Abstract
The repair of subcutaneous tendon ruptures can be stimulated by a single application of one of several growth factors [e.g. platelet-derived growth factor (PDGF), transforming growth factor (TGF)-beta, insulin-like growth factor (IGF)-1, vascular endothelial growth factor (VEGF), bone morphogenetic proteins (BMPs) like growth differentiation factor (GDF)-5, -6, -7] or by a thrombocyte concentrate (PRP). The response to these measures is dependent on the mechanical microenvironment, which is crucial for repair. So far, almost all research has been limited to rodent models, mostly using the rat Achilles tendon. Ruptured human Achilles tendons appear to be mechanically loaded in spite of immobilisation. This suggests that the mechanical microenvironment might be favourable for the clinical use of growth factors or platelets for this indication. New methods to quantitate human Achilles tendon repair have been developed.
Résumé
La réparation de rupture tendineuse sous cutanée peut être améliorée par une simple application d’un ou de plusieurs facteurs de croissance (PDGF, TGF-beta, IGF-1, VEGF, BMPs, GDF like -5, -6, -7) ou par un concentré de plaquettes (PRP). Ce modèle a surtout été utilisé, au niveau du tendon d’Achille des rats. Cette étude nous montre que le micro environnement mécanique peut être amélioré par l’utilisation de facteurs de croissance et de concentrés de plaquettes. De nouvelles méthodes permettant de quantifier l’importance de la réparation du tendon d’Achille humain ont été développées.
Introduction
It is possible to make injured tendons heal faster. Surprisingly little research has been done to explore and exploit this possibility. When the field of fracture repair enhancement became increasingly popular in the 1990s, my group looked for a less explored area. The field of tendon repair turned out to be virgin soil. In spite of our efforts to plough it, it largely remains so.
This mini-review is limited to the repair of subcutaneous tendons, i.e. tendons that do not glide through synovial sheaths, responsible for their nutrition. Repair of intra-synovial tendons is complicated by the problems of space limitation and adhesion formation, which will not be discussed here. Subcutaneous tendons are easier. A typical subcutaneous tendon rupture is that of the Achilles tendon, which is a common clinical condition. Achilles tendon ruptures heal spontaneously, if immobilised; but operated on or not, it takes an annoyingly long time. Moreover, some sequelae usually remain. Research on stimulation of tendon repair is still at the stage of animal experimentation. The by far most common experimental model for tendon repair uses the Achilles tendon of the rat.
Tendon repair can be described as going through different phases. Often, an inflammatory and a formative phase are described, followed by remodelling. If one were not confined to use a poetic triad, one could divide the formative phase into proliferation and differentiation. The division into phases is important in this context, because various attempts to improve repair can have different effects on different phases.
In brief, the process starts with an haematoma, platelet activation and invasion of cells that form a granuloma (Fig. 1). Fibroblasts in the granuloma produce collagen (mostly type 3), which gradually increases its mechanical strength, so that loading can lead to elastic deformation, which allows mechanical signalling to start to influence the process. Production of collagen type 1 gradually takes over, and the callus reaches its largest size. The large transverse area compensates for tissue weakness, so that considerable traction forces can be sustained. By remodelling, the collagen is resorbed and replaced to produce better organisation, and cross-linking increases. Finally the callus transverse area gradually decreases as the mechanical tissue properties improve.
Fig. 1.
