Abstract
The role of the vomeronasal organ (VNO) in mediating neuroendocrine responses in female mice is well known; however, whether the VNO is equally important for sex discrimination is more controversial as evidence exists for a role of the main olfactory system in mate recognition. Therefore, we studied the effect of VNO removal (VNOx) on the ability of female mice to discriminate between volatile and non-volatile odours of conspecifics of the two sexes and in different endocrine states using Y-maze tests. VNOx female mice were able to reliably distinguish between male and female or male and gonadectomized (gdx) male volatile odours. However, when subjects had to discriminate between male and female or gdx male non-volatile odours, VNOx females were no longer able to discriminate between sex or different endocrine status. These results thus show that the VNO is primarily involved in the detection and processing of non-volatile odours, and that female mice can use volatile odours detected and processed by the main olfactory system for mate recognition. However, VNO inputs are needed to promote contact with the male, including facilitation of lordosis responses to his mounts. A single subcutaneous injection with gonadotropin-releasing hormone (GnRH) partially reversed the deficit in lordosis behaviour observed in VNOx females suggesting that VNO inputs may reach hypothalamic GnRH neurons to influence the display of sexual behaviour.
Keywords: accessory olfactory system, mate recognition, olfaction, sexual behaviour
Introduction
Mice use body odours to distinguish the sex, social and reproductive status, and kinship of individuals (Brown, 1979). These odours, in turn, induce behavioural and neuroendocrine changes in the recipient (Bruce, 1959; Whitten, 1959; Lombardi & Vandenbergh, 1977). The accessory olfactory system is thought to play a pivotal role in the detection and processing of these odours. Indeed, the vomeronasal organ (VNO), where the initial detection of odours takes place following direct contact with non-volatile components of various body secretions such as skin secretions, urine or scent marks (Halpern & Martinez-Marcos, 2003; Wysocki et al., 1980; Luo et al., 2003), has been shown to play a critical role in mediating pregnancy block (Bruce effect), acceleration of puberty (Whitten effect) and maternal aggression (e.g. Lloyd-Thomas & Keverne, 1982; Halpern & Martinez-Marcos, 2003). However, whether the VNO is equally important for sex discrimination is more controversial as evidence exists for a role of the main olfactory system in mate recognition. For instance, odours determined by the major histocompatibility complex (MHC), which has been shown to be instrumental for individual recognition among mice and other mammals (Yamaguchi et al., 1981; Beauchamp et al., 1985; Brown et al., 1987; Yamazaki et al., 1992, 2000; Singh, 2001; Brennan & Peele, 2003), are detected by the main olfactory system (Schaefer et al., 2001, 2002; Wysocki et al., 2004). By contrast, studies using different transgenic mouse models in which VNO function was manipulated by either mutating a cluster of V1r receptor genes (Del Punta et al., 2002) or the transient receptor potential 2 cation channel (Leypold et al., 2002; Stowers et al., 2002) suggested that the VNO is required for sex discrimination in male mice. A recent study using surgical lesions of the VNO could, however, not confirm such a role for the VNO in sex discrimination, although VNO-lesioned males failed to show a preference for urinary odours from oestrous females (Pankevich et al., 2004). Because all these studies have focused on male mice, we asked, in the present study, whether the VNO contributes directly to sex discrimination in female mice. Thus, we subjected female mice to either surgical removal of the VNO (VNOx) or sham surgery (VNOi). The ability of such females to discriminate volatile and non-volatile chemosignals from intact male and castrated male or oestrous female conspecifics was then compared in Y-maze tests. To provide subjects with non-volatile odours only, low- and high-molecular-weight (LMW and HMW) urinary fractions were prepared from male and female urine (Peele et al., 2003).
If the VNO is indeed critical for mate recognition, sexual behaviour may be affected by VNO removal as the female presumably no longer recognizes the male on the basis of his odours. Therefore, we also assessed the effects of VNO removal on lordosis behaviour. Furthermore, because male odours detected by the VNO have been proposed to act via gonadotropin-releasing hormone (GnRH) neurons to facilitate sexual behaviour (Mackay-Sim & Rose, 1986; Saito & Moltz, 1986), we also determined whether lordosis behaviour could be restored in VNOx female mice by GnRH treatment.
Materials and methods
Subjects
Adult (10–12 weeks), sexually naive female mice (n = 36) of the C57Bl6 inbred strain were obtained from a local breeding colony at the University of Liège. One week later, all females were ovariec-tomized under general anaesthesia using a mixture of ketamine (80 mg/kg per mouse) and medetomidine (Domitor, Pfizer, 1 mg/kg per mouse). Mice received atipamezole (Antisedan, Pfizer, 4 mg/kg per mouse, s.c.) at the end of the surgery in order to antagonize medetomidine-induced effects, thereby accelerating their recovery. During surgery, a Silastic capsule (inner diameter: 1.57 mm; outer diameter: 2.41 mm; length: 5 mm) filled with 17β-oestradiol (diluted 1:1 with cholesterol) was implanted s.c. in the neck. Then, 1 week later subjects underwent either bilateral removal of the VNO or sham surgery (VNOx or VNOi groups). VNO removal was carried out under a dissecting stereomicroscope. Removal of the VNO was accomplished via a midline incision in the root of the mouth under ketamine/domitor anaesthesia. Animals were placed on their back in a head-holder and the lower jaw was gently opened. A midline incision was made in the soft palette extending rostrally from behind the first palatal ridge to the incisors, and the underlying bone was exposed by blunt dissection. In VNOi animals, the incision was closed with reabsorbable sutures. For VNOx animals, the rostral end of the VNO was exposed by drilling, the caudal end of the vomer bone was cut, and the VNO was removed bilaterally with a gentle twisting motion. To ensure that the VNO was entirely removed, the area behind the incisors was cauterized. Gel foam was packed into the cavity, and the incision was closed with 7.0 Vicryl sutures. Bleeding was controlled using a blunted 18-gauge needle attached to a vacuum. Animals were carefully monitored after surgery for bleeding and/or breathing difficulties, and were allowed 1 week to recover before the onset of behavioural testing. After surgery, subjects were housed alone in macrolon cages and were placed at random in a climate-controlled (light, temperature, ventilation) animal housing unit (Iffa-Credo, L’Arbresle, France) on a reversed 12:12 h light:dark cycle. The air pressure in the housing units was higher than in the animal room, thereby avoiding the inflow of any odours from the room in which the housing unit was placed. Thus, care was taken that females were not exposed to any male-derived odours except when tested. Sawdust served as bedding and was not changed for at least 48 h before any behavioural test. Food and water were always available ad libitum. All of the behavioural tests were performed between 11.00 and 16.00 h, during the dark phase of the light:dark cycle, and female subjects were injected s.c. with 500 μg progesterone 2–4 h before each behavioural test to induce behavioural oestrus (Bakker et al., 2002).
All of the procedures were conducted in accordance with the guidelines set forth by the National Institutes of Health Guiding Principles for the Care and Use of Research Animals, and were approved by the Ethical Committee for Animal Use of the University of Liège.
Behavioural tests
Y-maze used for olfactory discrimination tests
All olfactory discrimination tests were conducted in an enclosed, Plexiglas Y-maze (see Bakker et al., 2002 for a full description of the maze). When subjects were tested for mate recognition using volatile body odours as odour stimuli, removable opaque Plexiglas doors were placed at the distal end of each arm to separate the goal boxes from the rest of the maze. Volatile body odours were derived from placing anaesthetized stimulus animals behind these doors. It should be noted that the top of each opaque door was perforated to allow air to flow from the goal boxes into the maze. However, these holes were placed above ‘eye level’ so that subjects could not see the stimulus animals. The level of anaesthesia was checked regularly and adjusted, if necessary, between each trial. Also, stimulus animals were placed regularly on a heating pad to prevent hypothermia. When subjects were tested for mate recognition using volatile urinary odours, 30 μL urine was pipetted onto a glass slide and placed behind the opaque Plexiglas doors. When subjects were tested for mate recognition using non-volatile odours, the doors were removed to allow direct access to the odour stimuli, which were placed in the back of each goal box. Soiled bedding was placed in bowls whereas the different urine stimuli were pipetted onto glass slides. The time that the mouse spent investigating the stimulus in direct nasal contact was recorded.
