Abstract
Rats and humans avidly consume flavored foods that contain sucrose and fat, presumably due to their rewarding qualities. In this study, we hypothesized that the complex mixture of corn oil, sucrose, and flavor is more reinforcing than any of these components alone. We observed a concentration-dependent increase in reinforcers received of sucrose solutions (0, 3, 6.25, and 12.5%) in both fixed ratio and progressive ratio procedures, but with equicaloric corn oil solutions (0, 1.4, 2.8, and 5.6%) this finding was replicated only in the fixed ratio procedure. Likewise, addition of 1.4% oil to 3% or 12.5% sucrose increased fixed ratio, but not progressive ratio, reinforcers received relative to those of sucrose alone. Finally, addition of 3% vanilla flavoring did not change self-administration of 3% sucrose or 3% sucrose + 1.4% oil solutions. These data suggest that, calorie-for-calorie, sucrose is the dominant reinforcing component of novel foods that contain a mixture of fat, sucrose, and flavor.
Keywords: self-administration, sucrose, fat, reinforcement, macronutrients, flavor
Introduction
What makes us drink a vanilla milkshake? Is it the sugar, the fat, the flavor, or the unique combination of all three? Rewarding properties of foods are partially attributed to their fat and carbohydrate content (Levine et al., 2003). Increases in the content of these nutrients in the modern food supply may contribute to the obesity problem the developed world is experiencing (Drewnowski, 2003) and therefore it is important that we understand their impact on our intake and our desire to obtain and consume them. Although some milkshake-like beverages are used as a meal replacement for weight loss (Drewnowski & Bellisle, 2007), sodas and other high-calorie beverages are consumed in addition to regular meals, and studies show that consumers do not fully compensate for the additional calories, leading to weight gain (Raben et al., 2002; Tordoff & Alleva, 1990). In addition to their abundance and relative low cost (Drewnowski & Bellisle, 2007), these beverages are also easy to consume, requiring no food preparation or even chewing. One needs expend very little work to consume these calories.
Many studies indicate that fat and carbohydrates (particularly sugars) are rewarding in humans and in animals (Levine et al., 2003; Sclafani, 2004). However, fat and sugar are rarely, if ever, consumed in isolation from other nutrients or flavors and most palatable foods are not mixtures of nutrients alone, but have some flavor added to them. This can be studied using rats working for delivery of nutrient solutions. For example, we have observed in our laboratory that rats consistently consume more, and work harder for, chocolate Ensure than they do for pure sucrose solutions (unpublished data). Ensure differs from sucrose solutions in both macronutrient composition and oral qualities (i.e. texture) that resemble a milkshake. We became curious about which component(s) of complex highly palatable liquid foods evoke the most motivation in the rats. Nutrients and their rewarding properties have been extensively studied in animal models. Sucrose and fat have both been shown independently to be rewarding and motivating in several paradigms, including self administration (Freed & Green, 1998; Grimm et al., 2005), conditioned place preference (Figlewicz et al., 2004; Figlewicz et al., 2001), conditioned flavor preferences (Ackroff et al., 2004; Sclafani et al., 1994) and intake tests (Corwin, 2004; Zhang & Kelley, 1997).
In the present study, we aimed to determine whether fat, sucrose, a mixture, or a flavored mix is most reinforcing, using the self-administration procedure. Flavor preferences can be conditioned by intra-gastric infusion of either sweet or fat solutions. This finding suggests that macronutrient nutritive qualities alone can be positively associated with oral experiences of flavor (Sclafani, 2004). Other studies show that the nutrients’ pre-absorptive properties contribute to consumption, including some showing rats’ positive responses to non-nutritive fats (Ackroff et al., 1990; Elizalde & Sclafani, 1990). While these studies demonstrate that there are various ways in which individual macronutrients confer reward, they do not explain which part of a mixture of macronutrients is most reinforcing. This kind of analysis requires systematic dismantling of a rewarding solution into its components. In such a study using bottle-choice tests, Kimura and colleagues (Kimura et al., 2003) showed that rats prefer a mix of sucrose and fat in a liquid diet, relative to either sucrose or fat alone. In this study, rats habituated to an evaporated milk-based diet were subjected to one-bottle and two-bottle tests of evaporated milk solutions enriched with corn oil only, sucrose only, or a mixture of corn oil and sucrose. In both one- and two-bottle intake tests, rats consumed significantly more of the fat+sucrose solution than of the sucrose-only solution. When the fat-only solution was presented with the sucrose-only solution, the sucrose was preferred. This study and others indicate that fat alone is less preferred than sucrose, but that it has a facilitative effect on ad-libitum intake when added to sucrose (Takeda et al., 2000).
No studies have yet tested whether flavor added to the mixture makes a solution more rewarding, although this may seem intuitively obvious. In the Kimura study above, nutrients were added to milk, which may have confounded results, considering the sugars, fats and flavors inherent to milk. Furthermore, it seems possible that milk resembles mothers’ milk closely enough for rats that it recalls reward experienced in early life. In order to completely differentiate between responses to fat, sucrose, and flavor, we used emulsions of corn oil, sucrose and/or vanilla flavoring in water, rather than in milk. This design allowed us to test the relative reinforcing properties of each individual macronutrient, rather than the complex combination of milk plus nutrient.
We also wanted to determine the amount of work fully-sated animals were willing to expend in order to obtain fat, sucrose, a mix, or a flavored mix. This is a different measure than mere consumption of each nutrient. Self-administration chambers, in which rats press a lever to obtain a small amount of reinforcer, allow measurement of the degree to which they will work for such a reinforcer. Using fixed ratio (FR) reinforcement (FR1: one sucrose reinforcer for each lever press), we were able to ascertain the amount of each solution animals consumed. Using a progressive ratio (PR) schedule, in which each successive sucrose reinforcer requires more lever presses than the previous one, we determined the degree of motivation rats expressed for each solution. The PR schedule is thought to be the best way to determine the relative reinforcing strength of different reinforcers (Arnold & Roberts, 1997; Hodos, 1961; Richardson & Roberts, 1996). Note that we will use the term “motivation” here to refer to the amount of work animals will expend for a limited amount of reinforcer, as in our PR procedure. See reviews by Wise and Hoffman (1992) and Arnold and Roberts (1997) for expanded discussion of the concepts of reward, reinforcement, and motivation.
