Abstract
The combination of recombinant human bone morphogenetic protein-2 (rhBMP-2) on an absorbable collagen sponge (ACS) carrier has been shown to induce bone formation in a number of preclinical and clinical investigations. In 2002, rhBMP-2/ACS at a 1.5-mg/cc concentration (INFUSE® Bone Graft, Medtronic Spinal and Biologics, Memphis, TN) was FDA-approved as an autograft replacement for certain interbody spinal fusion procedures. In 2004, INFUSE® Bone Graft was approved for open tibial fractures with an intermedullary (IM) nail fixation. Most recently, in March 2007, INFUSE® Bone Graft was approved as an alternative to autogenous bone grafts for sinus augmentations, and for localised alveolar ridge augmentations for defects associated with extraction sockets. The culmination of extensive preclinical and clinical research and three FDA approvals makes rhBMP-2 one of the most studied, published and significant advances in orthopaedics. This review article summarises a number of clinical findings of rhBMP-2/ACS, including the FDA-approved investigational device exemption (IDE) studies used in gaining the aforementioned approvals.
Résumé
L’utilisation de la BMP (rhBPM-2) sur une éponge de collagène a des effets positifs sur l’ostéogénèse. En 2002, ce produit avec un dosage de 1.5 mg/cc (INFUSE® Bone Graft, Medtronic Spinal and Biologics, Memphis, Tenn), a été approuvé par la FDA comme un supplétif de l’auto greffe pour, certains cas d’arthrodèses rachidiennes. En 2004 ce produit (INFUSE®) a également été approuvé par la FDA pour les fractures ouvertes de jambes, traitées par clous centro-médullaires. De façon plus récente, en mars 2007, ce produit a été également autorisé comme une alternative au traitement des ostéolyses et défects osseux associés à l’ablation d’une cupule cotyloïdienne. L’association de ces autorisations et des recherches cliniques et pré-cliniques sur la rhBMP-2 permettent une avance significative des connaissances en chirurgie orthopédique ainsi que la revue des différents travaux et des autorisations de la FDA.
Introduction
Surgeons conducting bone grafting procedures have been searching for a bone graft substitute to avoid harvesting autogenous bone because of its associated complications. The identification and development of recombinant human bone morphogenetic protein-2 (rhBMP-2) has lead to the commercial availability for the first time of an osteoinductive autograft replacement (INFUSE® Bone Graft, Medtronic Spinal and Biologics, Memphis, TN). Prior to this, only osteoconductive ceramic-based materials were commercially available but they have limited clinical utility, since they have to be used in combination with autograft bone to be effective. INFUSE® Bone Graft (Fig. 1) is cleared for use in interbody spine fusion, fresh tibial fractures, and oral maxillofacial bone grafting procedures. rhBMP-2 is the most researched and published bone graft material and is arguably one of the most significant advances in orthopaedics. This review article summarises the clinical findings of rhBMP-2 in spine, orthopaedic and oral maxillofacial applications. All of these studies involved the use of INFUSE® Bone Graft, which contains rhBMP-2 at a concentration of 1.5 mg/cc delivered on an absorbable collagen sponge (ACS) (Table 1).
Fig. 1.
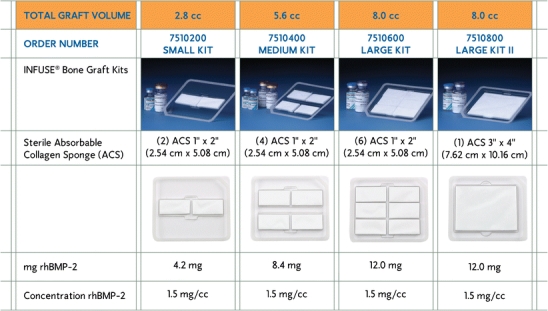
INFUSE® Bone Graft kits and contents. All four kits (small, medium, large and large II) are FDA-approved for the spine and oral maxillofacial applications, while only the large II kit is approved for orthopaedic trauma applications
Table 1.