At the beginning of each test, the subject was placed in the start box with the door closed to adapt for 1 min. The test began when the door was removed and the subject could freely move around in the Y-maze. The time the subject spent investigating each odour stimulus was recorded with a stopwatch. In the case of volatile odour stimuli, the time was recorded that the subject spent poking her nose in the holes of the door or actively sniffing the door. The maze was cleaned with 70% ethanol between trials. All Y-maze tests lasted 5 min (not including the 1-min adaptation period) and were separated by at least 3 days. Finally, care was taken that subjects were never exposed to any male-derived odours except when tested. For each test, cages were taken randomly out of the housing unit to prevent the same animals always being tested first or last.
Female sexual behaviour
All lordosis tests were conducted in a Plexiglas aquarium (35 cm long × 25 cm high × 19 cm wide) whose floor was covered with fresh sawdust. At the beginning of each test, a sexually experienced male of the NMRI strain was placed alone in the aquarium and allowed to adapt for 15 min. Subsequently, an experimental female was placed in the aquarium and the lordosis responses of the female to the mounts of the stimulus male were recorded. The test lasted until the female had received 20 mounts or 15 min had elapsed.
Preparation of odour stimuli
Urine collection
Urine was collected from 10 gonadally intact C57Bl6 males, which were either left gonadally intact or were castrated under general anaesthesia at least 2 weeks prior to urine sampling. Oestrous female urine was collected from 10 ovariectomized female mice who had an oestradiol implant and were injected with 500 μg progesterone 2–6 h prior to urine sampling. Urine was collected by holding the mouse by the scruff of the neck over a funnel, taking care that no faecal contamination of the urine occurred. Same urine stimulus samples were pooled and subsequently aliquoted in 500-μL Eppendorf and stored at −80 °C until use.
Soiled bedding
Groups of gonadally intact males (n = 4), castrated males (n = 4) or oestrous females (n = 4; the latter being ovariectomized and treated with oestradiol by Silastic implant and progesterone by s.c. injection) were placed in clean cages containing fresh sawdust. Bedding was collected 10 h later. All bedding was stored in plastic freezer bags at −80 °C until used in the experiment.
LMW and HMW urine fractions
To separate volatile from non-volatile urinary components, LMW and HMW fractions were prepared from intact male and oestrous female urine. The LMW fraction consisted of volatile molecules whereas the HMW fraction contained the non-volatile major urinary proteins (MUPs) stripped of their volatile ligands. Separation of the HMW from the LMW fraction was achieved by dialysis, as described by Peele et al. (2003). Briefly, a 6-mL volume of phosphate-buffered saline (PBS) was placed in dialysis tubing with a 12–14 kDa molecular mass cut-off (Fisher scientific, Belgium), and dialysed against 12 mL urine overnight. The sample inside the tube formed the LMW fraction. The urine remaining outside the tubing was transferred to fresh dialysis tubing and dialysed against 2 L PBS overnight. The PBS was then replaced and the dialysis repeated overnight. The fraction remaining in the dialysis tubing was then dialysed against 95 mL of a menadione solution [25 mL of 4 mg/mL menadione (Sigma) in 100% ethanol and 70 mL PBS] to competitively displace the ligands, which are bound tightly to the MUPs. The dialysis was performed overnight to ensure displacement of the majority of the ligands. The remaining fraction was then dialysed twice more overnight, against 2 L PBS, to remove excess menadione. The fraction remaining in the dialysis tubing formed the HMW fraction. All fractions were aliquoted and stored at −80 °C until use. Samples of the urine and each fraction were analysed by standard techniques of sodium dodecyl sulphate–polyacrylamide gel electrophoresis to determine the presence of MUPs in the LMW and HMW fractions.
Gel electrophoresis revealed a similar content of proteins between urine and HMW samples (Fig. 1). MUPs can be identified according to their molecular weight (about 16–18 kDa; Robertson et al., 1996; Armstrong et al., 2005). It is noticeable that female urine and HMW fractions contain a lower amount of protein in comparison to male urine samples, reflecting the sexual dimorphic expression of MUPs (Beynon & Hurst, 2004). No protein band could be detected in the LMW fractions of both male and female origin. If these urinary fractions contained any MUPs it would certainly have been at a considerably lower concentration than in the HMW fractions. Moreover, the finding that none of the VNOi females showed any significant olfactory investigation when the HMW fraction was used without direct access excludes any possible contamination with volatile ligands.
Fig. 1.
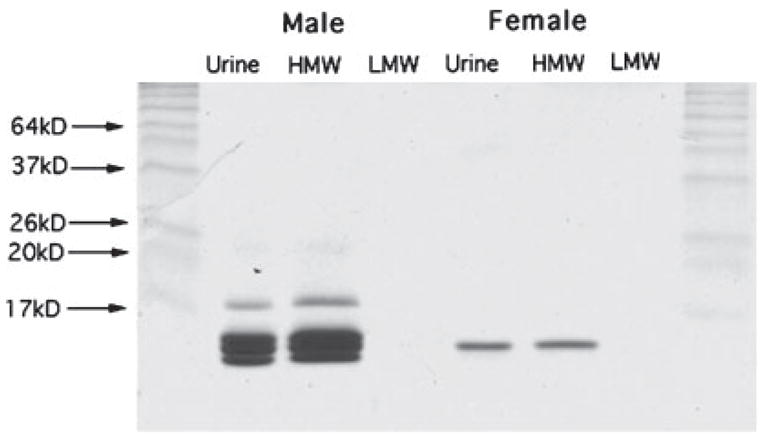
Samples of male and oestrous female urine and the HMW (high molecular weight) and LMW (low molecular weight) fractions run on a standard 15% sodium dodecyl sulphate–polyacrylamide gel electrophoresis. The molecular weight of the major urinary proteins is approximately 18 kDa.
Experimental procedures
Groups of VNOx (n = 10) and VNOi (n = 8) females were tested for their ability to discriminate between a series of different odour stimuli in the Y-maze, whereas other groups of VNOx (n = 10) and VNOi (n = 8) females were tested for their lordosis behaviour with a sexually experienced male. All female subjects were tested for odour preferences or lordosis behaviour while they received oestradiol (by Silastic implant) and following an injection with progesterone (500 μg) 2–4 h prior to testing.
Role of the VNO in sex discrimination
Role of the VNO in detecting volatile odours
To determine whether female mice in which the VNO was removed were still capable of using volatile odours for mate recognition, VNOx and VNOi females were first tested for their ability to discriminate between various volatile odour stimuli. Subjects were first tested for 5 min in the Y-maze with no stimulus animals in the goal boxes to adapt to the testing apparatus and to determine whether they would develop any side preferences. Then subjects were offered the choice between volatile body odours (Test 1) followed by volatile urinary odours (Test 2) from a gonadally intact male vs. those from a castrated (gdx) male. Next, subjects were offered the choice between volatile odour stimuli from a gonadally intact male vs. those of an oestrous female (Tests 3 and 4).
