Abstract
Reduced cerebrospinal fluid (CSF) histamine levels were found in human hypersomnia. To evaluate the functional significance of changes in CSF histamine levels, we measured the levels in rats across 24 h, after the administration of wake-promoting compounds modafinil, amphetamine, and thioperamide, and after sleep deprivation and food deprivation.
Thioperamide significantly increased CSF histamine levels with little effects on locomotor activation. Both modafinil and amphetamine markedly increased the locomotor activity, but had no effects on histamine. The levels are high during active period and are markedly elevated by sleep deprivation, but not by food deprivation.
Our study suggests that CSF histamine levels in rats reflect the central histamine neurotransmission and vigilance state changes, providing deeper insight into the human data.
Keywords: cerebrospinal fluid histamine, modafinil, amphetamine, thioperamide, sleep deprivation, food deprivation
Introduction
Recent animal and human studies suggested that impaired histaminergic neurotransmission is involved in narcolepsy and other hypersomnias [10, 14]. Histamine is one of the wake-active amines. Histaminergic neurons are located exclusively in the tuberomammillary nucleus (TMN) in the posterior hypothalamus and project to various brain regions involved in the regulation of the sleep-wake cycle, such as the cerebral cortex, thalamus, anterior hypothalamus and brainstem cholinergic and monoaminergic structures. Together with other wake-active amines, such as norepinephrine, histaminergic neurons in the TMN fire slowly and regularly most during waking state, less during slow wave sleep, and cease their firing during rapid eye movement (REM) sleep [20]. The histaminergic neurotransmission plays a pivotal role in cortical activation through their direct projection into the cortex and excitatory interactions with the cholinergic neurons.
The histaminergic system also interacts with other hypothalamic wake and sleep regulatory systems; hypocretin is an important wake-promoting neuropeptide involved in the pathophysiology of narcolepsy, and hypocretin neurons project to and excite the TMN histamine neurons [3]. Cessation of TMN histamine neuron activity is mediated by inhibitory GABAergic inputs from the ventrolateral preoptic area (VLPO) [18]. Non-neuronal histamine also exists in the brain, mostly produced by the neuronal mast cells [17].
Four types (H1-4) of histamine receptor have been identified, and H1-3 receptors are expressed in the central nervous system (CNS) [1]. The H1 receptor is Gq/11 coupled, the H2 receptor is Gs coupled. Both have widespread distributions in the brain, are located postsynaptically, and mainly mediate excitatory neurotransmission. Although H1 and H2 receptors are also expressed peripherally, H3 receptors are expressed mainly in the brain. H3 receptors are Gi/o coupled, predominantly located presynaptically, and they act as an autoreceptor to inhibit the release of histamine [1]. Consistent with wake-promoting roles of histamine, pharmacological experiments had demonstrated that H1 antagonists induce sleep while H3 antagonists enhance wakefulness [1].
Histamine is also involved in food intake; increase in histaminergic neurotransmission suppresses feeding, whereas decrease of histaminergic neurotransmission increases food intake [1].
Involvement of the histaminergic system in sleep disorders was first demonstrated in the canine model of narcolepsy; histamine contents in the cortex and thalamus in narcoleptic dogs were significantly reduced [10]. Subsequent human studies of histamine measures in the lumbar cerebrospinal fluid (CSF) had shown that reduced histamine levels are not limited to hypocretin-deficient narcolepsy; the levels are also reduced in hypocretin non-deficient narcolepsy and idiopathic hypersomnia [8, 12]. Thus, CSF histamine may be a new biomarker for hypersomnias of central origin. However, the origin of histamine in the CSF and the functional significance of changes in CSF histamine levels in various physiological conditions remain elusive. This information is critical to interpret the human results.
In order to evaluate the functional significance of CSF histamine levels, we measured CSF histamine levels in rats across 24 h, after the administration of wake-promoting compounds modafinil, amphetamine, and histamine H3 receptor antagonist thioperamide, as well as after 6 h sleep deprivation and 48 h food deprivation. In order to compare CSF histamine and hypocretin-1 levels, we also measured hypocretin-1 levels in the same CSF samples.
