Abstract
We have recently shown that human recombinant BMP-6 (rhBMP-6), given systematically, can restore bone in animal models of osteoporosis. To further elucidate the underlying mechanisms of new bone formation following systemic application of BMPs, we conducted gene expression profiling experiments using bone samples of oophrectomised mice treated with BMP-6. Gene set enrichment analysis revealed enrichment of insulin-like growth factor-I and epidermal growth factor related pathways in animals treated with BMP-6. Significant upregulation of IGF-I and EGF expression in bones of BMP-6 treated mice was confirmed by quantitative PCR. To develop an in vitro model for evaluation of the effects of BMP-6 on cells of human origin, we cultured primary human osteoblasts. Treatment with rhBMP-6 accelerated cell differentiation as indicated by the formation of mineralised nodules by day 18 of culture versus 28–30 days in vehicle treated cultures. In addition, alkaline phosphatase gene expression and activity were dramatically increased upon BMP-6 treatment. Expression of IGF-I and EGF was upregulated in human osteoblast cells treated with BMP-6. These results collectively indicate that BMP-6 exerts its osteoinductive effect, at least in part, through IGF-I and EGF pathways, which can be observed both in a murine model of osteopenia and in human osteoblasts.
Résumé
Nous avons récemment pu mettre en évidence que la BMP-6 (rhBMP-6), administrée de façon systématique, pouvait améliorer la restauration du capital osseux de modèles animaux avec ostéoporose. Nous avons conduit une expérimentation utilisant des souris ovarieactomisées traitées par BMP-6. L’analyse a montré qu’il y avait un apport d’insulin like growth factor et d’épidermal growth factor chez les animaux traités par BMP-6. Pour développer un modèle in vitro nous avons étudié l’effet de la BMP-6 sur les cellules de type ostéoplasties d’origine humaine. Le traitement par BMP-6 accélère la différenciation cellulaire au 18ème jour alors que normalement cette différence est notée aux alentours du 28ème et 30ème jour. De plus, l’expression du gène de la phosphatase alkaline et l’activité sont augmentées par le traitement par la BMP-6, de même en ce qui concerne l’IGF-1 et l’EGF. Ces résultats nous permettent de penser que le BMP-6 a un effet ostéo conducteur notamment pour les pathologies intéressant IGF-1 et EGF. Nous avons observé ces effets dans un modèle animal avec ostéoplastie et sur les ostéoblastes humains.
Introduction
Bone morphogenetic proteins (BMP) are potent local factors that have been shown to have specific functions during organogenesis and embryogenesis, regulate the differentiation of mesenchymal progenitor cells, and promote bone and cartilage formation in bone and cartilage defects, fracture repair, and periodontal diseases [3, 5, 11, 15–17]. BMPs are members of the transforming growth factor-β (TGF-β) superfamily and were first identified by their ability to induce ectopic bone formation in vivo [11].
The function of individual BMPs has been evaluated in vitro using various cell lines, e.g., multipotent progenitor cells, osteoprogenitor cells, osteoblasts, chondroblasts, and osteosarcoma cells [22]. BMPs were shown to be important in osteoblast differentiation [23, 24] and bone formation in vivo, where they, in concert with other cytokines and matrix components, induce sequential cascade of events for chondro-osteogenesis, consisting of chemotaxis, mesenchymal proliferation and differentiation, angiogenesis, and controlled synthesis of extracellular matrix [11, 18, 24].
Recently, we have shown that human recombinant BMP-6 (rhBMP-6), administered systematically, induces new bone formation in both osteoporotic rats and mice [19, 20]. In order to elucidate the underlying mechanism of new bone formation following systemic application of BMP-6, we conducted gene expression profiling in bone tissue of oophrectomised mice following BMP-6 therapy. Primary human osteoblasts served as an in vitro model for evaluation of the in vivo effect of BMP-6.
