Abstract
Postoperative shed blood retransfusion (autotransfusion) is a commonly used salvage method following major surgical operations, such as total knee arthroplasty (TKA). The systemic effects of shed blood are still unclear. We studied the effect of residual substances in the retransfused shed blood, on lung perfusion after TKA. Fifteen unilateral and one bilateral TKAs were performed with autotransfusion (the study group) and 15 unilateral and three bilateral TKAs were performed in a control group. Lung X-rays, arterial blood gases (ABG), D-dimer values, and lung perfusion scintigraphies were performed preoperatively and postoperatively. A mean of 300.0 ± 335.6 ml of bank blood was needed in the autotransfusion group and a mean of 685.7 ± 365.5 ml of bank blood was needed in the control group (p=0.001). There was a postoperative segmental perfusion defect at the lateral segment of the superior lobe of the left lung in one patient of the control group and he also had risk factors for thrombosis. Although both groups had a decrease in lung perfusion postoperatively, there were no significant differences among the groups regarding the lung perfusion scintigraphy, chest X-rays, ABG, and D-dimer values. In conclusion, although pulmonary perfusion diminishes following TKA, shed blood retransfusion does not add any risk to pulmonary perfusion.
Résumé
La retransfusion post-opératoire est souvent utilisée dans les interventions majeures comme les prothèses de genou. L’effet systémique du sang récupéré n’est pas très connu. Nous avons étudié les effets de cette transfusion sur la perfusion pulmonaire. 15 prothèses de genou unilatérales et une bilatérale étaient réalisées en autotransfusion avec un groupe de contrôle de 15 unilatérales et 3 bilatérales. Des radiographies pulmonaires, les gaz du sang, la valeur des D-dimer et des scintigraphies de la perfusion pulmonaire étaient faites avant et après l’opération. Une moyenne de 300,0+−335,6 ml de sang de banque était nécessaire dans le groupe autotransfusion et une moyenne de 685,7+−365,5 ml était nécessaire dans le groupe de contrôle (p = 0,001). Il y avait un déficit segmentaire de perfusion chez un patient du groupe de contrôle et il avait aussi des facteurs de risque thrombotique. Bien que les 2 groupes avaient une diminution de la perfusion pulmonaire, il n’y avait pas de différences entre les 2 groupes pour la scintigraphie pulmonaire, les radiographies, les gaz du sang et les valeurs des D-dimer. En conclusion, bien que la perfusion pulmonaire diminue à la suite de l’arthroplastie totale de genou, la retransfusion du sang répandu n’entraine pas de risque particulier.
Introduction
Blood transfusion is frequently mandatory after major orthopaedic operations. Preventing the immunological reactions of bank blood and decreasing the risk of disease transmission, shed blood retransfusion (in other words, “autotransfusion”) is increasingly preferred following orthopaedic operations. The transfusion of salvaged blood in orthopaedic surgery decreases the need for allogenic transfusions [15, 18, 20, 27], but there are concerns, as salvaged shed blood may contain fat particles, bone matrix, locally administered antibiotics, methylmethacrylate, and tissue debris [12]. Entrance of these materials into the circulation may block an artery and cause pulmonary embolus (PE). Shed blood is filtered using 40-μm filters prior to retransfusion to prevent this complication, but these filters are not enough to eliminate the fat particles, together with white blood cells (WBC) and microaggregates [4, 19]. These particles are not known to cause a clinical effect and are probably eliminated rapidly from the circulation [3, 8], but their effect on the pulmonary perfusion is still unknown.
Pulmonary complications are frequent following arthroplasty. Clinical findings, chest X-ray, arterial blood gas (ABG) analysis, and scintigraphic studies are used to make a proper differential diagnosis. In the event of embolism, there is dyspnoea, tachypnoea with accompanying decreases in blood PO2 levels. Most patients (88%) who have PE have abnormal, but non-specific chest radiographic findings, including atelectasis and parenchymal areas of increased opacity [25]. So, chest X-ray is not an accurate diagnostic tool [10]. The perfusion lung scan has been a safe, non-invasive technique to evaluate regional pulmonary perfusion [26]. As a result, the diagnosis of pulmonary embolus requires a combination of laboratory findings.
