Abstract
Prostaglandins, PGE2 in particular, have diverse actions on various organs, including inflammation, bone healing, bone formation, embryo implantation, induction of labour and vasodilatation, among others. However, systemic side effects have limited their clinical utility. The pharmacological activities of PGE2 are mediated through four G protein-coupled receptor subtypes, EP1–EP4. Recent studies have shown that EP2 and EP4 receptors play important roles in regulating bone formation and resorption. EP2 and EP4 receptor-selective agonists have been shown to stimulate local or systemic bone formation, augment bone mass and accelerate the healing of fractures or bone defects in animal models upon local or systemic administration, thus, potentially offering new therapeutic options for enhancing bone formation and bone repair in humans. This review will focus on the studies related to bone formation and bone healing in the EP receptor knockout (KO) mice and the EP2 or EP4 receptor-selective agonist treated animal models.
Résumé
Les prostaglandines en particulier la PGE2 ont des actions relativement diverses sur différents organes, notamment en termes d’inflammation, de réparation osseuse, de régénération osseuse, d’implantation embryonnaire, d’induction du travail et de vasodilatation. Ces études ont montré que les récepteurs EP2 et EP4 avaient donc un rôle important dans la régulation de formation osseuse et dans sa résorption. On a pu démontrer que les récepteurs EP2 et EP4 stimulaient de façon locale ou systémique la formation osseuse, augmentaient la masse osseuse et accéléraient la guérison des fractures ou la réparation des défects osseux chez les animaux. Ceci nous offre un nouveau potentiel thérapeutique concernant l’amélioration de la régénération osseuse et la réparation des lésions osseuses chez l’homme. Cette revue permet de mettre en valeur les études relatives à la formation et à la cicatrisation osseuse avec le récepteur EP chez la souris et les récepteurs ajustés EP2, EP4 chez les animaux modèles.
Introduction
Prostaglandins are enzymatically derived metabolites of polyunsaturated fatty acids, such as arachidonic acid. PGE2 in particular is the most widely produced prostanoid in the human body and has diverse actions on various organs, including inflammation, bone healing, bone formation, embryo implantation, induction of labour and vasodilatation, among others. Given such a widespread involvement, PGE2 and its signalling pathway has been the target of clinical utility for a variety of diseases/patho-physiological conditions, including fracture, osteoporosis and kidney failure, as suggested by animal studies [4, 23, 24]. The traditional pharmaceutical approach has been to target enzymes involved in the metabolism of PGE2, such as COX-1 or COX-2, which has been done either by non-selective agents, such as non-steroidal anti-inflammatory drugs (NSAIDs), or by selective COX-2 inhibitors (COXBs). This has mainly been the approach where one wants to limit the level of PGE2, such as in chronic inflammation.
Currently, few therapeutic options exist for the enhancement of bone repair. Pharmacological intervention in fracture healing or bone repair is still limited to bone morphogenetic proteins (BMP2 and BMP7) [18, 21]. The cost effectiveness, degree of clinical benefit and long-term safety of these therapies have not been fully delineated. Non-peptide, small molecules may provide advantages over peptides or proteins as pharmacological agents for initiating or enhancing bone repair. Prostaglandins, including prostaglandin E1 (PGE1), prostaglandin E2 (PGE2) and prostaglandin F2α, have been demonstrated to stimulate both bone resorption and bone formation but in favour of bone formation, thus, increasing bone mass and bone strength [4, 6]. Endogenous PGE2 increases locally after fracture [3] and the inhibition of PGE2 production impairs bone healing [8]. In contrast, the local administration of PGE2 stimulates bone formation and callus development in animal models [9, 23]. However, due to side effects, including diarrhoea, lethargy and flushing, PGE2 is an unacceptable therapeutic option for skeletal disorders in humans. The identification of the receptor subtypes has greatly facilitated the investigation of the roles for specific receptors in human pathophysiology and provides the opportunities to separate the beneficial and side effects of PGE2. It is now known that the pharmacological activities of PGE2 are mediated through four G protein-coupled receptor subtypes, EP1–EP4 [2], of which two, the EP2 and EP4 receptors, act by stimulating cAMP production. Both receptors are expressed in bone cells and marrow stromal cells. Although it is not completely understood which receptor subtype(s) is associated with the anabolic effect of PGE2, studies have shown that both EP2 and EP4 receptors play important roles in regulating bone formation and resorption [4, 13, 20]. Recent findings in mice lacking EP2 and EP4 receptors and the effects of EP2 and EP4 receptor-selective small molecule agonists have suggested a therapeutic potential of these agents for enhancing bone formation and bone healing.