Materials and methods
Animals
Wild type CD-1 mice were purchased from Charles River, Budapest, Hungary. Animals were maintained in accordance with the NIH Guide for the Care and Use of Laboratory Animals [14]. All protocols were approved by the Institutional Ethics Committee and Ministry of Science Grant Review Committee. In sham mice at 3 months of age ovaries were exteriorised but replaced intact, while bilateral OVX with the dorsal approach was performed in the remaining mice. Animals were left untreated for 3 weeks following surgery to await the development of osteopenia, and then divided into the following groups: (1) sham (n = 10), (2) OVX (n = 10), (3) OVX + BMP-6 (10 μg/kg intravenously, 3 times per week) (n = 10). Human mature BMP-6 was produced in CHO cells and purified as previously described [6, 20]. Therapy started 3 weeks following OVX and continued for the next 9 weeks for bone densitometry measurements (n = 15), or 4 weeks for gene expression analysis (n = 15) when the animals were sacrificed by cervical dislocation.
Bone mineral density (BMD) measurements
To determine the anabolic effect of BMP-6 on bone tissue, ex vivo BMD measurements of lumbar spine, femurs, and tibiae were performed by a PIXImus apparatus (Norland, Medizintechnik, Pforzheim, Germany) according to manufacturer’s instructions (using the mouse-specific software version 1.43).
Cell culture
Normal human osteoblasts were purchased from Cambrex Bioscience (Cambrex, Walkersville, MD, USA). Cells were plated in 12-well dishes at a density of 5,000 cell/cm2 in osteoblast basal growth medium (OBGM) containing 10% foetal bovine serum, ascorbic acid (100 μg/ml), and antibiotics. Media were replaced every 2 days until cells were confluent, and then media were replaced every 2 days until the completion of experiment with differentiation media (OBGM additionally supplemented with 1 μM dexamethasone and 10 mM β-glycerolphosphate). BMP-6 was added at plating and at every feeding in a concentration of 100 ng/ml.
Cell number, alkaline phosphatase activity and in vitro mineralisation
Cells were plated and cultured ±BMP-6 and at subsequent time points (1, 2, 3, 6, 10, 18, 28 and 37 days) were trypsinised, collected, rinsed with PBS and counted on a haemacytometer. Alkaline phosphatase (AP) activity was measured over a 28 day period at specific time points (6, 10, 14, 19, 23 and 28 days) as previously described [22] and expressed as product produced/min/μg protein. Adherent cell cultures from day 19 through day 28 were stained for the presence of minerals using the method of Von Kossa as previously described [4]. Mineralised nodules appeared dark brown and were photographed using a Spot digital camera (Diagnostic Instruments, Inc., Sterling Heights, MN, USA).
RNA isolation and reverse transcription
Total RNA was isolated from the whole murine femur samples using both TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and RNeasy Mini Kit (Qiagen, Hilden, Germany), and from the primary human osteoblasts using only RNeasy Mini Kit. The quality of RNA was analysed on a 2100 Bioanalyser (Agilent Technologies, Palo Alto, CA, USA). One-step quantitative RT-PCR was performed on 2.5 μg (bone samples) or 1 μg (cell culture) of total RNA using the Superscript First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA).
Microarray and gene set enrichment analysis
Microarray analysis was performed using Mouse Genome 430 2.0 GeneChips (Affymetrix, Santa Clara, CA, USA), which contain 45,000 25-mer probe sets. The RNA from murine samples was processed using the standard Affymetrix protocol. Usual quality measures and normalisation for the Affymetrix GeneChip were used in the experiments. All arrays were run in the same core facility at the Center for Functional Genomics, School of Medicine, University of Zagreb.
In an effort to test the functional correlation of gene sets that might be systemically altered in mice treated with BMP-6, we applied a statistical method called gene set enrichment analysis [21]. Using gene expression profiles of OVX mice treated with BMP-6 we calculated the enrichment scores (ES) of 472 predefined gene sets (MSigDB1, Broad Institute, Boston, USA) containing genes involved in specific metabolic and signalling pathways, and 50 sets containing genes coregulated in response to genetic and chemical stimuli. Estimated statistical significance (nominal P value) of the ES was also calculated by using an empirical phenotype-based permutation test procedure that preserves the complex correlation structure of the gene expression data. A nominal P value of P < 0.05 was taken as a cut-off value in the analysis.