In our attempt to “deconstruct the vanilla milkshake”, we hypothesized that a combination of fat and sucrose (in the form of emulsified corn oil and sucrose in water) would elicit more self-administration responding than either nutrient alone. In addition, we hypothesized that adding flavor to the mix would make the solution even more rewarding. Using standard self-administration chambers, we analyzed FR and PR responding for a series of solutions containing combinations of sucrose, corn oil, and vanilla.
Methods
Subjects
Subjects were male Albino rats (350–450 g) from Simonsen (Gilroy, CA). Rats were maintained on water and chow ad libitum at all times. They were maintained on a 12:12 h light-dark cycle with lights on at 6 AM. All procedures performed on the rats followed the NIH guidelines for animal care, and were approved by the Animal Care and Use Sub-Committee of the Research and Development Committee at the VA Puget Sound Health Care System or the WWU Animal Care and Use Committee. Each subject only underwent one round of training and testing, as described below.
Apparatus
Med Associates (Georgia, VT) self-administration chambers, controlled by a Med Associates integrator system, had two levers, but only one lever (an active, retractable lever) activated the infusion pump. Presses on the other lever (an inactive, stationary lever) were also recorded. For all experiments, the number of presses on the inactive lever was very low (less than 10 presses/session) and no experimental manipulation altered inactive lever pressing. The sucrose solution was delivered into a liquid drop receptacle for oral consumption.
General procedures
Procedures were based upon our published methodology (11,17). The experiment included four phases: autoshaping, FR training (10 days) followed by experimental FR testing (Day 11); PR training (3 days) followed by experimental PR testing (Day 15), using the PR algorithm of Richardson and Roberts (1997). This algorithm requires 1, 2, 4, 6, 9, 12, 16, 20, 28, 36, 48, 63, 83, 110, 145, 191, 251, 331, 437, 575, 759, 999, 999(etc) lever presses for succeeding reinforcer deliveries within a session (Richardson & Roberts, 1996). Rats were trained to self-administer 10% sucrose (0.6 ml reinforcer) delivered into a liquid drop receptacle. FR training was conducted during one 1-h session per day for 10 days under a continuous reinforcement schedule (FR1: each lever press was reinforced), with a maximum possible of 50 sucrose reinforcers delivered per session. Each session began with the insertion of the active lever and the illumination of a white house light that remained on for the entire session. A 5-s tone (2900 Hz, 20 dB above background) + light (7.5 W white light above the active lever) discrete compound cue accompanied each reinforcer delivery. Responses during reinforcer delivery (10 seconds) and the subsequent 10 seconds were recorded but had no consequences. This effectively served as a 20-second “time out”. PR training was similar to FR training, but was held for three hours each day and reinforcers were increasingly difficult to obtain, according to the schedule described above. In addition, the reinforcer volume was 0.4 ml and the lever was retracted for the duration of reinforcer delivery. There was no time out beyond the duration of the reinforcer delivery (6.2 seconds). PR sessions ended after 30 minutes of no active lever press responding, at which point the house light was turned off and the active lever retracted. Here we report number of presses on the active lever (active presses) and number of droplets received (reinforcers).
Experimental Procedures
Experiment 1
We first tested animals’ willingness to work for various dilutions of sucrose or corn oil, matched for caloric density. We tested FR and PR responding for 0%, 3%, 6.25%, and 12.5% sucrose, and for 0%, 1.4%, 2.8%, and 5.6% corn oil. Oil was emulsified in water with 7.5% Tween-80, which was added to all solutions, including controls. As described previously, data from our lab indicate that Tween has no effect on intake compared to water (Sipols et al., 2000). Separate groups of rats were used for each nutrient, but within each nutrient, concentration was the randomized repeated measure. Thus, 15 rats were given all four concentrations of sucrose, and 23 rats were given all four concentrations of oil. In this experiment, animals in the oil condition were trained to press for 4.7% oil, rather than 10% sucrose.
Experiment 2
Next, we sought to determine whether animals would work harder for sucrose if oil were added to it. The sucrose/oil combinations were: 0% sucrose + 0% oil; 0% sucrose + 1.4% oil; 3% sucrose + 0% oil; 3% sucrose + 1.4% oil; 12.5% sucrose + 0% oil; and 12.5% sucrose + 1.4% oil. These combinations tested whether the response to the lowest or highest concentrations of sucrose could be enhanced by the addition of oil. Twenty-four naïve rats were included in this study. A follow-up to this study, in 16 naïve animals, compared work for 3% sucrose with either 1.4% oil or 5.6% oil, to determine if the oil effect was concentration dependent. Doses were given in a randomized, repeated-measures design.
Experiment 3
To test the hypothesis that flavor would enhance the reinforcing properties of a sucrose or sucrose+oil solution, we added 3% alcohol-free vanilla flavoring (Durkee, ACH Food Companies) to each solution. This concentration was chosen based on initial taste preference tests conducted with separate animals. These animals preferred sweetened 3% vanilla over 1.5% vanilla, and preferred the 3% vanilla over a similarly diluted sweetened chocolate flavoring (Torani Syrups, www.torani.com), leading us to choose vanilla as our test flavor. As in Experiment 1, Tween-80 was used to emulsify the oil and was part of the vehicle for all solutions. The combinations were: vehicle alone; 3% sucrose alone; 3% sucrose + 3% vanilla; 3% sucrose + 1.4% oil; and 3% sucrose + 3% vanilla + 1.4% oil. As above, subjects received each combination in a randomized, repeated-measures design. Thirty naïve rats were included.
In a follow-up experiment, we tested whether a lower concentration (1.5%) of an all-natural vanilla (www.naturesflavors.com; ingredients are water, guar gum, and flavor) flavoring would be more rewarding than the 3% concentration of the artificial vanilla. We observed identical results when we repeated this experiment with the natural vanilla. For example, PR active presses for sucrose + oil were 81.7± 9.1 without vanilla and 83.5±10.5 with vanilla [paired t-test, p = 0.85].
