation were not significant for the NFL family. There were no significant correlations defined for either family for ulnar, peroneal and tibial nerve conduction times.
Motor evoked potentials (MEP) were studied in three subjects of NFL+AN. MEP and compound motor action potentials from stimulation of the nerve roots over the back could not be elicited in patient III-1 with clinical signs of the pyramidal tract affection because of severe wasting of both hand and tibialis anterior muscles. Two other subjects (IV-1 and IV-2) were not clinically spastic but had mildly prolonged abductor digiti quinti muscle central motor latencies (patient IV-1 11.8 and 12.0 ms and patient IV-2 13.0 and 9.9 ms on the right and left side, the upper normal value 11.0 ms), while MEPs from their tibialis anterior muscles could not be elicited. Of note is that peripheral nerve and back stimulation revealed normal CMAP amplitudes in hand and leg muscles of both patients localizing the deficit to the central pyramidal tract.
Genetic studies
Members of both families were genotyped at marker loci spanning all known CMT1 and CMT2 loci. Linkage and haplotype analyses supported linkage of the mutation on the CMT2E locus and sequence analysis of the NFL gene revealed the corresponding mutations, as described by Georgiou et al. (2002) and Zuchner et al. (2004).
4. Discussion
The results of this study showed that a family with NFL+AN mutation had many of the criteria of auditory neuropathy (abnormalities of ABRs, absent stapedius reflexes) but were without symptoms of an auditory temporal processing disorder (normal threshold of gap detection and normal speech comprehension). Since the ABR reflects activity of a limited portion of the auditory pathway, the lack of correspondence between ABR measures and perceptual functions of “hearing” can be expected. We studied two Slovenian families with an axonal autosomal dominant form of Charcot-Marie-Tooth disease caused by mutations in the NF-L gene. CMT2 in the family without AN is caused by the Pro22Ser (Georgiou et al., 2002) mutation and in NFL+AN family by the Glu397Lys (Zuchner et al., 2004) mutation. The genetic defects in these two families, occurring in the same gene, have different effects on auditory nerve, auditory brainstem, and central motor pathways. Different point mutations occur commonly for many genes (e.g., the MPZ gene) and are accompanied by different phenotypic variants of the disorder. The clinical features of the disease in the two families of the present report were similar but with some distinctive features. Both exhibited slowly progressive muscular atrophy and mild sensory loss in distal limbs with members of NFL being generally affected earlier and less severely than members of NFL+AN. Nerve conduction abnormalities were similar in both families. Only some of the affected members of NFL+AN showed clinical or electrophysiological signs of pyramidal tract involvement.
The clinical heterogeneity between these two families was especially revealed by auditory nerve function tests that were impaired only in affected members of NFL+AN and were consistent with the clinical diagnosis of auditory neuropathy. The ABR abnormalities in NFL+AN are consistent with a disorder affecting both the ganglion cells and auditory axons that is progressive with age. The ABR abnormalities in NFL+AN are consistent with a disorder affecting both the ganglion cells and auditory axons that is progressive with age. ABRs in young family members showed slowing of conduction of auditory nerve manifested as a lengthening of conduction time between wave I (generated distally in auditory nerve) and wave II (generated proximally in auditory nerve). In older family members there was a loss of waves I and II and in the oldest family members waves III, IV and V were also lost (Table 3). Three NFL+AN members also had slowing of conduction in the central auditory pathway – suggesting an additional brainstem auditory pathway involvement. Acoustic middle ear reflexes were absent in all but the youngest affected members who had preserved waves I. In contrast to these abnormalities of objective measures of auditory temporal processing, subjective measures of gap detection threshold and speech comprehension in quiet were preserved.
Subjects in NFL+AN had normal audiological evaluations (otoacoustic emissions), audiograms, speech comprehension, and threshold for detecting brief silent intervals in noise (gap detection). The mild high frequency hearing loss in both families is common in the general population (Robinson, 1988) and would not account for the abnormality of ABRs. The loss of middle ear muscle reflexes in five members of NFL+AN is typical of AN (Starr et al., 1996). We also did not find evidence of vestibular disorders in either family using a limited clinical caloric test suggesting that vestibular nerve is not affected in contrast to certain other types of CMT diseases (Butinar et al., 1999; Starr et al., 2003).
