Abstract
Three years ago we published a book chapter on the role of bone morphogenetic proteins (BMPs) in cartilage repair. Since that time our understanding of the function of osteogenic protein-1 (OP-1) or BMP-7 in cartilage homeostasis and repair has substantially improved and therefore we decided to devote a current review solely to this BMP. Here we summarise the information accumulated on OP-1 from in vitro and ex vivo studies with cartilage cells and tissues as well as from in vivo studies of cartilage repair in various animal models. The primary focus is on articular chondrocytes and cartilage, but data will also be presented on nonarticular cartilage, particularly from the intervertebral disc. The data show that OP-1 is a unique growth factor which, unlike other members of the same BMP family, exhibits in addition to its strong pro-anabolic activity very prominent anti-catabolic properties. Animal studies have demonstrated that OP-1 has the ability to repair cartilage in vivo in various models of articular cartilage degradation, including focal osteochondral and chondral defects and osteoarthritis, as well as models of degeneration in intervertebral disc cartilage. Together our findings indicate a significant promise for OP-1 as therapeutic in cartilage repair.
Résumé
Il y a trois ans a été publié le chapitre d’un livre sur le rôle des BMP dans la réparation cartilagineuse. Depuis cette époque, notre compréhension du mécanisme d’action de l’OP-1 ou BMP-7 dans la régulation cartilagineuse et sa réparation ont été améliorées. Nous avons décidé de passer en revue les différents travaux portant sur cette BMP. Nous avons résumé les informations accumulées sur l’OP-1, les études in-vitro et ex-vivo sur les cellules cartilagineuses et sur les tissus à partir de travaux sur la réparation cartilagineuse chez différents modèles d’animaux. Ces travaux ont été essentiellement centrés sur les chondrocytes du cartilage articulaire mais l’on peut également trouver des données sur le cartilage non articulaire particulièrement au niveau du disque inter vertébral. Ces données montrent que l’OP-1 est le seul facteur de croissance. Dans la famille des autres BMP les activités pro-anaboliques sont plus importantes que les propriétés anti anaboliques. Les études animales démontrent que l’OP-1 permet une réparation cartilagineuse parmi les différents modèles de lésions de ce cartilage, outre les lésions cartilagineuses, les lésions d’arthrose et les lésions de dégénérescence des disques intervertébraux. Tout cela nous fait entrevoir une sérieuse avancée thérapeutique grâce à l’OP-1 dans les champs des réparations cartilagineuses.
Introduction
Cartilage regeneration and repair are one of the major obstacles in current orthopaedics. The importance is enormous since osteoarthritis (OA) is a major cause of disability among the adult population in the United States and degenerative disc disease (DDD) is responsible for a significant amount of the chronic back pain. In recent times members of the bone morphogenetic protein (BMP) family, particularly osteogenic protein-1 (OP-1) (also called BMP-7), have demonstrated a great potential as cartilage anabolic factors because of their ability to induce matrix synthesis and promote repair in cartilage.
BMPs are structurally related to the transforming growth factor-β (TGF-β) superfamily [49] and are found in species ranging from worms and insects to mammals. BMPs have wide-ranging biological activities, including the regulation of cellular proliferation, apoptosis, differentiation and migration, embryonic development and the maintenance of tissue homeostasis during adult life [37, 49]. They were originally purified and identified in bone as proteins capable of inducing ectopic endochondral bone formation in subcutaneous implants [47]. However, it is now clear that they are expressed in a variety of tissues including adult articular cartilage [8, 40, 44, 46].
Since the OP-1 gene was identified in the late 1980s, recombinant OP-1 has been produced and extensively characterised both biochemically and biologically. A variety of animal models have been used to evaluate the therapeutic potential in bone repair applications. These studies led to the demonstration of bone repair in humans and resulted in OP-1 receiving regulatory approval as the first commercial BMP.
The past ten years have seen a new era opening for OP-1 in cartilage repair. The data have demonstrated that OP-1/BMP-7 is a cartilage repair factor in addition to its well-known bone induction activity. This article will review the information accumulated thus far from in vitro studies as well as from studies of repair in various animal models. The data show significant promise for OP-1/BMP-7 in cartilage repair and suggest that both articular and disc cartilage applications could become very important for OP-1/BMP-7 in orthopaedics.
In vitro studies
Here we provide an update on what we have learned about OP-1 in articular cartilage, its role in normal and OA tissue homeostasis, and the interaction OP-1 displays with various anabolic and catabolic pathways. Unlike TGF-β and other BMPs, OP-1 up-regulates chondrocyte metabolism and protein synthesis without creating uncontrolled cell proliferation and formation of osteophytes [7, 15, 16, 34, 42]. In chondrocytes, OP-1 stimulates only cartilage-specific extracellular proteins: collagens type II and VI, aggrecan, decorin, fibronectin, hyaluronan [HA], etc. [5, 16, 33, 42]. Furthermore, OP-1/BMP-7 generated normal, functional proteoglycans (PGs), with a hydrodynamic size unaltered by the treatment [32]. It induced similar anabolic responses in normal and OA chondrocytes from both young and old donors and did not cause chondrocyte hypertrophy or changes in chondrocytic phenotype [4, 7, 34, 40].
The anabolic effect of OP-1 extends beyond stimulation of cartilage extracellular proteins and their receptors. OP-1 also modulates the expression of various growth factors (insulin-like growth factor-1 [IGF-1], TGF-β/BMPs) and catabolic mediators (IL-6 family of proinflammatory cytokines) [9, 23]. Moreover, it regulates the synthesis of chondrocyte cytoskeleton proteins (talin, paxillin and focal adhesion kinase; [48]) and enhances gene expression of the anabolic molecule tissue inhibitor of metalloproteinase (TIMP) in normal and OA chondrocytes [9, 15, 50]. There was no stimulating effect of OP-1/BMP-7 on matrix degrading enzymes (MMP-1,3,13 and ADAMTS-4) observed in either normal or OA cells. Contrarily, the expression of these enzymes was inhibited by OP-1/BMP-7 in human chondrocytes [9] and in rat nucleus pulposus, annulus fibrosis and end-plate in disc degeneration model [6].