In this study, we aimed to determine the effect of shed blood transfusion on lung perfusion following total knee arthroplasty (TKA) and compared clinical findings, lung perfusion scintigraphy, chest X-ray blood gases, and D-dimer values in autologous and homologous transfused patient groups.
Methods
Patient selection and preoperative assessments
This is a prospective study which was approved by the Ethics Committee of the Faculty of Medicine, Afyon Kocatepe University, Turkey. The patients undergoing bi- or tricompartmental TKAs with a diagnosis of primary osteoarthritis were included in the study. The patients with a history of lung or thromboembolic problems, and patients having infection, rheumatoid, or malignant disease were excluded from the study. The 32 patients (30 female, two male) who gave informed consent were included in the study. For randomisation, the group of the first patient was determined by drawing lots and the following patients were distributed into groups one by one. Two randomised groups were formed, each consisting of 16 patients. The demographic data of both groups can be seen in Table 1. All patients had a chest X-ray, D-dimer and ABG analyses, and lung perfusion scintigraphies prior to the operation. The results were expressed as the alveolar-arterial gradient (A-a) in order to estimate the degree of shunting in the lungs. The A-a gradient was calculated by the formula: A-a=(148−(PaCO2/0.8))−PaO2.
Table 1.
Demographic patient data
| Study group | Control group | p value | |||
|---|---|---|---|---|---|
| Sex (female/male) | 16/0 | 14/2 | 0.483 | ||
| Age (years) | 66.9 ± 9.1 | 66.2 ± 7.1 | 0.625 | ||
| BMI | 32.6 ± 4.3 | 34.3 ± 8.3 | 0.830 | ||
| Preoperative risk factors | Hypertension | 5 | Hypertension | 6 | – |
| Diabetes mellitus | 2 | Varicosity+CAD+smoking | 1 | ||
| Anaesthesia time | <2 h | 14 | <2 h | 10 | 0.220 |
| >2 h | 2 | >2 h | 6 | ||
| Anaesthesia type | General | 11 | General | 10 | 0.710 |
| Spinal+epidural | 5 | Spinal+epidural | 6 | ||
| Arthroplasty type | Unilateral | 15 | Unilateral | 13 | 0.600 |
| Bilateral | 1 | Unilateral | 3 | ||
| Hospital stay duration (days) | 21.2 ± 11.3 | 16.5 ± 6.9 | 0.163 | ||
| Autologous transfusion (ml) | 509.7 ± 186.7 | – | – | ||
| Homologous transfusion (ml) | 300.0 ± 335.6 | 685.7 ± 365.5 | 0.001* | ||
| Total drainage (ml) | 725.6 ± 309.8 | 888.5 ± 497.8 | 0.385 | ||
*The need for bank blood was significantly lower in the autotransfusion group. BMI=body mass index. CAD=coronary artery disease
Surgery and postoperative assessments
All patients were given dalteparin sodium 5000 IU/once a day subcutaneously for thrombosis prophylaxis. It was given daily, commencing the night before the operations, and was continued until the end of the third postoperative week. A tourniquet was used in all patients. All patients underwent perioperative prophylaxis against infection using cephazolin sodium 2 g intravenously. The first IV dose was administered 30 min prior to inflation of the tourniquet. Continuing doses of antibiotic, which was 1 g of cephazolin sodium, was given every consecutive 8 h for two days. General or combined epidural spinal anaesthesia was performed in all patients (according to the preference of our department of anaesthetics). The tourniquet was released just before closure of the wound. All patients underwent cemented bi- or tricompartmental TKAs. Two drains for shed blood drainage, one subfascial and one subcutaneous, were placed prior to closure of the wound. During the postoperative 24 h, all patients were observed in the postoperative intensive care unit. Pain control was managed either with an epidural patient-controlled analgesia (PCA) device or with an IV PCA device. Cold pack, anti-inflammatory treatment, and antiembolic stockings were also used. Oxygen saturations were recorded with the aid of pulse oximeters and the number of pulses and the respiration rate were recorded every hour by nursing staff. Oxygen saturation below 90% was accepted as low saturation and a respiratory rate above 20/min was accepted as tachypnoea. If the blood haemoglobin level was below 9 g/dl and there was evident clinical signs of anaemia, the patients were given additional homologous blood. The drainage amount was recorded continuously and the drains were removed at the 48th hour postoperatively. Continuous passive motion exercises were started the day following the operation and the patients were mobilised the next day. On the postoperative first day, ABG, chest X-ray, D-dimer, and lung perfusion scintigraphies were repeated. The preoperative and postoperative chest X-rays were evaluated by a blinded chest diseases specialist (FF).