The pathophysiology of auditory neuropathy accompanying various forms of CMT has been proposed to be secondary to demyelination affecting synchrony of discharge and/or to axonal loss affecting the number of axons that can fire synchronously (Starr et al., 2001). The analysis of hearing in specific forms of CMT may help identify specific underlying pathophysiological mechanisms associated with symptoms of auditory neuropathy. CMT is classified into two major types by electrophysiological and histopathological criteria. The primary demyelinating or CMT1 type is characterized by slow nerve conduction velocities (NCV) with relative preservation of the number of axons (Dyck and Lambert, 1968). The axonal or CMT2 type is characterized by fiber loss but normal or slightly reduced NCVs (Dyck and Lambert, 1968). The terms demyelinating or axonal, as used to characterize the underlying pathology of peripheral neuropathies, do not accurately reflect the changing features of the disorders. Some of the pathological processes (e.g. dysimmune state in acute inflammatory postinfectious polyneuropathy – AIDP) may simultaneously initiate both demyelination and axonal loss. Moreover, the integrity of both the myelin and axons are interdependent and clinical expression of CMT1A, where the genetic mutation impairs PMP22 function through a “dosage effect” (the alteration of a phenotype by an increased dosage, or amount, of the product of the gene), occurs only after the pathogenic processes affect also axons (Krajewski et al., 2000). It may also be important to note that different mutations in same genes (e.g. NF-L, MPZ, Cx32) may cause features of either demyelinative or axonal type of polyneuropathy (Senderek et al., 2000; Hattori et al., 2003; Zuchner et al., 2004). Even the demyelination, that has been considered “secondary ” to axonal loss in different degenerative and metabolic axonal disorders is probably an essential feature of the pathology (Dyck, 1975). In our patients both sensory (sural and median nerves) and motor nerve studies was consistent with axonal loss. There was absence of sensory action potentials, low amplitude of M waves (the whole muscle compound muscle action potential), and borderline motor nerve conduction velocities (>38 m/s for median nerves). Hattori and coworkers (2003) in studies of CMT families with deafness (PMP22, MPZ, Cx32) found electrophysiological and anatomical evidence for the presence of both demyelinating and axonal features.
The perceptual consequences of auditory nerve dysfunction affect primarily temporal processes such as gap detection and speech comprehension. These deficits are present in AN independent of their etiologies (Zeng et al., 2004). Both demyelination and axonal disorders have several features that affect synchrony of discharge and the number of fibers that are active. Demyelination may result in the reduction of conduction velocity causing increased temporal dispersion, reduced ability to transmit trains of impulses, spontaneous generation of action potentials, and in axonal loss (Brown and Watson, 2002). Axonal lesion may also result in slow conduction velocities particularly pronounced at the nerve terminal and increased temporal dispersion (regenerating and dying axons, secondary demyelination, loss of fastest fibers), in block of conduction, but primarily in the reduced number of axons (characteristic is preferential involvement of certain types of axons), and in ectopic impulse generation (Brown and Watson, 2002). Both types of pathology in auditory nerve would interfere with auditory nerve functions in rather similar manner.
The two families of this report demonstrate that there can be profound differences in auditory nerve function when the peripheral nerve disorder is quite similar. The NFL family had normal auditory nerve functions whereas the NFL+AN family had physiological evidence (ABRs, middle ear muscle reflexes) of auditory neuropathy. Signs of the axonal loss in peripheral nerves (e.g. reduction in the compound muscle action potential amplitude) are usually accompanied clinically by muscle weakness. It may be that the disorder of the auditory nerve in our patients is sufficiently “mild” to be asymptomatic due perhaps to the slow evolution of nerve changes that may allow central processes to compensate for the altered auditory input. Physiological recordings of auditory nerve, brainstem, and cortical responses to sound provide quantitative measures of brain functions contributing to “hearing” and may provide clues as to the site(s) of auditory impairments without necessarily being objective measures of “hearing”.
Acknowledgements
We acknowledge and thank Assoc. Prof. Jagoda Vatovec, MD, PhD, from the Clinic for Otorhinolaryngology and Cervicofacial Surgery, University Medical Center, Ljubljana, who performed the auditory function tests, Assist. Prof. Marko Korošec, MD PhD, who collected the clinical data, Ignac Zidar-Nacek and Miloš Kogej from Institute of Clinical Neurophysiology who made many of the illustrations and tables, and electronic version of the manuscript, respectively. This work was supported by grants #DC-02168 from the National Institutes of Health (Starr) and #3311-01-838072 from the Slovenian Ministry of Education, Science and Sport (Butinar) and by a grant from the Muscular Dystrophy Association of the United States (Christodoulou).