Materials and Methods
Adult male Sprague-Dawley rats (n = 20; total number used for all experiments) were used for repeated CSF collections [5]. The animals were maintained under constant temperature (23°C) and controlled 12:12 h light:dark cycles (lights on at 07:00 h; zeitgeber time [ZT] 0) for at least 2 weeks prior to the beginning of the study. The CSF taps were performed under anesthesia [a mixture of isoflurane (2-3%) and oxygen gas]. The details of the method for CSF taps were previously described [5]. All collected CSF samples were put into siliconized micro-centrifuge tubes (0.5 ml), were immediately frozen and were stored in a -80°C freezer until high-performance liquid chromatography (HPLC) and radioimmunoassay (RIA) measurements were conducted. All experiments were carried out in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Pharmacological manipulations
Modafinil 200 mg/kg (Lafon, France, n = 11), d-amphetamine 5 mg/kg (Sigma, St. Louis, MO, n = 11), histamine H3 antagonist thioperamide 5 mg/kg (Sigma, n = 6) dissolved in 2-hydroxypropyl-β-cyclodextrin (β-CD) and β-CD alone as a vehicle (n = 8) were administered intraperitoneally (i.p.) at ZT2, and cisternal CSF collections were performed at ZT4. In the separate sessions, the home-cage activity of rats was monitored during pharmacological sessions using a MicroMax single axis home cage monitoring system (Accuscan Instruments, Inc., Columbus, OH). The animals were placed in the monitoring system for at least 1 week prior to the beginning of the recording. The recording was performed for 6 h after the administration of the drugs [modafinil 200 mg/kg (n = 9), amphetamine 5 mg/kg (n = 9), thioperamide 5 mg/kg (n = 9), and vehicle (β-CD, n = 9)] at ZT2. Period length analyses for recorded infrared beam breaks were performed.
Since i.p. injection (i.e. vehicle) non-specifically activates locomotor activity in rats for about 30 min, the period from 30 min to 350 min after injection was analyzed.
Diurnal fluctuation
We divided rats into group 1 (n = 10) and group 2 (n = 10). Cisternal CSF collection times for group 1 were at ZT0, 4, 8, 12, 16 and 20, and for group 2 were at ZT2, 6, 10, 14, 18 and 22. There were at least 3-day intervals between each collection date for enough recovery, and the order of the collections was randomized.
Sleep deprivation
The rats were kept awake for 6 h (ZT0-ZT6) by gently tapping the cage, stroking their backs using a brush whenever the rats showed behavioral signs of sleep [23]. Immediately after 6 h of sleep deprivation, the rats were anesthetized, and CSF samples were collected (n = 10). As controls, CSF samples were collected at ZT6 from the rats without sleep deprivation (n = 10).
Sleep was not monitored during sleep deprivation in this study, but our previous study suggested that this procedure produced 85 % of wakefulness compared to the baseline condition (15 %) [23].
Food deprivation
After the 48 h food deprivation (water available ad libitum), CSF samples were collected from the rats (n = 12) at ZT10. The entire experiment was repeated in the same 12 rats with taps being carried out at ZT22. As controls, CSF samples were collected at ZT10 (n = 10) or ZT22 (n = 10) from the rats without food deprivation.
Histamine and hypocretin-1 measurements
Histamine content in the CSF was determined by HPLC-fluorometry technique established by Yamatodani et al. [22]. CSF samples (50 μl) were injected directly into a column packed with the TSKgel SP2SW Cation Exchanger (150 × 3.0 mm i.d. Tosoh, Tokyo, Japan). The histamine eluted with 0.25 M potassium phosphate, at a flow rate of 0.3 ml/min was post-labeled with o-phthalaldehyde in an alkaline condition, and was detected fluorometrically in an F1080 Fluorometer (Hitachi, Tokyo, Japan).
CSF Hypocretin-1 was measured with commercially available 125I RIA kits (hcrt-1: Phoenix Pharmaceuticals, Burlingame, CA). CSF samples (25μl) were directly applied for measurement. All samples were measured in a single assay and the intra-assay variability was within 5%.
Statistics
All values are expressed as mean ± SEM. For multiple group comparison, the one-way analysis of variance (ANOVA) was applied to determine the significance followed by the Scheffe or Bonferroni-Dunn multiple comparison test. For two-group comparison, the unpaired t-test was applied. For the comparison of locomotor activity, repeated measures of ANOVA with Bonferroni-Dunn multiple comparison test was used. P < 0.05 was considered statistically significant.