Our recent findings highlight novel important aspects of the anabolic activity of OP-1/BMP-7: it promotes cell survival [5, 33, 34] and regulates various anabolic pathways active in articular cartilage. Addition of OP-1 into chondrocyte cultures caused an activation of the IGF-1 signalling pathway, namely expression of IGF-1, IGF-1 receptor, IGF-1 binding proteins and other downstream molecules. We believe such an effect on the IGF-1 pathway leads (at least in part) to the restoration of the responses to IGF-1 lost by human chondrocytes with aging [23, 34]. Furthermore, combined application of OP-1 and IGF-1 had a synergistic effect on cell survival and matrix synthesis and triggered a two-fold increase in chondrocyte proliferation rate [34], a new response not observed under the treatment with each individual factor. Unexpectedly, addition of basic FGF to these same two growth factors caused an opposite effect: it inhibited PG synthesis induced by OP-1, IGF-1 or their combination [33]. OP-1 also modulated its own TGF-β/BMP signalling pathway by regulating gene expression of related growth factors, their receptors, binding proteins and transcription factors [9].
One of the most critical properties of OP-1/BMP-7 is its anti-catabolic activity. OP-1/BMP-7 effectively counteracts chondrocyte catabolism (inhibition of PG and HA synthesis) induced by various catabolic mediators, such as proinflammatory cytokines (IL-1 [20] and IL-6) and fragments of cartilage matrix proteins (fibronectin fragments [29] or HA hexasaccharides [43]); inhibits endogenous expression of cytokines [especially IL-6 family of chemokines: IL-6, IL-8, IL-11, leukemia inhibitory factor, (LIF)] and their downstream signalling molecules (receptors, transcription factors, and mitogen-activated kinases [9]); and blocks both a baseline and cytokine-induced expression of MMP-1 and MMP-13 [23]. A novel anti-catabolic effect of OP-1 was recently shown in acute cartilage trauma model in sheep, where the injection of OP-1 significantly reduced the number of apoptotic cells [22], which correlated with the overall improvement in joint morphology and cartilage structural integrity. To our knowledge, OP-1 is the only BMP studied thus far in cartilage that exhibits both broad pro-anabolic and anti-catabolic activities. As we found OP-1 is a better stimulator of PGs than BMP-2, 4, 6 and cartilage-derived morphogenetic proteins (CDMPs) 1 and 2 [44]. Detailed analyses suggest that the inhibition of transcription factors NF-κB and AP-1 by OP-1/BMP-7 leading to the inhibition of MMP-1 and MMP-13 [23], or up-regulation of inhibitors of matrix proteinases [9, 50] may be part of the underlying mechanisms responsible for the anti-catabolic activity of OP-1. Furthermore, in our inflammation model (chondrocyte cultures in high doses of IL-1β) we also discovered a novel mechanism by which OP-1 counteracts IL-1β signalling. It appears that OP-1 has a potential to reverse MAPK signalling via the inhibition of IL-1β-induced P38 phosphorylation.
Endogenous OP-1/BMP-7
Although clinical application of recombinant OP-1 still remains the primary focus, the understanding of the regulation and function of endogenously expressed OP-1 offers significant supporting data. About a decade ago we identified that OP-1 or BMP-7 is synthesised by human articular chondrocytes [7, 8, 44] of different age (foetal, newborn, young and adult) and various morphological appearances (from normal to early and advanced degeneration including OA). OP-1/BMP-7 has also been detected in adult bovine, rabbit, sheep and goat tissues [14, 22, 41, 44]. Its gene and protein expressions are dramatically reduced with cartilage aging and degeneration [7, 40]. With the newly developed in our laboratory ELISA method [7] we found that in normal cartilage the concentration of OP-1/BMP-7 protein is around 50 ng/g dry tissue, which is within the physiological range of the anabolic activity of recombinant human OP-1/BMP-7 (50–200 ng/ml) [7, 44]. OP-1/BMP-7 protein was also detected in synovial fluid (SF) from normal joints and from patients with OA and rheumatoid arthritis (RA) [3], in synovium, ligament, tendon and menisci [44].
The detection of endogenously expressed and synthesised OP-1/BMP-7 in adult articular cartilage of different species provides support for the concept that this growth factor is not only important for skeletal development and bone healing, but it also has a functional role in the maintenance of normal cartilage homeostasis. This was proved by our recent studies with the OP-1/BMP-7 antisense probe [46], where transfection of human adult articular chondrocytes with OP-1/BMP-7 antisense oligos led to significant inhibition of aggrecan mRNA expression and about 50% decrease in PG synthesis. Recovery (add-back) experiments with recombinant OP-1/BMP-7 were able to restore, at least partially, PG synthesis, indicating a direct role of autocrine OP-1/BMP-7 in the regulation of PG metabolism. Histological evaluation of cartilage explants cultured in the presence of OP-1/BMP-7 antisense oligonucleotides revealed a remarkable depletion of PGs, paucity of chondrocytes, initial fibrillation of cartilage surface and the decrease in Safranin O staining in the upper and middle cartilage zones. These data, together with previous results, provide strong evidence for endogenous OP-1/BMP-7 being a critical factor that controls cartilage matrix integrity and is involved in the maintenance of normal cartilage homeostasis.