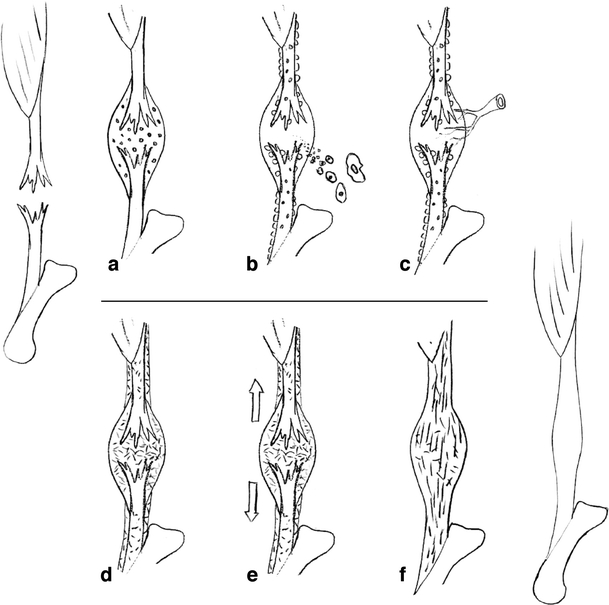
Scheme of tendon repair. a Haematoma with platelet activation. b Invasion of cells and proliferation of paratenon. c Vascular and neuronal ingrowth. d Loose collagenous callus formation. e Mechanical stimulation. f Maturation and remodelling
Mechanical stimulation
It is well described that mechanical stimulation improves tendon repair, or rather that lack of mechanical stimulation is detrimental. Most animal models for evaluation of the effect of disuse on tendon apply external fixation, hindlimb suspension or surgical interventions such as denervation and disarticulation [3, 19, 20, 29, 30]. These are often technically demanding procedures, and immobilisation could have undesired side effects, due to loss of joint movement. Denervation of the muscle also denervates the tendon and the surrounding tissues responsible for its nutrition. This could influence tendon metabolism, and innervation of the tendon callus may be important for the repair process [1]. I believe it is better to paralyse the gastrocnemius and soleus muscle complex with botulinum toxin to create disuse without surgery or external fixation devices. This is a less time-consuming procedure and probably results in fewer side effects on the animals, as the botulinum toxin is specific for the acetylcholine signalling of the motor end plates.
We have used botulinum toxin treatment in experiments with Achilles tendon transection in rats, and also in rats with intact tendons. In the latter, the gastrocnemius and soleus muscle complex had shrunk to a negligible size after 6 weeks. Moreover, the muscle remnants appeared to remain fully pliable, as tested by dorsi-flexion of the foot [9]. In rats with transected Achilles tendons, the botulinum toxin treatment had a drastic negative effect on tendon callus strength: 2 weeks after transection, force at failure was reduced to less than 30% of transected but loaded controls [25]. Unpublished data from rats with tibial nerve denervation indicate that on longer follow-up tendon healing ceases completely, so that the callus even loses strength with time. Rats in captivity have a sedentary lifestyle. If they are given an environment that stimulates them to more physical activity, the healing tendon callus becomes bigger and stronger [25]. Interestingly, increased activity also leads to shortening of the tendon callus, obviously via increased myofibroblastic activity, so that the separated tendon stumps come closer together (unpublished data).
The importance of mechanical signalling raises two questions: (1) Will patients whose tendons are immobilised and load-protected in plaster have suboptimal repair? (2) Can mechanical signalling be replaced by drugs?
The role of mechanical signalling in immobilised patients is largely unknown. However, there are two studies of electromyography (EMG) activity in patients with a lower leg plaster, one with uninjured subjects [2] and one with Achilles tendon ruptures [7]. It appears that most people contract their muscles and load their tendons also in a plaster and that somehow the loading of the healing Achilles tendon in a plaster is adapted to its strength. Thus, it is not certain that a controlled loading regime during the immobilisation period would be of much use (motion is a different thing: it prevents joint stiffness). The other question of whether mechanical signalling can be simulated or increased by drugs requires knowledge about gene and protein expressions following loading, which we do not have. Research in this field has just started. Preliminary data from our laboratory, comparing tendon repair with and without loading, suggest dramatic differences in the expression of several commonly studied genes, especially in the remodelling phase. Expression of CDMP-2, which has been our special interest (see below), was decreased in normal tendons that were protected from loading. It showed a short-lived increase after transection, but was back at low levels at days 8 and 14 of healing.
In contrast, loaded tendons showed a remaining threefold increase in CDMP-2 expression 8 and 14 days after transection compared to uninjured but loaded tendons.