Gene expression analysis by quantitative PCR
For experiments performed on murine samples, the gene expression of interest was measured using the LightCycler FastStart DNA Master SYBR Green kit (Roche Diagnostics, Mannheim, Germany) in the LightCycler instrument (Roche Diagnostics, Mannheim, Germany). The comparative CT method (ΔΔCT) was used for relative quantification of gene expression [10]. Expression of four housekeeping genes was analysed and glyceraldehyde-3-phosphate dehydrogenase was chosen as the most stable housekeeping gene. Results are represented as fold change of comparative expression level to OVX group of animals. Sequences of primers are shown in Table 1.
Table 1.
List of primers used in quantitative PCR reactions for gene expression analysis in murine femurs
| Gene | Forward Primer | Reverse primer |
|---|---|---|
| AP | 5′-CGGACATCATGAGGGTAAGG-3′ | 5′-GAG ACA TTT TCC CGT TCACC-3′ |
| BMP 4 | 5′-GACTTCGAGGCGACACTTCTA-3′ | 5′-GCCGGTAAAGATCCCTCATGTAA-3′ |
| BMP 6 | 5′-CAACGCCCTGTCCAATGAC-3′ | 5′-ACTCTTGCGGTTCAAGGAGTG-3′ |
| EEF1 | 5′-ACCGCACCCCTGAATTTCTC-3′ | 5′-CTGGCGTACTTCCTCGCAG-3′ |
| EGF | 5′-TTGGTATGCAAGGATGTGTC-3′ | 5′-CCACTTTGCGAAGTAACTTGGTA-3′ |
| GAPDH | 5′-AGGTCGGTGTGAACGGATTTG-3′ | 5′-TGTAGACCATGTAGTTGAGGTCA-3′ |
| IGF2bp3 | 5′-GTTGAGCACTCGGTCCCTAAA-3′ | 5′-CCGTTTCCGAATCCGTGTT-3′ |
| IGF-I | 5′-GACCGAGGGGCTTTTACTTCA-3′ | 5′-GGACGGGGACTTCTGAGTCTT-3′ |
| Osteocalcin | 5′-CTGACCTCACAGATCCCAAGC-3′ | 5′-TGGTCTGATAGCTCGTCACAAG-3′ |
| Collagen type I | 5′-TGTGTGCGATGACGTGCAAT-3′ | 5′-GGGTCCCTCGACTCCTACA-3′ |
| β-2-microglobulin | 5′-TTCTGGTGCTTGTCTCACTGA-3′ | 5′-CAGTATGTTCGGCTTCCCATTC-3′ |
| β-actin | 5′-GTGGGCCGCTCTAGGCACCAA-3′ | 5′-CTCTTTGATGTCACGCACGATTTC-3′ |
AP alkaline phosphatase, BMP bone morphogenetic protein, EE1G eukaryotic translation elongation factor 1 gamma, GAPDH glyceraldehyde-3-phosphate dehydrogenase, IGF insulin growth factor, bp binding protein
Total RNA from human osteoblast cultures, isolated at various time points (1, 2, 6, 10, 14, 18, 23, 28 days), was analysed using different q-PCR methods to compensate for lower RNA yield and accordingly weaker gene expression. Human primer/probe sets (assay numbers available on demand) were purchased as a 20X assay-on-demand mix from Applied Biosystems (Foster City, CA, USA). Data were normalised to the expression of Acidic Ribosomal Binding Protein which showed no change in mRNA expression with BMP-6 treatment. Data are represented as fold change of comparative expression level as compared either to the first day of culture or to the untreated cells.
Statistical analysis
Densitometric data measurement and changes in gene expression in mice were analysed with one way ANOVA with one-sided Dunett-t post hoc test against the OVX animals test (p < 0.05). Changes in gene expression in the cell culture were analysed with the student t-test (p < 0.05).