Bone phenotype of mice lacking EP receptors
Mice lacking each of the four EP receptor subtypes have been generated. The role of EP2 in the formation of osteoclast-like tartrate-resistant acid phosphatase-positive multi-nucleated cells has been studied using cells from the EP2 receptor knockout (KO) mice [12]. The results showed that osteoclastogenesis was impaired in EP2 KO mice and the major defect appeared to be in the capacity of osteoblastic cells to stimulate osteoclast formation and in the response of osteoclastic lineage to PGE2, suggesting that EP2 receptors play an important role in osteoclast formation. A study using EP1 and EP2 receptor KO mice demonstrated that EP1 receptors had minimal influence on skeletal strength or size, while EP2 receptors had a major influence on the biomechanical properties of bone, since the absence of EP2 receptors resulted in weaker bones when compared with the wild type animals [1].
Studies using cell cultures derived from EP4 KO mice have showed that EP4 receptor mediated both bone resorption and formation. Cultured calvarial cells from EP4 KO mice had no increase in bone resorption when exposed to PGE2 [16]. In the bone marrow cell cultures, mineralised nodule formation was absent and could not be increased by treatment with PGE2 [25]. When PGE2 was infused onto the periosteal surfaces of femurs, it caused local bone formation in the wild type but not in EP4 KO mice [26]. The lack of skeletal phenotype in the adult EP4 KO mice suggests that EP4 receptor is not essential for the bone growth and maturation in mice [17]. However, at 12 months of age [11], theses animals exhibited low bone mass, accompanied with deteriorated trabecular architecture and thinner cortex. The lower bone formation in the EP4 KO mice was primarily due to the defect in osteoblastogenesis. When the femurs of these mice fractured, callus formation was significantly delayed as both intramembraneous and endochondral calcification were impaired at the early stage of fracture healing in the absence of the EP4 receptor. With the progression of healing, the conversion of cartilaginous component of the callus to bone and forming a bony bridge at the fracture site was slower in the EP4 KO mice. These findings in the mice lacking EP2 or EP4 receptors indicate that these receptors play important roles in bone metabolism.
Angiogenesis is crucial for the wound healing of both soft and hard tissues. A recent study [5] in the EP1, EP2, EP3 and EP4 respective receptor KO mice with full-thickness skin wounds showed that wound closure and re-epithelisation in EP3 but not in the other EP receptor KO mice were significantly delayed compared with their wild type controls. Wound-induced angiogenesis estimated with CD31 immunohistochemistry was significantly inhibited and the immuno-reactive vascular endothelial growth factor (VEGF) in wound granulation tissues was markedly reduced in EP3 KO mice. In addition, wound-induced angiogenesis and wound closure were also significantly suppressed in wild type mice transplanted with bone marrow cells from EP3 KO mice compared with those in mice transplanted with bone marrow cells from wild type mice. These were accompanied with the reductions in accumulation of VEGF-expressing cells in wound granulation tissues and in the mobilisation of VEGF receptor 1-expressing leukocytes in peripheral circulation. These results indicate a critical role of EP3 receptor in wound healing and encourage investigation on the role of EP3 in bone repair.
EP2 receptor-selective agonist and bone formation and healing
More recently, based upon the understanding of EP receptor signalling, various laboratories have developed receptor-selective agents that are either agonists or antagonists of the receptor. One of these compounds is CP-533,536, a non-prostanoid EP2 receptor agonist [10, 19]. The effects of this compound in local bone formation and bone healing have been evaluated in several animal models. The findings in these studies could exemplify the potential efficacy of EP2 receptor agonists in bone formation and healing.