The inflammatory phase of tendon repair seems to pave the way for the formation of a fibrous callus. If early inflammation is inhibited by e.g. a cox inhibitor like indomethacin, or a specific one, such as valdecoxib, the fibrous callus can lose a third of its strength, due to inferior material properties (lower stress at failure). This detrimental effect of early treatment survives the inflammatory phase and can still be seen after 2 weeks in the rat [28]. If, on the other hand, cox inhibitor treatment is started on the 6th day after injury, when the inflammatory phase is mostly over, there is instead a positive effect on the mechanical properties, so that we get a thinner tendon with better material properties and intact overall strength (force at failure) [14, 28]. During the inflammatory phase, there is little mechanical strength and mostly plastic deformation. Any loading will only risk a failure of suture threads in operated cases, or permanently lengthen the callus in unoperated cases. As mechanical stimulation is dependent on cyclic or intermittent events, no stimulation of repair can be expected from such plastic deformation. Once some elasticity has been obtained in the early fibrous callus, however, deformed tissues will resume their pre-load shape, and cyclic loading will lead to biological signals.
Stimulation with growth and differentiation factors (CDMPs)
Several growth factors have been shown to stimulate tendon repair after exogenous protein application (Table 1). One of the problems with local application of growth factors is their short half-life and the associated need for a slow-release carrier. In the beginning, we implanted a collagen sponge as a carrier [4]. Others had the smart idea of using a suture thread as a carrier [22]. We then tried direct injections into the haematoma of the factor CDMP-2, after a suggestion from Slobodan Vukicevic (editor of this issue). This worked remarkably well, but we do not know why a carrier was not necessary [13]. The explanation might be that the growth factor is almost insoluble at neutral pH. It was injected in an acid solution and may have stayed in place because it precipitated when buffered by the tissue to neutral pH. Another possibility may be that it binds to the exposed collagen of the transected tendons. Anyway, it works: a local injection of 10 µg of CDMP-1, -2, or -3 into the haematoma 6 h after Achilles tendon transection leads to about 30% increase in total strength after 1 week in the rat [15]. In the rabbit, similar effects were seen at 2 weeks with 20 µg [12]. These two models using unsutured tendon defects allowed full weight bearing.
Table 1.
Papers on applying growth factor protein to healing tendon or ligament
| Growth factor | Doses | Model | Evaluation | Time | Results | Reference |
|---|---|---|---|---|---|---|
| PDGF | 0.5, 1 and 5 μg | Rat MCL | Mech. | 12 days | Dose-dependent beneficial effect if administrated within 24 h | Batten et al. 1996 [6] |
| PDGF-BB | 400 ng, 20 μg | Rabbit MCL | Mech., hist. | 6 weeks | Improved ultimate load, energy and elongation | Hildebrand et al. 1998 [16] |
| PDGF-BB+TGF-beta-1 | 400 ng + 4 ng, 20 μg + 200 ng | Rabbit MCL | Mech., hist. | 6 weeks | TGF-beta-1 did not lead to additional improvement | Hildebrand et al. 1998 [16] |
| FGF-2 | 0, 10, 100 and 1000 ng | Rat patellar tendon, 1 × 4 mm window defect | Mech., IHC and pyridinoline cross-link analyses | 7 and 14 days | Increased cell proliferation and type III collagen expression at 3 days | Chan et al. 2000 [8] |
| VEGF | 100 μl (50 μg/ml) | Rat AT repaired with suture | Mech. | 1, 2 and 4 weeks | Higher early tensile strength; increased TGF-β expression | Zhang et al. 2003 [31] |
| IGF-1 | 25 μg | Rat AT transection | Mech., functional | 15 days | Reduced maximum functional deficit and accelerated recovery | Kurtz et al. 1999 [18] |
| TGF-beta-1 | 10 ng, 100 ng | Rats AT, transected and sutured | Mech., in situ hybridization | 1, 2 and 4 weeks | A Dose-dependent increase in the expression of procollagen I and III mRNA; the failure load and stiffness increased by TGF-beta-1 at 2 and 4 weeks | Kashiwagi et al. 2004 [17] |