Results
BMP-6 induces new bone formation in OVX mice and enhances differentiation of human osteoblasts
Bone mineral density (BMD) measurements showed that systemic administration of BMP-6 for a period of 9 weeks restored bone volume of both the peripheral and the axial skeleton of OVX mice. BMD values of tibiae, femora and vertebrae were increased 12.8%, 10% and 7.4%, respectively, in mice treated with BMP-6 (Fig. 1).
Fig. 1.
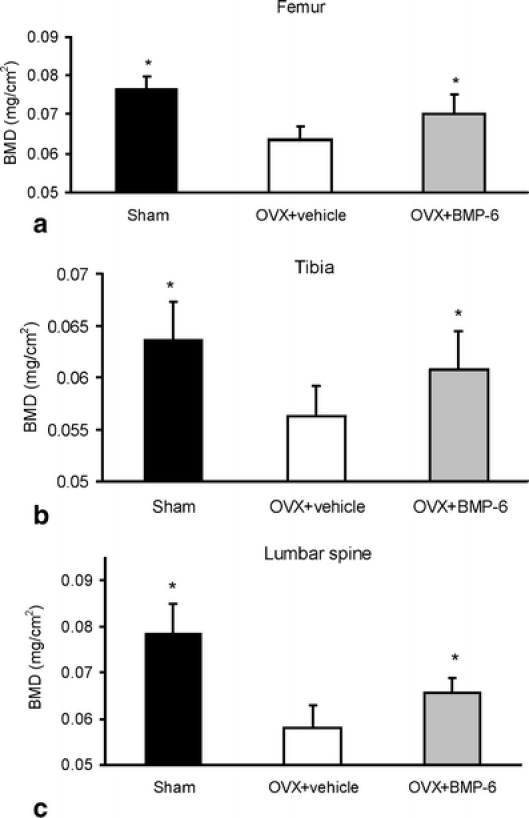
Ex vivo BMD values of sham, OVX and BMP-6 treated mice as analysed by PIXImus. Within 9 weeks of therapy BMP-6 restored bone volume of tibiae (a), femurs (b) and lumbar spine (c). *Significantly different from OVX control mice (p < 0.05, ANOVA, Dunnett test)
To develop an in vitro model for evaluation of the effect of BMP-6 on the cells of human origin, we used the primary human osteoblasts (HOB). After an intensive proliferation period (first 10 days of culture), cell number remained relatively stable between day 10 and day 28 of culture. No statistically significant difference was seen between BMP-6 and untreated cultures indicating that BMP-6 does not effect the cell growth (Fig. 2a). In order to determine the effect of BMP-6 treatment on the differentiation of HOBs, alkaline phosphatase activity was measured and increased from day 14 to day 23 from 2- to 2.7-fold in BMP-6 treated cultures when compared to untreated cells (Fig. 2b). Since numerous osteoblastic cell lines spontaneously form mineralised nodules during the process of cell differentiation and matrix mineralisation, we tested whether BMP-6 enhances the nodule formation in the primary human osteoblasts. We found that following BMP-6 treatment the cells formed mineralised nodules between day 18 and 23 of culture (Fig. 2c). Cell cultures not exposed to BMP-6 did not show the formation of mineralised nodules until day 30 of culture (data not shown).
Fig. 2.