Statistical analyses
Number of reinforcers and active lever responses were analyzed using a repeated-measures analysis of variance (ANOVA), with each animal receiving each combination of sucrose, oil, and/or vanilla. Fisher’s post-hoc analysis (significant differences indicated by different colors on graphs) and paired t-tests (Tables 1 and 2) were used to determine differences between treatments. Group data are presented as the mean ± SEM in the text and Figures. Significance is defined as p ≤ 0.05.
Results
Experiment 1
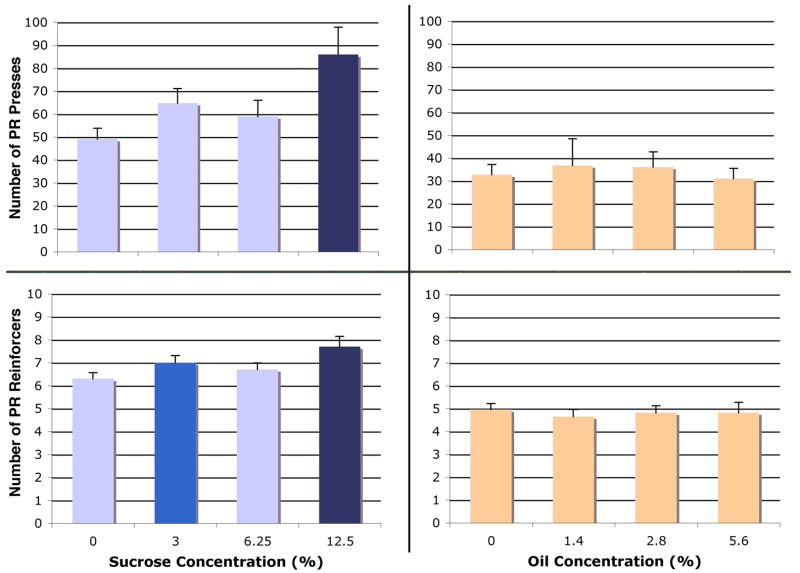
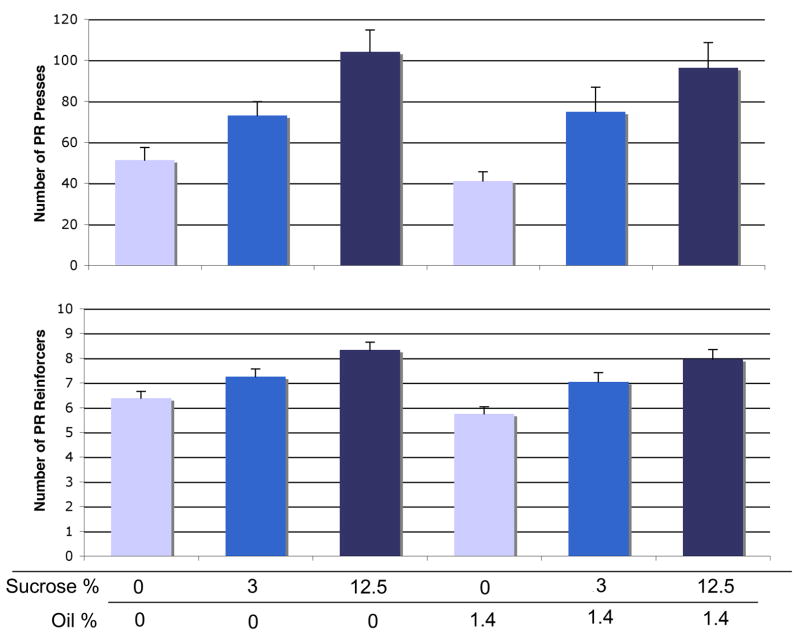
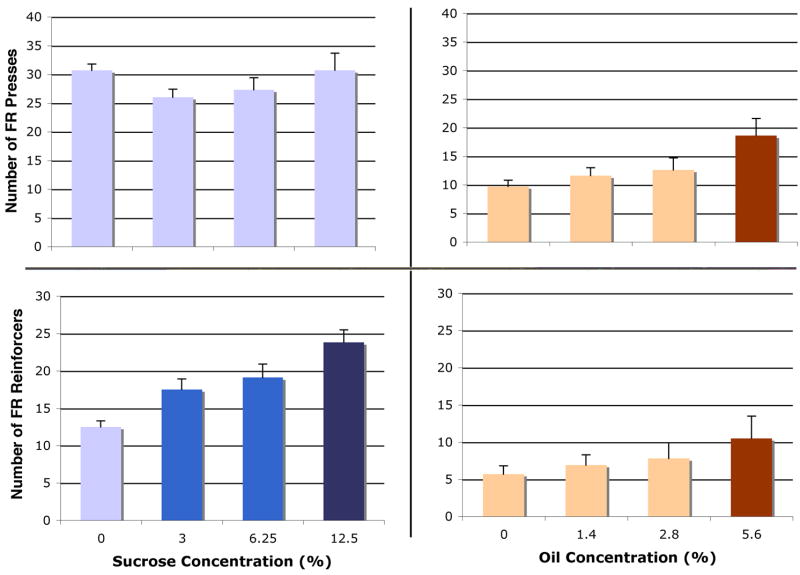
In our initial concentration-response test for sucrose, we observed concentration-dependent responding for sucrose in both the FR and PR procedures, but only for oil in the FR procedure. There was no significant effect of sucrose concentration on FR active presses [F(14,42) = 0.86, p = 0.47], but we did find a main effect of concentration on reinforcers [F(14,42) = 16.13, p < 0.0001] (Figure 2, left panel). We observed a significant effect of corn oil concentration for both FR active presses [F(22,66) = 4.24, p = 0.008] and for reinforcers [F(22,66) = 6.67, p = 0.0005] (Figure 2, right panel). We similarly observed a main effect of concentration on PR active presses for sucrose [F(14,42) = 7.59, p = 0.0004] as well as for PR sucrose reinforcers [F(14,42) = 6.69, p = 0.0009] (Figure 3, left panel). However, we observed no effect of oil concentration on PR active presses [F(22,66) = 0.29, p = 0.83] or reinforcers [F(22,66) = 0.28, p = 0.84] (Figure 3, right panel).