Transfusion procedure
We used ConstaVac CBC II (Stryker, Kalamazoo, MI, USA) to collect the blood from wound drainage. Each was connected at the end of the operation. The drain fluid was collected during the first 6 h. No anticoagulant was used in the collection system. Collected bloods were transfused at the end of the sixth hour. Reinfusion was performed using a standard 40-μm blood filter between the collection bag and the intravenous site. This particular system prevents the top layer of blood drainage (100 ml) from being collected in the reinfusion bag, so that the majority of collected fat particles are not transfused. After the six hours, any blood collected from the reinfusion drain was discarded.
Lung perfusion scans
Perfusion lung scintigraphy was performed using 5 mCi (185 MBq) 99mTc-labeled macro aggregated albumin (99mTc-MAA). The tracer was injected during the maximum inspiration and expiration phase, followed by holding the breath for about 20 s in a supine position. Just after tracer administration, lung scan was performed using a single head gamma-camera system (Philips Medical Systems gamma Diagnost, 1997, Holland) equipped with a low-energy high-resolution collimator. Image acquisition parameters were 256×256 matrix and a 20% window centered at the 99mTc photo peak energy (140 keV). Anterior, posterior, right and left lateral, and four oblique views (RAO, LAO, RPO, and LPO) were collected. Each view was acquired for 60 s.
The analyses of lung perfusion scans were performed using lung quantitation menu (Pegasys, Euro Custom Menu Version 2.8, Adac Laboratories Europe B.V. Netherlands). This program calculates pixel and counts data in six segments of the anterior, the posterior, and the conjugated view of a lung study. After background activity subtraction, each lung image was divided from top to base into three segments, upper, middle, and lower, and the count/pixel rate was calculated for each segment. Percentages per region and calculations as a total of the view are shown for the anterior, posterior, and conjugated views in preoperative and postoperative scans also (Fig. 1).
Fig. 1.
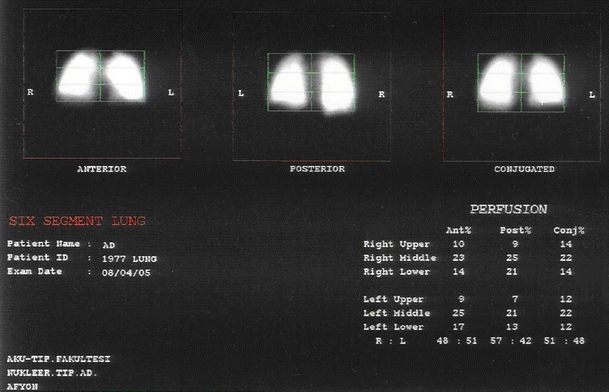
Percentages of regional distribution of 99mTc-macro aggregated albumin (MAA) perfusion
Statistical analysis
The data are reported as means and standard deviations. All statistical analysis was performed on SPSS Ver. 11.5 for windows. Shapiro-Wilk’s test was used to determine the distribution of data. Paired-samples t-test was used for the comparison of preoperative and postoperative data. When the differences among the groups were compared to each other, independent samples t-test was used. The Chi-square test was used for non-parametric values. A p value <0.05 was considered to be statistically significant.
Results
Patient groups and autotransfusion
The demographic data of both groups and the statistical differences among groups were shown in Table 1. Both groups were similar in terms of sex, age, body mass index, type and duration of anaesthesia, bi- or unilaterality of the operation, and length of stay at the hospital. None of the patients had postoperative dyspnoea. Five patients in the study group and nine patients in the control group had a decrease in oxygen saturation, but this was not found to be significant (p=0.233). Thirteen patients in the study and 13 patients in the control groups had tachypnoea. The total amount of drainage was not significantly different, but the study group receiving autologous transfusion needed much less homologous transfusion (Table 1).
Blood gas and D-dimer values
Both groups had postoperative alkalosis and hypoxia, but there were no significant differences among groups. Alkalosis and hypocarbia was more marked in the control group (Table 2).
Table 2.