Role of the VNO in detecting non-volatile odours
To determine whether female mice in which the VNO was removed were capable of using non-volatile odours for mate recognition, VNOx and VNOi females were tested for their ability to discriminate between various non-volatile odour stimuli. Subjects were first offered the choice between soiled bedding (Test 5) followed by urinary odours (Test 6) from a gonadally intact male vs. those from a castrated male. Next, subjects were offered the choice between non-volatile odour stimuli from a gonadally intact male vs. those of an oestrous female (Tests 7 and 8). Because bedding and urine contain both volatile and non-volatile components, we only recorded the time when the animal made direct nasal contact with the odour stimulus. Previous studies have shown that direct contact with non-volatile odours is needed to activate the VNO (Wysocki et al., 1980; Halpern & Martinez-Marcos, 2003; Luo et al., 2003).
When subjects were tested for olfactory discrimination of the LMW urine fractions, doors were used to prevent direct access to the urine stimuli (Test 9). When subjects were tested using the HMW urine fractions as odour stimuli, they were first tested with direct access and then without access (i.e. with the odour stimuli placed behind the opaque doors, Tests 10 and 11), the latter to ensure that the HMW fraction was not contaminated by LMW compounds, i.e. in case the animals respond to the HMW fraction in the absence of physical access, it probably still contained some volatile ligands.
Role of the VNO in the display of female sexual behaviour
VNOi (n = 10) and VNOx (n = 8) females were tested for female sexual behaviour with a sexually experienced male. Female receptivity was assessed by quantifying the ratio of the number of lordosis responses to the number of mounts by a stimulus male (lordosis quotient). Because a s.c. injection with GnRH has been shown to restore the detrimental effects of VNO removal on lordosis behaviour in the female hamster (Mackay-Sim & Rose, 1986) and rat (Saito & Moltz, 1986), suggesting that chemosensory signals from the male act via GnRH neurons to facilitate the triggering of lordosis, we determined whether the same phenomenon would occur in the female mouse. Therefore, female VNOi and VNOx mice were injected s.c. in the neck with either 5 μg of GnRH (Sigma) dissolved in saline or saline only in a counterbalanced paradigm, i.e. half of the animals (VNOi and VNOx) were tested first with GnRH and then re-tested with saline, the other half vice versa, thus animals served as their own control. Please note that animals were not injected with progesterone in order to better assess any stimulatory effects of GnRH on lordosis behaviour. Three hours after the GnRH or saline injection, animals were tested for lordosis behaviour. The two tests were 4 days apart.
Assessment of surgical lesion of the VNO
When behavioural testing was completed, the efficacy of the VNO lesion was assessed by histological procedures using soybean agglutinin (SBA) and Fos immunocytochemistry. Thus, VNOi and VNOx animals were stimulated by direct application of 30 μL of either male urine (positive control; VNOi, n = 4; VNOx, n = 4), male urine-derived LMW fraction (VNOi, n = 5; VNOx, n = 6), male urine-derived HMW fraction (VNOi, n = 4; VNOx, n = 5) or water (negative control; VNOi, n = 3; VNOx, n = 3) stimuli on the oronasal groove. Ninety minutes after stimulation, animals were anaesthetized and perfused transcardially with saline followed immediately by 4% cold paraformaldehyde. Brains were removed and postfixed in 4% paraformaldehyde for 2 h. Then brains were cryoprotected in 30% sucrose/PBS solution and when sunken, frozen on dry ice and kept at −80°. Sections of 30 μm were cut on a Leica cryostat. Olfactory bulbs were cut sagittally and alternate sections were stained for SBA conjugated with horseradish peroxidase (SBA–HRP) or Fos. Forebrains were cut coronally and processed for c-fos only. Each brain section was saved in antifreeze solution and maintained at −20 °C for later immunocytochemistry.
AOB morphology using SBA
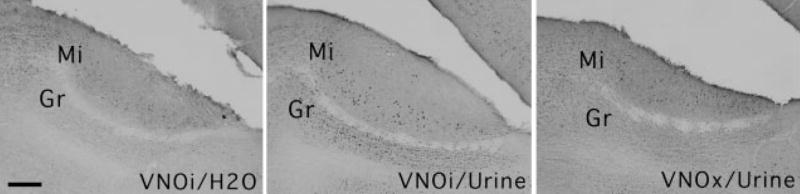
SBA–HRP stains the axons of VNO neurons that project to the glomerular layer of the accessory olfactory bulb (AOB) and serves as a useful marker for intact VNO neurons in mice and rats (Key & Giorgi, 1986; Wysocki & Wysocki, 1995). The lack of SBA staining in the AOB provides evidence that the VNO was successfully removed. SBA–HRP staining was performed to ensure successful VNO removal, as described by Pankevich et al. (2004). Sagittal sections of the olfactory bulbs were first incubated in 3% normal goat serum (NGS)/1% H2O2/PBS for 2 h, followed by washes in 0.1 M PBS. Sections were then incubated in SBA–HRP (15 μg/mL; Sigma) for 40 min at room temperature, followed by washes in PBS. Sections were reacted with nickel–3,3′-diaminobenzidine (DAB) for 5 min. Sections were then mounted onto gelatin-coated slides and coverslipped using Permount. After examination of the SBA–HRP-labelled sections (Fig. 2), two VNOx females who belonged to the group that was tested for lordosis behaviour were omitted from the experiment, as for both animals AOB labelling was seen in some of the sections examined. Only females with complete VNO lesions have been included in any of the behavioural analyses.
Fig. 2.
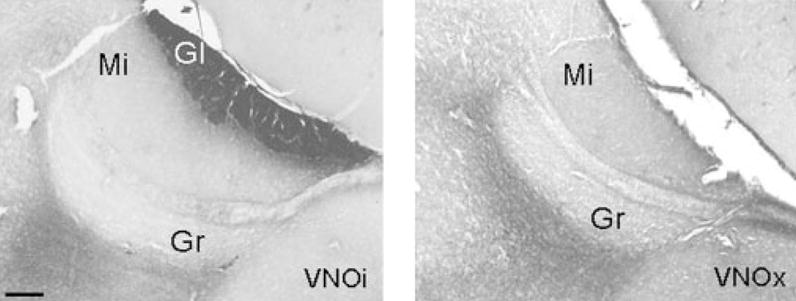
Representative photomicrographs showing sagittal sections stained with SBA–HRP of the AOB of female mice who had either undergone sham removal of (VNOi) or a bilateral surgical removal of the vomeronasal organ (VNOx). The absence of SBA–HRP staining in the glomerular layer of VNOx animals was taken as evidence of successful VNO removal. Gl, glomerular cell layer; Gr, granular cell layer; Mi, mitral cell layer. Scale bar, 100 μm.
Functional assessment of the accessory olfactory pathway using the expression of c-fos following VNO removal
Every fourth section was processed for Fos immunoreactivity as previously described (Halem et al., 2001; Pankevich et al., 2004). All incubations were carried out at room temperature and all washes of tissue sections were performed using Tris-buffered saline (TBS) or PBS. Briefly, sections were preincubated for 3 h in 7.5% NGS in TBS containing 0.1% Triton X-100 (TBST). Then, sections were incubated overnight with a rabbit polyclonal anti-c-fos antibody (Santa Cruz SC-52; 1:3000 in TBST/2% NGS) followed by an incubation for 1 h in a goat-anti-rabbit biotinylated antibody (Dako Cytomation, Denmark; 1:200 in TBST/2% NGS). To eliminate endogenous peroxidase activity, sections were incubated for 30 min in PBS containing H2O2 at a final concentration of 3%. Sections were then incubated for 45 min in avidin–biotin complex (ABC; Vector Laboratory) and reacted for 5 min with DAB containing nickel chloride (Vector Laboratory). Sections were washed, mounted on gelatin-coated slices, dried, dehydrated through graded alcohol, cleared in toluene and cover-slipped using Permount. The numbers of Fos-immunoreactive cells were quantified throughout the accessory olfactory pathway, i.e. in the AOB, medial amygdaloid nucleus (anterior, posterodorsal and posteroventral parts), medial preoptic area, bed nucleus of the stria terminalis and ventromedial hypothalamic nucleus, using a microscope with a camera lucida attachment. For the mitral and granular cell layers of the AOB, three sections (from lateral to medial) from both bulbs were analysed for numbers of Fos-immunoreactive neurons. For the other brain regions, all Fos-immunoreactive neurons in a field of view under the ×20 objective were counted in three sections (from rostral to caudal; for both sides of the brain).
