Abstract
Stress conditions inhibit mRNA export, but mRNAs encoding heat shock proteins continue to be efficiently exported from the nucleus during stress. How hsp mRNAs bypass this stress-associated export inhibition was not known. Here we show that HSF1, the transcription factor that binds hsp promoters after stress to induce their transcription, interacts with the nuclear pore-associating Tpr protein in a stress-responsive manner. Tpr is brought into proximity of the hsp70 promoter after stress and preferentially associates with mRNAs transcribed from this promoter. Disruption of the HSF1-Tpr interaction inhibits the export of mRNAs expressed from the hsp70 promoter, both endogenous hsp70 mRNA and a luciferase reporter mRNA. These results suggest that hsp mRNA export escapes stress inhibition via HSF1-mediated recruitment of the nuclear pore-associating protein Tpr to hsp genes, thereby functionally connecting the first and last nuclear steps of the gene expression pathway, transcription and mRNA export.
INTRODUCTION
The up-regulation of heat shock proteins such as hsp70 that occurs in response to exposure to elevated temperature and many other stress conditions is vital for the ability of cells to survive these stresses. Because of their crucial cytoprotective function, it is very important that up-regulation of hsp expression after stress occur as rapidly and as efficiently as possible. An intriguing finding of past studies is that stress conditions inhibit the export of many mRNAs from the nucleus, but mRNAs encoding heat shock proteins continue to be efficiently exported during stress (1–7). However, the mechanism by which hsp mRNAs bypass this stress-associated export inhibition was not known.
HSF1 is the transcription factor responsible for up-regulating transcription of the hsp70 and other genes in response to elevated temperature and other stress conditions. HSF1 performs this function by undergoing stress-induced trimerization to the DNA-binding form, and then interacting with heat shock elements in the promoters of these genes to increase their transcription (8, 9). Tpr is a 270 kDa polypeptide that is associated with the nuclear basket on the nucleoplasmic face of the nuclear pore complex (10–17). Previous data suggest that Tpr is involved in the export of both mRNAs and proteins from the nucleus (13, 16, 18–20). During the course of yeast two-hybrid experiments in our laboratory we identified the existence of an interaction between HSF1 and the Tpr protein. The results presented in this paper suggest that the HSF1-Tpr interaction could be important for the export of hsp mRNAs during stress conditions.
EXPERIMENTAL PROCEDURES
Yeast two-hybrid assay
The interactions between the pGBD-HSF1 and pVP16-Tpr 14–117 and pVP16-Tpr 1218–1320 were characterized by streaking yeast (strain pJ694A) containing these constructs or pGBD-HSF1 bait and empty pVP16 plasmid (as negative control) on -TL, -HTL, and -ATL plates.
In vitro binding assay
35S-labeled in vitro translated Tpr(14–117) or Tpr(1218–1320) were incubated with GST-HSF1 and GST bound to glutathione-agarose. After washing, bound proteins were analysed by SDS-PAGE and autoradiography to detect the 35S-labeled Tpr proteins. Amounts of GST-HSF1 and GST proteins bound to the beads were determined by SDS-PAGE followed by Western blot using goat polyclonal anti-GST antibody (Amersham).
Immunoprecipitation analysis
HeLa cells were heat-shocked at 42°C for 1 hr, and then extracts of these cells were subjected to immunoprecipitation using a rabbit anti-HSF1 polyclonal antibody (21) or rabbit non-specific IgG followed by Western blot using a mouse monoclonal anti-Tpr antibody (Oncogene Research Products).
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assays were performed with Jurkat cells as previously described (22), using the mouse monoclonal anti-Tpr antibody (Oncogene Research Products) and mouse non-specific IgG (as negative control). The primers used to amplify the promoter regions of the stress-inducible hsp70i gene and histone H4 gene were: hsp70i 5′-ctcagggtccctgtccc-3′ and 5′-tgagccaatcaccgagc 3′; histone H4 5′-gagagggcggggacaattga-3′ and 5′-ttggcgtgctcggtgtaggt-3′. PCR products were then separated on polyacrylamide gels and stained with ethidium bromide. ChIP samples were also analyzed by quantitative real-time PCR as described below.