First, CP-533,536 at doses of 0, 0.3, 1 or 3 mg/kg was delivered into the bone marrow of the proximal tibial metaphysis in 6-week-old male rats by a single injection on day one [10]. The injected tibia was analysed using peripheral quantitative computed tomography (pQCT), bone histomorphometry and biomechanical testing on day 7. CP-533,536 dose-dependently stimulated new bone formation and increased bone mass in the injected site. The increased bone mass translated into significantly increased bone strength in these animals. Next, the ability of CP-533,536 to stimulate bone formation on the periosteum, a likely source of chondrocyte and osteoblast precursor cells involved in bone healing, was tested in 3-week-old male Sprague-Dawley rats [10]. To provide a high and extended local drug exposure without giving multiple injections, the compound was incorporated into an injectable and biodegradable polymer matrix. A single dose (0.3 mg) of CP-533,536 in the matrix was injected onto the periosteum of the right femurs on day one. On day 15, a well defined area of new bone formation was detected by radiograph at the site of injection in every femur treated with CP-533,536 and their total bone area and bone mineral content were significantly increased. The newly formed bone consisted of mineralised trabecular bone, without evidence of cartilage formation. This effect in rats with CP-533,536 is similar to the effect of a continuous delivery of PGE1 or PGE2 onto the periosteum of dogs [15] or mice [26]. The data suggest that the stimulatory effects of CP-533,536 on periosteal bone formation of an uninjured long bone are mainly through intramembraneous osteogenesis but not chondrogenesis.
With the evidence that CP-533,536 stimulates marrow and periosteal bone formation upon local delivery, the capability of this compound to enhance fracture healing was evaluated in a rat femoral fracture model [10]. A single injection of CP-533,536 at doses of 0.05, 0.5, and 5 mg formulated in the matrix dose-dependently stimulated callus formation when delivered at the fracture site. The histological evaluation of the site of fracture repair showed more extensive endochondral and intramembraneous ossification in the fractured femurs treated with CP-533,536, resulting in a smaller fracture gap, indicating more advanced healing. More importantly, CP-533,536 significantly increased energy absorption and force to failure of the healing fracture callus, indicating superior mechanical properties. These results demonstrate that CP-533,536 accelerated fracture healing and improved the biomechanical integrity of the fracture site in rats.
Lastly, the ability of CP-533,536 in enhancing bone repair was examined in a tibial osteotomy and an ulnar critical defect (1.5 cm) canine model [19 and unpublished studies]. A single dose of CP-533,536 was applied directly to the fracture or defect site immediately after the surgeries. Bone healing was monitored and evaluated radiographically over time and histologically at the end of the studies. Significantly advanced healing was observed in the tibia (Fig. 1) or ulnae treated with CP-533,536 during the course of the study. In the critical defect model, the newly formed bone started at both proximal and distal ends of the defect ulnar and progressed towards the centre of the defect and resulted in full rebridgement by week 24. The newly formed bone at the rebridgement site was gradually remodelled with cortices and the medullar cavity was fully restored.
Fig. 1.