Statistical analysis
All data were analysed using repeated measures analysis of variance (ANOVA). When appropriate, all ANOVAs were followed by Tukey highest signification difference (HSD) post-hoc comparisons adapted for repeated measures ANOVA. Only significant (P < 0.05) effects detected by the ANOVAs are mentioned in detail in the Results.
Results
Role of the VNO in sex discrimination
Role of the VNO in detecting volatile odours
When volatile body or urinary odours were used as odour stimuli (Tests 1–4), female mice always showed a significant preference for male-derived stimuli over those derived from a gdx male or an oestrous female, regardless of whether the VNO was intact or lesioned (Fig. 3A). Indeed, two-way ANOVA with VNO status as independent factor and odour stimulus as the repeated factor revealed a significant effect of the odour stimulus (volatile body odours: male vs. gdx male: F1,16 = 22.8, P = 0.0002; male vs. female: F1,16 = 12.4, P = 0.0028; volatile urinary odours: male vs. gdx male: F1,16 = 10.4, P = 0.0.005; male vs. female: F1,16 = 18.0, P = 0.0006). No effect of VNO lesion or any interaction was found, with one exception when animals had to discriminate male from female volatile body odours (Test 3) where a small effect of the VNO lesion was observed (F1,16 = 5.2, P = 0.037). Then VNOx females spent less time investigating the odour stimuli compared with VNOi females (Test 3; Fig. 3A).
Fig. 3.
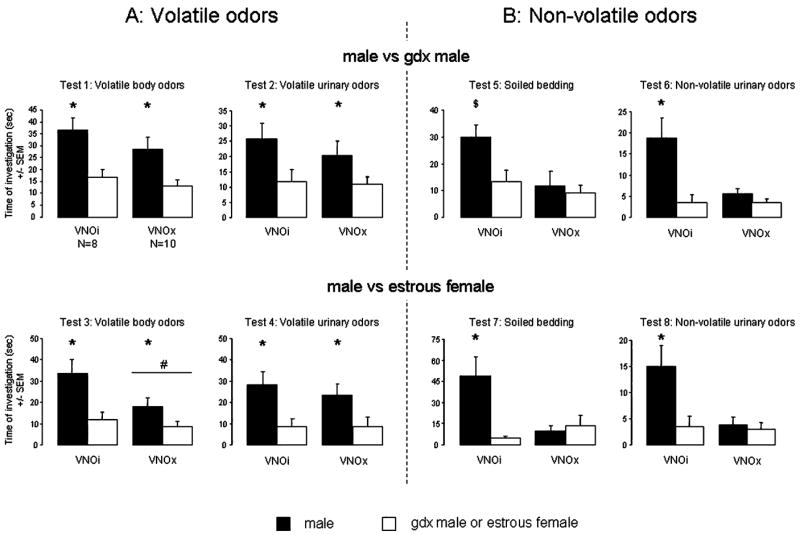
Mean (± SEM) amount of time spent by VNOi or VNOx females investigating (A) volatile (no access) and (B) non-volatile (direct access) olfactory cues when given a choice between intact male odours and gonadectomized male odours or between intact male odours and oestrous female odours in a Y-maze. *P < 0.05, effect of odour stimulus; #P < 0.05, effect of the VNO lesion.
Role of the VNO in detecting non-volatile odours
When female subjects were given direct access to the various odour stimuli, i.e. soiled bedding or urine from either male vs. gdx male or male vs. oestrous female, in the Y-maze, VNOi females always showed a preference for male soiled bedding or urine, whereas VNOx females did not show any odour preference (Fig. 3B). This was confirmed by two-way ANOVA that revealed a significant effect of the VNO lesion when females were provided with urine (male vs. gdx male: F1,16 = 6.5, P = 0.021; male vs. oestrous female: F1,16 = 8.9, P = 0.09), whereas the lesion effect was only marginally significant for soiled bedding (male vs. gdx male: F1,16 = 3.9, P = 0.066; male vs. female: F1,16 = 4.2, P = 0.058; Fig. 3B). Furthermore, a significant effect of odour stimulus (soiled bedding: male vs. gdx male: F1,16 = 11.4, P = 0.003; male vs. female: F1,16 = 6.7, P = 0.020; urine: male vs. gdx male: F1,16 = 13.6, P = 0.002; male vs. female: F1,16 = 5.1, P = 0.038) as well as significant interactions between the VNO lesion and the odour stimulus were observed for each test (soiled bedding: male vs. gdx male: F1,16 = 7.8, P = 0.012; male vs. female: F1,16 = 4.7, P = 0.042; urine: male vs. gdx male: F1,16 = 6.0, P = 0.026; male vs. female: F1,16 = 9.2, P = 0.008). Post-hoc comparisons revealed that the time spent investigating male-derived stimuli was significantly higher in VNOi compared with VNOx females, with the exception when females had to discriminate soiled bedding from males and gdx males, then no differences were observed in the time of investigation (Test 5). Finally, the total amount of time spent investigating the odour stimuli was significantly reduced in VNOx females compared with VNOi females (Fig. 3B).
LMW and HMW urinary fractions
With the LMW fraction placed behind the opaque doors, two-way ANOVA with VNO lesion as independent factor and LMW fraction as repeated factor revealed a significant preference for the male-derived LMW fraction for both VNOi and VNOx animals (F1,16 = 17.9, P = 0.007; Fig. 4). When given direct access to the HMW urinary fraction, only VNOi females showed a preference for male-derived odour stimuli (VNO lesion: F1,16 = 8.9, P = 0.009; odour stimulus: F1,16 = 5.1, P = 0.038; and an almost significant interaction: F1,16 = 3.89, P = 0.065). By contrast, when direct access to the HMW was prevented, no preference for male over oestrous female HMW urinary fractions was observed in either VNOi or VNOx group.
Fig. 4.
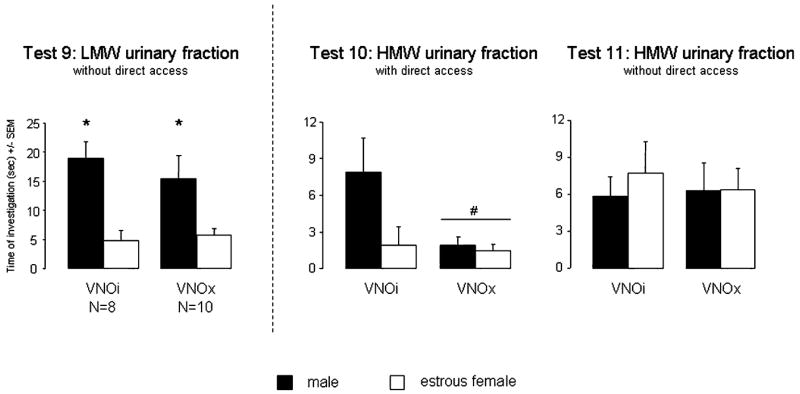
Mean (± SEM) amount of time spent by VNOi or VNOx females investigating the low-molecular-weight (LMW) urinary fraction or high-molecular-weight (HMW) urinary fraction, when given a choice between intact male odours and oestrous female odours in a Y-maze. *P < 0.05, effect of odour stimulus; #P < 0.05, effect of the VNO lesion.