Hsp mRNA export analysis
pEGFP-Tpr(14–117) and pEGFP-Tpr(1218–1320) were generated using a PCR-based strategy to amplify the relevant regions while adding restriction sites to each end to allow ligation into the pEGFP-C2 vector. Cloning junctions were checked by sequencing. One μg of each plasmid or empty vector was co-transfected with either hsp70i-luciferase (21) (luciferase expressed from the stress-inducible human hsp70i promoter) or RSV-luciferase plasmids using Effectene (Qiagen) following the manufacturer’s instructions. The RSV-luciferase plasmid was a kind gift of Dr. Dan Noonan, and was generated by replacing the CAT coding sequence from RSV-CAT (23) with the luciferase coding sequence. Cells were heat-shocked at 42°C for 1 hour, and then cytoplasmic and nuclear fractions were prepared using hypotonic lysis, as described in the following. Cells were swollen in 5 packed cell volumes of 10 mM HEPES (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 1 mM DTT, 1 mM PMSF (Buffer A) for 10 minutes on ice. Cells were then centrifuged at 2000 rpm for 10 minutes, resuspended in 2 packed cell volumes of Buffer A, and lysed by 20 strokes of a Dounce homogenizer (type B pestle). Nuclei and cytoplasm were separated by 2000 rpm centrifugation for 10 minutes. Separation was verified by viewing fractions with a microscope. mRNA was extracted from each fraction using Trizol reagent following the manufacturer’s instructions. To analyze mRNA concentrations, each pool was subjected to an RNAse protection assay using Super Signal RPA III (Ambion) following the manufacturer’s instructions, using a probe for either luciferase or L32 ribosomal protein mRNA. The probe for luciferase mRNA was constructed via in vitro transcription using MaxiScript (Ambion) and biotinylated UTP (Roche). The template for in vitro transcription was created using PCR with the hsp70-luciferase plasmid and the following primers: 5′-cacggaaagacgatgacg-3′ and 5′-taatacgactcactataggttgggtaacgccaggg-3′. This PCR product contained the 3′ end of the luciferase mRNA, ending with the polyadenylation signal (yielding a protected fragment of 325 basepairs) and untranscribed vector sequence (resulting in an unprotected fragment of 438 basepairs). The construction of the L32 probe was previously described (24).
To determine if expression of the two HSF1-binding regions of TPR affects endogenous hsp70 mRNA levels, GFP-Tpr(14–117) and GFP-Tpr (1218–1320) or empty vector were transfected into HeLa ATCC according to the manufacturer’s protocol. Cells were heat-shocked and fractionated as described above. Total RNA was extracted with Trizol (Invitrogen) according to the manufacturer’s instruction. RNA was resuspended in 10mM Tris-HCl, pH 7.5, 2.5mM MgCl2, 0.5mM CaCl2 and incubated with RNAse-free DNAse I to remove possible genomic contamination. cDNA was prepared from samples using ImProm II reverse transcriptase (Promega) and poly-d(T)16 primers. cDNA samples were assayed using quantitative real-time PCR as described below.
RNA immunoprecipitation analysis
1.5 × 106 HeLa ATCC cells were transfected with 2 μg of either the Hsp70i-luc or RSV-luc plasmid using Effectene (Qiagen) following the manufacturer’s instructions. Cells were then heat-shocked for 1 hour at 42°C, washed once with ice-cold PBS, and crosslinked with 2% paraformaldehyde for 12 minutes while rotating. Cross-linking was quenched with 125 mM glycine for 5 minutes, and cells were washed twice with ice-cold PBS, harvested by scraping and snap-frozen in liquid nitrogen. Cells were resuspended in 2 ml low-stringency RIPA buffer (50 mM Tris-HCl, pH 7.5, 1% NP-40, 0.5% sodium deoxycholate, 0.05% SDS, 1 mM EDTA, 150 mM NaCl, 1X Protease Inhibitor Cocktail (Roche) and 80U RNAseOUT (Invitrogen), pipetted 20X and incubated on ice for 10 minutes. Cells were then sonicated 3X, 80–90% output, for 20 seconds. Cells were centrifuged at 16,000×g for 10 minutes at 4°C. The supernatant was precleared with 20 μl Protein G-sepharose (GE Healthcare) washed in low-stringency RIPA buffer and 100 μg/ml yeast tRNA (Ambion) for 2 hours at 4°C. During the pre-clear, low-stringency washed Protein G-sepharose beads were coated with 5 μg either Tpr antibody or mouse IgG (Sigma) in low-stringency RIPA buffer. Pre-cleared supernatant was then incubated with the antibody-coated beads for 90 minutes at 4°C with rotation. Complexes were washed 5X 10 minutes at room-temperature with 1 ml high-stringency RIPA buffer (50 mM Tris-HCl pH 7.5, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, 1 M NaCl, 1 M Urea, 0.2 M PMSF. Beads were then resuspended in 100 μl 50 mM Tris-HCl pH 7.5, 5 mM EDTA, 10 mM DTT, 1% SDS and crosslinks were reversed for 1 hour at 70°C. Messenger RNA was isolated as described above, and then samples were analyzed by Q-PCR as described in the following section.