Sources of support: DC-02168 from the National Institutes of Health (Starr), 3311-01-838072, Slovenian Ministry of Education, Science and Sport (Butinar), and Muscular Dystrophy Association of the United States (Christodoulou).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boerkoel CF, Takashima H, Garcia CA, Olney RK, Johnson J, Berry K, et al. Charcot-Marie-Tooth disease and related neuropathies: mutation distribution and genotype-phenotype correlation. Ann Neurol. 2002;51(2):190–201. doi: 10.1002/ana.10089. [DOI] [PubMed] [Google Scholar]
- Brown WF, Watson BV. Pathophysiology of conduction in peripheral neuropathies. In: Brown WF, Bolton CF, Aminoff MJ, editors. Neuromuscular function and disease basic, clinical, and electrodiagnostic aspects. W B Saunders Company; Philadelphia: 2002. pp. 56–95. [Google Scholar]
- Butinar D, Zidar J, Leonardis L, Popovic M, Kalaydjieva L, Angelicheva D, et al. Hereditary auditory, vestibular, motor, and sensory neuropathy in a Slovenian Roma (Gypsy) kindred. Ann Neurol. 1999;46:3644. doi: 10.1002/1531-8249(199907)46:1<36::aid-ana7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Cassandro E, Mosca F, Sequino L, De Falco FA, Campanella G. Otoneurological findings in Friedreich's ataxia and other inherited neuropathies. Audiology. 1986;25:84–91. doi: 10.3109/00206098609078373. [DOI] [PubMed] [Google Scholar]
- Ceranic B, Luxon LM. Progressive auditory neuropathy in patients with Leber's hereditary optic neuropathy. J Neurol Neurosurg Psychiatry. 2004;75:626–30. doi: 10.1136/jnnp.2003.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CW, Lien IN. Estimate for motor conduction in human spinal cord: slowed conduction in spinal cord injury. Muscle Nerve. 1991;14:990–6. doi: 10.1002/mus.880141010. [DOI] [PubMed] [Google Scholar]
- Chapon F, Latour P, Diraison P, Schaeffer S, Vandenberghe A. Axonal phenotype of Charcot-Marie-Tooth disease associated with a mutation in the myelin protein zero gene. J Neurol Neurosurg Psychiatry. 1999;66:779–82. doi: 10.1136/jnnp.66.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jonghe P, Timmerman V, Ceuterick C, Nelis E, De Vriendt E, Lofgren A, et al. The Thr124Met mutation in the peripheral myelin protein zero (MPZ) gene is associated with a clinically distinct Charcot-Marie-Tooth phenotype. Brain. 1999;122:281–90. doi: 10.1093/brain/122.2.281. [DOI] [PubMed] [Google Scholar]
- Dubourg O, Tardieu S, Birouk N, Gouider R, Leger JM, Maisonobe T, et al. Clinical, electrophysiological and molecular genetic characteristics of 93 patients with X-linked Charcot-Marie-Tooth disease. Brain. 2001;124:1958–67. doi: 10.1093/brain/124.10.1958. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Lambert EH. Lower motor and primary sensory neuron diseases with peroneal muscular atrophy. I. Neurologic, genetic, and electrophysiologic findings in hereditary polyneuropathies. Arch Neurol. 1968;18:603–18. doi: 10.1001/archneur.1968.00470360025002. [DOI] [PubMed] [Google Scholar]
- Dyck PJ. Pathological alterations of the peripheral nervous system of man. In: Dyck PJ, Thomas PK, Lambert EH, editors. Peripheral neuropathy. W B Saunders; Philadelphia: 1975. pp. 296–336. [Google Scholar]
- Fujikawa S, Starr A. Vestibular neuropathy accompanying auditory and peripheral neuropathies. Arch Otolaryngol Head Neck Surg. 2000;126:1453–6. doi: 10.1001/archotol.126.12.1453. [DOI] [PubMed] [Google Scholar]
- Furst M, Aharonson V, Levine RA, Fullerton BC, Tadmor R, Pratt H, et al. Sound lateralization and interaural discrimination. Effects of brainstem infarcts and multiple sclerosis lesions. Hear Res. 2000;143:29–42. doi: 10.1016/s0378-5955(00)00019-8. [DOI] [PubMed] [Google Scholar]
- Georgiou DM, Zidar J, Korosec M, Middleton LT, Kyriakides T, Christodoulou K. A novel NF-L mutation Pro22Ser is associated with CMT2 in a large Slovenian family. Neurogenetics. 2002;4:93–6. doi: 10.1007/s10048-002-0138-4. [DOI] [PubMed] [Google Scholar]