Selected studies examining INFUSE® Bone Graft in spine, orthopaedic trauma and oral maxillofacial indications
| Author | Journal | Year | |
|---|---|---|---|
| Spine | |||
| Anterior lumbar interbody fusion (ALIF) | Burkus et al. [8] | J Bone Joint Surg Am | 2005 |
| Burkus et al. [7] | J Spinal Disord Tech | 2003 | |
| Burkus et al. [6] | J Spinal Disord Tech | 2002 | |
| Posterior lumbar interbody fusion (TLIF) | Haid et al. [11] | Spine J | 2004 |
| Transforaminal lumbar interbody fusion (TLIF) | Schwender et al. [19] | J Spinal Disord Tech | 2005 |
| Mummaneni et al. [16] | J Neurosurg Spine | 2004 | |
| Anterior cervical discectomy and fusion (ACDF) | Baskin et al. [1] | Spine | 2003 |
| Posterolateral fusion | Glassman et al. [10] | Spine J | 2007 |
| Singh et al. [20] | J Spinal Disord Tech | 2006 | |
| Orthopaedic trauma | |||
| Open tibia fractures | BESTT Study Group [2] | J Bone Joint Surg Am | 2002 |
| Swiontkowski et al. [21] | J Bone Joint Surg Am | 2006 | |
| Jones et al. [12] | J Bone Joint Surg Am | 2006 | |
| Segmental defects | Schwartz and Hicks [18] | J Orthopaedics | 2006 |
| Other | Riedel and Valentin-Opran [17] | Orthopedics | 1999 |
| Oral maxillofacial | |||
| Sinus augmentation | Boyne et al. [3] | Int J Periodontics Restorative Dent | 1997 |
| Boyne et al. [5] | J Oral Maxillofac Surg | 2005 | |
| Alveolar ridge augmentation | Fiorellini et al. [9] | J Periodontol | 2005 |
Spinal applications
rhBMP-2 has been studied extensively in preclinical spine fusion models in several species, including non-human primates [13, 14]. These studies consistently showed rhBMP-2 to be equivalent and, in many cases, superior to autogenous bone. In 2002, INFUSE® Bone Graft was approved by the US Food and Drug Administration (FDA) as a replacement for autogenous bone graft in anterior lumbar interbody fusion (ALIF), used in combination with the LT-CAGE® Lumbar Tapered Fusion Device (Medtronic Spinal and Biologics, Memphis, TN). Its approval was based on the results of a prospective, randomised, multi-centre clinical trial. The trial involved 279 patients with degenerative disc disease (DDD), randomised to receive either rhBMP-2/ACS or autogenous bone from the iliac crest. Based on computed tomography (CT) evaluation, Burkus et al. [6] reported 2-year fusion rates of 94.5% versus 88.7% for rhBMP-2/ACS and autograft, respectively. There was no statistical difference at any of the follow-up intervals in Oswestry, back pain and leg pain scores, and neurological status improved in both treatment groups with similar outcomes. In the autograft control group, a 5.9% adverse event rate related to the autograft harvest site occurred and 32% reported graft site discomfort at 2 years. A subsequent integrated analysis by Burkus et al. [7] of two separate ALIF LT-CAGE® clinical studies was performed using an analysis of covariance to adjust for preoperative variables in a total of 679 patients. Of these patients, 277 had their cages implanted with rhBMP-2/ACS and the rest with iliac crest autograft. The patients treated with rhBMP-2/ACS had statistically superior outcomes with regards to length of surgery, blood loss, hospital stay, reoperation rate, median time to return to work, Oswestry Disability Index scores, physical component scores and pain index of the SF-36 (Short Form, 36 questions) scale, and fusion rate at 6, 12 and 24 months (P < 0.05).