Results
We carried out a total of 280 taps in 20 animals (12-16 times in each animal). The turnover of the CSF is approximately 6 hours, and a 3-day interval between each collection time was long enough to allow sufficient CSF regeneration. The repeated tap did not cause changes in any behavior or in any neurological signs in any rats. We observed no obvious inflammatory reactions and/or tubercle around the back cervix, even after 16 taps in a single animal.
However, blood contamination was sometime observed, and we evaluated the influence of the blood contamination on histamine levels in the CSF using a separate set of baseline CSF samples (n = 19). We divided these CSF samples into four groups according to the level of blood contamination (clear <5%, ± 5-10%, + 10-20%, ++ >20% of blood contamination, v/v) and CSF histamine levels among 4 groups were compared. We found that histamine levels of group ++ (n = 5, 7.91 ± 1.95 pg/μl) is significantly higher than those of other groups (n = 14, 3.98 ± 0.36 pg/μl). We therefore decided not to include the CSF samples of group ++ in the histamine data analysis. The influence of the blood contamination (>20% v/v contamination) on the CSF hypocretin-1 levels was excluded previously.
Pharmacological manipulation
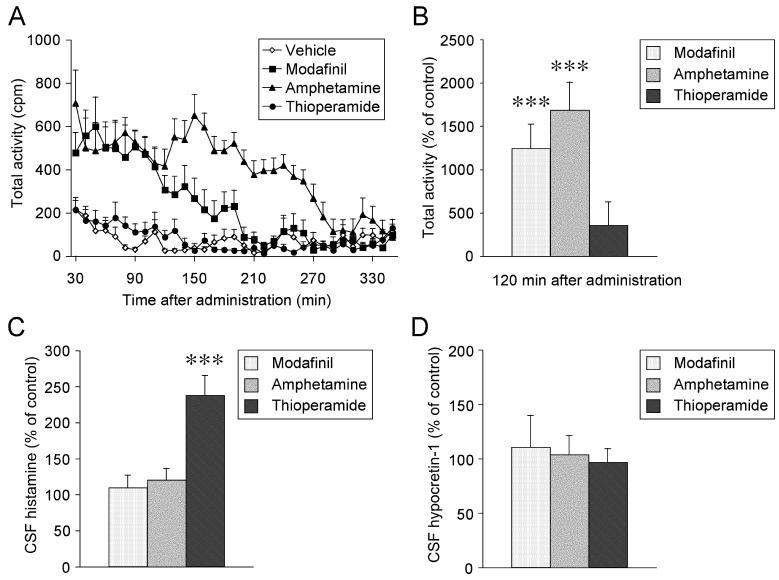
Amphetamine administered at ZT2 induced a robust increase in the locomotor activity of the rats compared with the vehicle administration (until 260 min after injection, p < 0.0001). Modafinil had also significantly activated locomotor activity (until 190 min after injection, p = 0.003). On the other hand, thioperamide induced only a small increase on locomotor activity between 70 min to 130 min (p = 0.99, Fig 1A). Since CSF was collected 2 h after the drug administrations, effects on cumulative locomotor counts until 120 min were compared (Fig 1B). Amphetamine (p < 0.0001) and modafinil (p = 0.0002) elicited profound increase on locomotor activity while the increase of activity by thioperamide administration was not significant (p = 0.44).
Figure 1.
The effects of i.p. injection of modafinil, amphetamine, and thioperamide on locomotor activity and CSF histamine and hypocretin-1 levels of rats. Values are means ± SEM. ***p < 0.001 vehicle-treated.
(A) Average values count per minute (cpm) for locomotor activity of rats after i.p. administrations of vehicle (n = 9), modafinil (200 mg/kg, n = 9), amphetamine (5 mg/kg, n = 9), and thioperamide (5 mg/kg, n = 9).
(B) The effects of i.p. administration of modafinil, amphetamine, and thioperamide on locomotor activity of rats at 120 min after administration compared to vehicle.
(C) CSF histamine levels 2 h after i.p. administration of modafinil (200 mg/kg, n = 11), amphetamine (5 mg/kg, n = 11), and thioperamide (5 mg/kg n = 6).