- Hallpike CS, Harriman DG, Wells CE. A case of afferent neuropathy and deafness. J Laryngol Otol. 1980;94:945–64. doi: 10.1017/s0022215100089696. [DOI] [PubMed] [Google Scholar]
- Hattori N, Yamamoto M, Yoshihara T, Koike H, Nakagawa M, Yoshikawa H, et al. Demyelinating and axonal features of Charcot-Marie-Tooth disease with mutations of myelin-related proteins (PMP22, MPZ and Cx32): a clinicopathological study of 205 Japanese patients. Brain. 2003;126:134–51. doi: 10.1093/brain/awg012. [DOI] [PubMed] [Google Scholar]
- Infante J, Garcia A, Combarros O, Mateo JI, Berciano J, Sedano MJ, et al. Diagnostic strategy for familial and sporadic cases of neuropathy associated with 17p11.2 deletion. Muscle Nerve. 2001;24:114955. doi: 10.1002/mus.1126. [DOI] [PubMed] [Google Scholar]
- Joo IS, Ki CS, Joo SY, Huh K, Kim JW. A novel point mutation in PMP22 gene associated with a familial case of Charcot-Marie-Tooth disease type 1A with sensorineural deafness. Neuromuscul Disord. 2004;14:325–8. doi: 10.1016/j.nmd.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Kalaydjieva L, Gresham D, Gooding R, Heather L, Baas F, de Jonge R, et al. N-myc downstream-regulated gene 1 is mutated in hereditary motor and sensory neuropathy-Lom. Am J Hum Genet. 2000;67:47–58. doi: 10.1086/302978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaydjieva L, Nikolova A, Turnev I, Petrova J, Hristova A, Ishpekova B, et al. Hereditary motor and sensory neuropathy – Lom, a novel demyelinating neuropathy associated with deafness in gypsies. Clinical, electrophysiological and nerve biopsy findings. Brain. 1998;121:399–408. doi: 10.1093/brain/121.3.399. [DOI] [PubMed] [Google Scholar]
- Kovach MJ, Campbell KC, Herman K, Waggoner B, Gelber D, Hughes LF, et al. Anticipation in a unique family with Charcot-Marie-Tooth syndrome and deafness: delineation of the clinical features and review of the literature. Am J Med Genet. 2002;108:295–303. doi: 10.1002/ajmg.10223. [DOI] [PubMed] [Google Scholar]
- Krajewski KM, Lewis RA, Fuerst DR, Turansky C, Hinderer SR, Garbern J, et al. Neurological dysfunction and axonal degeneration in Charcot-Marie-Tooth disease type 1 A. Brain. 2000;123:1516–27. doi: 10.1093/brain/123.7.1516. [DOI] [PubMed] [Google Scholar]
- Lopez-Bigas N, Olive M, Rabionet R, Ben-David O, Martinez-Matos JA, Bravo O, et al. Connexin 31 (GJB3) is expressed in the peripheral and auditory nerves and causes neuropathy and hearing impairment. Hum Mol Genet. 2001;10:947–52. doi: 10.1093/hmg/10.9.947. [DOI] [PubMed] [Google Scholar]
- Misu K, Yoshihara T, Shikama Y, Awaki E, Yamamoto M, Hattori N, et al. An axonal form of Charcot-Marie-Tooth disease showing distinctive features in association with mutations in the peripheral myelin protein zero gene (Thr124Met or Asp75Va1) J Neurol Neurosurg Psychiatry. 2000;69:806–11. doi: 10.1136/jnnp.69.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T, Strenzke N, Meyer A, Lesinski-Schiedat A, Lenarz T, Beutner D, et al. Diagnosis and therapy of auditory synoptopathy/neuropathy. HNO. 2006;54:833–9. doi: 10.1007/s00106-006-1450-3. [DOI] [PubMed] [Google Scholar]
- Petit C. From deafness genes to hearing mechanisms: harmony and counterpoint. Trends Mol Med. 2006;12:57–64. doi: 10.1016/j.molmed.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Pratt H, Aminoff M, Nuwer R, Starr A. Short latency auditory evoked potentials. Electroenceph. Clin. Neurophysiol. 1999;(Suppl 52):69–78. [PubMed] [Google Scholar]
- Raglan E, Prasher DK, Trinder E, Rudge P. Auditory function in hereditary motor and sensory neuropathy (Charcot-Marie-Tooth disease) Acta Otolaryngol. 1987;103:505. doi: 10.3109/00016488709134697. [DOI] [PubMed] [Google Scholar]
- Robinson DW. Threshold of hearing as a function of age and sex for the typical unscreened population. Br J Audiol. 1988;22:5–20. doi: 10.3109/03005368809077793. [DOI] [PubMed] [Google Scholar]