Burkus et al. [8] also studied rhBMP-2/ACS in ALIF procedures using structural cortical allografts. This study was a prospective, randomised, multi-centre study of 131 patients randomised to receive threaded allograft dowels filled with either rhBMP-2/ACS or autograft bone from the iliac crest. Fusion rates (P < 0.001), Oswestry Disability Index scores, SF-36 physical component summary scores and low back and leg pain scores (P < 0.05) were significantly better in the rhBMP-2/ACS group.
Spine surgeons began to develop surgical techniques for conducting interbody fusion procedures through posterior lumbar interbody fusion (PLIF) and, later, transforaminal lumbar interbody fusion (TLIF) procedures. Haid et al. [11] reported on a series of 67 patients with single-level DDD treated with cylindrical threaded cages filled with either rhBMP-2/ACS or iliac crest autograft using a PLIF procedure. At 2 years, the rhBMP-2/ACS fusion rate was 92.3% versus only 77.8% for the autograft control patients. Significantly more bone was found outside the disc space in the rhBMP-2/ACS-treated patients but it was not correlated to a recurrence or increase in leg pain from the postoperative state. All other clinical outcome parameters were the same between the two groups. Mummaneni et al. [16] reported on their experience with rhBMP-2/ACS using a TLIF procedure. Of the 40 patients enrolled, 19 received iliac-crest-filled interbody cages and 21 received rhBMP-2/ACS-filled cages. For the rhBMP-2/ACS group, they placed only 1.4 cc of rhBMP-2/ACS in the anterior portion of the prepared disc space before inserting an additional 1.4 cc of rhBMP-2/ACS in the interbody cage. The mean follow-up period was 9 months and only one patient in each group was determined not to be fused. Schwender et al. [19] obtained a 100% fusion rate in a series of 49 patients using a minimally invasive TLIF procedure. Two patients developed radiculopathies postoperatively, one because of graft dislodgement and a second from contralateral neural foraminal stenosis. Both were resolved with reoperations.
Baskin et al. [1] reported on a multi-centre, prospective, randomised cervical fusion clinical study with the CORNERSTONE SR® allograft ring (Medtronic Spinal and Biologics, Memphis, TN) filled with either rhBMP-2/ACS or iliac crest autograft and an ATLANTIS® anterior cervical plate (Medtronic Spinal and Biologics, Memphis, TN). The study consisted of 33 patients with DDD requiring one- or two-level fusions. In this study, only 0.4 cc of the rhBMP-2/ACS was placed inside each allograft ring to ensure filling without over-packing the sponge. All of the patients evaluated had solid fusions. At 2 years, the rhBMP-2/ACS group had mean improvement superior to that of the autograft control group in neck disability and arm pain scores (P < 0.03). No difference in soft tissue swelling was found between the two study groups when matching the rhBMP-2/ACS volume to the small internal volume of the interbody spacer.
More recently, Glassman et al. [10] reported on the use of rhBMP-2/ACS in single- and multi-level posterolateral spine fusions. They reported retrospectively on 91 patients treated with rhBMP-2/ACS combined with a bulking agent to give the ACS sponge some compression resistance. A large INFUSE® kit (8.0 cc) was wrapped around either local autograft bone or a graft extender. Two independent orthopaedic spine surgeons determined the fusion rate to be 93.4% based on CT evaluations. When they compared the fusion rate of just the primary one-level cases to a comparison group of 35 one-level-autograft-treated patients, the fusions rates were 95.8% versus 88.6%, respectively. Singh et al. [20] also used one large INFUSE® kit in combination with iliac crest autograft in posterolateral spine fusions. They reported on a prospective, single-institution, clinical case-matched, radiographic, cohort study of 52 patients. Based on 2-year CT evaluation, 97% of the rhBMP-2/ACS supplemented autograft patients were classified as fused compared to only 77% of the autograft-only control patients (P < 0.05).
Orthopaedic trauma applications
The approval of INFUSE® Bone Graft for use in open tibia fractures in 2004 was the culmination of over a decade of preclinical and clinical development. By the early 1990s, preclinical research had clearly demonstrated the capability of rhBMP-2/ACS to induce new bone formation in a number of different orthotopic locations in animal models ranging from rats to non-human primates. With compelling preclinical data firmly established, the next challenge was to identify an appropriate treatment group for clinical investigations.