| OP-1 | 100 μg | Rats AT, transected, with or without denervation of the calf muscle or forefoot amputation | Mech., hist. | 2 weeks | Reduced strength of tendon with or without mechanical unloading; lots of bone | Forslund and Aspenberg 1998 [10] |
| CDMP-1, CDMP-2 | 1 μg, 10 μg | Rats AT defect, denervation of the calf muscle | Mech., hist. | 2 weeks | Tensile strength increased dose-dependently | Aspenberg and Forslund 1999 [4] |
| CDMP-2 | 10 μg | Rabbits AT defect | Mech. | 8 days | CDMP-2 improved tendon repair; no bone or cartilage | Forslund and Aspenberg 2001 [13] |
| CDMP-1 | CDMP-1-coated suture | Rats AT transected and sutured | Mech., hist. | 1, 2, 4 and 8 weeks | Improved repair | Rickert et al. 2001 [22] |
| CDMP-2 | 0, 2, 10 or 50 μg | Rats AT defect | Mech. | 8 days | CDMP-2 improved tendon repair | Forslund and Aspenberg 2003 [12] |
| CDMP-1, 2 and 3 | 0, 0.4, 2 and 10 μg | Rats AT transection | Mech., hist. | 8 days, 4 weeks | Improved healing dose-dependently; some bone and cartilage | Forslund et al. 2003 [14, 15] |
PDGF platelet-derived growth factor, MCL medial collateral ligament, TGF transforming growth factor, FGF fibroblast growth factor, IHC immunohistochemistry, VEGF vascular endothelial growth factor, CDMP cartilage-derived morphogenetic protein, IGF insulin-like growth factor, OP osteogenic protein
The experiments with CDMPs aimed at clinical use. We were therefore worried that the CDMP would induce bone formation, as it belongs to the bone morphogenetic protein (BMP) group, and has been shown to stimulate bone formation and repair [24]. Again, it turned out that mechanical signalling was involved. When the healing rat tendons were loaded, i.e. subjected to traction and shearing forces, minimal or no bone formation occurred as a consequence of CDMP treatment. If, however, the tendons were unloaded, CDMP-2 led to an increased formation of cartilage or bone [11]. We then showed that collagen sponges with CDMP-2 implanted in a mechanically quiescent area (subcutaneously on top of the head) regularly induced bone formation, whereas similar sponges in the loaded tendon did not [11]. How, then, in larger species?
We unloaded the patellar tendon in the rabbit by connecting the patella with the tibial tubercle with steel wires and then transected the tendon. A single injection of 20 µg CDMP into the haematoma increased the strength by more than 65% [26]. For the first time, this occurred without an increase in callus transverse area; the increase in force at failure at mechanical testing was entirely due to improved material properties. No cartilage or bone was found, in spite of load protection. This contrasts to our rat findings. Either there are environmental factors other than mechanics, which guide tissue differentiation away from cartilage or bone, or the unloading was incomplete. By flexing and extending the rabbit knee, the unloading wires bent a little, causing changes in length of the construct by 1 or 2 mm, which might have yielded some mechanical stimulation.
Anyway, the success of these rat and rabbit experiments with single injections of small to moderate doses has convinced me that CDMPs might become useful for enhancement of tendon repair in humans.
Platelet concentrate (PC)
Another option would be platelets. These cells (or cell fragments) contain a wide variety of growth factors. Although seldom mentioned, this includes BMPs [23]. Several of these factors have been shown to stimulate repair in various soft tissue models. At the site of an injury, the platelets are activated and release their growth factors. This has been exploited by several medical technical companies, who sell centrifuge systems for separation of the patient’s platelets in the operating room, mostly for stimulation of bone healing. The evidence for a positive effect on bone healing is scant, however. This is perhaps not so surprising, considering the very specific nature of bone healing, in contrast to scar formation, which is what platelets normally initiate. Tendon healing, on the other hand, can be regarded as a refined form of scar formation, and it therefore seems reasonable to assume that the addition of an increased number of platelets might improve the process.