In addition, the data further suggest that the lack of endogenous OP-1/BMP-7 could predispose cartilage to degenerative processes and make tissue more susceptible to the influence of catabolic agents. The results of gene array analysis on chondrocytes treated with OP-1 antisense [9] clearly demonstrated that the lack of OP-1 expression negatively affected a number of matrix proteins and anabolic pathways (mostly those that were upregulated by recombinant OP-1) and stimulated factors associated with cartilage catabolism (proteinases with various mode of action including caspases and cathepsins, inflammatory cytokines and their signalling mediators).
In summary, knowledge accumulated in our laboratory over the last decade unequivocally points to the crucial role OP-1 plays in the regulation of overall cartilage homeostasis (Fig. 1). In addition to its pro-anabolic properties as a repair factor, it acts as an anti-catabolic agent affecting numerous catabolic pathways. The new information developed over the years significantly advanced our understanding of the mechanisms of OP-1 action in cartilage and provided a strong scientific basis for considering this member of the BMP family as a proper therapeutic approach for cartilage repair and regeneration in post-traumatic and degenerative OA.
Fig. 1.
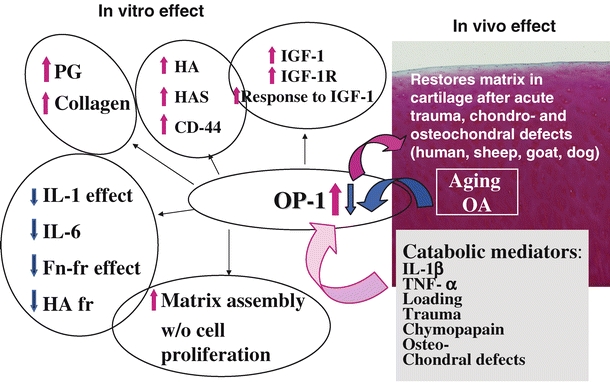
Schematic summary of the in vitro and in vivo effects of the recombinant OP-1 and the changes endogenous OP-1 undergoes with aging, OA and under the influence of various catabolic mediators
Animal studies
The pivotal role of BMPs in the development and regeneration process of the skeleton had originally suggested a role in articular cartilage repair. Furthermore, the accumulation of data from numerous in vitro studies (described above) clearly demonstrated that certain BMPs, in particular BMP-7, have an important role in chondrocyte differentiation and extracellular matrix production as well as the maintenance of adult chondrocyte phenotype. In the light of these data the testing of BMP-7 in animal models of articular cartilage repair was begun with the objective to determine if the protein could improve the healing in osteochondral defects or, most importantly, promote repair in chondral defects. More recently, studies have shifted to animal models of osteoarthritis and non-articular cartilage repair, particularly involving degenerative disc disease. This section summarises the variety of studies which have evaluated the therapeutic potential of OP-1/BMP-7 in rat, rabbit, dog, sheep, goats and horse models.
Articular cartilage repair
Focal osteochondral defect models
Repair of osteochondral defects, which involves both the cartilage tissue and the underlying bone, is known to occur to a limited extent promoted by the presence of both stem cells and growth and differentiation factors brought into the defect by the blood/marrow. In animal studies these defects undergo repair with formation of a new layer of bone and cartilage, but the macromolecular organisation and the bio-chemical characteristics of the cartilage matrix are imperfect. High levels of type I instead of type II collagen and proteoglycans that are not cartilage specific, such as dermatan sulphate containing proteoglycans, make up the repair tissue and result in fibrillations and degenerative changes over time.
Early studies were done in rabbit models using recombinant OP-1/BMP-7 delivered on bone-derived type I collagen particles press-fitted into large focal defects [17]. The results showed that the repair of both the bone and cartilage tissues were improved over controls. A later study showed that this repair could be duplicated using delivery of the OP-1/BMP-7 gene via periosteal-derived allogenic mesenchymal stem cells embedded in a polymer scaffold [36]. Recently a study was done in a rabbit model to evaluate using OP-1/BMP-7 to augment the microfracture procedure [30]. It was concluded that microfracture and OP-1/BMP-7 act synergistically to stimulate cartilage repair, and it is a technically simple procedure that could easily be adopted into clinical practice.
The encouraging results from multiple rabbit studies provided the impetus to extend the studies on osteochondral defect repair to larger animal models [12, 35, 45]. These studies have been done in dog, goat or sheep and have, for the most part, also evaluated OP-1/BMP-7 delivered on bone-derived type I collagen particles press-fitted into defects. Similar to results seen in the rabbit studies, histological assessment demonstrated improved healing in both cartilage and bone in the OP-1/BMP-7- treated sites compared to control sites. More recently a sheep study was conducted to ascertain whether OP-1/BMP-7 could be used to augment the mosaicplasty (osteochondral autograft) procedure [45]. This investigation determined that the addition of the OP-1/BMP-7 collagen material to the defect sites could improve the histological outcome of: (1) the interface between transplanted and host cartilage, (2) the interface between the transplanted and host bone and (3) donor site healing. It was concluded from the data that mosaicplasty augmentation was another promising clinical application for OP-1/BMP-7.
Focal chondral defect models
Chondral defect repair studies have more clearly demonstrated that BMP-7 has the ability to be a cartilage anabolic factor in vivo. In contrast to the osteochondral defects, the repair of chondral defects (defects that do not penetrate into the subchondral bone) is not believed to occur even to a limited extent. The studies with OP-1/BMP-7 have shown that hyaline-like tissue repair can be induced in a model where the defects are not exposed to stem cells and factors from the blood/marrow. However, the models are technically more difficult than the osteochondral defect models; creation of defects that do not penetrate the subchondral bone is challenging and methods for containment of the BMP-7 in the shallow defects have proven difficult. As a result these studies have used large animals where the cartilage is much thicker than in the rabbit and have involved novel techniques to deliver OP-1/BMP-7.