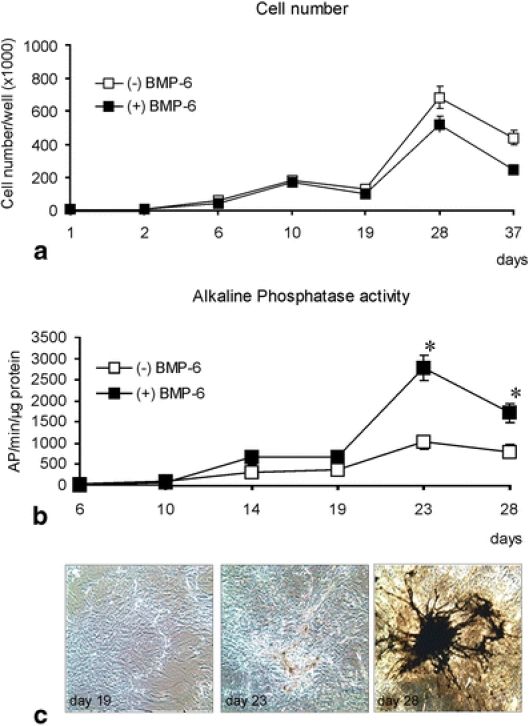
Primary human osteoblasts upon BMP-6 treatment. (a) Cell number during the culture period. BMP-6 did not significantly affect cell growth when compared with untreated cultures. (b) An increase in alkaline phosphatase activity is seen beginning at day 10 of culture in BMP-6 treated cultures. This activity appeared to peak at day 23 of culture. *Significantly different from untreated cell cultures (p <0.05, student t-test). (c) In vitro human osteoblast cultures were Von Kossa stained. High magnification views represent BMP-6 treated human osteoblasts at days 19, 23 and 28 days of culture. Areas of mineralisation appear brown. Mineralisation usually appeared at approximately day 19 of culture
Effect of BMP-6 on markers of osteogenic activity
Gene expression analysis of common markers of osteogenic activity (AP, collagen I and BMPs) confirmed that BMP-6 induces osteoblast differentiation both in vivo and in vitro. In mice, 4 weeks of BMP-6 therapy induced expression of AP above the sham value (Fig. 3a). No change in AP gene expression was observed when the comparison was made between BMP-6 treated cultures and untreated cultures until approximately day 19 of culture where the difference was 3.2-fold higher in the BMP-6 treated cultures as compared to control cultures. By day 23 of culture, the difference in AP mRNA levels had risen to 4.3-fold and to 6-fold by day 28 of culture (Fig. 3e).
Fig. 3.
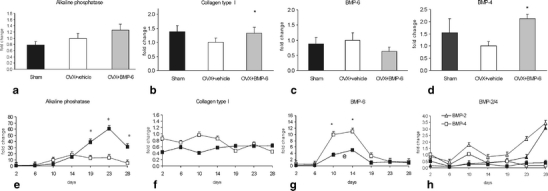
Gene expression of osteogenic markers in mice and human primary osteoblast treated by BMP-6. Total RNA was isolated from femurs of mice (n = 15) (a–d) following 4 weeks of BMP-6 therapy and from human osteoblasts at certain time points (day 2,6, 10, 14, 19, 23 and 28) of culture (e–h). Results are represented as fold change of comparative expression level to OVX group of animals (a–d) or as fold change as compared to the first day of culture period (e–h). *Significantly different from OVX control mice (p < 0.05, ANOVA, Dunnett test) (a–d) or control cultures (p<0.05, student test) (e–h). HOBs were either left untreated (□,▵) or were BMP-6 treated (■,▴)
In mice, collagen type I expression was increased upon BMP-6 treatment (Fig. 3b). On the contrary, expression of this common marker of differentiated osteoblasts showed no difference in the expression levels when untreated and BMP-6 treated cultures were compared (Fig. 3f). In mice, BMP-6 expression was not changed following OVX, but was reduced 4 weeks after BMP-6 therapy (Fig. 3c). Endogenous BMP-6 expression increased upon differentiation of HOBs, and addition of exogenous BMP-6 resulted in a decrease of BMP-6 transcripts expression (Fig. 3g) indicating the ability of BMP-6 to auto-regulate its own expression level. Expression of BMP-4 was reduced in OVX animals and significantly increased after BMP-6 therapy (Fig. 3d). On the contrary, in HOBs no change in the expression of BMP-4 was observed in either treated or untreated cells during the period of 30 days (Fig. 3h). We observed no change in the BMP-2 expression in OVX and BMP-6 treated mice (data not shown). Similarly, in HOBs there was an analogous pattern of increased BMP-2 expression with BMP-6 treatment as compared to control cultures (Fig. 3h).