Quantitative real-time PCR
This was performed with the Mx 4000 system (Stratagene) using Brilliant SYBR Green QPCR master mix (Stratagene). Samples were checked for specific amplification using dissociation curves analysis included with the software. PCR products were also assayed on polyacrylamide gels with ethidium bromide staining to ensure they were of the expected size. For the Q-PCR analysis of the Tpr-hsp70 promoter ChIP assay the primers used were hsp70 5′-caacacccttcccaccgccactc-3′ and 5′-ctgattggtccaaggaaggctgg-3′; histone H4 5′-gagagggcggggacaattga-3′ and 5′-ggtcatgtccggctgtggaaag-3′. The Ct values were normalized to input DNA (DNA before immunoprecipitation step) and IgG controls. Data is represented as fold-differences above control mouse IgG relative to input DNA using the formula 2 [(Ct IgG-Ct Input)-(Ct Tpr-Ct Input)]. For the experiments analyzing luciferase cDNA, primers used for Q-PCR analysis were: 5′-gtctgaattccagtcgatgtacacgttcg-3′ and 5′-cacgaagcttgcatgcgagaactccacgc-3′. The Ct values were normalized to input cDNA (cDNA made from total RNA before immunoprecipitation step) and IgG controls, which were set as 1 unit. Data is represented as fold-differences relative to these two values using the formula 2 [(Ct lIgG-Ct Input)-(Ct Tpr-Ct Input)].
For experiments analyzing endogenous hsp70 levels the following primers were used: hhsp70 5′-caccttgccgtgttgga-3′ and 5′-ttctcgcggatccagtg-3′; hL325′-catctccttctcggcatca-3′ and 5′-aaccctgttgtcaatgcctc-3′. Data presented represents two independent experiments in which values obtained utilizing the formula 2−ΔΔCt were averaged. Results are graphed as relative differences between cytoplasmic and nuclear hsp70 mRNA levels compared to the control samples (GFP alone), which were set to a value of 1.
RESULTS
In order to further understanding of the regulation and function of HSF1, we performed a yeast two-hybrid screen using the HSF1 protein as a bait. Two of the interacting clones that were obtained from this screen represented two different regions of the Tpr protein. One of the HSF1-interacting regions of Tpr identified by the yeast two-hybrid screen comprises a sequence near the amino terminus (amino acids 14–117), while the other is located close to the middle of the protein (amino acids 1218–1320) (Fig. 1A and 1B). As an independent test of the interaction between HSF1 and these two regions of Tpr, and to determine whether the interaction was direct, in vitro binding experiments were performed in which 35S-labelled in vitro translated Tpr(14–117) and Tpr(1218–1320) were incubated with GST-HSF1 or GST bound to glutathione-agarose beads. The results confirm the ability of both regions of Tpr to interact with HSF1 (Fig. 1C).
FIGURE 1. HSF1 interacts with the Tpr protein.
A, Yeast strain pJ694A transformed with pGBD-HSF1 and pVP16-Tpr(14–117), pVP16-Tpr(1218–1320), or pVP16 alone were streaked on plates lacking tryptophan and leucine (-TL), tryptophan, leucine, and histidine (-TLH), or tryptophan, leucine, and alanine (-TLA). B, Schematic depicting the location of the N-terminal and centrally-located Tpr segments identified as HSF1-interacting regions. C, 35S-labeled in vitro translated Tpr(14–117) or Tpr(1218–1320) were incubated with GST-HSF1 or GST that were bound to glutathione-agarose beads, and then after washing the amount of bound 35S-labeled Tpr(14–117) or Tpr(1218–1320) was determined by SDS-PAGE and autoradiography. Amounts of GST-HSF1 and GST bound to beads were determined by GST Western blot.
To determine whether endogenous HSF1 and Tpr proteins interact, and if so whether the interaction between these proteins is regulated in a stress-dependent manner, immunoprecipitation analysis was performed using extracts of cells kept at 37°C or cells subjected to heat stress at 42°C for one hour. The results indicate that endogenous HSF1 and Tpr do associate, and that more HSF1-Tpr complex is observed in extracts of stressed cells than those of non-stressed cells (Fig. 2).
FIGURE 2. HSF1 interacts with the Tpr protein in a stress-regulated manner.

Extracts of non-stressed (37°C) or stressed (42°C, 60 min.) HeLa cells were immunoprecipitated using anti-HSF1 antibodies or non-specific IgG and the immunoprecipitates subjected to anti-Tpr Western blot.
In multi-cellular eukaryotes, HSF1 binds to heat shock gene promoters in response to stress conditions. Therefore, the data presented above in Figure 2 indicating that HSF1 interacts with Tpr in a stress-induced manner prompted the question of whether Tpr might also associate with the promoter of the stress-inducible hsp70 gene when cells are exposed to stress. We tested this hypothesis using the Chromatin Immunoprecipitation assay. The results demonstrated that a low level of Tpr association is detected within cross-linking distance of the hsp70 promoter in cells kept at 37°C, and that a higher level of Tpr is associated with the hsp70 promoter in cells that were subjected to stress treatment at 42°C for one hour (Fig. 3A, upper set of panels). Tpr was not found to associate with the promoter region of the histone H4 gene, indicating the specificity of its hsp70 promoter association (Fig. 3A, lower set of panels). As a complementary approach, we repeated this experiment and used quantitative real-time PCR for the analysis. The results, shown in Figure 3B, are consistent with the finding of increased association between Tpr and the hsp70 promoter in response to exposure to stress conditions.