(D) CSF hypocretin-1 levels 2 h after i.p. administration of modafinil (200 mg/kg, n = 6), amphetamine (5 mg/kg, n = 7), and thioperamide (5 mg/kg, n = 7).
The CSF histamine levels were profoundly increased after administration of thioperamide compared with vehicle (p < 0.0001), but modafinil (p = 0.78) and amphetamine (p = 0.58) had no effects (Fig. 1C). CSF hypocretin-1 levels had no significant changes after the administration of modafinil (p = 0.99), amphetamine (p = 0.99), or thioperamide (p = 0.99) (Fig. 1D).
Diurnal fluctuation
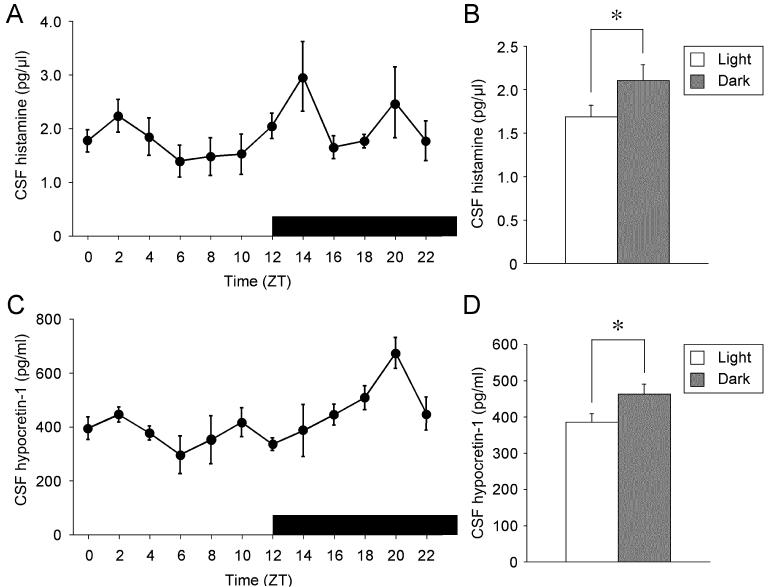
Consistent with the results reported previously, CSF hypocretin-1 levels fluctuated significantly over a 24 h period (p = 0.003, Fig 2C). CSF hypocretin-1 levels were low at the second half of the light period (ZT6 to ZT12), and then increased gradually and reached the highest levels at ZT20. The mean of the CSF hypocretin-1 levels during the dark period was significantly higher than that during the light period (p = 0.035, Fig 2D). CSF histamine levels were also low at the second half of the light period, and high at some time points during dark period, such as at ZT14 and ZT20, although the fluctuation across 24 h was not statistically significant (p = 0.19) (Fig. 2A). However, the mean of the CSF histamine levels during the dark period was significantly higher than that during the light period (p = 0.049, Fig 2B).
Figure 2.
Diurnal fluctuation of CSF histamine and hypocretin-1 in rats.
(A) Each point represents mean value (± SEM, n = 6-10) of CSF histamine across 24 h. The horizontal black bar indicates the dark period.
(B) Mean value (± SEM) of CSF histamine during light period (n = 49) and during dark period (n = 49).
(C) Each point represents mean value (± SEM, n = 5-10) of CSF hypocretin-1 across 24 h.
(D) Mean value (± SEM) of CSF hypocretin-1 during light period (n = 48) and dark period (n = 49).