Figure 2.
Concentration-dependent increase in PR motivation for sucrose but not oil. Left panel: Top, PR active presses for sucrose. Bottom, sucrose reinforcers received. Right panel: Top, PR active presses for oil. Bottom, oil reinforcers received. Within each graph, each bar color indicates significant difference (p < 0.05) from bars of other colors. Bars represent means ± SEM; repeated-measures, n = 15 in sucrose condition, n = 23 in oil condition.
Figure 3.
FR consumption of sucrose + oil combinations. Upper panel: FR active presses. Lower panel: Reinforcers received. Within each graph, each bar color indicates significant difference (p < 0.05) from bars of other colors. Bars represent means ± SEM; repeated-measures, n = 24.
Experiment 2
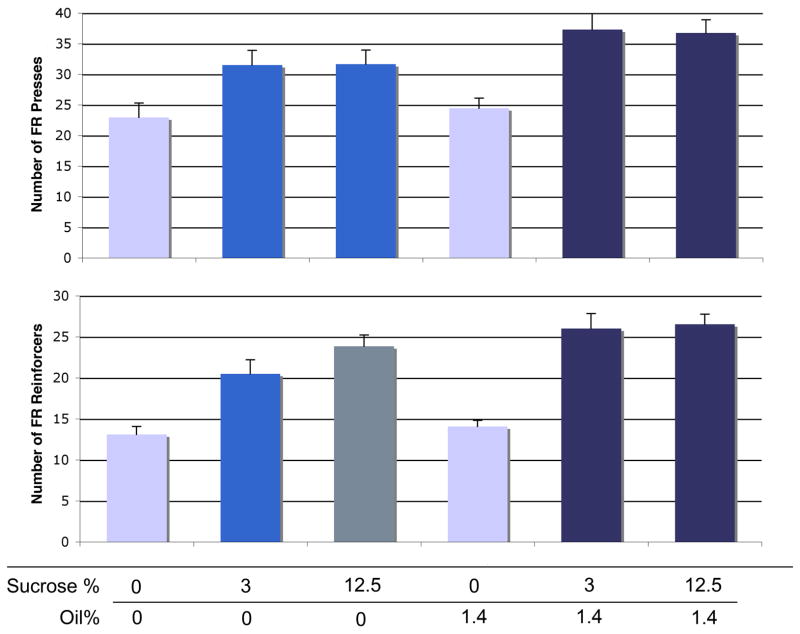
This experiment was designed to determine whether a combination of oil and sucrose is more rewarding or motivating than either nutrient alone. While oil alone did not increase responding over the control solution, it did increase responding for sucrose. We found a significant overall effect of nutrient for active lever presses in the FR procedure [F(23,115) = 12.2, p < 0.0001] (Figure 4, top). Oil alone did not increase responding above control levels [p = 0.54]. However, both the 3% and 12.5% concentrations of sucrose alone increased responding on the active lever by about 37% [p < 0.001 for both, compared to control]. The 12.5% concentration of sucrose did not increase lever presses above those for 3% sucrose. Oil combined with either sucrose concentration increased active lever presses by about 17% above responding with the sucrose concentration alone [p < 0.05 for both, above both sucrose concentrations]. The oil/sucrose combinations increased pressing by about 60% above control conditions [p < 0.0001 for both combinations compared to control]. See Table 1 for paired t-tests comparing addition of oil to sucrose for FR active lever presses. We also observed a significant overall effect of nutrient for number of reinforcers received in the FR procedure [F(23,115) = 32.7, p < 0.0001] (Figure 4, bottom). Again, oil alone had no effect on reinforcers received [p = 0.49]. The 3% sucrose solution increased reinforcers by 58% [p < 0.0001] compared to control; the 12.5% solution increased reinforcers by 82% [p < 0.0001]. In addition, the 12.5% solution increased reinforcers by 16% above the 3% solution [p = 0.02]. Addition of 1.4% oil to the 3% sucrose further increased reinforcers by 27% [p = 0.0003], and addition of oil to 12.5% sucrose further increased reinforcers by 11% [p = 0.04]. Table 1 further demonstrates the facilitative effect of oil on FR pressing for sucrose.
Figure 4.
PR motivation for sucrose + oil combinations. Upper panel: PR active presses. Lower panel: Reinforcers received. Within each graph, each bar color indicates significant difference (p < 0.05) from bars of other colors. Bars represent means ± SEM; repeated-measures, n = 24.
In the PR procedure, we again observed a significant overall effect of nutrient on active lever presses [F(23,115) = 10.2, p < 0.0001] (Figure 5, top). Oil alone had no effect on pressing [p = 0.35]. The 3% sucrose increased pressing by 42% [p = 0.48]; 12.5% sucrose increased pressing by 103% above control [p < 0.0001] and by 43% above 3% sucrose pressing [p = 0.005]. Adding oil to either of these sucrose concentrations did not increase pressing above that obtained with sucrose alone. See Table 1 for paired t-tests comparing addition of oil to sucrose for PR active lever presses. There was likewise a significant overall effect of nutrient on reinforcers received in the PR procedure [F(23,115) = 14.7, p < 0.0001] (Figure 5, bottom). The 3% sucrose increased reinforcers by 12% [p = 0.02]. The 12.5% sucrose increased reinforcers by 30% above control [p < 0.0001] and by 14% above the 3% concentration [p = 0.003]. As for active lever presses, addition of oil to either of these concentrations did not change number of reinforcers received from that of the sucrose concentration alone. See Table 1 for paired t-tests comparing addition of oil to sucrose for PR reinforcers received. The PR portion of this experiment thus demonstrated that the addition of a sweet component to a fat solution increases its motivational value, but addition of fat to an already motivating sweet solution does not enhance its value.
Figure 5.
FR consumption of sucrose + oil + vanilla combinations. Upper panel: FR active presses. Lower panel: Reinforcers received. Within each graph, each bar color indicates significant difference (p < 0.05) from bars of other colors. Bars represent means ± SEM; repeated-measures, n = 30.