A new era in cartilage repair began with the publication of a sheep study where liquid OP-1/BMP-7 was delivered into the knee joint to heal a chondral defect [25]. In this study large (10 mm) defects were created such that the subchondral bone was not damaged and liquid OP-1/BMP-7 in acetate buffer was infused over a two-week period into the synovial fluid via an extraarticularly positioned mini-osmotic pump connected to the joint by a polyethylene tubing. At three months following surgery defects treated with OP-1/BMP-7 were partially filled with newly formed cartilage. At six months these defects showed additional new cartilage and this tissue was well fused to the old cartilage and stained positive for type II collagen. None of the control defects showed healing with any kind of tissue. In this regard it is interesting that the repair process continued to progress between three and six months, even though OP-1/BMP-7 was delivered for only the first two weeks and without a scaffolding material. It was hypothesised that the continuous presence of OP-1/BMP-7 throughout the initial weeks following surgery may have attracted sufficient mesenchymal-like cells originating from the synovium into the defect area. Subsequently, OP-1/BMP-7 could stimulate these cells to differentiate into chondrocytes which would then produce the appropriate extracellular matrix for filling the defect site. The actual filling of the defect site continues at a slow process long after the OP-1/BMP-7 has been cleared from the joint.
Another study has been reported using the OP-1/BMP-7 gene to repair a focal chondral defect in a horse model [19]. The OP-1/BMP-7 gene on an adenoviral vector was delivered to the defect site via transfected allogenic chondrocytes embedded in a fibrin clot. In comparison with the control cells without OP-1/BMP-7, the BMP-treated defects showed significantly better healing at four weeks and markedly more hyaline-like morphology. However by eight months both the control and OP-1/BMP-7 treated defects had similarly healed with cartilage repair tissue and it was concluded that the advantage of OP-1/BMP-7 appears to be limited to an acceleration of cartilage healing in this model using modified allogenic chondrocytes.
Osteoarthritis models
The anti-catabolic, anabolic and reparative activity of BMPs described in the previous sections makes these molecules ideal candidates for osteoarthritis prevention and treatment. The chondroprotective potential of BMPs in arthritis and joint injury is also supported by in vitro studies, but additional proof in animal models and naturally occurring disease is needed where the entire synovial environment and immune system are intact.
BMPs may be beneficial in sports injuries when necrosis, apoptosis and areas of acellular matrix are found overlying femoral condyle bone bruises identified by MRI [10]. These lesions may progress to acellular areas, partial and full thickness defects and promote osteoarthritic degeneration. In such cases BMP-7 might be protective since this protein enhanced the viability and proteoglycan production of aged and osteoarthritis chondrocytes in alginate cultures [5, 34]. This appears to be due to suppression of apoptosis associated with NFκB activation [23], which leads to improved viability as well as suppression of proteoglycan loss after injury [21]. Nishida et al. [43] showed that BMP-7 (100 ng/ml) dramatically improved cell-associated proteoglycan deposition and prevented matrix degradation caused by hyaluronan hexasaccharide depletion of the CD44 receptor cartilage explants. These data suggest that BMPs may promote chondrocyte viability and facilitate repair after sub-lethal mechanical or biochemical injury; however, cartilage repair may also be needed.
OP-1/BMP-7 may be a part of the normal reparative response because increased levels of BMP-7 were found in synovial fluid and tissues after joint injury [21, 22], an arthrotomy incision [14] or induction of osteoarthritis [41]. Kaps et al. [26] showed that BMP-7 expressing chondrocytes suppressed ingrowth of destructive fibrous connective tissue (pannus) so this protein may also be useful in inflammatory arthritis. Repair of bone should also be considered.
Bone oedema and microdamage to the subchondral bone plate may create pain, but can be catastrophic if the bone is sufficiently compromised to allow the cartilage surface to collapse [13]. The evidence for BMP-7 promoting bone remodelling and repair in long bone fractures and osteochondral defects is strong [11]. Repair and remodelling at bone-tendon or bone ligament interfaces may also play a role in arthritis, and BMP-7 has been shown to play a role in an ACL repair model in rabbits where these proteins were associated with osseous integration of the transplanted tendon.
Data from the authors’ laboratories indicate that reliable delivery of therapeutic proteins to the synovial environment is possible and a series of appropriately timed injections can maintain therapeutic levels. Chondroprotective BMP therapy may be quite useful in the context of sports injuries in combination with restoration of joint stability and protection against further injury until a population of competent chondrocytes can be restored.
With this in mind, recent data from an ACL transection model in rabbits demonstrated that BMP-7 had a protective effect on cartilage degeneration [2]. Significant improvements in histological and morphometric scores and expression of type II collagen were found in addition to suppression of aggrecanase activity. BMP-7 prevented post-traumatic osteoarthritis in a sheep model when two intra-articular injections were given at the time of injury and one week later [22]. Macroscopic (Fig. 2) and histological damage to the articular surface (Fig. 3) was reduced, as was the C3/4 short collagen epitope immunostaining, indicating that there was protection against metalloproteinase-mediated collagen degradation. Similar results were seen with two doses of BMP-7 injected three and four weeks after injury, but not when therapy was delayed for 12 weeks [21].
Fig. 2.
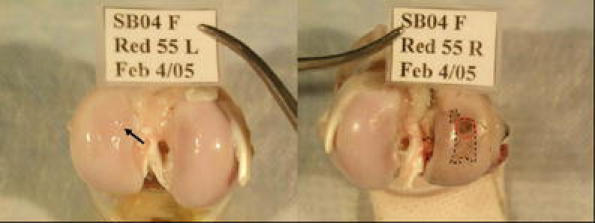
BMP-7 treated (left) and control (right) knee joints from sheep in the post-traumatic osteoarthritis model. In the control joint India ink staining of the joint surface reveals the anterior central impact zone (red circle) as well as areas of progression from the original injury site (dashed line). In the OP-1 treated joint there is a some loss of sheen (arrow) that represents partial loss of the superficial layer of cartilage zone, but little ink uptake
Fig. 3.