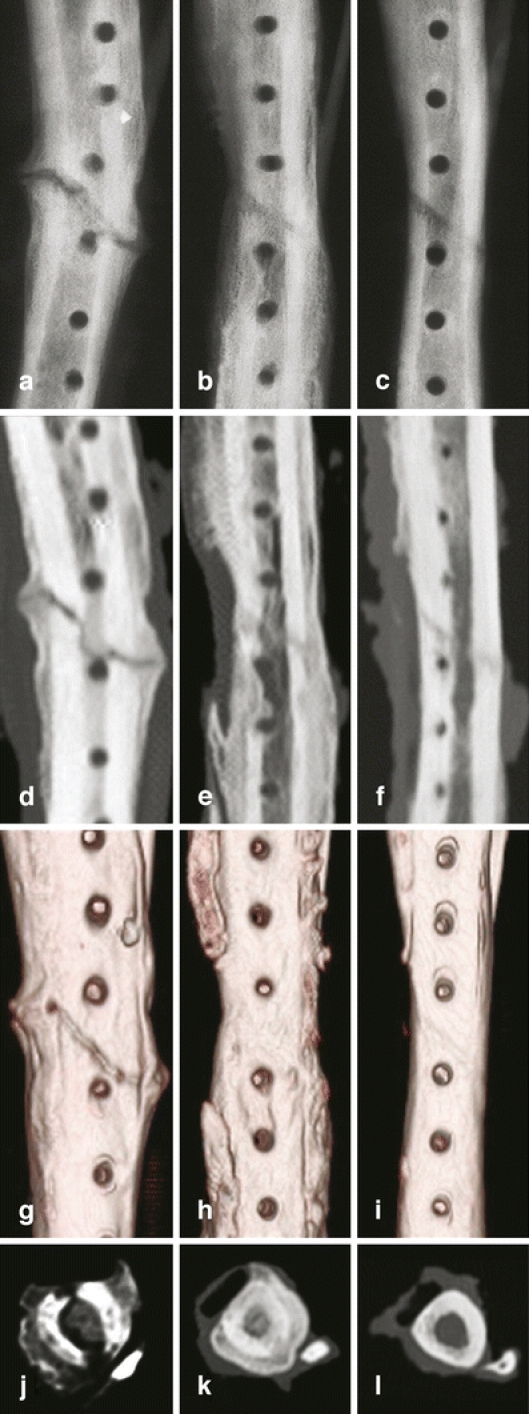
Ex vivo X-ray and computerised tomography (CT) images of canine tibia at 10 weeks post oblique osteotomy. A, B and C: X-ray images; D, E and F: 2D CT images; G, H and I: 3D CT images; J, K and L: 2D cross-sectional CT images. No rebridgement of the defect in the tibia treated with carrier alone was seen (a, d, g and j). In contrast, the tibial oblique defects treated with 1 mg (b, e, h and k) and 0.1 mg (c, f, i and l) of CP-533,536 exhibit a dose-dependent formation of new bone with full rebridgement of the defect
These results indicated that EP2 receptor plays an important role in PGE2’s local bone anabolic activity. CP-533,536, an EP2 receptor-selective agonist, stimulates new bone formation in marrow cavity and on the periosteal surface upon being locally delivered to the sites. Further, CP-533,536 stimulates callus formation and enhances fracture healing in rat and canine fracture models, and heals a critical-sized defect in the canine ulna. These data demonstrate that the EP2 receptor-selective agonists may provide therapeutic potentials for local bone augmentation, bone repair and bone healing in humans.
EP4 receptor-selective agonist and bone formation and healing
The anabolic effects of EP4 receptor agonists on bone have been demonstrated in a number of animal studies via local or systemic administrations. A selective EP4 agonist (AE1-329) dose-dependently and markedly increased local bone formation when the compound was continuously infused onto the periosteal surface of mouse femurs for 6 weeks [26]. AE1-329 was then modified to increase its chemical stability, resulting in compound ONO-4891. Daily injections (three times per day) of ONO-4891 not only fully prevented bone loss but also restored bone mass and strength in the ovariectomised (OVX) rats, an animal model of oestrogen deficiency induced bone loss or postmenopausal osteoporosis. In addition, it completely blocked bone loss induced by disuse in immobilised rats by infused systemically 2 h twice per day for 14 days [26]. Histomorphometric analyses of cancellous bone in both studies revealed that the positive effects of ONO-4891 in the OVX or immobilised rats were through the stimulation of bone formation. However, ONO-4891 at the higher doses then that required for bone formation caused diarrhoea, hypotension and the thickening of intestinal epithelium in rodents.