We have tried out platelets for stimulation of tendon repair in our rat Achilles model. A single injection of a moderate amount of activated platelets into the haematoma 6 h after transection increased force at failure after 1 week [5]. Moreover, the effect on tissue mechanics was largest as late as 3 weeks after transection. At this time, a significantly better tissue organisation could be demonstrated by histology. The PC-treated tendons reached 84% of the force at failure of unoperated tendons at 3 weeks. The controls had reached 63% at this time, and 70% at 4 weeks. The platelets stimulated repair also if they were implanted as a clot during the surgery [27]. Similar time sequences have not been performed with CDMPs, which have mostly been studied at 1 week.
How can it be possible that a single injection of thrombocytes can have effects on repair that lasts for several weeks? It seems counterintuitive, considering that the release of growth factors, and their survival, may be over in minutes or hours. We made a series of experiments with combinations of thrombocyte injections and different loading regimes and times, which appears to produce an explanation [25]. If the healing tendon was unloaded with botulinum toxin, no stimulatory effect of a thrombocyte injection could be seen at 2 weeks. Thus, loading is required for a lasting effect. However, thrombocytes increased callus strength at 5 days, and the size of the callus at 3 days, regardless of the loading regime. Thus, it seems that the thrombocyte injection caused a short-lived proliferative response. We concluded that this early response created a tissue, which allowed for mechanical stimulation to begin exerting its effects at an earlier stage. The thrombocyte-treated rats got a head start, and then kept ahead of the controls.
An important finding in our thrombocyte experiments was that the thrombin, which was used for activation of the platelets, also has a small but significant stimulatory effect of its own. Indeed, thrombin can act as a growth factor, not only via its proteolytic effects, but also as a ligand for cell surface receptors. This leads to the suspicion that inhibition of thrombin might have a deleterious effect on tendon repair. We have unpublished rat data to support that this is really the case (Virchenko et al., submitted manuscript 1). Continuous inhibition of thrombin activity decreases the size and strength of the tendon callus by about a third. This is important, because there have been discussions on whether patients with Achilles tendon ruptures should have routine prophylaxis against venous thrombosis. This prophylaxis acts through inhibition of thrombin (via factor Xa activity) and might have a negative effect on repair.
Conclusion and clinical possibilities
At least in rodent models, it is clear that tendon repair leaves room for improvement by externally applied factors. If this were to be tested in humans, where should one start? The first choice to make would be between mechanical stimulation and growth factors in some form. The mechanical influence is huge, and at first thought this would allow for more powerful clinical tools. However, we know very little of how to dose mechanical stimulation. We would need to know amplitude, frequency and duration, and also the appropriate time point during the repair process for loading to start, and how to avoid overload. Moreover, EMG studies [7] suggest that patients already do apply loads to their healing Achilles tendons. The maximum force in these studies is similar to the maximum voluntary force the patients have produced in our unpublished measurements. It seems that the loading is tuned by proprioceptive control mechanisms. Possibly, appropriate loading is already in place. Therefore, I believe chances for successful stimulation might be greater with local growth factors, especially if it holds true that an early stimulatory effect can have late consequences. The goal of human treatment would be to shorten the immobilisation time, and perhaps indirectly, through this, obtain a better functional end result. We have measured the mechanics of the healing human Achilles tendon 6 weeks after surgery via implanted markers, correlating strain with stress (Schepull et al., submitted manuscript 1). It turns out that the variation between patients is very large. This seems to be the case also in other species with similar-sized Achilles tendons, such as sheep, where force at failure showed an unexplained variation, much larger that in rodents (Virchenko et al., submitted manuscript 2). The variation in humans suggests that there is room for improvement in at least some patients, but unfortunately it also means that clinical studies will have to be large to have a satisfactory statistical power.
A possible explanation for the weak interest in stimulation of tendon repair might be the difficulty of measuring any beneficial effect in clinical studies. In bone, the repair process can be followed on radiographs. Although this says little about the mechanical competence of the healing bone, imaging of healing tendons says almost nothing (Schepull et al., submitted manuscript 2). There are, however, methods at hand. As mentioned, we are currently using roentgen stereometric analysis (RSA). This method uses tantalum beads, which can be inserted into the tendon with needles. The diameter of the beads is only 0.8 mm, and they are harmless, but still visible on radiographs. With these markers, and stereoradiographs, three-dimensional measurements of tendon strain per load can be achieved, which allows calculation of e.g. modulus of elasticity of the tendon callus. Measurement of mechanical properties might open the way for earlier plaster removal following stimulation of repair. Moreover, we found that the modulus at the time of plaster removal predicts the functional result 1 year later. Another option for measuring treatment effects is to use validated, patient-administered scores. One such score has recently been developed to evaluate how the patients rate their functional end result after Achilles tendon injury [21].