Sleep deprivation
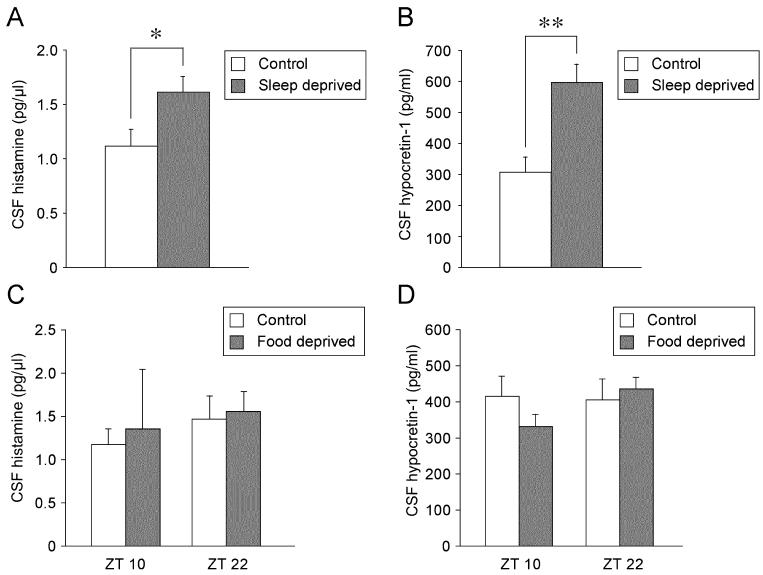
After 6 h sleep deprivation from ZT0, CSF histamine levels increased significantly in comparison with controls whose sleep were not deprived (p = 0.028, Fig 3A). CSF hypocretin-1 levels significantly increased after sleep deprivation compared to control (p = 0.003, Fig 3B). The magnitude of elevation of CSF histamine levels was modest (144% compared to control) and less prominent than that of hypocretin-1 levels (195%).
Figure 3.
The effects of 6 h sleep deprivation (A and B) and 48 h food deprivation (C and D) on CSF histamine and hypocretin-1 levels. The effects of 48 h food deprivation on CSF histamine and hypocretin-1 levels were evaluated at ZT10 and ZT22. Values are means ± SEM. **p < 0.01, *p < 0.05 versus control.
Food deprivation
CSF histamine levels were not affected by 48 h of food deprivation at ZT10 (p = 0.41) or ZT22 (p = 0.82, Fig 3C). Furthermore, the CSF hypocretin-1 levels were also not changed significantly by the 48 h food deprivation at ZT10 (p = 0.21) or ZT22 (p = 0.52, Fig 3D).
Discussion
This is the first study that demonstrated how CSF histamine levels in rats fluctuate across 24 h, by sleep and food deprivations, and by wake-promoting compounds. Histamine is released in the brain not only from TMN histamine neurons but also from neuronal mast cells. Especially in the rat brain, mast cells are highly concentrated in the thalamus and also enriched in the choroids plexus [17]. Although roles of mast cells in sleep regulation are not known, histamine from mast cells may affect CSF histamine levels significantly.
We observed that systemic administration of H3 antagonist thioperamide significantly increased CSF histamine levels. Since thioperamide increases neuronal histamine release in the brain, increased CSF histamine levels after thioperamide administration possibly reflect the increased activity of central histamine neurotransmission.
We found that wake-promoting doses of thioperamide [9] exhibit only negligible enhancement of locomotor activity, suggesting that changes in CSF histamine levels are not associated with locomotor changes. This is further supported by the results that both modafinil and amphetamine did not have any influences on CSF histamine nor hypocretin-1 levels, but induced significant increase in locomotor activity. It has been reported that modafinil and amphetamine induces Fos expression in the hypocretin neurons in the rat [4, 16]. Furthermore, modafinil was reported to enhance Fos expression in the TMN histamine neurons [16], and i.p. or intracerebroventricular (i.c.v.) administration of modafinil and i.p. administration of methamphetamine enhance histamine release in the anterior hypothalamus [6] and striatum [7], respectively. These effects of modafinil and methamphetamine may however, be secondary to the wake enhancement or indirect effects mediated by other neurotransmission since a time delay (1∼2 h) before the increase in histamine levels was observed and since modafinil injected directly to the TMN has no effect on histamine levels. In contrast, H3 antagonists are known to act directly on presynaptic histamine receptors and enhance histamine release from the histaminergic terminals. This taken together with the fact that histamine neurons project to the area close the ventricle (i.e. the mammillary recess) [2] may explain a rapid and significant enhancement of histamine levels in the CSF after thioperamide administration. A dissociation between the Fos expressions of hypocretin neurons and release of hypocretin-1, to the CSF may exist, and Fos expressions may be much more sensitive to neuronal or behavioral changes.