This challenge was addressed through a prospective 86-patient observational study to understand and document the standard of care and outcomes in the surgical management of tibial fractures [17]. The analysis of patients who had open tibia fractures treated with intermedullary (IM) nails revealed that 41% of the patients required a second surgical intervention. Based on the potential for clinical benefit, this treatment group was selected for follow-up studies with rhBMP-2/ACS. The first of these was a pilot study to assess the safety and feasibility of applying rhBMP-2/ACS in open tibial fractures. Fractures healed in 9 of the 12 patients treated with rhBMP-2/ACS without the need for further intervention. This included 8 of 8 patients who received an IM nail for fracture fixation. These data were used to design and support a larger pivotal clinical trial.
The pivotal study was an international investigation performed by a group of surgeons collectively named the BESTT (BMP-2 Evaluation in Surgery for Tibial Trauma) Study Group [2]. A total of 450 patients from 11 countries were enrolled in this prospective, randomised, controlled study. Patients with an open tibia fracture were assigned to one of three treatment groups: (1) standard care (IM nailing with routine soft tissue management), (2) standard care plus 0.75 mg/cc rhBMP-2/ACS or (3) standard care plus 1.5 mg/cc rhBMP-2/ACS (INFUSE® Bone Graft). The rhBMP-2/ACS was placed as an onlay over the fracture at the time of definitive wound closure. The primary endpoint measurement was the proportion of patients who required a secondary intervention within 12 months of wound closure. The definition of secondary intervention was conservative with self-dynamisation by screw breakage or recommendation of secondary intervention with or without actual treatment counted as treatment failures.
Outcomes from the pivotal study revealed a dose-dependent decrease in the rate of secondary interventions, with a 44% reduction for patients who received INFUSE® Bone Graft, relative to control patients. Overall, 74% of INFUSE® Bone Graft patients healed without secondary intervention compared to 54% of control patients. Further analyses showed that INFUSE® Bone Graft patients had fewer hardware failures and significantly faster fracture healing than control patients. Finally, in the most severe fracture cases (Gustilo-Anderson type-III), there was a reduced rate of infection in the INFUSE® treatment group. The data from this study led to EMEA (European Agency for the Evaluation of Medicinal Products) approval of rhBMP-2/ACS in 2002 and FDA approval in 2004 for open tibial fractures stabilised with an IM nail [2].
In a follow-up to the BESTT study, Swiontkowski et al. [21] combined the results of the BESTT study with results from a previously unpublished study conducted in the US using the same protocol and conducted a subgroup analysis patients from 59 trauma centres. The subgroup analysis examined both results for patients with the most severe fractures (Gustilo-Anderson type-IIIA or IIIB) and results from patients treated with reamed IM nailing. The results indicated that rhBMP-2/ACS led to a significant reduction in the number of severe fracture patients needing autologous bone grafting and in the number of patients receiving an invasive secondary procedure of any type. This analysis also confirmed the BESTT study results that a demonstrated decreased rate of infection in patients receiving rhBMP-2/ACS.
With the demonstrated ability of rhBMP-2/ACS to induce bone formation in preclinical models and prospective randomised clinical investigations of open tibial fractures, there has been interest in applying rhBMP-2/ACS to other orthopaedic trauma applications. Schwartz and Hicks [18] reported on a retrospective analysis of 18 patients with 19 segmental bone defects treated with rhBMP-2/ACS combined with a calcium sulphate or calcium phosphate bone void filler. Bony union occurred in 16 of 19 defects, with the three failures attributed to patient non-compliance in one case and premature resorption of the calcium sulphate in two cases. In a randomised, controlled, prospective clinical investigation, Jones et al. [12] compared rhBMP-2/ACS combined with cancellous allograft to iliac crest autograft in tibial fractures with critical bone loss. The fractures healed in 13 of 15 patients in the rhBMP-2 group and 10 of 15 in the autograft group without any type of secondary intervention. Adverse events consisted of a higher rate of soft-tissue swelling and erythema in the rhBMP-2 group. However, the rhBMP-2 group had less blood loss and avoided complications associated with iliac crest graft harvest while still achieving a high rate of healing.