In conclusion: tendon repair can be stimulated in animals, and methods for measuring tendon repair and function in humans have recently been developed. What are we waiting for?
Acknowledgement
Dr. Olena Virchenko helped making Table 1.
References
- 1.Ackermann PW, Li J, Lundeberg T, Kreicbergs A (2003) Neuronal plasticity in relation to nociception and healing of rat achilles tendon. J Orthop Res 21:432–441 [DOI] [PubMed]
- 2.Akizuki KH, Gartman EJ, Nisonson B, Ben-Avi S, McHugh MP (2001) The relative stress on the Achilles tendon during ambulation in an ankle immobiliser: implications for rehabilitation after Achilles tendon repair. Br J Sports Med 35:329–333, discussion 333–324 [DOI] [PMC free article] [PubMed]
- 3.Almeida-Silveira MI, Lambertz D, Perot C, Goubel F (2000) Changes in stiffness induced by hindlimb suspension in rat Achilles tendon. Eur J Appl Physiol 81:252–257 [DOI] [PubMed]
- 4.Aspenberg P, Forslund C (1999) Enhanced tendon healing with GDF 5 and 6. Acta Orthop Scand 70:51–54 [DOI] [PubMed]
- 5.Aspenberg P, Virchenko O (2004) Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand 75:93–99 [DOI] [PubMed]
- 6.Batten ML, Hansen JC, Dahners LE(1996) Influence of dosage and timing of application of platelet-derived growth factor on early healing of the rat medial collateral ligament.J Orthop Res 14(5):736–741 [DOI] [PubMed]
- 7.Benum P, Berg V, Fretheim OJ (1984) The strain on sutured Achilles tendons in walking cast. An EMG analysis. Eur Surg Res 16(Suppl 2):14–21 [DOI] [PubMed]
- 8.Chan PB, Fu S, Qin L, Lee K, Rolf CG, Chan K(2000) Effects of basic fibroblast growth factor (bFGF) on early stages of tendon healing: a rat patellar tendon model.Acta Orthop Scand 71(5):513–518 [DOI] [PubMed]
- 9.Eliasson P, Fahlgren A, Pasternak B, Aspenberg P (2007) Unloaded rat Achilles tendons continue to grow, but lose viscoelasticity. J Appl Physiol, e-pub ahead of print [DOI] [PubMed]
- 10.Forslund C, Aspenberg P(1998) OP-1 has more effect than mechanical signals in the control of tissue differentiation in healing rat tendons.Acta Orthop Scand 69(6):622–626 [DOI] [PubMed]
- 11.Forslund C, Aspenberg P (2002) CDMP-2 induces bone or tendon-like tissue depending on mechanical stimulation. J Orthop Res 20:1170–1174 [DOI] [PubMed]
- 12.Forslund C, Aspenberg P (2003) Improved healing of transected rabbit Achilles tendon after a single injection of cartilage-derived morphogenetic protein-2. Am J Sports Med 31:555–559 [DOI] [PubMed]
- 13.Forslund C, Aspenberg P (2001) Tendon healing stimulated by injected CDMP-2. Med Sci Sports Exerc 33:685–687 [DOI] [PubMed]
- 14.Forslund C, Bylander B, Aspenberg P (2003) Indomethacin and celecoxib improve tendon healing in rats. Acta Orthop Scand 74:465–469 [DOI] [PubMed]
- 15.Forslund C, Rueger D, Aspenberg P (2003) A comparative dose-response study of cartilage-derived morphogenetic protein (CDMP)-1, -2 and -3 for tendon healing in rats. J Orthop Res 21:617–621 [DOI] [PubMed]
- 16.Hildebrand KA, Woo SL, Smith DW, Allen CR, Deie M, Taylor BJ, Schmidt CC(1998) The effects of platelet-derived growth factor-BB on healing of the rabbit medial collateral ligament. An in vivo study.Am J Sports Med 26(4):549–554 [DOI] [PubMed]