Arterial blood gas (ABG) values
| ABG | Study group | Control group | p* value | ||||
|---|---|---|---|---|---|---|---|
| Preop | Postop | p value | Preop | Postop | p value | ||
| PH | 7.46 ± 0.05 | 7.49 ± 0.04 | 0.024 | 7.47 ± 0.06 | 7.51 ± 0.07 | 0.003 | 0.945 |
| pO2 | 80.2 ± 6.6 | 66.2 ± 10.1 | 0.002 | 80.1 ± 5.8 | 68.8 ± 9.1 | 0.001 | 0.546 |
| pCO2 | 35.2 ± 5.4 | 34.7 ± 7.5 | 0.692 | 30.2 ± 4.8 | 26.4 ± 6.2 | 0.012 | 0.126 |
| A-a | 23.8 ± 11.2 | 38.4 ± 14.1 | 0.001 | 30.1 ± 6.7 | 45.3 ± 9.6 | 0.001 | 1.000 |
| SO2% | 94.8 ± 2.0 | 92.3 ± 3.1 | 0.015 | 93.7 ± 2.9 | 93.3 ± 3.3 | 0.740 | 0.105 |
| BEb | 0.65 ± 3.37 | 3.38 ± 3.83 | 0.164 | −0.31 ± 3.85 | −1.00 ± 2.87 | 0.693 | 0.059 |
| HCO−3 | 25.6 ± 4.0 | 26.8 ± 4.8 | 0.308 | 21.7 ± 3.5 | 20.4 ± 4.1 | 0.803 | 0.375 |
p* = differences of between the groups. ABG=arterial blood gases analysis. A-a=alveolar-arterial gradient. BEb=base excess
The preoperative D-dimer levels of the study group were 0.46 ± 12.96 mg/dl, and rose significantly up to 47.16 ± 41.89 mg/dl postoperatively (p=0.001). The D-dimer levels in the control group rose to 31.84 ± 47.10 mg/dl from a preoperative value of 0.96 ± 2.01 mg/dl (p=0.008). The increases in both groups were not significantly different (p=0.235).
Chest X-ray and lung perfusion scintigraphy
The blinded evaluation of chest X-rays revealed unilateral elevation of the diaphragm in three patients in the study group and two patients in the control group. One patient in the control group preoperatively had left basal linear atelectasis, which persisted postoperatively. No other patient had signs of atelectasis. Five patients in both groups had cardiomegaly.
When compared to preoperative values, scintigraphic studies revealed significant postoperative decreases in perfusion scans in the AS-L (p = 0.039), AM-R (p = 0.028), PS-L (p = 0.040), and PM-L (p = 0.002) segments in the study group and AS-L (p = 0.012), PS-R (p = 0.042), PS-L (p = 0.036), and PI-L (p = 0.012) segments in the control group (Table 3). There was no difference between groups regarding perfusion changes (p > 0.05), except AS-L segment (p = 0.007). Neither of the groups showed differences regarding lung perfusions when the count/pixel ratios were compared in lung segments (Table 4).
Table 3.