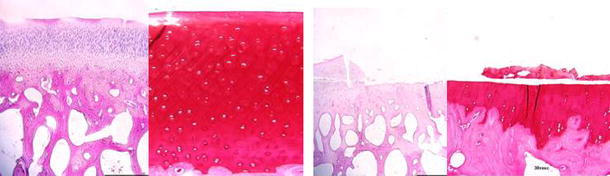
Histological sections of the impact area of OP-1 treated (left) and control sheep (right) femoral condyles 12 weeks after injury. In control sheep there is loss of most of the thickness of the cartilage due to delamination of necrotic cartilage. In OP-1-treated knees there was partial loss of the superficial zone and small fissures. H&E (×20) and safranin-O stained (×100) 5-micron-thick sections
This body of preclinical evidence supports a role for administration of BMPs in prevention and treatment of injuries and early osteoarthritis. These studies raise two interesting points. Firstly, the risk of intra-articular bone formation appears to be very low with BMP-7 despite the fact that in other applications (long bone repair and spine fusion) this is its major action. Secondly, since there was little or no evidence of intrinsic or extrinsic cartilage repair reported in these studies, it must be assumed that the beneficial effects observed are due to enhanced survival of a more functional chondrocyte population that is resistant to the environment associated with injury and inflammation that interferes with chondrocyte metabolism. The duration of exposure and concentration of BMP-7 needed to create this effect in patients are unknown, but based on the above evidence 50 to 100 ng/ml over two weeks may be sufficient to reverse or significantly reduce the size of early traumatic lesions and prevent progression of degeneration to osteoarthritis. The window of opportunity to address developing lesions may depend on many factors, but the above experiments indicate that treatment within the first four to six weeks after injury should be beneficial.
Non-articular cartilage repair
OP-1/BMP-7, as discussed above, has been known for some time to have potent anabolic effects in vitro on articular chondrocytes, particularly for the stimulation of the synthesis of proteoglycans and type II collagen. Similar studies using IVD cells and cells derived from meniscal and nasal cartilage have demonstrated the same ability of OP-1/BMP-7 to stimulate the production of extracellular matrix components [18, 31, 39]. Animal models for non-articular cartilage repair have primarily involved intervertebral disc (IVD) cartilage [28, 38], but a larynx cartilage repair study has also been reported [27].
The structure of the IVD is composed of an outer collagen-rich annulus fibrosus (AF) surrounding a central hydrated proteoglycan (PG)-rich gelatinous nucleus pulposus (NP). The AF is composed of concentric layers which contain variable proportions of type I and II collagen. The NP, in contrast, is a gelatinous tissue that provides weight-bearing properties to the disc. Endogenous cells in both the NP and AF are responsible for the synthesis and degradation of these matrix components to maintain tissue homeostasis. The IVD undergoes profound cellular and matrix changes with aging and degenerates much earlier than other weight-bearing cartilaginous tissues.
The stimulatory effect of OP-1/BMP-7 on IVD cells was first demonstrated in vivo by the intradiscal injection in normal rabbits [1]. A single injection of OP-1/BMP-7 increased the mean disc height index and this corresponded to a significant increase in the PG content of the NP. Models using degenerated discs were subsequently used to evaluate the therapeutic effects of OP-1/BMP-7. Initially a rabbit model was developed where a needle puncture of the AF induced degeneration of the disc [38]. Four weeks after puncture and development of disc degeneration, liquid OP-1/BMP-7 was injected into the NP. Six weeks after the injection a complete restoration of the disc height was observed and maintained for up to 24 weeks and this restoration did not occur in the control discs. Histological scoring after safranin-O staining showed less degeneration for the OP-1 treated discs and biomechanical measurements demonstrated that OP-1 also restored the viscoelastic properties of the disc to the level of non-punctured control discs. Similar restoration was seen in another rabbit model using chondroitinase-ABC to induce degeneration of the disc [24].
A rat model has also been used to study the therapeutic efficacy of intradiscal injection of OP-1 [6, 28]. This model used chronic compression of tail vertebrae with an Illizarov-type apparatus to induce degeneration. After four weeks liquid OP-1/BMP-7 was injected into the discs and the discs were harvested four weeks later. The results showed that OP-1/BMP-7 was able to reverse the damage as well as to reduce discogenic pain. Immunohistochemical analysis demonstrated an anti-catabolic effect through reduced levels of aggrecanase, MMP-13, TNF-α, IL-1β and substance P. Because substance P is a neuropeptide linked with inflammation and pain, the observed reduction supports the lower level of pain-related behavior of the OP-1 treated rats. These results were particularly important because this was the first demonstration of an inhibitory effect on pain by OP-1/BMP-7.
In summary in vitro studies had suggested an important role for OP-1/BMP-7 in cartilage repair and a variety of animal studies have now demonstrated such a role in vivo. The application of OP-1/BMP-7 has used implantable and injectable formulations to heal a variety of defects in both articular and non-articular cartilage.
Discussion
The purpose of this article is to review the current knowledge on OP-1/BMP-7 in cartilage repair from the standpoint of both in vitro and animal repair studies. The data clearly show that OP-1/BMP-7 has an important role in cartilage, both in normal homeostasis and in repair. The animal study results predict a bright future for the use of OP-1/BMP-7 in the engineering of cartilage.
