Abstract
Inhalation anesthetics are effective chemical preconditioning agents in experimental cerebral ischemia. However, previous work has been performed exclusively in male animals. We determined if there is a gender difference in ischemic outcome after isoflurane preconditioning (IsoPC), and if this sex-specific response is linked to differences in Akt phosphorylation or expression of neuronal inducible cell-death putative kinase (NIPK), a negative modulator of Akt activation. Young and middle-aged male and female mice were preconditioned for 4 h with air (sham PC) or 1.0% IsoPC and recovered for 24 h. Cortices were subdissected from preconditioned young male and female mice for measurement of Akt phosphorylation (Western blot) and NIPK mRNA (quantitative polymerase chain reaction). Additional cohorts underwent 2 h of reversible middle cerebral artery occlusion. Lastly, male and female Akt1+/+ and Akt1−/− mice were studied to determine if gender differences in ischemic outcome after IsoPC is Akt1-dependent. Infarction volume was determined at 22 h reperfusion (2,3,5-triphenyltetrazolium chloride). As expected, IsoPC decreased ischemic damage as compared with sham PC in young and middle-aged male mice. In contrast, IsoPC markedly increased infarction in young female mice and had no effect in middle-aged female mice. Cortical phospho-Akt was increased by IsoPC versus sham PC only in male mice. No increase was observed in IsoPC female mice. NIPK mRNA was higher in female mice than in male mice regardless of preconditioning status. Male IsoPC neuroprotection was lost in Akt1-deficient male mice. We conclude that IsoPC is beneficial only in ischemic male brain and that sex differences in IsoPC are mediated through Akt activation and basal NIPK expression.
Keywords: Akt, focal ischemia, gender, neuroprotection, preconditioning
Introduction
Perioperative stroke remains a devastating procedural complication after cardiovascular surgical procedures like carotid endarterectomy or coronary artery bypass grafting (Kelley, 2001). Women, however, may have a greater perioperative stroke risk than men for these procedures (Bond et al, 2005; Koch et al, 2004; Ozatik et al, 2005; Weise et al, 2004). Anesthetic selection is an important consideration for at risk patients since agents like isoflurane are known to affect experimental ischemic outcome (for a review, see Kitano et al, 2006). Anesthetic preconditioning may therefore prevent or delay neurologic complications from perioperative stroke. Experimental brain studies examining anesthetic preconditioning and its neuroprotective mechanisms have utilized primarily male animals, leaving open the question of the effect of gender and the role of sex steroids.
Akt (protein kinase B) activation is a key neuronal survival-signaling pathway that is triggered during brain development and neurodegenerative diseases and controls the balance between survival and death signals in brain (Brunet et al, 2001; Dudek et al, 1997). Akt activation via phosphorylation is increased after cerebral ischemia (Friguls et al, 2001; Kitagawa et al, 1999; Ouyang et al, 1999; Noshita et al, 2001; Shibata et al, 2002) and appears to play an important role in mediating ischemic-induced tolerance in brain (Garcia et al, 2004; Hashiguchi et al, 2004; Nakajima et al, 2004; Yano et al, 2001). Three Akt isoforms (Akt1, Akt2 and Akt3) have been described, with Akt1 and Akt3 being more prevalent in brain (Yang et al, 2004). Only the Akt1 isoform has been examined in cerebral ischemia (Janelidze et al, 2001). Neuronal cell death-inducible putative kinase (NIPK) has been reported to block Akt activation by binding directly to the kinase, suggesting that NIPK is a negative modulator of Akt (Du et al, 2003; Koo et al, 2004).
Using a mouse isoflurane preconditioning (IsoPC) model, we determined if IsoPC neuroprotection is gender-specific in young and middle-aged mouse ischemic brain. This study also examined whether IsoPC alters NIPK expression and consequent Akt activation and how gender modifies this response in IsoPC brain. We chose to focus on effects owing to preconditioning rather than effects that occur in response to preconditioning and ischemia, our premise being that ischemic sensitivity is already determined by the time the preconditioned animal undergoes cerebral ischemia. We also investigated whether the gender-specific response to IsoPC in ischemic brain is Akt1-dependent.