Activation of IGF-I and EGF pathways upon BMP-6 treatment
To elucidate the underlying mechanisms of the new bone formation following systemic application of BMP-6 we analysed the gene expression profile in OVX mice receiving BMP-6. In order to ascertain whether BMP-6 can activate formation related pathways in OVX animals, we performed BMP-6>OVX analysis using GSEA (Table 2). BMP-6 treatment specifically enriched IGF-I related pathways, including the IGF-I MTOR pathway and insulin signalling. The epidermal growth factor (EGF) pathway was also highly enriched, indicating that BMP-6 might at least in part achieve its effects through the activation of IGF-I and EGF. OVX>BMP-6 comparison revealed increased enrichment of the interleukin-1 receptor pathway, which indicates that BMP-6 treatment reduced the cytokine production in OVX animals. This could also be substantiated by an increased enrichment of cell adhesion molecule activity pathway in OVX, which includes known effectors of immune response, such as intercellular adhesion molecule 1 (ICAM-1). WNT signalling was also highly enriched, specifically the WNT beta catenin and WNT signalling pathways.
Table 2.
Functional sets of genes specifically altered in BMP-6 treated mice
| Pathway name | ES | |
|---|---|---|
| OVX vs BMP-6 | Wnt_Signaling | 0.348 |
| il1rPathway | 0.330 | |
| ST_Wnt_beta_catenin_Pathway | 0.322 | |
| p38mapkPathway | 0.310 | |
| cell_adhesion | 0.306 | |
| lairPathway | 0.300 | |
| FRASOR_ER_UP | 0.239 | |
| BMP-6 vs OVX | carm-erPathway | 0.399 |
| igf1mtorPathway | 0.239 | |
| etsPathway | 0.216 | |
| egfPathway | 0.170 | |
| igf1rPathway | 0.147 |
GSEA results of functional sets of genes altered following BMP-6 therapy. n = 5 per group. ES enrichment score. Estimated statistical significance of p < 0.05 was taken as a cut-off value in the analysis
Effect of BMP-6 on expression of IGF-I and EGF was subsequently determined by quantitative PCR. Analysis of the gene expression in murine bones confirmed the results obtained by microarray. It was shown that IGF-I, IGF-II binding protein 3 and EGF expression was reduced following OVX (Fig. 4a–c) and that BMP-6 therapy corrected altered gene expression levels. In human osteoblasts it was confirmed that BMP-6 induces expression of both EGF and IGF-I. The peak of the IGF-I expression was observed in the beginning of the treatment (day 7), followed by decline, but the expression was still elevated compared to vehicle cultures. Expression of EGF was also increased upon BMP-6 treatment, and again on days 12 and 23 of the culture period (Fig. 4d,e).
Fig. 4.
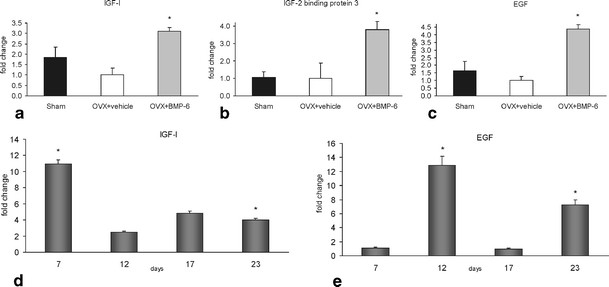
Gene expression of IGF-I (a), EGF (b) and IGFbp3 (c) in mice, and IGF-I (d) and EGF (e) in human primary osteoblast treated with BMP-6. Total RNA was isolated from femurs of mice (n = 15) (a–c) following 4 weeks of BMP-6 therapy and from human osteoblasts at certain culturing time points (day 7, 12, 17, 23) (d, e). Results are represented as fold change of comparative expression level to OVX group of animals (a–c) or as fold change as compared to the untreated cultures (d, e). *Significantly different from OVX control mice (p < 0.05, ANOVA, Dunnett test)
Discussion
In this study we explored the mechanisms by which BMP-6 induces new bone formation in OVX mice and effects the differentiation of human osteoblasts. It was shown that BMP-6 induced enrichment of IGF-1 and EGF related pathways in both experimental models.