Oral maxillofacial applications
In March 2007, INFUSE® Bone Graft was approved by the FDA as an alternative to autogenous bone graft for sinus augmentations, and for localised alveolar ridge augmentations for defects associated with extraction sockets. These applications are the third FDA-approved indication for INFUSE® Bone Graft.
Prior to FDA approval, extensive preclinical and clinical research was performed to examine the feasibility, safety and efficacy of using rhBMP-2/ACS for treating common oral maxillofacial defects. Similar to spine and trauma preclinical research, these studies were performed in a number of animal species [4, 22]. This work demonstrated that rhBMP-2/ACS was effective at inducing viable de novo bone formation capable of implant osseointegration and functional loading. After demonstrating feasibility and efficacy in preclinical studies, clinical investigations were performed.
Boyne et al. [3] performed a feasibility sinus floor augmentation study (n = 12) examining 0.43 mg/cc rhBMP-2 concentration on ACS, which was successful at inducing bone formation in a non-human primate segmental defect model. CT evaluations and histology demonstrated a significant change in height and normal bone formation with the use of rhBMP-2/ACS. Boyne et al. [5] followed up this feasibility sinus floor augmentation study with a larger randomised, controlled, rhBMP-2 dosing study for staged maxillary sinus floor augmentation. In this study, patients were treated with either bone graft (n = 13), 0.75 mg/cc rhBMP-2/ACS (n = 18) or 1.5 mg/cc rhBMP-2/ACS (n = 17). The results demonstrated mean bone height changes from a baseline of 11.29 mm, 9.47 mm and 10.16 mm for the bone graft, 0.75 mg/cc and 1.5 mg/cc treatment groups, respectively. Histological bone core biopsies taken at the time of dental implant placement demonstrated normal bone growth and CT scan bone density readings were comparable between all treatment groups following 6 months of functional loading.
After identifying 1.5 mg/cc of rhBMP-2 as the most effective concentration, a randomised, multi-centre, pivotal study was performed examining the safety and efficacy of INFUSE® Bone Graft in sinus floor augmentations [15]. A total of 160 patients were treated with either 1.5 mg/cc rhBMP-2/ACS (n = 82) or bone graft (n = 78). The bone graft group consisted of autogenous bone alone or in combination with allogeneic bone. The treatment course included the insertion of INFUSE® Bone Graft followed by 4 to 12 months of bone formation, dental implant placement followed by 12 months of osseointegration, and prosthesis placement followed by 12 months of functional loading. CT scans prior to and following implant placement and bone core biopsies for histological analysis were obtained and analysed.
At 6 months post-op, mean changes in the bone height from baseline were 7.83 mm and 9.46 mm for the INFUSE® Bone Graft and bone graft groups, respectively [15]. Histology demonstrated that both groups experienced significant formation of new trabecular bone that was biologically and structurally similar to the host site. After 6 months of functional loading, the INFUSE® Bone Graft resulted in an implant survival rate of 79%, exceeding the study protocol target success rate of 73%. At 12 months of functional loading, the implant success rates for both groups were comparable (P > 0.05). Furthermore, no clinically significant adverse events resulted from the use of INFUSE® Bone Graft.
Another large clinical study performed by Fiorellini et al. [9] examined the efficacy of two doses of rhBMP-2/ACS in 80 patients requiring extraction socket augmentation. An empty control and rhBMP-2/ACS at 0.75 mg/cc or 1.5 mg/cc concentrations were examined. The results demonstrated that the 1.5 mg/cc rhBMP-2/ACS treated sites had about two times the amount of bone compared to the empty control group, preserving ridge height and significantly increasing width at 75%, 50% and 25% of the extraction socket length (ESL). In addition, histology on core bone biopsies showed no differences between the rhBMP-2-induced bone and native bone.