FIGURE 3. Tpr associates with the hsp70 promoter in response to stress.
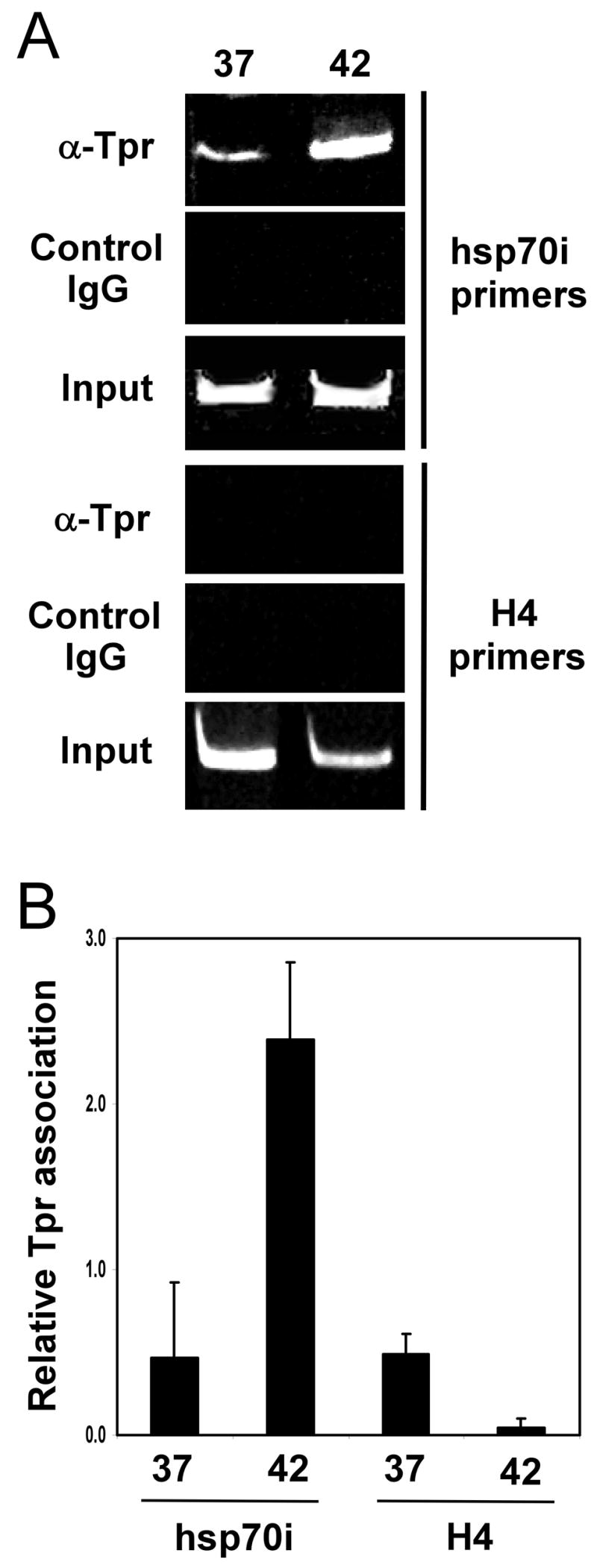
A, Chromatin immunoprecipitation (ChIP) assay was performed on non-stressed (37°C) or stressed (42°C, 60 min.) Jurkat cells using Tpr antibodies or control IgG antibodies and PCR primers specific to the promoter regions of the stress-inducible hsp70i gene (upper panel) or histone H4 gene (lower panel). PCR products were separated on a polyacrylamide gel and stained with ethidium bromide. B, Chromatin immunoprecipitation (ChIP) assay was performed as described above for panel A, but analysis was done using Quantitative real-time PCR assay. Data is from triplicate experiments.
Based on previous results indicating a role for Tpr in mRNA export (13, 18, 19), including the finding that the yeast Tpr ortholog mlp1 interacts with the mRNA export hnRNP nab2 (19), we hypothesized that the recruitment of Tpr to the hsp70 promoter might function as a way to specifically promote association between Tpr and the stress-induced transcripts that arise from this gene. We tested this hypothesis using an RNA-Immunoprecipitation approach. In this experiment HeLa cells were transfected with expression constructs in which the luciferase gene is transcribed either from the stress-inducible human hsp70 gene promoter or the RSV promoter. The transfected cells were subjected to one hour of heat shock treatment (42°C), after which they were incubated with the chemical cross-linking agent paraformaldehyde, and then extracts of these cells were immunoprecipitated using anti-Tpr antibodies. RNA isolated from the Tpr-containing complexes was reverse transcribed into cDNAs, which were then analysed by quantitative real-time PCR using a luciferase primer pair. The results of this experiment, shown in Figure 4, indicate that significantly more luciferase mRNA transcripts generated from the hsp70 promoter are associated with Tpr protein compared to luciferase mRNAs transcribed from the RSV promoter.