We observed a marked increase in CSF histamine and hypocretin-1 levels after 6 h sleep deprivation. This result, taken together with the facts that both histamine and hypocretin neurons are wake-active, suggests that forced wakefulness during resting period likely activates both histamine and hypocretin neurons. However, this result is quite a contrast to the results that modafinil and amphetamine did not increase CSF histamine and hypocretin-1 levels while they both enhance wakefulness and locomotion. Our interpretation is that multiple wake-prompting systems that are essential for physiological wakefulness may sufficiently be activated during forced wakefulness while modafinil and amphetamine may more specifically activate their target molecular systems to promote wakefulness and locomotion. As the major mode of action for the wake promotion of modafinil and amphetamine, the dopamine transporters (DAT)-mediated enhancement of dopaminergic release at the dopaminergic terminals is proposed [21], but also other mechanisms such as effects on norepinephrine transporters (NET) have been proposed [11]. NET may also be involved in the locomotor effects by amphetamine and modafinil. Since modafinil and amphetamine promote wakefulness in hypocretin-deficit narcoleptic canines [21] and in histidine decarboxylase knockout mice (lacks histamine synthesis) [13], activation of histamine and hypocretin neurotransmission is not required for the wake-promotion and locomotor enhancement of these compounds.
We found that hypocrestin-1 levels in the CSF are the lowest at the second half of the light period and they gradually increase during dark period when the animal spent most time in wake [5]. CSF histamine levels are also high during active period. This result is in line with several earlier studies found significant diurnal fluctuation of brain histamine contents [1]. The fluctuation of histamine in the CSF across 24 h was not statistically significant. This is due to the fact that histamine levels at several time points (such as ZT16, 18 and 22) during dark period were rather low and the fact that CSF histamine did not tightly correlated with CSF hypocretin-1 levels. This may partially be due to the fact that histamine levels fluctuate swiftly and are tightly correlated with the amount of wakefulness, while changes in hypocretin-1 levels are slower and cumulate toward the end of active period [23]. A recent electrophysiological study also demonstrated TMN histamine neurons are highly sensitive to changes in vigilance level [19]. Sleep/wake pattern is polyphasic with wake episodes interrupted by periods of sleep in rats, and this may induce some dissociations between CSF histamine and hypocretin-1 levels.
We observed that 48 h food deprivation did not alter histamine and hypocretin-1 levels in the CSF collected, regardless of the time of the day. In the earlier study, prepro-hypocretin mRNA levels showed 2.4-fold increase after 48 h food deprivation [15]. We also reported previously that CSF hypocretin-1 level was elevated by the 72 h food deprivation in rats. However, its elevation was modest and only 1.3 fold, and 24 h food deprivation did not alter the levels [5]. Therefore, much higher degree of the manipulation (48 h vs. 72 h) may be required before detection of the changes in CSF histamine and hypocretin-1 levels associated with starving.
Conclusion
The results of our present study suggest that CSF histamine levels in rats reflect the central histamine neurotransmission and vigilance state changes. This encourages us to further study the functional significance of reduced CSF histamine levels in human hypersomnia cases of central origin since it is likely that reduced levels reflect altered vigilance state in these conditions.
Acknowledgements
We appreciate Ms. Mari Matsumura for assisting hypocretin-1 measurement and editing the manuscript. This research was supported by NIH Grants R01MH072525 and R03MH079258, and the fellowship from The Naito Foundation (Japan).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Prog Neurobiol. 2001;63:637–672. doi: 10.1016/s0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 2.Ericson H, Watanabe T, Kohler C. Morphological analysis of the tuberomammillary nucleus in the rat brain: delineation of subgroups with antibody against L-histidine decarboxylase as a marker. J Comp Neurol. 1987;263:1–24. doi: 10.1002/cne.902630102. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson KS, Sergeeva O, Brown RE, Haas HL. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2001;21:9273–9279. doi: 10.1523/JNEUROSCI.21-23-09273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujiki N, Yoshida Y, Ripley B, Honda K, Mignot E, Nishino S. Changes in CSF hypocretin-1 (orexin A) levels in rats across 24 hours and in response to food deprivation. Neuroreport. 2001;12:993–997. doi: 10.1097/00001756-200104170-00026. [DOI] [PubMed] [Google Scholar]