Anterior and posterior activity counts of the six segments of the lungs
| Lung segment | Study group | Control group | p* value | |||||
|---|---|---|---|---|---|---|---|---|
| Preop (count) | Postop (count) | p value | Preop (count) | Postop (count) | p value | |||
| AS | R | 168.98 ± 24.86 | 151.63 ± 34.81 | 0.092 | 169.90 ± 26.73 | 145.73 ± 96.97 | 0.572 | 0.391 |
| L | 204.22 ± 24.83 | 187.04 ± 38.34 | 0.039 | 222.71 ± 47.28 | 147.73 ± 54.99 | 0.012 | 0.007 | |
| AM | R | 212.61 ± 30.02 | 185.19 ± 47.86 | 0.028 | 221.15 ± 71.52 | 161.17 ± 76.89 | 0.056 | 0.281 |
| L | 136.26 ± 43.46 | 125.17 ± 35.41 | 0.378 | 137.65 ± 47.35 | 129.95 ± 65.73 | 0.692 | 0.877 | |
| AI | R | 90.20 ± 31.93 | 72.07 ± 27.70 | 0.082 | 73.28 ± 22.69 | 60.71 ± 34.61 | 0.414 | 0.749 |
| L | 68.03 ± 27.15 | 63.34 ± 17.40 | 0.836 | 62.51 ± 14.44 | 61.71 ± 31.66 | 0.945 | 0.763 | |
| PS | R | 84.25 ± 22.54 | 84.01 ± 30.92 | 0.977 | 99.29 ± 25.36 | 71.81 ± 29.40 | 0.042 | 0.064 |
| L | 84.49 ± 19.59 | 71.66 ± 18.24 | 0.040 | 85.00 ± 19.55 | 56.35 ± 20.81 | 0.036 | 0.188 | |
| PM | R | 93.22 ± 26.18 | 89.26 ± 29.77 | 0.660 | 100.88 ± 20.64 | 79.94 ± 31.60 | 0.103 | 0.683 |
| L | 84.01 ± 20.93 | 65.65 ± 19.58 | 0.002 | 80.83 ± 15.01 | 64.75 ± 26.24 | 0.233 | 0.856 | |
| PI | R | 111.45 ± 36.51 | 85.91 ± 33.39 | 0.066 | 138.91 ± 85.70 | 72.73 ± 33.41 | 0.068 | 0.668 |
| L | 99.85 ± 33.53 | 96.56 ± 20.86 | 0.918 | 106.30 ± 22.44 | 72.61 ± 25.85 | 0.012 | 0.142 | |
p* = differences between the groups. AS=antero-superior, AM=antero-medial, AI=antero-inferior, PS=postero-superior, PM=postero-medial, PI=postero-inferior, preop=preoperative, postop=postoperative
Table 4.
Amount (in each pixel) of anterior and posterior activities in six lung segments
| Lung segment | Study group | Control group | p* value | |||||
|---|---|---|---|---|---|---|---|---|
| Preop (count/pixel) | Postop (count/pixel) | p value | Preop (count/pixel) | Postop (count/pixel) | p value | |||
| AS | R | 22.00 ± 2.56 | 21.69 ± 2.18 | 0.703 | 21.00 ± 1.20 | 21.50 ± 1.69 | 0.381 | 0.409 |
| L | 26.00 ± 4.89 | 27.00 ± 2.22 | 0.899 | 27.50 ± 2.27 | 26.88 ± 1.96 | 0.435 | 0.497 | |
| AM | R | 30.94 ± 3.70 | 30.25 ± 3.26 | 0.368 | 31.50 ± 3.07 | 28.75 ± 4.71 | 0.222 | 0.256 |
| L | 19.06 ± 4.11 | 20.38 ± 2.68 | 0.233 | 20.75 ± 6.02 | 21.62 ± 2.97 | 0.570 | 0.812 | |
| AI | R | 12.56 ± 3.18 | 11.31 ± 3.53 | 0.249 | 10.50 ± 2.56 | 11.00 ± 3.70 | 0.582 | 0.288 |
| L | 9.31 ± 2.36 | 10.19 ± 2.07 | 0.115 | 8.88 ± 1.36 | 10.50 ± 2.07 | 0.048 | 0.405 | |
| PS | R | 10.37 ± 2.45 | 12.88 ± 2.30 | 0.006 | 11.88 ± 2.03 | 12.88 ± 1.81 | 0.275 | 0.162 |
| L | 10.81 ± 1.97 | 10.06 ± 1.95 | 0.216 | 10.25 ± 1.28 | 10.00 ± 0.78 | 0.914 | 0.398 | |
| PM | R | 13.19 ± 2.71 | 14.06 ± 3.38 | 0.453 | 14.75 ± 1.28 | 14.50 ± 2.73 | 0.809 | 0.530 |
| L | 11.88 ± 1.78 | 10.44 ± 2.25 | 0.027 | 11.88 ± 2.59 | 11.38 ± 1.60 | 0.664 | 0.122 | |
| PI | R | 14.56 ± 4.00 | 12.06 ± 3.55 | 0.090 | 13.13 ± 2.80 | 12.62 ± 2.62 | 0.920 | 0.267 |
| L | 13.00 ± 3.35 | 13.50 ± 1.63 | 0.584 | 13.25 ± 2.12 | 13.00 ± 1.60 | 0.048 | 0.439 | |
p = differences in number of pixels in each group in preoperative and postoperative period. p* = differences in number of pixels between the groups. AS=antero-superior, AM=antero-medial, AI=antero-inferior, PS=postero-superior, PM=postero-medial, PI=postero-inferior, preop=preoperative, postop=postoperative
Pulmonary embolus was diagnosed in one patient who had a unilateral bicompartmental knee replacement in the control group. This patient had coronary artery disease, varicosities of the lower extremities, and a history of smoking, which increased the risk factors for thrombosis. Despite the lack of clinical findings, the laboratory studies showed a drop of oxygen saturation, hypoxia, hypocarbia, and alkalosis (PO2: 46.60, SO2: 83.90, PCO2: 26.60, pH: 7.60). Although there were no signs of ventilation problems, there was a segmental perfusion defect at a lateral segment of the superior lobe of the left lung (Fig. 2a,b).