Role of the VNO in the display of female sexual behaviour
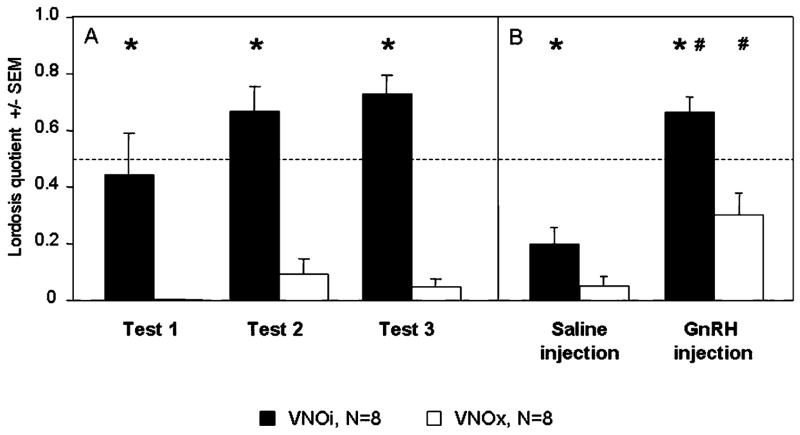
VNOi females showed high levels of sexual receptivity whereas VNOx females were significantly less receptive (Fig. 5A). Two-way ANOVA with VNO lesion as independent factor and tests as repeated factor showed a significant effect of VNO lesion (F1,14 = 40.2, P < 0.001) as well as a significant effect of repeated testing (F2,28 = 5.4, P = 0.010), but no interaction suggesting that the level of sexual receptivity increased significantly in all female subjects.
Fig. 5.
(A) Mean (± SEM) lordosis quotient expressed by VNOi and VNOx females in response to the male’s mount during three consecutive tests. *P < 0.05; effect of the VNO lesion. (B) Mean (± SEM) lordosis quotient expressed by VNOi and VNOx females in response to the male’s mount after injection of GnRH or saline. *P < 0.05, effect of the lesion; #P < 0.05, effect of GnRH (vs. saline) injection.
A s.c. injection with GnRH (instead of progesterone) partially restored lordosis behaviour in VNOx females (Fig. 5B). Two-way ANOVA revealed a significant effect of VNO lesion (F1,28 = 20.1, P < 0.0001) and GnRH treatment (F2,28 = 21.2, P < 0.001). In VNOi animals, GnRH treatment induced high levels of receptivity that were similar to those observed when tested after priming with progesterone, whereas saline injections did not induce lordosis behaviour. A similar stimulatory effect of GnRH on lordosis behaviour was observed in VNOx females, although their lordosis quotients remained lower than those of VNOi females.
Functional assessment of the accessory olfactory pathway using the expression of c-fos following VNO removal
Male urine as well as the LMW fraction clearly stimulated the expression of c-fos in the AOB of VNOi but not VNOx females (Fig. 6). Two-way ANOVA revealed a significant effect of the VNO lesion (mitral cell layer: F1,14 = 13.7, P = 0.0005; granular cell layer: F1,14 = 15.6, P = 0.0005), of the odour stimulus (mitral cell layer: F2,28 = 7.7, P = 0.0007; granular cell layer: F2,28 = 3.4, P = 0.032), as well as a significant interaction between these two factors (mitral cell layer: F2,28 = 9.3, P = 0.002; granular cell layer: F2,28 = 3.0, P = 0.048; Table 1). Post-hoc analysis showed that exposure to either male urine or the male-derived LMW urinary fraction significantly induced c-fos in the mitral and granular cell layers of the AOB in VNOi but not in VNOx females. Furthermore, neither male-derived HMW urinary fraction nor water induced any significant c-fos responses in the AOB. At more central levels in the accessory olfactory pathway, i.e. the amygdala and the hypothalamus, odour-induced Fos responses were less pronounced. In the anterior medial amygdala (MeA), a significant Fos response was observed in VNOi but not in VNOx females following exposure to either male urine or male-derived LMW urinary fraction. Two-way ANOVA revealed an effect of the VNO lesion (F1,14 = 23.6, P < 0.0001), of odour stimulus (F2,28 = 9.8, P = 0.0002) and a significant interaction (F2,28 = 5.2, P = 0006). Again, neither HMW nor water induced any significant Fos-immunoreactivity in the MeA. In the posterodorsal medial amygdala (MePD), exposure to male urine and male-derived LMW urinary fraction but not HMW urinary fraction or water induced Fos-immunoreactivity in VNOi but not VNOx females (effect of lesion: F1,14 = 9.2, P = 0.005; odour stimulus: F2,28 = 4.6, P = 0.011). However, no significant effects of VNO lesion, odour stimulus or any significant interactions were observed for the other brain regions, i.e. the posteroventral medial amygdala (MePV), the medial preoptic area (MPOA), the bed nucleus of the stria terminalis (BNST) or the ventromedial nucleus (VMN) (Table 1).
Fig. 6.
Representative sagittal sections showing Fos-immunoreactive protein in the AOB of VNOi and VNOx females following stimulation with 30 μL of male urine (VNOx and VNOi) or water (VNOi). Scale bar, 100 μm.
Table 1.
Mean (± SEM) number of Fos-immunoreactive cells in various brain structures of VNOi and VNOx females after stimulation with 30 μL of either male urine, male urine-derived LMW fraction, male urine-derived HMW fraction or water on the oronasal groove
| Urine
|
LMW
|
HMW
|
Water
|
|||||
|---|---|---|---|---|---|---|---|---|
| Structure | VNOi (n = 4) | VNOx (n = 4) | VNOi (n = 5) | VNOx (n = 6) | VNOi (n = 4) | VNOx (n = 5) | VNOi (n = 3) | VNOx (n = 3) |
| AOB (mitral layer) | 25 ± 2.5*,† | 7 ± 3 | 18 ± 3*,† | 6 ± 2 | 7 ± 2 | 7 ± 2 | 5 ± 2 | 8 ± 2 |
| AOB (granular layer) | 43 ± 14*,† | 2 ± 0.6 | 36 ± 13* | 3 ± 1 | 5 ± 2 | 1 ± 0.4 | 6 ± 3 | 1 ± 1 |
| MeA | 14 ± 1*,† | 5 ± 0.6 | 13 ± 2*,† | 6 ± 1 | 6 ± 1 | 4 ± 1 | 4 ± 1 | 4 ± 1 |
| MePD | 11 ± 2 | 5 ± 0.6 | 12 ± 2* | 8 ± 1 | 7 ± 2 | 4 ± 1 | 6 ± 1 | 6 ± 0.4 |
| MePV | 8 ± 0.3 | 4 ± 1 | 10 ± 3 | 5 ± 1 | 4 ± 1 | 3 ± 1 | 4 ± 1 | 4 ± 2 |
| MPOA | 10 ± 2 | 6 ± 3 | 10 ± 2 | 3 ± 1 | 4 ± 2 | 4 ± 1 | 4 ± 2 | 5 ± 2 |
| BNST | 7 ± 3 | 7 ± 4 | 11 ± 2 | 4 ± 2 | 8 ± 4 | 7 ± 2 | 7 ± 3 | 6 ± 4 |
| VMN | 4 ± 2 | 1 ± 1 | 3 ± 2 | 4 ± 2 | 4 ± 2 | 3 ± 1 | 6 ± 1 | 4 ± 2 |
P < 0.05, comparison between VNOi and VNOx animals for the same odour stimulus,
P < 0.05, comparison with VNOi animals stimulated with HMW urinary fraction or water. AOB, accessory olfactory bulb; MeA, anterior medial amygdala; MePD, posterodorsal medial amygdala; MePV, posteroventral medial amygdala; BNST, bed nucleus of the stria terminalis; VMN, ventromedial nucleus.