The activities of bone formation and resorption are regulated by systemic hormones and local factors produced in bone [2]. IGFs are among the most important regulators of bone cell function because of their abundance and their proven anabolic effects on the skeleton [1, 12, 13]. IGF-I regulates bone formation and remodelling by induction of early osteoblast gene expression in the differentiation of mature osteoblastic and preosteoblastic cells [8]. It was shown that BMP-7 can act locally by modulating the IGF regulatory system, suggesting that the mitogenic/differentiative effect of BMP-7 on human bone cells may in part be mediated via IGF-II [7]. Activation of IGF-I and markers of early osteoblastic differentiation by BMP-6 in both mice and cell cultures of human osteoblasts indicates that BMP-6 might act on bone at least partially via the IGF-I-related pathways and that this mechanism might be regulated by mutual interactions.
In addition, BMP-6 upregulation of EGF in bones and osteoblasts has not been previously demonstrated and serves as an additional collaboration pathway for transmitting its anabolic bone effect. Recently, the importance of EGF and EGF-like ligands in regulating bone growth and modelling has been discovered [25], including the fact that EGF stimulates the differentiation of human mesenchymal stem cells into bone-forming cells as a single molecule picked by a full scale proteomic analysis [9]. This finding might explain how BMP-6 forms bone in the bone marrow of OVX mice lacking the BMP-6 gene [19]. Thus, EGF might contribute to the BMP-6 anabolic effect on bone in vivo, which has also been confirmed in this study on human osteoblasts.
These results collectively indicate that BMP-6 exerts its osteoinductive effect at least in part through the IGF-1 and EGF pathways, which was observed both in a murine model of osteopenia and in the human osteoblastic cell line and could serve as a basis for developing new bone-forming drugs based on BMP-6 biology.
Acknowledgments
The authors acknowledge the great help of Djurdjica Car and Mirjana Palcic for performing animal studies.
Footnotes
W.A. Grasser and I. Orlic equally contributed to the results of this manuscript.
Contributor Information
S. Vukicevic, Phone: +385-1-4566812, Email: vukicev@mef.hr
V. M. Paralkar, Phone: +1-860-4411947, Email: Vishwas.M.Paralkar@pfizer.com
References
- 1.Bagi C, van der Meulen M, Brommage R, Rosen D, Sommer A (1995) The effect of systemically administered rhIGF-I/IGFBP-3 complex on cortical bone strength and structure in ovariectomized rats. Bone 16:559–565 [DOI] [PubMed]
- 2.Baylink DJ, Finkelman RD, Mohan S (1993) Growth factors to stimulate bone formation. J Bone Miner Res 2:S565–S572 [DOI] [PubMed]
- 3.Bilic R, Simic P, Jelic M, Stern-Padovan R, Dodig D, van Meerdervoort HP, Martinovic S, Ivankovic D, Pecina M, Vukicevic S (2006) Osteogenic protein-1 (BMP-7) accelerates healing of scaphoid non-union with proximal pole sclerosis. Int Orthop 30:128–134 [DOI] [PMC free article] [PubMed]
- 4.Franceschi RT, Iyer BS, Cui Y (1994) Effects of ascorbic acid on collagen matrix formation and osteoblast differentiation in murine MC3T3-E1 cells. J Bone Miner Res 9:843–854 [DOI] [PubMed]
- 5.Jelic M, Pecina M, Haspl M, Kos J, Taylor K, Maticic D, McCartney J, Yin S, Rueger D, Vukicevic S (2001) Regeneration of articular cartilage chondral defects by osteogenic protein-1 (bone morphogenetic protein-7) in sheep. Growth Factors 19:101–113 [DOI] [PubMed]