- 6.Ishizuka T, Sakamoto Y, Sakurai T, Yamatodani A. Modafinil increases histamine release in the anterior hypothalamus of rats. Neurosci Lett. 2003;339:143–146. doi: 10.1016/s0304-3940(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 7.Ito C, Onodera K, Sakurai E, Sato M, Watanabe T. Effects of dopamine antagonists on neuronal histamine release in the striatum of rats subjected to acute and chronic treatments with methamphetamine. J Pharmacol Exp Ther. 1996;279:271–276. [PubMed] [Google Scholar]
- 8.Kanbayashi T, Kodama T, Kondo H, Satoh S, Miyazaki N, Kuroda K, Abe M, Nishino S, Inoue Y, Shimizu T. CSF histamine and noradrenaline contents in narcolepsy and other sleep disorders. Sleep. 2004;27:A236. [Google Scholar]
- 9.Monti JM, Jantos H, Boussard M, Altier H, Orellana C, Olivera S. Effects of selective activation or blockade of the histamine H3 receptor on sleep and wakefulness. Eur J Pharmacol. 1991;205:283–287. doi: 10.1016/0014-2999(91)90911-9. [DOI] [PubMed] [Google Scholar]
- 10.Nishino S, Fujiki N, Ripley B, Sakurai E, Kato M, Watanabe T, Mignot E, Yanai K. Decreased brain histamine content in hypocretin/orexin receptor-2 mutated narcoleptic dogs. Neurosci Lett. 2001;313:125–128. doi: 10.1016/s0304-3940(01)02270-4. [DOI] [PubMed] [Google Scholar]
- 11.Nishino S, Mao J, Sampathkumaran R, Shelton J. Increased dopaminergic transmission mediates the wake-promoting effects of CNS stimulants. Sleep Res Online. 1998;1:49–61. [PubMed] [Google Scholar]
- 12.Nishino S, Sakurai E, Nevisimalova S, Vankova J, Yoshida Y, Watanabe T, Yanai K, Mignot E. CSF histamine content is decreased in hypocretin-deficient human narcolepsy. Sleep. 2002;25:A476. [Google Scholar]
- 13.Parmentier R, Anaclet C, Guhennec C, Brousseau E, Bricout D, Giboulot T, Bozyczko-Coyne D, Spiegel K, Ohtsu H, Williams M, Lin JS. The brain H3-receptor as a novel therapeutic target for vigilance and sleep-wake disorders. Biochem Pharmacol. 2007;73:1157–1171. doi: 10.1016/j.bcp.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Parmentier R, Ohtsu H, Djebbara-Hannas Z, Valatx JL, Watanabe T, Lin JS. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control. J Neurosci. 2002;22:7695–7711. doi: 10.1523/JNEUROSCI.22-17-07695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 16.Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS, Saper CB. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20:8620–8628. doi: 10.1523/JNEUROSCI.20-22-08620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silver R, Silverman AJ, Vitkovic L. Lederhendler, II, Mast cells in the brain: evidence and functional significance. Trends Neurosci. 1996;19:25–31. doi: 10.1016/0166-2236(96)81863-7. [DOI] [PubMed] [Google Scholar]
- 18.Steininger TL, Stevens DR, Haas HL, McGinty D, Symusiak R. Preoptic area inhibition of histaminergic tuberomammillary neurons in vitro. Soc Neurosci Abs. 1997 302.6. [Google Scholar]
- 19.Takahashi K, Lin JS, Sakai K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J Neurosci. 2006;26:10292–10298. doi: 10.1523/JNEUROSCI.2341-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanni-Mercier G, Sakai K, Jouvet M. [Specific neurons for wakefulness in the posterior hypothalamus in the cat] C R Acad Sci III. 1984;298:195–200. [PubMed] [Google Scholar]
- 21.Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21:1787–1794. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamatodani A, Fukuda H, Wada H, Iwaeda T, Watanabe T. High-performance liquid chromatographic determination of plasma and brain histamine without previous purification of biological samples: cation-exchange chromatography coupled with post-column derivatization fluorometry. J Chromatogr. 1985;344:115–123. doi: 10.1016/s0378-4347(00)82012-5. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida Y, Fujiki N, Nakajima T, Ripley B, Matsumura H, Yoneda H, Mignot E, Nishino S. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur J Neurosci. 2001;14:1075–1081. doi: 10.1046/j.0953-816x.2001.01725.x. [DOI] [PubMed] [Google Scholar]