Discussion
Role of the VNO in sex discrimination
Our results confirm the well-established view of the VNO and accessory olfactory system being used to process – when in close contact – non-volatile components of body and urinary odorants that may trigger behavioural or endocrine responses (Powers et al., 1979; O’Connell & Meredith, 1984). In addition, our results show that not the VNO but the main olfactory system is used to localize and identify the sex and endocrine status of conspecifics on the basis of their volatile odours. After VNO removal, female mice were still able to reliably discriminate between volatile odours derived from intact males and those from gdx males or oestrous females, thereby confirming previous results showing that the VNO is not involved in the detection and processing of volatile odours (Petrulis et al., 1999) and that male mice can use volatile odours for sex discrimination (Pankevich et al., 2004). However, when animals were provided with direct access to the odour stimuli, the effects of VNO lesions became visible: VNOi but not VNOx females showed odour preferences as well as significant amounts of olfactory investigation of these non-volatile odour stimuli, suggesting that the VNO is necessary to prolong olfactory investigation of the odour stimuli (Luo et al., 2003; Pankevich et al., 2004). Thus, our results further confirm that the VNO primarily detects non-volatile components, whereas the main olfactory system detects volatile components of urine.
The view that the VNO is only involved in the detection of non-volatile odours has recently been challenged. Using both electrophysiological and optical imaging methods, several researchers have demonstrated that VNO neurons can respond in a very sensitive and specific way to volatile substances contained in male urine, such as farnesenes, brevicomin, dimethylpirazine and thiazole (Leinders-Zufall et al., 2000; Sam et al., 2001; Boschat et al., 2002; Del Punta et al., 2002). However, it is generally thought that volatile ligands need to be carried into the VNO by transport proteins, such as the MUPs. Such MUPs have been shown to bind small volatile molecules such as brevicomin and thiazole, thereby prolonging their release from dried urine marks and playing a vital role in transporting these chemosignals into the VNO (Hurst et al., 1998, 2001).
At present the only indirect behavioural evidence for a role of the VNO in the detection of volatile molecules comes from the study by Trinh & Storm (2003), who used transgenic mice with a nonfunctional cAMP signalling pathway in the olfactory epithelium due to a mutation in type 3 adenylyl cyclase (AC3). Such AC3 −/− mice were able to detect several volatile odorants, including putative pheromones, presumably via the VNO. By contrast, more recently it was shown that mice defective in the olfactory cAMP signalling pathway can detect some volatile odorants through their olfactory epithelium using alternative transduction pathways (Lin et al., 2004). Our results of VNOx females showing no olfactory deficits when provided with volatile odours suggest that such odours are primarily detected and processed by the main olfactory system and thus not by the VNO. On the other hand, exposure to the LMW urinary fraction, which consists of volatile ligands, significantly induced c-fos in the mitral and granular cell layers of the AOB of VNOi but not VNOx females, suggesting that the VNO had been activated by this odour stimulus. Perhaps the fact that the LMW urinary fraction was directly applied onto the oronasal groove could have facilitated the direct access of volatile ligands to the VNO and thus activated the mitral and granular cell layers of the AOB. In contrast with the LMW urinary fraction, exposure to the HMW urinary fraction, which contains the MUPs stripped of their ligands, did not induce any c-fos in the AOB, suggesting that MUPs alone do not stimulate the accessory olfactory system. This is in line with previous findings by Brennan et al. (1999). This result is surprising in light of our findings of VNOi but not VNOx females showing odour preferences and a significant amount of olfactory investigation when presented with the HMW fraction, suggesting that the VNO is essential in responding to these odours. The odour preference observed in VNOi females did not result from the HMW urinary fraction still being contaminated with volatile ligands, because when the HMW odour stimuli were placed behind the opaque doors to prevent access, the VNOi females no longer displayed any significant odour preferences. It should be noted, however, that the absence of a significant induction of c-fos in the AOB after applying the HMW urinary fraction onto the oronasal groove does not necessarily mean that neurons in the AOB were not activated by these odours. It could simply mean that they did not express c-fos. Our results are, however, consistent with the study of Peele et al. (2003) who showed that neither MUPs isolated from urine nor recombinant MUPs produced in an artificial yeast system could induce the expression of the immediate-early gene, egr-1, in the AOB. In addition, the HMW fraction was ineffective in blocking pregnancy in recently mated females, whereas the LMW fraction did induce egr-1 in the mitral and granular cell layers of the AOB as well as being effective in blocking pregnancy (Peele et al., 2003). Pregnancy block in this study was probably caused by the presence of small peptides related to MHC in the LMW fraction (Leinders-Zufall et al., 2004).
Finally, the finding of an overall reduced level of olfactory investigation of non-volatile odour stimuli in VNOx females may also reflect a motivational deficit rather than an inability to discriminate between the two odour stimuli. It has been reported that VNO removal eliminates a reinforcement mechanism that normally operates to sustain high levels of chemoinvestigation or other behavioural responses to socially relevant odours in several species, such as mice and hamsters (Beauchamp et al., 1982, 1985; Wysocki et al., 1982; Labov & Wysocki, 1989; Petrulis et al., 1999; Pankevich et al., 2004).
Role of the VNO in sexual behaviour
Removal of the VNO clearly disrupted lordosis behaviour in ovariectomized, hormonally primed, female mice. A reduction in sexual receptivity by VNO removal has also been reported in various other rodent species, such as rats (Rajendren et al., 1990; Saito & Moltz, 1986), hamsters (Mackay-Sim & Rose, 1986) and voles (Lepri & Wysocki, 1987; Curtis et al., 2001). Intriguingly, studies on the role of the VNO in the Bruce effect showed that VNO ablation did not prevent female mice from becoming pregnant (Lloyd-Thomas & Keverne, 1982), suggesting that the VNO may not mediate female sexual receptivity in mice. Because we only measured lordosis behaviour in three tests of 20 min, it is possible that VNOx females become sexually receptive after long-term exposure to the male. It has been shown in rats that the lordosis quotient of VNOx females increased after prolonged exposure to the male (Rajendren et al., 1990). Whether females are sexually experienced or not prior to VNO removal may also play a role. It has been shown in male hamsters that once animals gain sexual experience, either olfactory system could sustain sexual behaviour and only lesioning both olfactory systems disrupted sexual performance (Winans & Powers, 1977; Meredith, 1986; Pfeiffer & Johnston, 1994). The females in the present study had no mating experience prior to the first mating test.
Interestingly, a single s.c. injection of GnRH (given instead of progesterone to oestradiol-primed subjects) partially reversed the effects of VNO removal on lordosis behaviour. This result is in line with previous experiments performed in female rats and hamsters (Saito & Moltz, 1986; Mackay-Sim & Rose, 1986), and thus suggests that chemosensory signals from the male detected by the accessory olfactory system act via GnRH neurons to facilitate lordosis behaviour. GnRH neurons and fibres have been found in the accessory olfactory system as well as the ventromedial hypothalamus (Dudley et al., 1996; Meredith, 1998), the latter being important in mediating oestrogen–progesterone-induced lordosis behaviour (e.g. Rubin & Barfield, 1983). Furthermore, it was recently shown that GnRH neurons receive pheromone signals from both main and accessory olfactory relays in the brain (Boehm et al., 2005). There is some evidence for a role of GnRH in female sexual behaviour. Peripheral or central injection of GnRH in the third ventricle has been reported to facilitate lordosis behaviour in ovariectomized or in ovariectomized, hypophysectomized rats primed with oestrogen (Moss & McCann, 1973; Pfaff, 1973; Foreman & Moss, 1977; Dudley & Moss, 1988). Furthermore, VNOi but not VNOx females exhibited a luteinizing hormone surge following mating, suggesting a VNO-mediated GnRH release (Rajendren et al., 1990). More research is needed to determine whether activation of VNO receptor neurons directly induces GnRH release.