FIGURE 4. Tpr interacts with mRNAs transcribed from the hsp70 promoter.
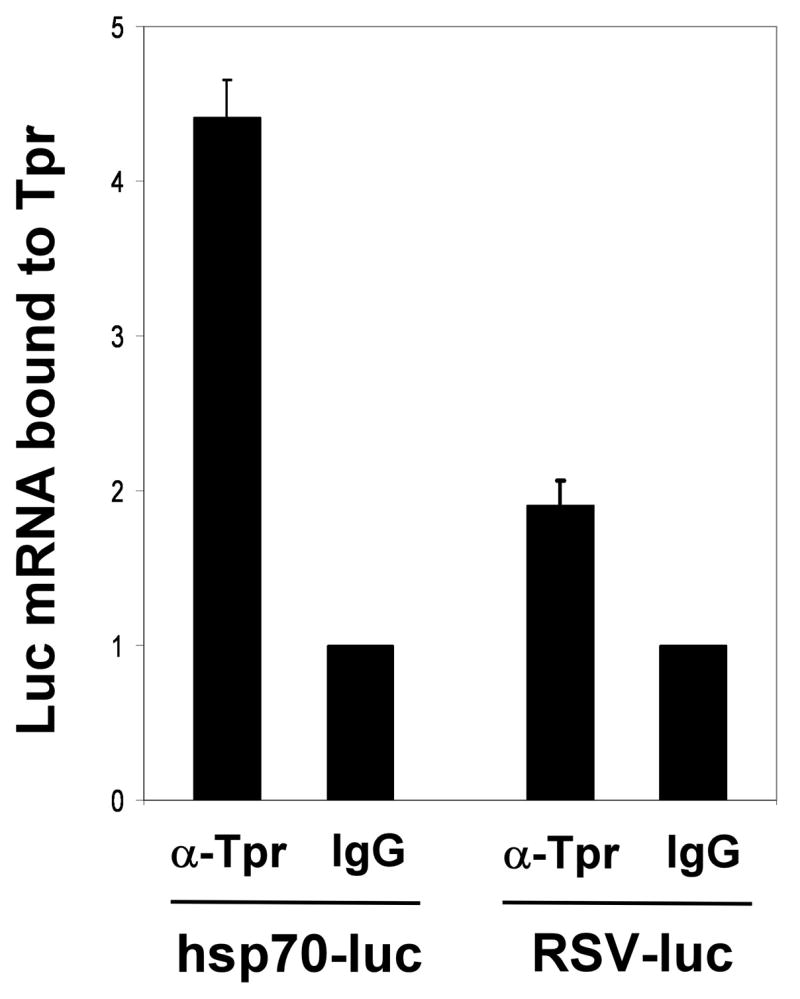
RNA Immunoprecipitation analysis was performed to measure the amounts of luciferase mRNA transcribed from the hsp70 promoter vs. the RSV promoter that are associated with Tpr protein. Results were normalized to levels of each mRNA, and to IgG control samples (whose values were set to 1). Data is from triplicate experiments.
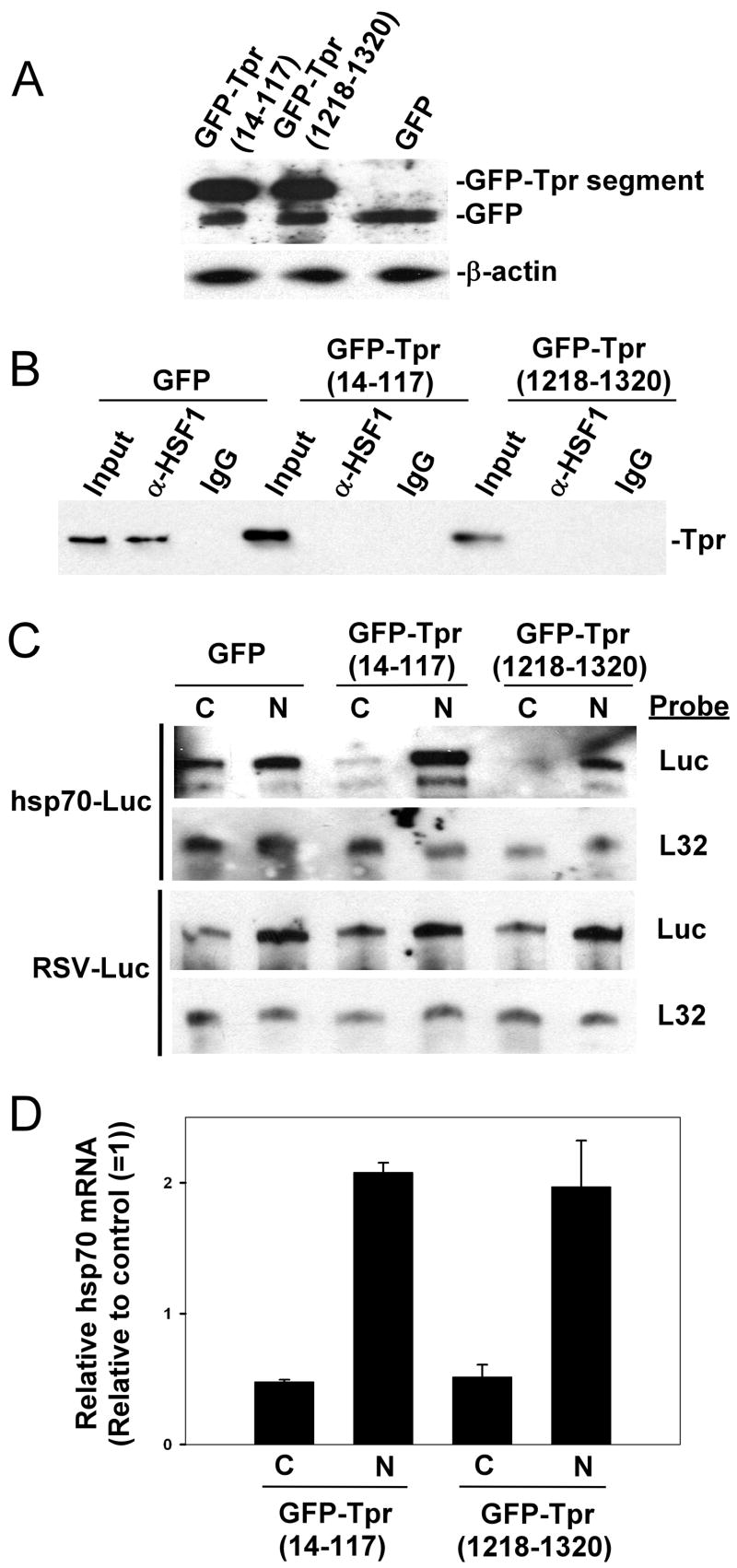
The results described above indicate that stress conditions result in increased interaction between HSF1 and Tpr, increased association of Tpr with the hsp70 promoter, and the preferential association of Tpr with mRNAs arising from transcription from the hsp70 promoter. In light of the data suggesting a role for Tpr in mRNA export (13, 18, 19), we hypothesized that these events could be part of a mechanism for specifically enhancing the export of mRNAs transcribed from heat shock gene promoters. To test this hypothesis we sought to determine whether export of the hsp70 promoter-driven luciferase mRNAs described above is affected by co-transfecting the cells with expression constructs encoding the two regions of the Tpr protein (aa’s 14–117 and 1218–1320) that were shown by our data to interact with HSF1 (Figure 1 above). We reasoned that if HSF1-Tpr interaction is important for export of mRNAs expressed from hsp gene promoters, then expressing either of these two HSF1-binding regions of Tpr could inhibit export of these mRNAs expressed from hsp gene promoters by decreasing the ability of HSF1 and Tpr to associate. The data in Figure 5A shows that both GFP-fusion constructs are expressed at levels similar to that of GFP alone. Fluorescence microscopy analysis of cells transfected with these constructs reveals that a significant proportion of each GFP-Tpr fragment fusion construct is found in the nuclei of these cells, where they would need to be to exert their effects in this experiment (shown in Figure 1 of Supplementary Data). Co-immunoprecipitation analysis confirms that transfection of the GFP-Tpr(14–117) and GFP-Tpr(1218–1320) constructs, but not the GFP expression construct, results in decreased levels of the HSF1-Tpr complex in stressed cells (Fig. 5B).