Clinical studies in both maxillary sinus floor augmentations and alveolar ridge augmentation demonstrated that rhBMP-2/ACS at 1.5 mg/cc, INFUSE® Bone Graft, induced significant bone formation suitable for implant placement. The bone induced by rhBMP-2/ACS was found to be biologically similar to native bone and is capable of implant osseointegration and supporting the functional loading of dental prostheses.
Conclusions
Following three separate prospective, Level 1 clinical trials, rhBMP-2/ACS is now commercially available for three FDA-approved clinical indications as INFUSE® Bone Graft (Fig. 2). These trials have demonstrated that rhBMP-2/ACS at a 1.5-mg/cc concentration is equivalent to autogenous bone in both its ability to form de novo bone and as well as clinical outcomes if prepared and used as studied. The tibia fresh fracture and sinus elevation clinical trials both showed the importance of the rhBMP-2 concentration, with the 1.5-mg/cc concentration being more effective than the 0.75-mg/cc concentration. Furthermore, overfilling a contained defect or device with rhBMP-2/ACS can result in unexpected increases in local rhBMP-2 concentration above 1.5 mg/cc and undesired local effects. When used properly, rhBMP-2/ACS can eliminate the need to harvest autogenous bone for grafting procedures, benefiting both the surgeon and patient.
Fig. 2.
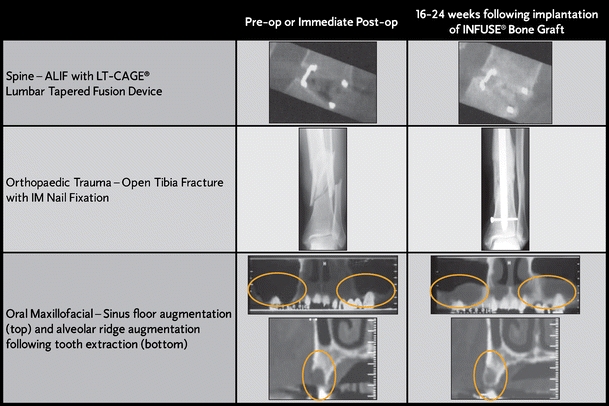
Sample computed tomography (CT) scans showing the efficacy of INFUSE® Bone Graft in each of the three approved indications
References
- 1.Baskin DS, Ryan P, Sonntag V, Westmark R, Widmayer MA (2003) A prospective, randomized, controlled cervical fusion study using recombinant human bone morphogenetic protein-2 with the CORNERSTONE-SR allograft ring and the ATLANTIS anterior cervical plate. Spine 28(12):1219–1225 [DOI] [PubMed]
- 2.BESTT Study Group, Govender S, Csimma C, Genant HK, Valentin-Opran A et al (2002) Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am 84(12):2123–2134 [DOI] [PubMed]
- 3.Boyne PJ, Marx RE, Nevins M, Triplett G, Lazaro E, Lilly LC, Alder M, Nummikoski P (1997) A feasibility study evaluating rhBMP-2/absorbable collagen sponge for maxillary sinus floor augmentation. Int J Periodontics Restorative Dent 17(1):11–25 [PubMed]
- 4.Boyne PJ (2001) Application of bone morphogenetic proteins in the treatment of clinical oral and maxillofacial osseous defects. J Bone Joint Surg Am 83:146–150 [PubMed]
- 5.Boyne PJ, Lilly LC, Marx RE, Moy PK, Nevins M, Spagnoli DB, Triplett RG (2005) De novo bone induction by recombinant human bone morphogenetic protein-2 (rhBMP-2) in maxillary sinus floor augmentation. J Oral Maxillofac Surg 63:1693–1707 [DOI] [PubMed]
- 6.Burkus JK, Gornet MF, Dickman C, Zdeblick TA (2002) Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech 15(5):337–349 [DOI] [PubMed]
- 7.Burkus JK, Heim SE, Gornet MF, Zdeblick TA (2003) Is INFUSE Bone Graft superior to autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J Spinal Disord Tech 16(2):113–122 [DOI] [PubMed]