- Roux I, Safieddine S, Nouvian R, Grati M, Simmler MC, Bahloul A, et al. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127:277–89. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Rouillon I, Marcolla A, Roux I, Marlin S, Feldmann D, Couderc R, et al. Results of cochlear implantation in two children with mutations in the OTOF gene. Int J Pediatr Otorhinolaryngol. 2006;70:689–96. doi: 10.1016/j.ijporl.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Satya-Murti S, Cacace A, Hanson P. Auditory dysfunction in Friedreich ataxia: result of spiral ganglion degeneration. Neurology. 1980;30:1047–53. doi: 10.1212/wnl.30.10.1047. [DOI] [PubMed] [Google Scholar]
- Senderek J, Hermanns B, Lehmann U, Bergmann C, Marx G, Kabus C, et al. Charcot-Marie-Tooth neuropathy type 2 and PO point mutations: two novel amino acid substitutions (Asp61Gly; Tyr119Cys) and a possible “hotspot” on Thr124Met. Brain Pathol. 2000;10:235–48. doi: 10.1111/j.1750-3639.2000.tb00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallop JK, Jin SH, Driscoll CL, Tibesar RJ. Characteristics of electrically evoked potentials in patients with auditory neuropathy/auditory dys-synchrony. Int J Audiol. 2004;43(Suppl 1):22–7. [PubMed] [Google Scholar]
- Spoendlin H. Optic cochleovestibular degenerations in hereditary ataxias. II. Temporal bone pathology in two cases of Friedreich's ataxia with vestibulo-cochlear disorders. Brain. 1974;97:41–8. doi: 10.1093/brain/97.1.41. [DOI] [PubMed] [Google Scholar]
- Stålberg E, Falck B. Clinical motor nerve conduction studies. Methods in Clinical Neurophysiology. 1993:61–80. [Google Scholar]
- Starr A, Achor J. Auditory brain stem responses in neurological disease. Arch Neurol. 1975;32:761–8. doi: 10.1001/archneur.1975.00490530083009. [DOI] [PubMed] [Google Scholar]
- Starr A, Michalewski HJ, Zeng FG, Fujikawa-Brooks S, Linthicum F, Kim CS, et al. Pathology and physiology of auditory neuropathy with a novel mutation in the MPZ gene (Tyr145->Ser) Brain. 2003;126:1604–19. doi: 10.1093/brain/awg156. [DOI] [PubMed] [Google Scholar]
- Starr A, Picton T, Kim R. Pathophysiology of auditory neuropathy. In: Sininger Y, Starr A, editors. Auditory neuropathy a new perspective on hearing disorders. Singular – Thomson Learning; San Diego: 2001. pp. 76–82. [Google Scholar]
- Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain. 1996;119:741–53. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- Starr A, Sininger Y, Winter M, Derebery MJ, Oba S, Michalewski HJ. Transient deafness due to temperature-sensitive auditory neuropathy. Ear Hear. 1998;19:169–79. doi: 10.1097/00003446-199806000-00001. [DOI] [PubMed] [Google Scholar]
- Varga R, Avenarius MR, Kelley PM, Keats BJ, Berlin CI, Hood LJ, et al. OTOF mutations revealed by genetic analysis of hearing loss families including a potential temperature sensitive auditory neuropathy allele. J Med Genet. 2006;43:576–81. doi: 10.1136/jmg.2005.038612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng FG, Oba S, Garde S, Sininger Y, Starr A. Temporal and speech processing deficits in auditory neuropathy. Neuroreport. 1999;10:3429–35. doi: 10.1097/00001756-199911080-00031. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Nie K, Liu S, Stickney G, Del Rio E, Kong YY, et al. On the dichotomy in auditory perception between temporal envelope and fine structure cues. J Acoust Soc Am. 2004;116:1351–4. doi: 10.1121/1.1777938. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Kong YY, Michalewski HJ, Starr A. Perceptual consequences of disrupted auditory nerve activity. J Neurophysiol. 2005;93:3050–63. doi: 10.1152/jn.00985.2004. [DOI] [PubMed] [Google Scholar]
- Zuchner S, Vorgerd M, Sindern E, Schroder JM. The novel neurofilament light (NEFL) mutation G1u397Lys is associated with a clinically and morphologically heterogeneous type of Charcot-Marie-Tooth neuropathy. Neuromuscul Disord. 2004;14(2):147–157. doi: 10.1016/j.nmd.2003.10.003. [DOI] [PubMed] [Google Scholar]