FIGURE 5. Expression of HSF1-interacting regions of Tpr inhibits export of endogenous hsp70 mRNA and a reporter transcript expressed from the hsp70 promoter.
(A) HeLa cells were transfected with GFP-Tpr(14–117), GFP-Tpr(1218–1320), or GFP alone, and then subjected either to Western blot analysis using anti-GFP antibodies. B, HeLa cells transfected with GFP-Tpr(14–117), GFP-Tpr(1218–1320), or GFP alone were subjected to heat treatment at 42°C, 60 minutes, and then HSF1 immunoprecipitates from extracts of these cells were subjected to anti-Tpr Western blot. C, HeLa cells were co-transfected with the GFP-Tpr(14–117), GFP-Tpr(1218–1320), or GFP alone constructs along with either a hsp70 promoter- (upper two panels) or RSV promoter-driven reporter plasmid (lower two panels), subjected to heat shock treatment at 42°C for 60 minutes, and then mRNA from the cytoplasmic and nuclear fractions of these transfected cells was analysed by RNAse protection assay using probes that detect luciferase mRNA or the mRNA of the L32 ribosomal protein (control). D, HeLa cells were transfected with the GFP-Tpr(14–117), GFP-Tpr(1218–1320), or GFP alone constructs, subjected to heat shock treatment at 42°C for 60 minutes, and then mRNA from cytoplasmic and nuclear fractions of the transfected cells was reverse transcribed into cDNA and analysed by quantitative real-time PCR using primers that amplify a segment of the hsp70i nucleotide sequence or the L32 ribosomal protein nucleotide sequence (normalizing control). The results of this experiment are presented as relative levels of endogenous hsp70 mRNA in the nucleus or cytoplasm, normalized for L32 mRNA levels, compared to the results for the control cells (transfected with GFP alone), whose values were set to 1. Data is from duplicate experiments.