- 8.Burkus JK, Sandhu HS, Gornet MF, Longley MC (2005) Use of rhBMP-2 in combination with structural cortical allografts: clinical and radiographic outcomes in anterior lumbar spinal surgery. J Bone Joint Surg Am 87(6):1205–1212 [DOI] [PubMed]
- 9.Fiorellini JP, Howell TH, Cochran D, Malmquist J, Lilly LC, Spagnoli D, Toljanic J, Jones A, Nevins M (2005) Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J Periodontol 76(4):605–613 [DOI] [PubMed]
- 10.Glassman SD, Carreon LC, Djurasovic M, Campbell MJ, Puno RM, Johnson JR, Dimar JR (2007) Posterolateral lumbar spine fusion with INFUSE Bone Graft. Spine J 7:44–49 [DOI] [PubMed]
- 11.Haid RW, Branch CL Jr, Alexander JT, Burkus JK (2004) Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J 4:527–539 [DOI] [PubMed]
- 12.Jones AL, Bucholz RW, Bosse MJ, Mirza SK, Lyon TR, Webb LX, Pollak AN, Golden JD, Valentin-Opran A (2006) Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects: a randomized, controlled trial. J Bone Joint Surg Am 88(7):1431–1441 [DOI] [PubMed]
- 13.McKay B, Sandhu HS (2002) Use of recombinant human bone morphogenetic protein-2 in spinal fusion applications. Spine 27:S66–S85 [DOI] [PubMed]
- 14.McKay WF, Peckham S, Scifert J (2006) Biologics to promote spinal fusion. In: Kurtz SM, Edidin AA (eds) Spine technology handbook. Elsevier Academic Press, Boston, Massachusetts, pp 241–279
- 15.Medtronic package insert no. M704819B001 (2007) INFUSE® Bone Graft for Certain Oral Maxillofacial and Dental Regenerative Uses
- 16.Mummaneni PV, Pan J, Haid RW, Rodts GE (2004) Contribution of recombinant human bone morphogenetic protein-2 to the rapid creation of interbody fusion when used in transforaminal lumbar interbody fusion: a preliminary report. J Neurosurg Spine 1:19–23 [DOI] [PubMed]
- 17.Riedel GE, Valentin-Opran A (1999) Clinical evaluation of rhBMP-2/ACS in orthopedic trauma: a progress report. Orthopedics 22(7):663–665 [PubMed]
- 18.Schwartz ND, Hicks BM (2006) Segmental bone defects treated using recombinant human bone morphogenetic protein. J Orthopaedics 3(2):e2
- 19.Schwender JD, Holly LT, Rouben DP, Foley KT (2005) Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech 18:S1–S6 [DOI] [PubMed]
- 20.Singh K, Smucker JD, Gill S, Boden SD (2006) Use of recombinant human bone morphogenetic protein-2 as an adjunct in posterolateral lumbar spine fusion: a prospective CT-scan analysis at one and two years. J Spinal Disord Tech 19(6):416–423 [DOI] [PubMed]
- 21.Swiontkowski MF, Aro HT, Donell S, Esterhai JL, Goulet J, Jones A, Kregor PJ, Nordsletten L, Paiement G, Patel A (2006) Recombinant human bone morphogenetic protein-2 in open tibial fractures: a subgroup analysis of data combined from two prospective randomized studies. J Bone Joint Surg Am 88(6):1258–1265 [DOI] [PubMed]
- 22.Wikesjö UME, Sorensen RG, Wozney JM (2001) Augmentation of alveolar bone and dental implant osseointegration: clinical implications of studies with rhBMP-2. J Bone Joint Surg Am 83(Suppl 1 Pt 2):S136–S145 [PubMed]