Finally, to test whether an even higher concentration of oil would increase responding more than our original 1.4% concentration, we compared FR and PR active lever presses and reinforcers received for 3% sucrose + 1.4% oil and 3% sucrose and 5.6% oil. We saw no differences between these two oil+sucrose concentrations (data not shown).
Experiment 3
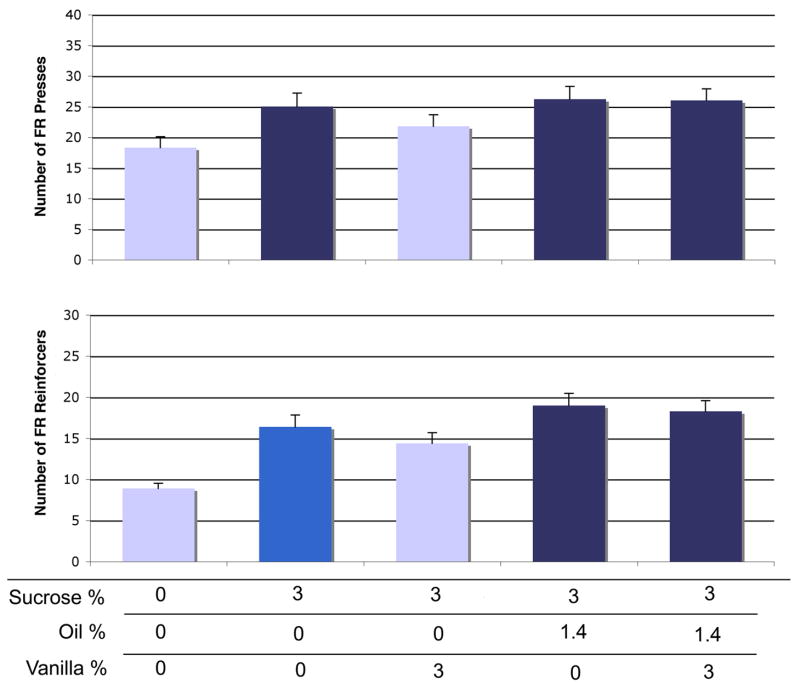
Addition of vanilla did not increase responding for oil, sucrose, or a combination of the two. We found a significant overall effect of solution on FR active lever presses [F(29,116) = 6.25, p = 0.0001] (Figure 6, top). The 3% sucrose alone increased pressing by 36% above control [p = 0.0007]. Addition of vanilla to sucrose had no effect on active presses. Addition of oil to sucrose, without vanilla, did not increase lever presses compared to sucrose alone [p = 0.52], although the increase above control was maintained. Addition of vanilla to the oil+sucrose solution did not increase FR pressing [p = 0.89]. There was also a significant overall effect of solution components on number of reinforcers received in the FR procedure [F(29,116) = 22.4, p < 0.0001] (Figure 6, bottom). Sucrose alone increased reinforcers by 84% [p < 0.0001]. Vanilla did not increase reinforcers for sucrose [p = 0.11]. As we observed in Experiment 2, oil did increase reinforcers when added to sucrose [p = 0.04]. Addition of vanilla to the oil+sucrose combination did not increase reinforcers received [p = 0.56], when compared to oil+sucrose alone. However, there was a significant increase in reinforcers received in the oil+sucrose+vanilla condition compared to the sucrose+vanilla combination alone [p = 0.002], suggesting that, while not rewarding itself, vanilla did not inhibit pressing for already-rewarding solutions. See Table 2 for paired t-tests comparing addition of vanilla to sucrose or sucrose+oil for FR active presses and reinforcers.
Figure 6.
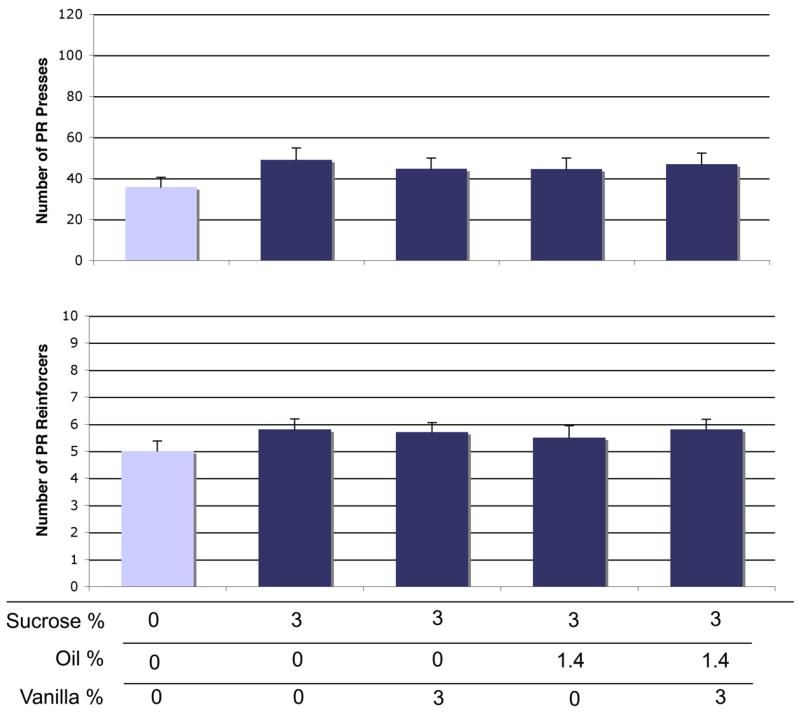
PR motivation for sucrose + oil + vanilla combinations. Upper panel: PR active presses. Lower panel: Reinforcers received. Within each graph, each bar color indicates significant difference (p < 0.05) from bars of other colors. Bars represent means ± SEM; repeated-measures, n = 30.
In the PR procedure, we again saw a significant overall effect of solution on active presses [F(29,116) = 2.82, p = 0.03] (Figure 7, top). Sucrose alone increased presses by 37% [p = 0.002]. Vanilla did not significantly increase responding when added to sucrose [p = 0.33]. When oil or oil + vanilla were added to sucrose, no increase was seen in PR active presses. Finally, in the PR procedure, while we did see a significant overall effect of solution on reinforcers received [F(29,116) = 3.7, p = 0.007] (Figure 7, bottom), this was entirely due to sucrose. Sucrose induced an increase in reinforcers received by 15% compared to control [p = 0.002], and this number of reinforcers was received under all other conditions. See Table 2 for paired t-tests comparing addition of vanilla to sucrose or sucrose+oil for PR active presses and reinforcers received.