Another non-prostanoid EP4 receptor-selective PGE2 agonist that has been characterised for its effects on bone is CP-734,432 [7]. It increases osteogenesis in vitro, as demonstrated by a dose-dependent increase in mineralised nodule formation in rat bone marrow cell cultures. Daily subcutaneous injection of CP-734,432 for 6 weeks for the ovariectomised (OVX) rats that were 12 months of age and 8.5 months post OVX completely restored both cancellous and cortical bone mass and strength to these animals with established osteopenia. It markedly increased the mineralising surface, mineral apposition rate and bone formation rate on the periosteal, endocortical and trabecular surfaces of OVX rats, demonstrating its ability to stimulate osteoblast recruitment and activity. The anabolic effects of these selective EP4 receptor agonists on rodent skeletons suggest a therapeutic potential of these agents for the prevention and treatment of osteoporosis.
The powerful effects of EP4 agonists on stimulating bone formation upon local and systemic administrations prompted scientists to investigate their potential in facilitating bone healing. Two such studies are particularly interesting. The first study was conducted in 12-week-old rats with drill-hole injury in their femoral diaphyses using ONO-4819 as the test agent [22]. The animals were subcutaneously injected with the compound at 0, 10 or 30 μg/kg twice a day for 0, 5, 7, 14, 21 and 28 days after the defect was created. The regenerated cortical bone volume and content were dose-dependently increased at 14 and 21 days following ONO-3819 treatment. The injured tissues in the animals treated with a high dose of ONO-3819 had increased expression levels of EP4, BMP2 and RANKL mRNAs at 7 days, and had higher values of osteoclast surface and expression levels of osteocalcin and TRAP mRNAs at 14 days after treatment. These data indicated that the systemic administration of EP4 agonist ONO-4819 accelerated cortical bone healing after drill-hole injury by upregulating the local turnover of the regenerating bone.
The second study investigated the utility of ONO-4819 in sternal healing after median sternotomy with removal of the bilateral internal thoracic arteries (BITA) in streptozotocin-induced diabetic rats [14]. The dose of 300 μg of ONO-4819 was delivered to the posterior table of the sternum after surgery in fibrin glue containing PLGA (copolylactic acid/glycolic acid) micro-spheres, which provides slow release of the compound for 4 weeks after a single application. Four weeks after surgery, sternal wound complications (SWCs) developed in 5 of 8 rats in the control group but only 1 of 8 rats in the ONO-4819 treated group. In the rats without SWCs, sternal union and almost complete bone healing was observed in the compound-treated group in contrast to the dehiscence of the separated original sternum in the control group. The bone mineral content and density of the regenerated sternum were higher in the compound-treated rats than those of controls. This study demonstrated the ability of an EP4 receptor-selective agonist in accelerating sternal healing, thus, reducing the incidence of SWCs after median sternotomy with the removal of BITAs in diabetic rats. Utilising a slow-release carrier for the delivery of EP4 receptor agonists may enable dose reduction and avoid side effects.
Conclusion
Collective data indicate that both EP2 and EP4 receptors mediate the anabolic effects of PGE2. Preclinical studies demonstrated that CP-533,536, an EP2 receptor-selective agonist, augmented local bone formation and enhanced fracture healing and bone repaired upon local administration. EP4 receptor agonists exampled by ONO-4819 and CP-734,432 stimulated bone formation on the periosteal, endocortical and trabecular surface and increased bone mass and strength. These compounds prevented cancellous and cortical bone loss and restored bone mass and strength in ovariectomised (OVX) and immobilised rat models by systemic application. In addition, EP4 agonists also accelerated bone repair in drill-hole injury and sternal healing rat models upon systemic and local administration, respectively. Therefore, the EP4 receptor agonists may provide therapeutic potentials for systemic use in the prevention and treatment of skeletal disorders, including osteoporosis, and both the EP2 and EP4 receptor-selective agonists may provide therapeutic potentials for local bone augmentation, bone healing and bone repair.