Fig. 2.
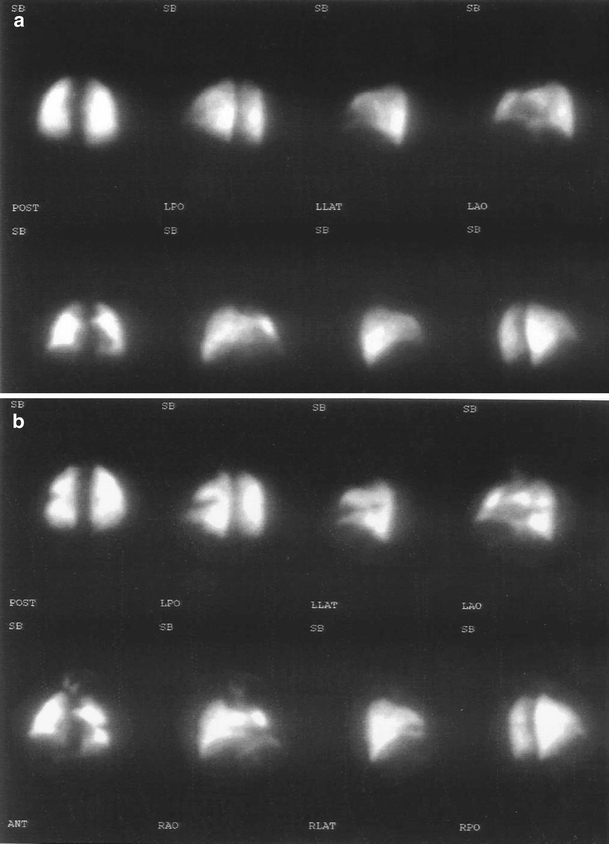
a The preoperative perfusion scan of the patient (with risk factors for thrombosis). b Postoperative perfusion scan of the same patient showing a segmental perfusion defect at a lateral segment of the superior lobe of the left lung
Discussion
Because it is easy, effective, safe enough, and applicable in every patient, nowadays, postoperative salvage of blood from wound drainage is preferred in TKA [7, 8, 11, 13, 23]. Since most of the bleeding occurs not during, but early after the operation, postoperative autotransfusion decreases the need to use allogenic bank blood [17, 18]. Similarly, in our study, the patients in the study group received 509.70 ± 186.65 ml autotransfusion, which decreased the need to use bank blood when compared to the control group.
Fat particles, bone matrix, methylmethacrylate, and tissue debris, the presence of which are open to debate in the salvaged blood, are thought to decrease lung perfusion by obliterating the lung vessels and, also, it is thought that autotransfusion can cause thromboembolism by increasing blood coagulation [2, 6]. After TKA, total pulmonary thromboemboli (PTE) is thought to occur in 0% to 12% (median, 4.6%), symptomatic PTE in 0% to 1.1% (median, 0.5%), and fatal PTE in 0% to 1.5% (median, 0.22%) patients [1, 5, 16]. Wolf et al. have extensively reviewed the available literature, especially examining serial scintigraphic perfusion scanning before and after arthroplasty. With the use of low-dose heparin, the incidence of PTE is between 0.8% to 4.7% [24]. Fat embolism occurred in 65% of cases with a bilateral TKA and 46% with a unilateral TKA. Bone marrow cell embolism occurred in 12% of the bilateral and in 4% of the unilateral cases [14]. Blevins et al. used autotransfusion in children after orthopaedic operations and studied the fat particles in shed blood. They found that there were many particles smaller than 9 μm and a few particles between 9–40 μm. He did not detect any particles larger than 40 μm. He also reported that the 9–40 μm and <9 μm fat particles were rapidly eliminated from the circulation [3]. On the other hand, despite the activation of coagulation, there is no evidence that fibrinogen-fibrin products in unwashed shed blood produce coagulopathy. Nor did we find any differences between the autologous and homologous transfusion groups regarding the activation of coagulation. But the possibility remains a cause for concern [8]. Martin et al. used autotransfusion in 197 cases to whom they had performed cementless total hip arthroplasty and did not face any perioperative mortalities, pulmonary embolism, or fat embolism syndrome [18]. This is also consistent with our results.