In cell culture studies there have been numerous demonstrations that BMP-7 is endogenously expressed in cartilage and that it acts as anabolic factor for chondrocytes in culture. BMP-7 has also been localised to synovial fluid, synovium, ligament, tendon and meniscus. In regard to the anabolic activity the role BMP-7 plays in stimulating chondrocyte differentiation, extracellular matrix production and maintenance of the adult chondrocytic phenotype is well documented. OP-1/BMP-7 has been shown to stimulate the synthesis of all the major cartilage extracellular matrix proteins and to counteract the degenerative effect of numerous catabolic mediators. Furthermore recent antisense studies have demonstrated that down-regulation of OP-1/BMP-7 mRNA induced a significant decrease in proteoglycan synthesis in articular chondrocytes in culture. Thus the data from in vitro studies have clearly demonstrated that at least one BMP, OP-1/BMP-7, is very important in articular cartilage homeostasis.
Data from studies in animals show that OP-1/BMP-7 clearly has a therapeutic potential for cartilage repair. In a large chondral defect study in sheep OP-1/BMP-7 was shown to induce significant repair in a model where no repair takes place in the controls. In several large osteochondral defect studies OP-1/BMP-7 was observed to induce a significant improvement in repair of both the cartilage and bone tissues over that observed in control defects. In studies evaluating models of osteoarthritis, OP-1/BMP-7 was shown to prevent development of damage and in some models reverse the damage. Finally in animal models of degenerated disc the injection of liquid formulations of OP-1 has been shown to stimulate restoration of the tissue. In conclusion these studies have demonstrated significant repair, but the goal of perfectly repaired cartilage remains to be achieved. Studies in the future will require evaluations of a variety of formulations, scaffolds, methods of administration and possibly combinations with other factors. However, the clinical demand is so great for new cartilage repair procedures that simply an improvement over the repair currently achieved can be an acceptable interim goal.
In addition to efficacy data the animal studies have also demonstrated that OP-1/BMP-7 can be safely administered to the joint or the disc. Recombinant OP-1/BMP-7 has been evaluated in various formulations, concentrations, frequencies of dosing and delivery routes for in rabbits, dogs, goats and sheep. In these studies there have been no reports of side effects, such as bone formation on the synovial or disc surface or free floating objects in the synovial fluid. Furthermore there have not been reports of inflammatory side effects such as synovitis, pannus formation or joint effusion.
In conclusion a BMP-7-based therapy for damaged cartilage would appear to have significant clinical potential. The BMP-7 could be delivered locally to a focal defect site on an appropriate scaffold material or possibly delivered to the joint without a scaffold as was demonstrated in the minipump delivery study. In the disc studies liquid OP-1 was demonstrated to be effective when directly injected into the disc. Specifically a BMP-7 therapy could be appropriate for augmentation of current joint repair procedures like mosaicplasty and microfracture, as well as a replacement for cell-based therapies, which involve removal of autologous cells derived from marrow or from cartilage. Furthermore, arthroscopic administration of a BMP-7 product should increase the attractiveness of the therapy enormously. Finally the goal of injectable formulations of a BMP-7 in a slow-release material would seem to be the ideal route of administration in order to extend the therapeutic potential dramatically, particularly in the area of treatment and, ultimately, prevention of osteoarthritis.
References
- 1.An HS, Takegami K, Kamada H, Nguyen CM, Thonar E, Singh K, Andersson GB, Masuda K (2005) Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine 30(1):25–32 [DOI] [PubMed]
- 2.Badlani N, Inoue A, Healey R, Coutts R, Amiel D (2007) The protective effect of OP-1 on articular cartilage in the development of osteoarthritis. Proceedings ICRS [DOI] [PubMed]
- 3.Chubinskaya S, Frank BS, Michalska M, Kumar B, Merrihew CA, Thonar EJ-MA, Lenz ME, Otten L, Rueger DC, Block JA (2006) Osteoarthritic protein-1 in synovial fluid from patients with rheumatoid arthritis or osteoarthritis: relationship to disease and levels of hyaluronan and antigenic keratan sulfate. Arthritis Res Ther 8(3):R73 [DOI] [PMC free article] [PubMed]
- 4.Chubinskaya S, Hakimiyan A, Otten L, Rappoport L, Rueger DC, Sobhy M, Cole B (2006) Response of human chondrocytes prepared for autologous chondrocyte implantation to growth factors. Trans 52nd ORS p.1390 [DOI] [PubMed]
- 5.Chubinskaya S, Hakimiyan A, Pacione C, Yanke A, Rappoport L, Aigner T, Rueger D, Loeser RF (2007) Synergistic effect of IGF-1 and OP-1 on matrix formation by normal and OA chondrocytes cultured in alginate beads. Osteoarthritis Cartilage 15:421–430 [DOI] [PMC free article] [PubMed]