Contributor Information
M. Li, Phone: +1-860-7150097, FAX: +1-860-6860170, Email: mei.li@pfizer.com
V. M. Paralkar, Phone: +1-860-4411947, FAX: +1-860-6860170, Email: vishwas.m.paralkar@pfizer.com
References
- 1.Akhter MP, Cullen DM, Gong G, Recker RR (2001) Bone biomechanical properties in prostaglandin EP1 and EP2 knockout mice. Bone 29:121–125 [DOI] [PubMed]
- 2.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD (2001) Prostanoid receptors: subtypes and signaling. Ann Rev Pharmacol Toxicol 41:661–690 [DOI] [PubMed]
- 3.Dekel S, Lenthall G, Francis MJ (1981) Release of prostaglandins from bone and muscle after tibial fracture. An experimental study in rabbits. J Bone Joint Surg Br 63:185–189 [DOI] [PubMed]
- 4.Hartke JR, Lundy MW (2001) Bone anabolic therapy with selective prostaglandin analogs. J Musculoskel Neuron Inteact 2:25–31 [PubMed]
- 5.Kamoshita E, Ikeda Y, Fujita M, Amano H, Oikawa A, Suzuki T, Ogawa Y, Yamashina S, Azuma S, Narumiya S, Unno N, Majima M (2006) Recruitment of a prostaglandin E receptor subtype, EP3-expressing bone marrow cells is crucial in wound-induced angiogenesis. Am J Pathology 169:1458–1472 [DOI] [PMC free article] [PubMed]
- 6.Kawaguchi H, Pilbeam CC, Harrison JR, Raisz LG (1995) The role of prostaglandins in the regulation of bone metabolism. Clinical Orthop Rel Res 313:36–46 [PubMed]
- 7.Ke HZ, Crawford DT, Qi H, Simmons HA, Owen TA, Paralkar VM, Li M, Lu B, Grasser WA, Cameron KO, Lefker BA, DaSilva-Jardine P, Scott DO, Zhang Q, Tian XY, Jee WSS, Brown TA, Thompson DD (2006) A nonprostanoid EP4 receptor selective prostaglandin E2 agonist restores bone mass and strength in aged, ovariectomized rats. J Bone Mineral Res 21:565–575 [DOI] [PubMed]
- 8.Keller J, Bünger C, Andreassen TT, Bak B, Lucht U (1987) Bone repair inhibited by indomethacin. Effects on bone metabolism and strength of rabbit osteotomies. Acta Orthop Scand 58:379–383 [DOI] [PubMed]
- 9.Keller J, Klamer A, Bak B, He SZ, Tidd L, Schwartz A, Sørensen S, Bünger C (1992) Short-term effect of local application of PGE2 on callus in rabbit osteotomy. Eur J Exp Musculoskeletal Res 1:86–92
- 10.Li M, Ke HZ, Qi H, Healy DR, Li Y, Crawford DT, Paralkar VM, Owen TA, Cameron KO, Lefker BA, Brown TA, Thompson DD (2003) A novel, non-prostanoid EP2 receptor-selective prostaglandin E2 agonist stimulates local bone formation and enhances fracture healing. J Bone Miner Res 18:2033–2042 [DOI] [PubMed]
- 11.Li M, Healy DR, Li Y, Simmons HA, Crawford DT, Ke HZ, Pan LC, Brown TA, Thompson DD (2005) Osteopenia and impaired fracture healing in aged EP4 receptor knockout mice. Bone 37:46–54 [DOI] [PubMed]
- 12.Li X, Okada Y, Pilbeam CC, Lorenzo JA, Kennedy CRJ, Breyer RM, Raisz LG (2000) Knockout of the murine prostaglandin EP2 receptor impairs osteoclastogenesis in vitro. Endocrinology 141:2054–2061 [DOI] [PubMed]
- 13.Machwate M, Harada S, Leu CT, Seedor G, Labelle M, Gallant M, Hutchins S, Lachance N, Sawyer N, Slipetz D, Metters KM, Rodan SB, Young R, Rodan GA (2001) Prostaglandin receptor EP4 mediates the bone anabolic effects of PGE2. Mol Pharmacol 60:36–41 [DOI] [PubMed]