The radionuclide imaging technique we used to determine the lung perfusion depends on trapping the radiolabeled particles (microembolisation) in the capillary bed of the lung [22]. Pulmonary capillaries have a diameter of about 8 μm and the precapillary arterioles have a diameter of 20–25 μm. 99mTc-MAA particles are, generally, in the range of 10–50 μm in diameter. 99mTc-MAA particles are physically trapped in the arteriocapillary beds of the lung and block the blood flow to distal regions [22]. The lung perfusion scan permits a better assessment of the integrity of the microcirculation than pulmonary angiography and also indicates the status of the entire pulmonary vasculature [9]. The main strength of the ventilation-perfusion scan is that it allows us to obtain quantitative information about the ventilation and perfusion of the lungs in a physiological way [21]. Quantitative assessment of lung perfusion provides useful information about the severity of diseases and the effect of therapy, in addition to the routine visual representation [9]. Although we took precautions such as antiembolic prophylaxis, antiembolic stockings, and early mobilisation, we had scintigraphically evident pulmonary embolus in only one (3.13%) of the cases in our control group and that case had risk factors for thrombosis (coronary artery disease, varicosities, and smoking).
In conclusion, although there is a risk of pulmonary embolus or fat embolism following total knee replacements, shed blood retransfusion does not increase the risk to lung perfusion.
References
- 1.Ansari S, Warwick D, Ackroyd CE, Newman JH (1997) Incidence of fatal pulmonary embolism after 1,390 knee arthroplasties without routine prophylactic anticoagulation, except in high-risk cases. J Arthroplasty 12(6):599–602 [DOI] [PubMed]
- 2.Biagini D, Filipucci E, Agnelli G, Pagliaricci S (2004) Activation of blood coagulation in patients undergoing postoperative blood salvage and re-infusion of unwashed whole blood after total knee arthroplasty. Thromb Res 113(3–4):211–215 [DOI] [PubMed]
- 3.Blevins FT, Shaw B, Valeri CR, Kasser J, Hall J (1993) Reinfusion of shed blood after orthopaedic procedures in children and adolescents. J Bone Joint Surg Am 75(3):363–371 [DOI] [PubMed]
- 4.Brooke M, Van Aken H, Strom M, Fritzsche F, Wirtz S, Hinder F (2001) Fat elimination from autologous blood. Anesthesia Analg 92(2):341–343 [DOI] [PubMed]
- 5.Brookenthal KR, Freedman KB, Lotke PA, Fitzgerald RH, Lonner JH (2001) A meta-analysis of thromboembolic prophylaxis in total knee arthroplasty. J Arthroplasty 16(3):293–300 [DOI] [PubMed]
- 6.Bruce W, Van der Wall H, Peters M, Liaw Y, Morgan L, Storey G (2001) Occurrence of pulmonary thromboembolism immediately after arthroplasty. Nucl Med Commun 22(11):1237–1242 [DOI] [PubMed]
- 7.Clements DH, Sculco TP, Burke SW, Mayer K, Levine DB (1992) Salvage and reinfusion of postoperative sanguineous wound drainage. A preliminary report. J Bone Joint Surg Am 74(5):646–651 [PubMed]
- 8.Faris PM, Ritter MA, Keating EM, Valeri CR (1991) Unwashed filtered shed blood collected after knee and hip arthroplasties. A source of autologous red blood cells. J Bone Joint Surg Am 73(8):1169–1178 [PubMed]
- 9.Fukuchi K, Hayashida K, Nakanishi N, Inubushi M, Kyotani S, Nagaya N, Ishida Y (2002) Quantitative analysis of lung perfusion in patients with primary pulmonary hypertension. J Nucl Med 43(6):757–761 [PubMed]