- 6.Jones WK, Richmond EA, White K, Sasak H, Kusmik W, Smart J, Oppermann H, Rueger DC, Tucker RF (1994) Osteogenic protein-1 (OP-1) expression and processing in Chinese hamster ovary cells: isolation of a soluble complex containing the mature and pro-domains of OP-1. Growth Factors 11:215–225 [DOI] [PubMed]
- 7.Knutsen R, Honda Y, Strong DD, Sampath TK, Baylink DJ, Mohan S (1995) Regulation of insulin-like growth factor system components by osteogenic protein-1 in human bone cells. Endocrinology 136:857–865 [DOI] [PubMed]
- 8.Koch H, Jadlowiec JA, Campbell PG (2005) Insulin-like growth factor-I induces early osteoblast gene expression in human mesenchymal stem cells. Stem Cells Dev 14:621–631 [DOI] [PubMed]
- 9.Kratchmarova I, Blagoev B, Haack-Sorensen M, Kassem M, Mann M (2005) Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science 308:1472–1477 [DOI] [PubMed]
- 10.Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408 [DOI] [PubMed]
- 11.Martinovic S, Simic P, Borovecki F, Vukicevic S (2004) Biology of bone morphogenetic proteins. In: Vukicevic S, Sampath K (eds) Bone morphogenetic proteins: from laboratory to clinical practice. Birkhäuser, Basel, pp 45–72
- 12.McCarthy TL, Centrella M, Canalis E (1989) Insulin-like growth factor (IGF) and bone. Connect Tissue Res 20:277–282 [DOI] [PubMed]
- 13.Mohan S (1993) Insulin-like growth factor binding proteins in bone cell regulation. Growth Reg 3:67–70 [PubMed]
- 14.National Academy of Sciences, Institute of Laboratory Animal Resources Commission on Life Sciences (1996) Guide for the care and use of laboratory animals. National Academy Press, Washington, DC
- 15.Pecina M, Giltaij LR, Vukicevic S (2001) Orthopaedic applications of osteogenic protein-1 (BMP-7). Int Orthop 25:203–208 [DOI] [PMC free article] [PubMed]
- 16.Pecina M, Haspl M, Jelic M, Vukicevic S (2003) Repair of a resistant tibial non-union with a recombinant bone morphogenetic protein-7 (rh-BMP-7). Int Orthop 27:320–321 [DOI] [PMC free article] [PubMed]
- 17.Pecina M, Jelic M, Martinovic S, Haspl M, Vukicevic S (2002) Articular cartilage repair: the role of bone morphogenetic proteins. Int Orthop 26:131–136 [DOI] [PMC free article] [PubMed]
- 18.Reddi AH (1997) Bone morphogenetic proteins: an unconventional approach to isolation of first mammalian morphogens. Cytokine Growth Factor Rev 8:11–20 [DOI] [PubMed]
- 19.Simic P, Buljan-Culej J, Orlic I, Borovecki F, Vukicevic S (2005) BMP-6 restores bone in osteoporotic aged rats and, unlike estradiol and PTH, restores trabecular bone in ovariectomized BMP-6 knockout mice. J Bone Miner Res 20:S8
- 20.Simic P, Culej JB, Orlic I, Grgurevic L, Draca N, Spaventi R, Vukicevic S (2006) Systemically administered bone morphogenetic protein-6 restores bone in aged ovariectomized rats by increasing bone formation and suppressing bone resorption. J Biol Chem 281:25509–25521 [DOI] [PubMed]
- 21.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102:15545–15550 [DOI] [PMC free article] [PubMed]
- 22.van der Horst G, van Bezooijen RL, Deckers MM, Hoogendam J, Visser A, Lowik CW, Karperien M (2002) Differentiation of murine preosteoblastic KS483 cells depends on autocrine bone morphogenetic protein signaling during all phases of osteoblast formation. Bone 31:661–669 [DOI] [PubMed]
- 23.Vukicevic S, Luyten FP, Reddi AH (1989) Stimulation of the expression of osteogenic and chondrogenic phenotypes in vitro by osteogenin. Proc Natl Acad Sci USA 86:8793–8797 [DOI] [PMC free article] [PubMed]
- 24.Wozney JM (1992) The bone morphogenetic protein family and osteogenesis. Mol Reprod Dev 32:160–167 [DOI] [PubMed]
- 25.Xian CJ (2007) Roles of epidermal growth factor family in the regulation of postnatal somatic growth. Endocr Rev 28:284–296 [DOI] [PubMed]