- 14.Marui A, Hirose K, Maruyama T, Arai Y, Huang Y, Doi K, Ikeda T, Komeda M (2006) Prostaglandin E2 EP4 receptor-selective agonist facilitates sternal healing after harvesting bilateral internal thoracic arteries in diabetic rats. J Thorac Cardiovasc Surg 131:587–593 [DOI] [PubMed]
- 15.Miller SC, Markers SC (1993) Local stimulation of new bone formation by prostaglandin E1: quantitative histomorphometry and comparison of delivery by minipumps and controlled-release pellets. Bone 14:143–151 [DOI] [PubMed]
- 16.Miyaura C, Inada M, Suzawa T, Sugimoto Y, Ushikubi F, Ichikawa A, Narumiya S, Suda T (2000) Impaired bone resorption to prostaglandin E2 in prostaglandin E receptor EP4-knockout mice. J Bio Chem 276:19819–19823 [DOI] [PubMed]
- 17.Pan LC, Crawford DT, Simmons HA, McCurdy SP, Nguyen MT, Tilley S, Koller HB, Ke HZ (1998) Mouse lines lacking EP2 or EP4 prostaglandin receptor subtypes exhibit different bone phenotypes. Bone 23:S168
- 18.Pecina M, Giltaij L, Vukicevic S (2001) Orthopaedic applications of osteogenic protein-1 (BMP-7). Int Orthop 25:203–208 [DOI] [PMC free article] [PubMed]
- 19.Paralkar VM, Borovecki F, Ke HZ, Cameron KO, Lefker BA, Grasser WA, Owen TA, Li M, DaSilva-Jardine P, Zhou M, Dunn RL, Dumont F, Korsmeyer R, Krasney P, Brown TA, Plowchalk D, Vukicevic S, Thompson DD (2003) An EP2 receptor-selective prostaglandin E2 agonist induces bone healing. Proc Natl Acad Sci USA 100:6736–6740 [DOI] [PMC free article] [PubMed]
- 20.Raisz LG, Woodiel FN (2003) Effects of selective prostaglandin EP2 and EP4 receptor agonists on bone resorption and formation in fetal rat organ cultures. Prostaglandins Other Lipid Mediat 71:287–292 [DOI] [PubMed]
- 21.Sakou T (1998) Bone morphogenetic proteins: from basic studies to clinical approaches. Bone 22:591–603 [DOI] [PubMed]
- 22.Tanaka M, Sakai A, Uchida S, Tanaka S, Nagashima M, Katayama T, Yamaguchi K, Nakamura T (2004) Prostaglandin E2 receptor (EP4) selective agonist (ONO-4819.CD) accelerates bone repair of femoral cortex after drill-hole injury associated with local upregulation of bone turnover in mature rats. Bone 34:940–948 [DOI] [PubMed]
- 23.Voegeli TL, Chapman MW (1985) Utilization of prostaglandin in fracture healing. Trans Orthop Res Soc 10:134
- 24.Vukicevic S, Simic P, Borovecki F, Grgurevic L, Rogic D, Grasser WA, Thompson DD, Paralkar VM (2006) An EP4 receptor selective agonist of the prostaglandin E2 (PGE2) prevents renal damage and inhibits progression of renal disease in rodent models of acute and chronic kidney disease. Kidney Int 70:1099–1106 [DOI] [PubMed]
- 25.Weinreb M, Machwate M, Shir N, Abramovitz M, Rodan GA, Harada S (2001) Expression of the prostaglandin PGE2 receptor subtype EP4 and its regulation by PGE2 in osteoblastic cell lines and adult rat bone tissue. Bone 28:275–281 [DOI] [PubMed]
- 26.Yoshida K, Oida H, Kobayashi T, Maruyama T, Tanada M, Katayama T, Yamaguchi K, Segi E, Tsubouama T, Matshushita M, Ito K, Ito Y, Sugimoto Y, Ushikubi F, Ohuchida S, Kondo K, Nakamura T, Narumiya S (2002) Stimulation of bone formation and prevention of bone loss by prostaglandin E EP4 receptor activation. Proc Natl Acad Sci USA 899:4580–4585 [DOI] [PMC free article] [PubMed]