- 10.Greenspan RH, Ravin CE, Polansky SM, McLoud TC (1982) Accuracy of the chest radiograph in diagnosis of pulmonary embolism. Invest Radiol 17(6):539–543 [DOI] [PubMed]
- 11.Han CD, Shin DE (1997) Postoperative blood salvage and reinfusion after total joint arthroplasty. J Arthroplasty 12(5):511–516 [DOI] [PubMed]
- 12.Jacobsson M, Bengtsson A (2004) Fat embolism and autologous blood transfusions in orthopaedic surgery. Curr Anaesth Crit Care 15(2):87–93 [DOI]
- 13.Jensen CM, Pilegaard R, Hviid K, Nielsen JD, Nielsen HJ (1999) Quality of reinfused drainage blood after total knee arthroplasty. J Arthroplasty 14(3):312–318 [DOI] [PubMed]
- 14.Kim YH (2001) Incidence of fat embolism syndrome after cemented or cementless bilateral simultaneous and unilateral total knee arthroplasty. J Arthroplasty 16(6):730–739 [DOI] [PubMed]
- 15.Kristensen PV, Sorensen LS, Thregod HC (1992) Autotransfusion of drainage blood in arthroplasty. A prospective, controlled study of 31 operations. Acta Orthop Scand 63(4):377–380 [DOI] [PubMed]
- 16.Lieberman JR, Sung R, Dorey F, Thomas BJ, Kilgus DJ, Finerman GA (1997) Low-dose warfarin prophylaxis to prevent symptomatic pulmonary embolism after total knee arthroplasty. J Arthroplasty 12(2):180–184 [DOI] [PubMed]
- 17.Lotke PA, Faralli VJ, Orenstein EM, Ecker ML (1991) Blood loss after total knee replacement. Effects of tourniquet release and continuous passive motion. J Bone Joint Surg Am 73(7):1037–1040 [PubMed]
- 18.Martin JW, Whiteside LA, Milliano MT, Reedy ME (1992) Postoperative blood retrieval and transfusion in cementless total knee arthroplasty. J Arthroplasty 7(2):205–210 [DOI] [PubMed]
- 19.Ramirez G, Romero A, Garcia-Vallejo JJ, Munoz M (2002) Detection and removal of fat particles from postoperative salvaged blood in orthopedic surgery. Transfusion 42(1):66–75 [DOI] [PubMed]
- 20.Thomas D, Wareham K, Cohen D, Hutchings H (2001) Autologous blood transfusion in total knee replacement surgery. Br J Anaesth 86(5):669–673 [DOI] [PubMed]
- 21.Tiel-van Buul MMC, Verzijlbergen JF (2004) Ventilation-perfusion lung scintigraphy. Imaging Decis 8(4):3–14 [DOI]
- 22.Vallabhajosula S (2001) Pathophysiology and mechanisms of radiopharmaceutical localization. In: Elgazzar AH (ed) The pathophysiologic basis of nuclear medicine. Springer, Berlin Heidelberg New York, p 29
- 23.Wixon RL, Kwaan HC, Spies SM, Zimmer AM (1994) Reinfusion of postoperative wound drainage in total joint arthroplasty. Red blood cell survival and coagulopathy risk. J Arthroplasty 9(4):351–358 [DOI] [PubMed]
- 24.Wolf LD, Hozak WJ, Rothman RH (1993) Pulmonary embolism in total joint arthroplasty. Clin Orthop Related Res 288:219–233 [PubMed]
- 25.Worsley DF, Alavi A, Aronchick JM, Chen JT, Greenspan RH, Ravin CE (1993) Chest radiographic findings in patients with acute pulmonary embolism: observations from the PIOPED study. Radiology 189(1):133–136 [DOI] [PubMed]
- 26.Worsley DF, Alavi A (2003) Radionuclide imaging of acute pulmonary embolism. Semin Nucl Med 33(4):259–278 [DOI] [PubMed]
- 27.Xenakis TA, Malzos KN, Dailiana Z, Koukoubis T, Zervou E, Golegou C, Soucacos PN (1997) Blood salvage after total hip and total knee arthroplasty. Acta Orthop Scand 275:135–138 [DOI] [PubMed]