- 17.Kashiwagi K, Mochizuki Y, Yasunaga Y, Ishida O, Deie M, Ochi M(2004) Effects of transforming growth factor-beta 1 on the early stages of healing of the Achilles tendon in a rat model.Scand J Plast Reconstr Surg Hand Surg 38(4):193–197 [DOI] [PubMed]
- 18.Kurtz CA, Loebig TG, Anderson DD, DeMeo PJ, Campbell PG(1999) Insulin-like growth factor I accelerates functional recovery from Achilles tendon injury in a rat model.Am J Sports Med 27(3):363–369 [DOI] [PubMed]
- 19.Lin TW, Cardenas L, Soslowsky LJ (2004) Biomechanics of tendon injury and repair. J Biomech 37:865–877 [DOI] [PubMed]
- 20.Matsumoto F, Trudel G, Uhthoff HK, Backman DS (2003) Mechanical effects of immobilization on the Achilles’ tendon. Arch Phys Med Rehabil 84:662–667 [DOI] [PubMed]
- 21.Nilsson-Helander K, Thomee R, Gravare-Silbernagel K, Thomee P, Faxen E, Eriksson BI, Karlsson J (2007) The Achilles tendon Total Rupture Score (ATRS): development and validation. Am J Sports Med 35:421–426 [DOI] [PubMed]
- 22.Rickert M, Jung M, Adiyaman M, Richter W, Simank HG (2001) A growth and differentiation factor-5 (GDF-5)-coated suture stimulates tendon healing in an Achilles tendon model in rats. Growth Factors 19:115–126 [DOI] [PubMed]
- 23.Sipe JB, Zhang J, Waits C, Skikne B, Garimella R, Anderson HC (2004) Localization of bone morphogenetic proteins (BMPs)-2, -4, and -6 within megakaryocytes and platelets. Bone 35:1316–1322 [DOI] [PubMed]
- 24.Spiro RC, Liu L, Heidaran MA, Thompson AY, Ng CK, Pohl J, Poser JW (2000) Inductive activity of recombinant human growth and differentiation factor-5. Biochem Soc Trans 28:362–368 [DOI] [PubMed]
- 25.Virchenko O, Aspenberg P (2006) How can one platelet injection after tendon injury lead to a stronger tendon after 4 weeks? Interplay between early regeneration and mechanical stimulation. Acta Orthop 77:806–812 [DOI] [PubMed]
- 26.Virchenko O, Fahlgren A, Skoglund B, Aspenberg P (2005) CDMP-2 injection improves early tendon healing in a rabbit model for surgical repair. Scand J Med Sci Sports 15:260–264 [DOI] [PubMed]
- 27.Virchenko O, Grenegard M, Aspenberg P (2006) Independent and additive stimulation of tendon repair by thrombin and platelets. Acta Orthop 77:960–966 [DOI] [PubMed]
- 28.Virchenko O, Skoglund B, Aspenberg P (2004) Parecoxib impairs early tendon repair but improves later remodeling. Am J Sports Med 32:1743–1747 [DOI] [PubMed]
- 29.Yamamoto N, Ohno K, Hayashi K, Kuriyama H, Yasuda K, Kaneda K (1993) Effects of stress shielding on the mechanical properties of rabbit patellar tendon. J Biomech Eng 115:23–28 [DOI] [PubMed]
- 30.Yasuda K, Hayashi K (1999) Changes in biomechanical properties of tendons and ligaments from joint disuse. Osteoarthritis Cartilage 7:122–129 [DOI] [PubMed]
- 31.Zhang F, Liu H, Stile F, Lei MP, Pang Y, Oswald TM, Beck J, Dorsett-Martin W, Lineaweaver WC (2003) Effect of vascular endothelial growth factor on rat Achilles tendon healing.Plast Reconstr Surg 112(6):1613–1619 [DOI] [PubMed]