Concluding remarks
In the present experiments, VNO removal did not affect the female’s ability to discriminate sex or endocrine status based on olfactory cues, suggesting that it is not critical for sex discrimination. By contrast, VNO removal clearly disrupted sexual behaviour, suggesting that non-volatile olfactory cues from males, which are detected by the VNO, are necessary in order for a female to become sexually receptive.
Acknowledgments
The authors would like to acknowledge Dr Peter A. Brennan for advising on how to prepare the urinary fractions. This work was supported by Fonds National de la Recherche Scientifique (1.5.082.04 to J.B.) and NICHD grant No. HD044897 to J.B. and M.J.B. J. Bakker is a research associate of the Fonds National de la Recherche Scientifique.
Abbreviations
- AC3
type 3 adenylyl cyclase
- AOB
accessory olfactory bulb
- DAB
3,3′-diaminobenzidine
- GnRH
gonadotropin-releasing hormone
- HMW
high-molecular-weight urinary fraction
- HRP
horseradish peroxidase
- LMW
low-molecular-weight urinary fraction
- MHC
major histocompatibility complex
- MUPs
major urinary proteins
- PBS
phosphate-buffered saline
- SBA
soybean agglutinin
- TBS
Tris-buffered saline
- TBST
TBS containing 0.1% Triton X-100
- VNO
vomeronasal organ
References
- Armstrong SD, Robertson DHL, Cheetham SA, Hurst JL, Beynon RJ. Structural and functional differences in isoforms of major urinary proteins: a male specific protein that preferentially binds a male pheromone. Biochem J. 2005;391:343–350. doi: 10.1042/BJ20050404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, Balthazart J. The aromatase knockout mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviours in adulthood. J Neurosci. 2002;22:9104–9112. doi: 10.1523/JNEUROSCI.22-20-09104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp GK, Martin IG, Wysocki CJ, Wellington JL. Chemoinvestigatory and sexual behavior of male guinea pigs following vomeronasal organ removal. Physiol Behav. 1982;29:329–336. doi: 10.1016/0031-9384(82)90022-1. [DOI] [PubMed] [Google Scholar]
- Beauchamp GK, Yamazaki K, Wysocki CJ, Slotnick B, Thomas L, Boyse EA. Chemosensory recognition of mouse major histocompatibility types by another species. Proc Natl Acad Sci USA. 1985;82:4186–4188. doi: 10.1073/pnas.82.12.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynon RJ, Hurst JL. Urinary proteins and the modulation of chemical scents in mice and rats. Peptides. 2004;25:1553–1563. doi: 10.1016/j.peptides.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Boehm U, Zou Z, Buck LB. Feedback loops link odor and pheromone signaling with reproduction. Cell. 2005;123:683–695. doi: 10.1016/j.cell.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Boschat C, Pelofi C, Randin O, Roppolo D, Luscher C, Broilet MC, Rodriguez I. Pheromone detection mediated by a V1r vomeronasal receptor. Nat Neurosci. 2002;5:1261–1262. doi: 10.1038/nn978. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Peele P. Towards an understanding of the pregnancy-blocking urinary chemosignals of mice. Biochem Soc Trans. 2003;31:152–155. doi: 10.1042/bst0310152. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Schellinck HM, Keverne EB. Patterns of expression of the immediate-early gene egr-1 in the accessory olfactory bulb of female mice exposed to pheromonal constituents of male urine. Neuroscience. 1999;90:1463–1470. doi: 10.1016/s0306-4522(98)00556-9. [DOI] [PubMed] [Google Scholar]
- Brown RE. Mammalian social odors. Adv Stud Behav. 1979;10:107–161. [Google Scholar]
- Brown RE, Singh PB, Roser B. The major histocompatibility complex and the chemosensory recognition of individuality in rats. Physiol Behav. 1987;40:65–73. doi: 10.1016/0031-9384(87)90186-7. [DOI] [PubMed] [Google Scholar]
- Bruce HM. An exteroceptive block to pregnancy in the mouse. Nature. 1959;184:105. doi: 10.1038/184105a0. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Liu Y, Wang Z. Lesions of the vomeronasal organ disrupt mating-induced pair bonding in female prairie voles (Microtus ochrogaster) Brain Res. 2001;18:167–174. doi: 10.1016/s0006-8993(01)02343-5. [DOI] [PubMed] [Google Scholar]
- Del Punta K, Leinders-Zufall T, Rodriguez I, Jukam D, Wysocki CJ, Ogawa S, Zufall F, Mombaerts P. Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature. 2002;419:70–74. doi: 10.1038/nature00955. [DOI] [PubMed] [Google Scholar]
- Dudley CA, Moss RL. Facilitation of lordosis in female rats by CNS-site specific infusions of an LH-RH fragment, Ac-LH-RH-(5–10) Brain Res. 1988;16:161–167. doi: 10.1016/0006-8993(88)91394-7. [DOI] [PubMed] [Google Scholar]
- Dudley CA, Rajendren G, Moss RL. Signal processing in the vomeronasal system: modulation of sexual behavior in the female rat. Crit Rev Neurobiol. 1996;10:265–290. doi: 10.1615/critrevneurobiol.v10.i3-4.10. [DOI] [PubMed] [Google Scholar]
- Foreman MM, Moss RL. Effects of subcutaneous injection and intrahypothalamic infusion of releasing hormones upon lordotic response to repetitive coital stimulation. Horm Behav. 1977;8:219–234. doi: 10.1016/0018-506x(77)90039-3. [DOI] [PubMed] [Google Scholar]
- Halem HA, Cherry JA, Baum MJ. Central forebrain Fos responses to familiar male odours are attenuated in recently mated female mice. Eur J Neurosci. 2001;13:389–399. [PubMed] [Google Scholar]
- Halpern M, Martinez-Marcos A. Structure and function of the vomeronasal system: an update. Prog Neurobiol. 2003;70:245–318. doi: 10.1016/s0301-0082(03)00103-5. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Payne CE, Nevison CM, Marie AD, Humphries RE, Robertson DHL, Cavaggioni A, Beynon JB. Individual recognition in mice mediated by major urinary proteins. Nature. 2001;414:631–634. doi: 10.1038/414631a. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Robertson DHL, Tolladay U, Beynon RJ. Proteins in urine scent marks of male house mice extend the longevity of olfactory signals. Anim Behav. 1998;55:1289–1297. doi: 10.1006/anbe.1997.0650. [DOI] [PubMed] [Google Scholar]
- Key B, Giorgi PP. Soybean agglutinin binding to the olfactory systems of the rat and mouse. Neurosci Lett. 1986;29:131–136. doi: 10.1016/0304-3940(86)90591-4. [DOI] [PubMed] [Google Scholar]
- Labov JB, Wysocki CJ. Vomeronasal organ and social factors affect urine marking by male mice. Physiol Behav. 1989;45:447–453. doi: 10.1016/0031-9384(89)90153-4. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Brennan P, Widmayer PSPC, Maul-Pavicic A, Jager M, Li XH, Breer H, Zufall F, Boehm T. MHC class I peptides as chemosensory signals in the vomeronasal organ. Science. 2004;306:1033–1037. doi: 10.1126/science.1102818. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Lane AP, Puche AC, Ma W, Novotny MV, Shipley MT, Zufall F. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature. 2000;405:792–796. doi: 10.1038/35015572. [DOI] [PubMed] [Google Scholar]
- Lepri JJ, Wysocki CJ. Removal of the vomeronasal organ disrupts the activation of reproduction in female voles. Physiol Behav. 1987;40:349–355. doi: 10.1016/0031-9384(87)90058-8. [DOI] [PubMed] [Google Scholar]