Discussion
In this study we tested the hypothesis that it is the unique combination of flavor, fat, and sucrose that makes nutrient mixtures, such as a milkshake, so rewarding. In fully-sated rats, we evaluated self-administration of increasing concentrations of sucrose and oil alone, the addition of oil to sucrose solutions, and the addition of vanilla flavoring to solutions of sucrose or sucrose + oil. We hypothesized that addition of oil would lead to increased responding for sucrose. We also expected that the addition of flavor would increase consumption above that of nutrients alone. In fact, we observed that sucrose was the strongest determinant of nutrient solution self-administration.
In the first experiment, all concentrations of sucrose elicited more FR and PR presses than oil. We chose concentrations of sucrose or corn oil that were calorically matched, so that self-administration would reflect reward value independent of caloric value. We observed a significant increase in FR reinforcers received as sucrose concentration increased. Others have also shown that rats will self-administer more sucrose reinforcers as concentration increases (Slawecki et al., 1997). We expected to see a similar concentration-dependent increase in FR lever presses; however, we observed an unusually high rate of pressing for vehicle in this experiment. These presses must have occurred during the time out, since they are not reflected in the reinforcers received. It should be noted here that in all of our experiments, animals were trained for ten days each of FR and PR pressing for 10% sucrose (or 4.7% oil for the oil group in Experiment 1), followed by testing with various concentrations of sucrose, oil, or control, in a randomized pattern. During testing, those receiving vehicle, oil, or 3% sucrose were then pressing for something other than what they had trained to press for. Therefore, we may have observed a burst of extinction responding when rats were presented with these solutions. Furthermore, the light and tone that served as secondary reinforcers were consistently present, which would have increased likelihood of responding even in the absence of the primary reinforcer. Nonetheless, sucrose proved to be more reinforcing than oil, which elicited half as many presses, even at the highest concentration tested. FR presses and reinforcers were significantly elevated above control levels for this high concentration of oil, however. PR lever pressing and reinforcers were elevated at the highest concentration of sucrose, but there was no increase in PR performance for oil.
In Experiment 2, we hypothesized that adding fat to sucrose would increase its reinforcing properties. This was confirmed in the FR procedure, with the low concentration of oil increasing presses and reinforcers of both 3% and 12.5% sucrose. However, similar to the results of Experiment 1, oil did not increase responding in the PR procedure. The observation that rats will press for oil in the FR but not the PR procedure suggests that oil may be rewarding enough for increased consumption, but adding an incremental cost to each oil reinforcer diminishes its motivational value. In support of this idea, Arnold and Roberts (1997) suggest that FR1 responding can be considered equivalent to the rate of consumption, while PR responding is a better measure of the relative reinforcing efficacy of different drug doses or, in our case, sucrose and oil concentrations. Another consideration is that the PR schedule chosen in this laboratory might have been too stringent for weak reinforcers such as dilute corn oil. The first reported PR schedule, described by Hodos (1961), was a ratio increased by an additive increment of two for a reinforcer of dilute sweetened milk. Yet, our stringent schedule is not too much work for chocolate Ensure, and is still effective for demonstrating differences in motivation for dilute sucrose. That it is too stringent only for dilute oil further illustrates the relatively higher reinforcing value of equicaloric sucrose compared to oil.
Data from the first two experiments indicate that, when matched for caloric density, the two reinforcing nutrients in a milkshake, fat and sucrose, are not equally powerful. This is an important finding, since increased consumption is one direct cause of weight gain, regardless of motivation. If, as has been discussed thoroughly in nutrition literature (Blundell et al., 1993; Blundell & MacDiarmid, 1997; Gerstein et al., 2004; Johnstone et al., 1996; Stubbs et al., 2000; Westerterp, 2006), fat is less satiating than other macronutrients, then its presence in the human diet could be the reason for overconsumption of vanilla milkshakes (among other fine foods). These data support the findings of Kimura et al., (2003) who showed that fat alone does not enhance intake of condensed milk, but that it does facilitate intake of sucrose in the same vehicle. Another study measuring consumption of emulsions in addition to chow in the home cage showed that more sucrose+fat solution was consumed than either sucrose or fat alone (Lucas & Sclafani, 1990). Our study now demonstrates the high relative reinforcing value of sucrose compared to oil. This is consistent with our previous report of performance in a 5-min lickrate task by rats. In that study, rats had five minutes to lick a series of equicaloric solutions of sucrose and corn oil (including the concentrations used here, as well as higher concentrations). This is a behavioral paradigm in which post-ingestive influences are minimal or absent, and is thought to reflect hedonic valuation of the solutions. We observed that lick rates for sucrose were higher than those for oil. Further, increasing oil concentration as high as 16% did not result in increased licking of oil solutions (Sipols et al, 2000). In the present study, a non-reinforcing concentration of oil did increase pressing for sucrose, suggesting that the presence of even a low amount of fat in foods makes them more rewarding.