- 6.Chubinskaya S, Kawakami M, Rappoport L, Matsumoto T, Migita N, Rueger DC (2007) Anti-catabolic effect of OP-1 in chronically compressed intervertebral discs. J Ortho Res 25(4):517–530 [DOI] [PubMed]
- 7.Chubinskaya S, Kumar B, Merrihew C, Heretis K, Rueger D, Kuettner KE (2002) Age-related changes in cartilage endogenous OP-1. Biochimica Biophysica Acta. Mol Basis Dis 1588(2):126–134 [DOI] [PubMed]
- 8.Chubinskaya S, Merrihew C, Cs-Szabo G, Mollenhauer J, McCartney J, Rueger DL, Kuettner KE (2000) Human articular chondrocytes express osteogenic protein-1. J Histochem Cytochem 48(2):239–250 [DOI] [PubMed]
- 9.Chubinskaya S, Otten L, Soeder S, Aigner T, Loeser RF, Rueger DC (2007) Regulation of anabolic and catabolic pathways by osteogenic protein-1: gene array data. Trans 53rd ORS p.546
- 10.Colwell CW Jr, D’Lima DD, Hoenecke HR, Fronek J, Pulido P, Morris BA, Chung C, Resnick D, Lotz M (2001) In vivo changes after mechanical injury. Clin Ortho Rel Res 391(Suppl):S116–S123 [DOI] [PubMed]
- 11.Cook SD, Barrack RL, Patron LP, Sakeld SL (2004) Osteogenic protein-1 in knee arthritis and arthroplasty. Clinical Ortho Rel Res 428:140–145 [DOI] [PubMed]
- 12.Cook SD, Patron LP, Salkeld SL, Rueger DC (2003) Repair of articular cartilage defects with osteogenic protein-1 (BMP-7) in dogs. J Bone Joint Surg 85-A(Supp 3):116–123 [DOI] [PubMed]
- 13.Costa-Paz M, Muscolo DL, Ayerza M, Makino A, Aponte-Tinao L (2001) Magnetic resonance imaging follow-up study of bone bruises associated with anterior cruciate ligament ruptures. Arthroscopy 17(5):445–449 [DOI] [PubMed]
- 14.Fahlgren A, Chubinskaya S, Messner K, Aspenberg P (2006) A capsular incision leads to a fast osteoarthrotic response, but also elevated levels of activated osteogenic protein-1 in rabbit knee joint cartilage. J Scand J Med Sci Sports 16:456–462 [DOI] [PubMed]
- 15.Fan Z, Chubinskaya S, Rueger DC, Bau B, Haag J, Aigner T (2004) Regulation of anabolic and catabolic gene expression in normal and osteoarthritic adult human articular chondrocytes by OP-1 (BMP-7). J Clin Exper Rheum 22(1):103–106 [PubMed]
- 16.Flechtenmacher J, Huch K, Thonar EJ-MA, Mollenhauer JA, Davies SR, Schmid TM, Puhl W, Sampath TK, Aydelotte MB, Kuettner KE (1996) Recombinant human osteogenic protein 1 is a potent stimulator of the synthesis of cartilage proteoglycans and collagens by human articular chondrocytes. Arthritis Rheum 39:1896–1904 [DOI] [PubMed]
- 17.Grgic M, Jelic M, Basic V, Basic N, Pecina M, Vukicevic S (1997) Regeneration of articular cartilage defects in rabbits by osteogenic protein-1 (bone morphogenetic protein-7). Acta Med Croatia 51(1):23–27 [PubMed]
- 18.Hicks DL, Sage AB, Shelton E, Schumacher BL, Sah RL, Watson D (2007) Effect of bone morphogenetic proteins 2 and 7 on septal chondrocytes in alginate. Otolaryngol Head Neck Surg 136(3):373–379 [DOI] [PubMed]
- 19.Hidaka C, Goodrich LR, Chen CT, Warren RF, Crystal RG, Nixon AJ (2003) Acceleration of cartilage repair by genetically modified chondrocytes over expressing bone morphogenetic protein-7. J Ortho Res 21:573–583 [DOI] [PubMed]
- 20.Huch K, Wilbrink B, Flechtenmacher J, Koepp HE, Aydelotte MB, Sampath TK, Kuettner KE, Mollenhauer JA, Thonar EJ-MA (1997) Effects of recombinant human osteogenic protein 1 on the production of proteoglycan, prostaglandin E2, and interleukin-1 receptor antagonist by human articular chondrocytes cultured in the presence of interleukin-1β. Arthritis Rheum 40:2157–2161 [DOI] [PubMed]
- 21.Hurtig MB (2004) Delayed administration of OP-1 reduces articular degeneration after contusive impact injury. Transactions ORS 52:1338
- 22.Hurtig MB, Chubinskaya S (2004) The protective effect of OP-1 in early traumatic osteoarthritis-animal studies. Trans 5th Combined ORS p.70
- 23.Im HJ, Pacione C, Chubinskaya S, Van Wijnen AJ, Sun Y, Loeser RF (2003) Inhibitory effects of insulin-like growth factor-1 and osteogenic protein-1 on fibronectin fragment- and interleukin-1beta-stimulated matrix metalloproteinase-13 expression in human chondrocytes. J Biol Chem 278: 25386–25394 [DOI] [PMC free article] [PubMed]
- 24.Imai Y, An H, Matsumoto T, Nguyen C, Andersson G, Thonar E (2002) Intervertebral disc regeneration with rhOP-1 following C-ABC Chemonucleolysis: An in vivo study using the rabbit model. In: Proceedings: The International Society for the Study of the Lumbar Spine. Proceedings 29th annual meeting, p 71, May 14–18
- 25.Jelic M, Pecina M, Haspl M, Kos J, Taylor K, Maticic D, McCartney J, Yin S, Rueger D, Vukicevic S (2001) Regeneration of articular cartilage chondral defects by osteogenic protein-1 (bone morphogenetic protein-7) in sheep. Growth Fact 19:101–113 [DOI] [PubMed]
- 26.Kaps C, Bramlage C, Smolian H, Haisch A, Ungethum U, Burmester GR, Sittinger M, Gross G, Haupl T (2002) Bone morphogenetic proteins promote cartilage differentiation and protect engineered artificial cartilage from fibroblast invasion and destruction. Arthritis Rheum 46(1):149–162 [DOI] [PubMed]
- 27.Katic V, Majstorovic L, Maticic D, Pirkic B, Yin S, Kos J, Martinovic S, McCartney JE, Vukicevic S (2000) Biological repair of thyroid cartilage defects by osteogenic protein-1 (bone morphogenetic protein-7) in dog. Growth Fact 17:221–232 [DOI] [PubMed]