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behavior in trp2 mutant mice. Proc Natl Acad Sci USA. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Arellano J, Slotnick B, Restrepo D. Odors detected by mice deficient in cyclic nucleotide-gated channel subunit A2 stimulate the main olfactory system. J Neurosci. 2004;24:3703–3710. doi: 10.1523/JNEUROSCI.0188-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Thomas A, Keverne EB. Role of the brain and accessory olfactory system in the block to pregnancy in mice. Neuroscience. 1982;7:907–913. doi: 10.1016/0306-4522(82)90051-3. [DOI] [PubMed] [Google Scholar]
- Lombardi JR, Vandenbergh JC. Pheromonally induced sexual maturation in females: regulation by the social environment of the male. Science. 1977;196:545–546. doi: 10.1126/science.557838. [DOI] [PubMed] [Google Scholar]
- Luo M, Fee MS, Katz LC. Encoding pheromonal signal in the accessory olfactory bulb of behaving mice. Science. 2003;299:1196–1201. doi: 10.1126/science.1082133. [DOI] [PubMed] [Google Scholar]
- Mackay-Sim A, Rose JD. Removal of vomeronasal organ impairs lordosis in female hamsters: effect is reversed by luteinising hormone-releasing hormone. Neuroendocrinology. 1986;42:489–493. doi: 10.1159/000124492. [DOI] [PubMed] [Google Scholar]
- Meredith M. Vomeronasal organ removal before sexual experience impairs male hamster mating behavior. Physiol Behav. 1986;36:737–743. doi: 10.1016/0031-9384(86)90362-8. [DOI] [PubMed] [Google Scholar]
- Meredith M. Vomeronasal, olfactory, hormonal convergence in the brain. Cooperation or coincidence? Ann NY Acad Sci. 1998;30:349–361. doi: 10.1111/j.1749-6632.1998.tb10593.x. [DOI] [PubMed] [Google Scholar]
- Moss RL, McCann SM. Induction of mating behavior in rats by luteinizing hormone-releasing factor. Science. 1973;13:177–179. doi: 10.1126/science.181.4095.177. [DOI] [PubMed] [Google Scholar]
- O’Connell RJ, Meredith M. Effects of volatile and nonvolatile chemical signals on male sex behaviors mediated by the main and accessory olfactory systems. Behav Neurosci. 1984;98:1083–1093. doi: 10.1037//0735-7044.98.6.1083. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci. 2004;24:9451–9457. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peele P, Salazar I, Mimmack M, Keverne EB, Brennan PA. Low molecular weight constituents of male mouse urine mediate the pregnancy block effect and convey information about the identity of the mating male. Eur J Neurosci. 2003;18:622–628. doi: 10.1046/j.1460-9568.2003.02790.x. [DOI] [PubMed] [Google Scholar]
- Petrulis A, Peng M, Johnston RE. Effects of vomeronasal organ removal on individual odor discrimination, sex-odor preference, and scent marking by female hamsters. Physiol Behav. 1999;66:73–83. doi: 10.1016/s0031-9384(98)00259-5. [DOI] [PubMed] [Google Scholar]
- Pfaff DW. Luteinizing hormone-releasing factor potentiates lordosis behavior in hypophysectomized ovarictomized female rats. Science. 1973;182:1148–1149. doi: 10.1126/science.182.4117.1148. [DOI] [PubMed] [Google Scholar]
- Pfeiffer CA, Johnston RE. Hormonal and behavioral responses of male hamsters to females and female odors: roles of olfaction, the vomeronasal system, and sexual experience. Physiol Behav. 1994;55:129–138. doi: 10.1016/0031-9384(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Powers JB, Fields RB, Winans SS. Olfactory and vomeronasal system participation in male hamsters’ attraction to female vaginal secretions. Physiol Behav. 1979;22:77–84. doi: 10.1016/0031-9384(79)90407-4. [DOI] [PubMed] [Google Scholar]
- Rajendren G, Dudley CA, Moss RL. Role of the vomeronasal organ in the male-induced enhancement of sexual receptivity in female rats. Neuroendocrinology. 1990;52:368–372. doi: 10.1159/000125619. [DOI] [PubMed] [Google Scholar]
- Robertson DHL, Cox KA, Gaskell SJ, Evershed RP, Beynon RJ. Molecular heterogeneity in the major urinary proteins of the house mouse Mus musculus. J Biochem. 1996;316:265–272. doi: 10.1042/bj3160265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, Barfield RJ. Induction of estrous behavior in ovariectomized rats by sequential replacement of estrogen and progesterone to the ventromedial hypothalamus. Neuroendocrinology. 1983;37:218–224. doi: 10.1159/000123546. [DOI] [PubMed] [Google Scholar]
- Saito TR, Moltz H. Sexual behavior in the female rat following removal of the vomeronasal organ. Physiol Behav. 1986;38:81–87. doi: 10.1016/0031-9384(86)90135-6. [DOI] [PubMed] [Google Scholar]
- Sam M, Vora S, Salnic B, Ma W, Novotny MV, Buck LB. Neuropharmacology. Odorants may arouse instinctive behaviors. Nature. 2001;412:142. doi: 10.1038/35084137. [DOI] [PubMed] [Google Scholar]
- Schaefer ML, Yamazaki K, Osada K, Restrepo D, Beauchamp GK. Olfactory fingerprints for major histocompatibility complex-determined body odors II: relationship among odor maps, genetics, odor composition, and behavior. J Neurosci. 2002;22:9513–9521. doi: 10.1523/JNEUROSCI.22-21-09513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer ML, Young DA, Restrepo D. Olfactory fingerprints for major histocompatibility complex-determined body odors. J Neurosci. 2001;21:2481–2487. doi: 10.1523/JNEUROSCI.21-07-02481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PB. Chemosensation and genetic individuality. Reproduction. 2001;121:529–539. doi: 10.1530/rep.0.1210529. [DOI] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Trinh K, Storm DR. Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nat Neurosci. 2003;6:519–525. doi: 10.1038/nn1039. [DOI] [PubMed] [Google Scholar]
- Whitten WK. Occurrence of anoestrus in mice cage in groups. J Endocrinol. 1959;18:102–107. doi: 10.1677/joe.0.0180102. [DOI] [PubMed] [Google Scholar]
- Winans SS, Powers JB. Olfactory and vomeronasal deafferentation of male hamsters: histological and behavioral analyses. Brain Res. 1977;126:325–344. doi: 10.1016/0006-8993(77)90729-6. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Nyby J, Whitney G, Beauchamp GK, Katz Y. The vomeronasal organ: primary role in mouse chemosensory gender recognition. Physiol Behav. 1982;29:315–327. doi: 10.1016/0031-9384(82)90021-x. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Wellington JL, Beauchamp GK. Access of urinary nonvolatiles to the mammalian vomeronasal organ. Science. 1980;207:781–783. doi: 10.1126/science.7352288. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Wysocki LM. Surgical removal of the vomeronasal organ and its verification. In: Speilman AI, Brand JG, editors. Experimental Cell Biology of Taste and Olfaction. CRC Press; New York: 1995. [Google Scholar]
- Wysocki CJ, Yamazaki K, Curran GH, Wysocki LM, Beauchamp GK. Mice (Mus musculus) lacking a vomeronasal organ can discriminate MHC-determined odortypes. Hom Behav. 2004;46:241–246. doi: 10.1016/j.yhbeh.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Yamazaki K, Beauchamp GK, Bard J, Thomas L, Boyse EA. Distinctive urinary odors governed by the major histocompatibility locus of the mouse. Proc Natl Acad Sci USA. 1981;78:5817–5820. doi: 10.1073/pnas.78.9.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Beauchamp GK, Curran M, Bard J, Boyse EA. Parent-progeny recognition as a function of MHC odortype identity. Proc Natl Acad Sci USA. 2000;97:10500–10502. doi: 10.1073/pnas.180320997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Beauchamp GK, Imai Y, Bard J, Boyse EA. Expression of urinary H-2 odortypes by infant mice. Proc Natl Acad Sci USA. 1992;89:2756–2758. doi: 10.1073/pnas.89.7.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]