Our final question was whether addition of a flavor increased the reinforcing qualities of sucrose or sucrose + fat solutions. In this experiment, sucrose and fat seemed to be the only reinforcing factors. Vanilla did not increase responding. Oil did increase the number of reinforcers received in the FR procedure, in a manner similar to what we observed in Experiment 2, although only FR reinforcers, not active presses, were significantly elevated above those for sucrose alone. Our main question in this experiment—whether vanilla increased responding—yielded a clear, negative, answer. Vanilla neither enhanced nor attenuated responding for sucrose + fat, and this was the case even for a lower concentration of an all-natural vanilla (data not shown). Previous studies have shown that rats and humans have different preferences for polycose versus sucrose (Feigin et al., 1987), and although sugars are detected by different receptors than are flavors, it seems reasonable to assume that rats may have different preference for vanilla than do humans. The rats had been exposed once to the vanilla solution before the study, to eliminate neophobia, but they had not had positive associative experiences with it, as have, for example, humans who enjoy vanilla. Our finding that vanilla does not alter consumption confirms that of Archer and colleagues (Archer et al., 2006), who tested rats’ consumption of four flavors of Ensure and found no difference in intake or body weight gain over two or three weeks. A human-subjects counterpart to these studies was conducted by Essed and colleagues (Essed et al., 2007), in which flavor and monosodium glutamate (MSG) were added to meals provided to elderly patients in a nursing home. The authors observed no increase in intake by the groups who received flavoring or MSG added to their food over 16 weeks. Other studies (Mathey et al, 2001; Schiffman and Warwick, 1993) have shown that flavor enhancement does increase intake in elderly subjects, but attribute their original diminished intake to a decline in flavor perception. Since our rats presumably have intact flavor perception, these studies may not be comparable. In an unpublished study by our group, we observed that rats prefer sucrose + vanilla in a two-bottle test, but do not consume more sucrose as a result of flavoring. These studies suggest that flavor alone is not an appetite stimulant to rats. We are currently exploring the role of experience and positive association in conferring reward value to flavors such as vanilla.
From all of these studies the relative value of sucrose is quite clear. For rats, this is the most important component of the nutrient mixtures we tested. Nevertheless, there is something in the composition of chocolate Ensure that compels our rats to work very hard. Two aspects of palatability that our experiments did not test are texture and past association. Ensure may offer both of these to rats, recalling the texture and bonding experience of milk while nursing. Our deconstruction did not attempt to replicate these factors.
Questions about the relative roles of macronutrients in food reward warrant future investigation. For example, many products targeted to dieters substitute sugar for fat calories in low-fat foods. Behavioral economic analysis tells us that this is an acceptable solution, since palatable alternatives are able to lure subjects away from a high-fat option (Freed & Green, 1998). Based on some of the present findings, this may be effective in minimizing caloric intake, since the addition of fat prolonged consumption in the FR1 test. However, based on our other findings, sucrose is the strongest reinforcer present in the solutions, and sucrose is often the substituted nutrient in low-fat human food. If the obesity problem results from excessive desire for palatable foods, then our results suggest that substituting sucrose for fat would not lead to a decrease in their consumption. We also must acknowledge that our methods are not specific enough to account for the pre- and post-ingestive reward effects of each solution. For example, as we increase the concentration of each solution, we simultaneously increase the reward value as well as the degree of satiation. These factors both affect self-administration. Therefore, this analysis is a first attempt at describing the reinforcing value of each nutrient, but remains limited in its ability to draw conclusions about psychological mechanisms involved in the intake of each nutrient.
Clearly, the reasons for human over-consumption of high-calorie foods are complex. The present results suggest that, while fat is rewarding and problematic for a healthy diet, an equivalent number of calories of sucrose is more motivating; therefore, the sucrose content of our foods must be considered as we try to limit our consumption.
Figure 1.
Concentration-dependent increase in FR consumption of sucrose or oil. Left panel: Top, FR active presses for sucrose. Bottom, sucrose reinforcers received. Right panel: Top, FR active presses for oil. Bottom, oil reinforcers received. Within each graph, each bar color indicates significant difference (p < 0.05) from bars of other colors. Bars represent means ± SEM; repeated-measures, n = 15 in sucrose condition, n = 23 in oil condition.
Acknowledgments
The authors thank Richard L. Martin, III, for his help in the execution of these studies. This work was supported by Contract 4442sc (DFL) and 4443sc (JWG), from the University of California at San Francisco. This publication was made possible through support from the Dean's Office of the School of Medicine, University of California at San Francisco, and the Resnick Family Foundation, and through the Exploratory Center for Obesity Research at the University of Washington, Seattle, NIH Grant P20RR020774. Dianne Figlewicz Lattemann is supported by NIH DK40963 and the Dept. of Veterans Affairs (Research Career Scientist Award). Dr. Naleid is supported by the University of Washington NIH F32 DA020315-01A2 and NIH Training Grant T32-AA007455. Dr. Sipols is supported by Latvian Council of Science Grant 04.1116. Dr. Grimm is supported by NIH/NIDA Grant R15 DA016285 and Western Washington University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackroff K, Rozental D, Sclafani A. Ethanol-conditioned flavor preferences compared with sugar- and fat-conditioned preferences in rats. Physiol Behav. 2004;81(4):699–713. doi: 10.1016/j.physbeh.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Ackroff K, Vigorito M, Sclafani A. Fat appetite in rats: The response of infant and adult rats to nutritive and non-nutritive oil emulsions. Appetite. 1990;15(3):171–188. doi: 10.1016/0195-6663(90)90018-4. [DOI] [PubMed] [Google Scholar]