- 28.Kawakami M, Matsumoto T, Hashizume H, Kuribayashi K, Chubinskaya S, Yoshida M (2005) Osteogenic protein-1 (osteogenic protein-1/bone morphogenetic protein-7) inhibits degeneration and pain-related behavior induced by chronically compressed nucleus pulposus in the rat. Spine 30(17):1933–1939 [DOI] [PubMed]
- 29.Koepp HE, Sampath KT, Kuettner KE, Homandberg GA (1999) Osteogenic protein-1 (OP-1) blocks cartilage damage caused by fibronectin fragments and promotes repair by enhancing proteoglycan synthesis. Inflamm Res 47:1–6 [DOI] [PubMed]
- 30.Kuo AC, Rodrigo JJ, Reddi AH, Curtiss S, Grotkopp E, Chiu M (2006) Microfracture and bone morphogenetic protein 7 (BMP-7) synergistically stimulate articular cartilage repair. Osteoarthritis Cart 14(11):1126–1135 [DOI] [PubMed]
- 31.Lietman SA, Hobbs W, Inoue N, Reddi AH (2003) Effects of selected growth factors on porcine meniscus in chemically defined medium. Orthopedics 26(8):799–803 [DOI] [PubMed]
- 32.Lietman S, Yanagishita M, Sampath TK, Reddi AH (1997) Stimulation of proteoglycan synthesis in explants of porcine articular cartilage by recombinant osteogenic protein-1 (bone morphogenetic protein-7). J Bone J Surg 79-A:1132–1137 [DOI] [PubMed]
- 33.Loeser R, Chubinskaya S, Pacione C, Im H-J (2005) Basic fibroblast growth factor inhibits the anabolic activity of insulin-like growth factor-1 and osteogenic protein-1 in adult human articular chondrocytes. Arthritis Rheum 52(12):3910–3917 [DOI] [PMC free article] [PubMed]
- 34.Loeser RF, Pacione CA, Chubinskaya S (2003) The combination of insulin-like growth factor-1 and osteogenic protein-1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis Rheum 48(8):2188–2196 [DOI] [PubMed]
- 35.Louwerse RT, Iheyligers IC, Klein-Nulend J, Sugiihara S, van Kampen GPJ, Semeins CM, Goei SW, de Koning MHMT, Wuisman PIJM, Burger EH (2000) Use of recombinant human osteogenic protein-1 for the repair of subchondral defects in articular cartilage in goats. J Biomed Mater Res 49(4):506–516 [DOI] [PubMed]
- 36.Mason JM, Breibart AS, Barcia M, Porti D, Pergolizzi RG, Grande DA (2000) Cartilage and bone regeneration using gene-enhanced tissue engineering. Clin Ortho Rel Res 379S:S171–S178 [DOI] [PubMed]
- 37.Massagué J, Chen YG (2000) Controlling TGF-β signaling. Genes Dev 14:627–644 [PubMed]
- 38.Masuda K, Imai Y, Okuma M, Muehleman C, Nakagawa K, Akeda K, Thonar E, Andersson G, An HS (2006) Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine 31(7):742–754 [DOI] [PubMed]
- 39.Masuda K, Takegami K, An H, Kumano F, Chiba K, Andersson GBJ, Schmid T, Thonar E (2003) Recombinant osteogenic protein-1 upregulates extracellular matrix metabolism by rabbit annulus fibrosus and nucleus pulposus cells cultured in alginate beads. J Orthop Res 21:922–930 [DOI] [PubMed]
- 40.Merrihew C, Kumar B, Heretis K, Rueger DC, Kuettner KE, Chubinskaya S (2003) Alterations in endogenous Osteogenic Protein-1 (OP-1) with degeneration of human articular cartilage. J Ortho Res 21(5):899–907 [DOI] [PubMed]
- 41.Muehleman C, Kuettner KE, Rueger DC, ten Dijke P, Chubinskaya S (2002) Immunohistochemical localization of osteogenic protein-1 and its receptors in rabbit articular cartilage. J Histochem Cytochem 50:1341–1350 [DOI] [PubMed]
- 42.Nishida Y, Knudson CB, Eger W, Kuettner KE, Knudson W (2000) Osteogenic protein-1 stimulates cell-associated matrix assembly by normal human articular chondrocytes: upregulation of hyaluronan synthase, CD 44 and aggrecan. Arthritis Rheum 43:206–214 [DOI] [PubMed]
- 43.Nishida Y, Knudson CB, Knudson W (2004) Osteogenic protein-1 inhibits matrix depletion in a hyaluronan hexasaccharide-induced model of osteoarthritis. Osteoarthritis Cart 12:374–382 [DOI] [PubMed]
- 44.Rueger DC, Chubinskaya S (2004) BMPs in articular cartilage repair. In: Vukicevic S, Sampath KT (eds) Bone morphogenetic proteins: regeneration of bone and beyond. Birkhauser Verlag AG, Basel, pp 109–132
- 45.Shimmin A, Young D, O’Leary S, Shih MS, Rueger DC Walsh WR (2003) Growth factor augmentation of an ovine mosaicplasty model. Trans ICRS
- 46.Soeder S, Hakimiyan A, Rueger D, Kuettner KE, Aigner T, Chubinskaya S (2005) Antisense inhibition of osteogenic protein-1 disturbs human articular cartilage integrity. Arthritis Rheum 52(2):468–478 [DOI] [PubMed]
- 47.Urist MR, Mikulski A, Lietze A (1979) Solubilized and insolubilized bone morphogenetic protein. Proc Natl Acad Sci USA 76:1828–1832 [DOI] [PMC free article] [PubMed]
- 48.Vinall RL, Lo SH, Reddi AH (2002) Regulation of articular chondrocyte phenotype by bone morphogenetic protein 7, interleukin 1, and cellular context is dependent on the cytoskeleton. Exp Cell Res 272:32–44 [DOI] [PubMed]
- 49.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA (1988) Novel regulators of bone formation: molecular clones and activities. Science 242:1528–1534 [DOI] [PubMed]
- 50.Yao J, Cole AA, Huch K, Kuettner KE (1996) The effect of OP-1 on IL-1beta induced gene expressions of matrix metalloproteinases and TIMP in human articular cartilage. Trans ORS 42:305