Next, cells were co-transfected with the GFP-Tpr(14–117), GFP-Tpr(1218–1320), or GFP constructs alone, along with constructs containing luciferase expressed either from an hsp70 promoter or from an RSV promoter (non-HSE-containing), subjected to heat shock treatment at 42°C for 60 minutes, and then mRNA from cytoplasmic and nuclear fractions of these cells was analyzed by RNAse protection assay using probes that detect luciferase mRNA or mRNA encoding L32 ribosomal protein (as a control). The results indicate that cells transfected with GFP-Tpr(14–117) or GFP-Tpr(1218–1320) exhibit a decrease in cytoplasmic levels of luciferase mRNA expressed from the HSE-containing hsp70 promoter compared to cells transfected with GFP (Fig. 5C, upper two panels). Transfection of GFP-Tpr(14–117) or GFP-Tpr(1218–1320) did not change the nuclear vs. cytoplasmic levels of luciferase mRNA expressed from the non-HSE-containing RSV promoter compared to GFP alone, indicating the hsp70 promoter selectivity of the effect (Fig. 5C, lower two panels).
Finally, to test whether expression of these two regions of the Tpr protein is able to inhibit export of endogenous hsp70i mRNAs, we performed an experiment similar to the one shown in Figure 5D except that the cells were only transfected with the GFP-Tpr(14–117), GFP-Tpr(1218–1320), or GFP expression constructs (no luciferase reporter constructs). After subjecting the transfected cells to heat shock treatment at 42°C for 60 minutes, mRNA from cytoplasmic and nuclear fractions of these cells was reverse transcribed and then analyzed by quantitative real-time PCR using primers that amplify the hsp70i sequence or primers that amplify the L32 sequence (normalizing control). The results of this experiment, shown in Figure 5D, are presented graphically as relative levels of endogenous hsp70 mRNA in the nucleus or cytoplasm, normalized for L32 mRNA levels, compared to the results for the control cells (transfected with GFP alone), whose values were set to 1. The results indicate that the export of endogenous hsp70i mRNA, like that of the HSE-driven luciferase mRNAs above, is decreased in cells transfected with GFP-Tpr(14–117) or GFP-Tpr(1218–1320) compared to cells transfected with GFP (Fig. 5D).
DISCUSSION
The results presented in this paper indicate that in response to stress the Tpr protein interacts with the stress gene transcriptional regulator HSF1, is recruited to the hsp70 promoter region, preferentially associates with mRNAs transcribed from this promoter compared to those expressed from a non-stress-induced promoter, and that the HSF1-Tpr interaction is required for efficient export of hsp mRNAs from the nucleus during stress. Tpr association with these mRNAs may be assisted by its interaction with mRNA-binding hnRNPs such as nab2 (19). The Tpr protein is known to be able to form homodimers (25). Thus, once it is complexed with hsp mRNAs, Tpr could facilitate their export by docking with Tpr found at the nucleoplasmic face of nuclear pore complexes (10–17).
These results reveal the existence of a direct functional connection between the first and last nuclear steps in the gene expression pathway, transcription and export of mRNAs from the nucleus. The HSF1-Tpr interaction and its downstream events could serve as a mechanism for bypassing the inhibition of mRNA export that occurs in response to stress, and/or to increase the kinetics of export of hsp mRNAs so that cells can express these crucial cytoprotective proteins as soon as possible. One intriguing question for future studies is whether the Tpr protein is recruited to other genes to aid in the export of their mRNAs from the nucleus.
Supplementary Material
FIGURE S1. HSF1-interacting regions of Tpr are found in the nucleus. HeLa cells were transfected with GFP-Tpr(14–117) or GFP-Tpr(1218–1320) and then subjected to fluorescence microscopy to detect GFP (green channel). DNA was visualized by DAPI staining.