- Archer ZA, Brown YA, Rayner DV, Stubbs RJ, Mercer JG. Effect of flavour of liquid ensure diet supplement on energy intake in male sd rats. Physiol Behav. 2006;89(3):414–419. doi: 10.1016/j.physbeh.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57(3):441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Blundell JE, Burley VJ, Cotton JR, Lawton CL. Dietary fat and the control of energy intake: Evaluating the effects of fat on meal size and postmeal satiety. Am J Clin Nutr. 1993;57(5 Suppl):772S–777S. doi: 10.1093/ajcn/57.5.772S. discussion 777S–778S. [DOI] [PubMed] [Google Scholar]
- Blundell JE, MacDiarmid JI. Fat as a risk factor for overconsumption: Satiation, satiety, and patterns of eating. J Am Diet Assoc. 1997;97(7 Suppl):S63–69. doi: 10.1016/s0002-8223(97)00733-5. [DOI] [PubMed] [Google Scholar]
- Corwin RL. Binge-type eating induced by limited access in rats does not require energy restriction on the previous day. Appetite. 2004;42(2):139–142. doi: 10.1016/j.appet.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Drewnowski A. The role of energy density. Lipids. 2003;38(2):109–115. doi: 10.1007/s11745-003-1039-3. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Bellisle F. Liquid calories, sugar, and body weight. Am J Clin Nutr. 2007;85(3):651–661. doi: 10.1093/ajcn/85.3.651. [DOI] [PubMed] [Google Scholar]
- Elizalde G, Sclafani A. Fat appetite in rats: Flavor preferences conditioned by nutritive and non-nutritive oil emulsions. Appetite. 1990;15(3):189–197. doi: 10.1016/0195-6663(90)90019-5. [DOI] [PubMed] [Google Scholar]
- Essed NH, van Staveren WA, Kok FJ, de Graaf C. No effect of 16 weeks flavor enhancement on dietary intake and nutritional status of nursing home elderly. Appetite. 2007;48(1):29–36. doi: 10.1016/j.appet.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Feigin MB, Sclafani A, Sunday SR. Species differences in polysaccharide and sugar taste preferences. Neurosci Biobehav Rev. 1987;11(2):231–240. doi: 10.1016/s0149-7634(87)80031-3. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett J, Evans SB, Kaiyala K, Sipols AJ, Benoit SC. Intraventricular insulin and leptin reverse place preference conditioned with high-fat diet in rats. Behav Neurosci. 2004;118(3):479–487. doi: 10.1037/0735-7044.118.3.479. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Higgins MS, Ng-Evans SB, Havel PJ. Leptin reverses sucrose-conditioned place preference in food-restricted rats. Physiol Behav. 2001;73(1–2):229–234. doi: 10.1016/s0031-9384(01)00486-3. [DOI] [PubMed] [Google Scholar]
- Freed DE, Green L. A behavioral economic analysis of fat appetite in rats. Appetite. 1998;31(3):333–349. doi: 10.1006/appe.1998.0170. [DOI] [PubMed] [Google Scholar]
- Gerstein DE, Woodward-Lopez G, Evans AE, Kelsey K, Drewnowski A. Clarifying concepts about macronutrients' effects on satiation and satiety. J Am Diet Assoc. 2004;104(7):1151–1153. doi: 10.1016/j.jada.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Fyall AM, Osincup DP. Incubation of sucrose craving: Effects of reduced training and sucrose pre-loading. Physiol Behav. 2005;84(1):73–79. doi: 10.1016/j.physbeh.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Johnstone AM, Stubbs RJ, Harbron CG. Effect of overfeeding macronutrients on day-to-day food intake in man. Eur J Clin Nutr. 1996;50(7):418–430. [PubMed] [Google Scholar]
- Kimura F, Okada R, Endo Y, Fujimoto K. Bottle-choice tests in sprague-dawley rats using liquid diets that differ in oil and sucrose contents. Biosci Biotechnol Biochem. 2003;67(8):1683–1690. doi: 10.1271/bbb.67.1683. [DOI] [PubMed] [Google Scholar]
- Levine AS, Kotz CM, Gosnell BA. Sugars and fats: The neurobiology of preference. J Nutr. 2003;133(3):831S–834S. doi: 10.1093/jn/133.3.831S. [DOI] [PubMed] [Google Scholar]
- Lucas F, Sclafani A. Hyperphagia in rats produced by a mixture of fat and sugar. Physiol Behav. 1990;47(1):51–55. doi: 10.1016/0031-9384(90)90041-2. [DOI] [PubMed] [Google Scholar]
- Mathey MF, Siebelink E, de Graaf C, Van Staveren WA. Flavor enhancement of food improves dietary intake and nutritional status of elderly nursing home residents. J Geronotol A Biol Sci Med Sci. 2001;56(4):M200–M205. doi: 10.1093/gerona/56.4.m200. [DOI] [PubMed] [Google Scholar]
- Raben A, Vasilaras TH, Moller AC, Astrup A. Sucrose compared with artificial sweeteners: Different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76(4):721–729. doi: 10.1093/ajcn/76.4.721. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66(1):1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Warwick ZS. Effect of flavor enhancement of foods for the elderly on nutritional status: food intake, biochemical indices, and anthropometric measures. Physiol Behav. 1993;53(2):395–402. doi: 10.1016/0031-9384(93)90224-4. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Oral and postoral determinants of food reward. Physiol Behav. 2004;81(5):773–779. doi: 10.1016/j.physbeh.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Nissenbaum JW, Ackroff K. Learned preferences for real-fed and sham-fed polycose in rats: Interaction of taste, postingestive reinforcement, and satiety. Physiol Behav. 1994;56(2):331–337. doi: 10.1016/0031-9384(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Sipols AJ, Stuber GD, Klein SN, Higgins MS, Figlewicz DP. Insulin and raclopride combine to decrease short-term intake of sucrose solutions. Peptides. 2000;21(9):1361–1367. doi: 10.1016/s0196-9781(00)00279-5. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Samson HH, Hodge CW. Differential changes in sucrose/ethanol and sucrose maintained responding by independently altering ethanol or sucrose concentration. Alcohol Clin Exp Res. 1997;21(2):250–260. [PubMed] [Google Scholar]
- Stubbs J, Ferres S, Horgan G. Energy density of foods: Effects on energy intake. Crit Rev Food Sci Nutr. 2000;40(6):481–515. doi: 10.1080/10408690091189248. [DOI] [PubMed] [Google Scholar]
- Takeda M, Imaizumi M, Fushiki T. Preference for vegetable oils in the two-bottle choice test in mice. Life Sci. 2000;67(2):197–204. doi: 10.1016/s0024-3205(00)00614-7. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Alleva AM. Effect of drinking soda sweetened with aspartame or high-fructose corn syrup on food intake and body weight. Am J Clin Nutr. 1990;51(6):963–969. doi: 10.1093/ajcn/51.6.963. [DOI] [PubMed] [Google Scholar]
- Westerterp KR. Perception, passive overfeeding and energy metabolism. Physiol Behav. 2006;89(1):62–65. doi: 10.1016/j.physbeh.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Wise RA, Hoffman DC. Localization of Drug Reward Mechanisms by Intracranial Injections. Synapse. 1992;10:247–263. doi: 10.1002/syn.890100307. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Opiate agonists microinjected into the nucleus accumbens enhance sucrose drinking in rats. Psychopharmacology (Berl) 1997;132(4):350–360. doi: 10.1007/s002130050355. [DOI] [PubMed] [Google Scholar]