Acknowledgments
We are grateful to Dan Noonan and Brian Finlin for providing plasmid constructs, and to other members of the laboratory for insightful discussions. This research was supported by NIH grants GM61053 and GM64606 to K.D.S., Training Grant T3sS007266 (Department of Toxicology) to H.S.S., and NIH postdoctoral support (HD50043) to D.C.W
References
- 1.Sadis S, Hickey E, Weber LA. J Cell Physiol. 1988;135:377–386. doi: 10.1002/jcp.1041350304. [DOI] [PubMed] [Google Scholar]
- 2.Gallouzi IE, Brennan CM, Stenberg MG, Swanson MS, Eversole A, Maizels N, Steitz JA. Proc Natl Acad Sci USA. 2000;97:3073–3078. doi: 10.1073/pnas.97.7.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saavedra C, Tung KS, Amberg DC, Hopper AK, Cole CN. Genes Dev. 1996;10:1608–1620. doi: 10.1101/gad.10.13.1608. [DOI] [PubMed] [Google Scholar]
- 4.Tani T, Derby RJ, Hiraoka Y, Spector DL. Mol Biol Cell. 1996;7:173–192. doi: 10.1091/mbc.7.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Liang S, Tartakoff AM. EMBO J. 1996;15:6750–6757. [PMC free article] [PubMed] [Google Scholar]
- 6.Krebber H, Taura T, Lee MS, Silver PA. Genes Dev. 1999;13:1994–2004. doi: 10.1101/gad.13.15.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bond U. FEMS Yeast Res. 2006;6:160–170. doi: 10.1111/j.1567-1364.2006.00032.x. [DOI] [PubMed] [Google Scholar]
- 8.Voellmy R. Cell Stress Chaperones. 2004;9:122–133. doi: 10.1379/CSC-14R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmberg CI, Tran SE, Eriksson JE, Sistonen L. Trends Biochem Sci. 2002;27:619–627. doi: 10.1016/s0968-0004(02)02207-7. [DOI] [PubMed] [Google Scholar]
- 10.Cordes VC, Reidenbach S, Rackwitz HR, Franke WW. J Cell Biol. 1997;136:515–529. doi: 10.1083/jcb.136.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimowska G, Aris JP, Paddy MR. J Cell Sci. 1997;110:927–944. doi: 10.1242/jcs.110.8.927. [DOI] [PubMed] [Google Scholar]
- 12.Shah S, Tugendreich S, Forbes D. J Cell Biol. 1998;141:31–49. doi: 10.1083/jcb.141.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bangs P, Burke B, Powers C, Craig R, Purohit A, Doxsey S. J Cell Biol. 1998;143:1801–1812. doi: 10.1083/jcb.143.7.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strambio-de-Castillia C, Blobel G, Rout MP. J Cell Biol. 1999;144:839–855. doi: 10.1083/jcb.144.5.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontoura BM, Dales S, Blobel G, Zhong H. Proc Natl Acad Sci USA. 2001;98:3208–3213. doi: 10.1073/pnas.061014698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frosst P, Guan T, Subauste C, Hahn K, Gerace L. J Cell Biol. 2002;156:617–630. doi: 10.1083/jcb.200106046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krull S, Thyberg J, Bjorkroth B, Rackwitz HR, Cordes VC. Mol Biol Cell. 2004;15:4261–4277. doi: 10.1091/mbc.E04-03-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vinciguerra P, Iglesias N, Camblong J, Zenklusen D, Stutz F. EMBO J. 2005;24:813–823. doi: 10.1038/sj.emboj.7600527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green DM, Johnson CP, Hagan H, Corbett AH. Proc Natl Acad Sci USA. 2003;100:1010–1015. doi: 10.1073/pnas.0336594100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornett J, Cao F, Wang CE, Ross CA, Bates GP, Li SH, Li XJ. Nat Genet. 2005;37:198–204. doi: 10.1038/ng1503. [DOI] [PubMed] [Google Scholar]
- 21.Sarge KD, Murphy SP, Morimoto RI. Mol Cell Biol. 1993;13:1392–1407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xing H, Wilkerson DC, Mayhew CN, Lubert EJ, Skaggs HS, Goodson ML, Hong Y, Park-Sarge OK, Sarge KD. Science. 2005;307:421–423. doi: 10.1126/science.1106478. [DOI] [PubMed] [Google Scholar]
- 23.Giguere V, Hollenberg SM, Rosenfeld MG, Evans RM. Cell. 1986;46:645–652. doi: 10.1016/0092-8674(86)90339-9. [DOI] [PubMed] [Google Scholar]
- 24.Hobbs MV, Weigle WO, Noonan DJ, Torbett BE, McEvilly RJ, Koch RJ, Cardenas GJ, Ernst DN. J Immunol. 1993;150:3602–3614. [PubMed] [Google Scholar]
- 25.Hase ME, Kuznetsov NV, Cordes VC. Mol Biol Cell. 2001;12:2433–2452. doi: 10.1091/mbc.12.8.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1. HSF1-interacting regions of Tpr are found in the nucleus. HeLa cells were transfected with GFP-Tpr(14–117) or GFP-Tpr(1218–1320) and then subjected to fluorescence microscopy to detect GFP (green channel). DNA was visualized by DAPI staining.