Materials and methods
Animal Model
The Oregon Health and Science University Animal Care and Use Committee approved all experiments. Young (8 to 14 weeks of age, 20 to 25 g, n = 108) and middle-aged (35 to 40 weeks of age, 26 to 39 g; n = 59) male and female C57BL/6 mice (Charles River Laboratories, Wilmington, MA, USA) were used for infarct volume, cortical Akt activation, and NIPK expression determinations. Infarct volumes were also determined in male (8 to 18 weeks of age, 20 to 27 g) and female (11 to 23 weeks of age, 17 to 25 g) Akt1 wild-type (WT) (n = 42) and Akt1-deficient (n = 39) mice.
Isoflurane Preconditioning Model
Femoral arterial catheters were placed in a separate group of male mice (n = 5) to determine mean arterial blood pressure, blood gases (pH, PaO2, PaCO2), and blood glucose values while delivering 1% isoflurane in oxygen-enriched air with a nonrebreathing system and a sealed face mask (Summit Medical Equipment Company, Bend, OR, USA). Body temperature was controlled as needed using a warming blanket. The effect of 1% IsoPC on systemic variables was evaluated after 1 and 4 h of preconditioning. Mice were then euthanized at the end of the 4 h preconditioning period. For survival preconditioning experiments, mice were placed for 4 h in an air-tight, temperature-controlled chamber flushed with 1.0% isoflurane (IsoPC) or no isoflurane (sham preconditioning or sham PC) in oxygen-enriched air. Twenty-four hours after preconditioning, mice subsequently underwent either transient focal cerebral ischemia (n = 172), or concurrent Western blot analysis for Akt activation (n = 48) and quantitative polymerase chain reaction (qPCR) for NIPK expression (n = 28).
Transient Focal Cerebral Ischemia
Preconditioned (sham PC, IsoPC) young and middle-aged male and female C57BL/6 mice were subjected to 2 h of reversible middle cerebral artery occlusion (MCAO) via the intraluminal filament technique as previously described (Ardelt et al, 2005). Mice were anesthetized with isoflurane (induction 4%; maintenance 1.5%) during surgery until left-sided MCAO was achieved and then allowed to awaken during ischemia. Towards the end of ischemia, mice were briefly reanesthetized with isoflurane and reperfusion was initiated by intraluminal filament withdrawal. Rectal temperatures were monitored throughout MCAO surgery and during manipulations to induce reperfusion. Anesthetized mice were warmed as needed using a warming blanket. Cortical blood flow was monitored throughout MCAO surgery, initial induction of MCAO, end-ischemia, and initiation of reperfusion by laser Doppler flowmetry (Moor Instruments Ltd, model MBF3D, Oxford, England) to confirm occlusion and reperfusion. Neurologic deficit scores were determined during ischemia and at 22 h reperfusion to confirm ischemia and the presence of ischemic injury, respectively, according to the following graded scoring system: 0, no deficit; 1, forelimb weakness, and torso turning to the ipsilateral side when held by tail; 2, circling to the affected side; 3, unable to bear weight on the affected side; and 4, no spontaneous locomotor activity or barrel rolling. Any animal without a deficit was excluded from the study. After 22 h of reperfusion, animals were euthanized, brains removed, and blood obtained for hormone analysis. Tissue infarction was identified by the lack of 2,3,5-triphenyltetrazolium chloride (Sigma, St Louis) staining in 2-mm thick coronal sections (5 slices total) (Bederson et al, 1986). Both sides of each stained coronal slice were photographed via a digital camera, then evaluated by digital image analysis (SigmaScan Pro, Jandel, San Rafael, CA, USA). Infarcted area was integrated across sections, and volume expressed as % of contralateral structure (cortex—CTX, caudate-putamen—CP). Plasma hormone levels (estradiol, progesterone, total testosterone) were measured in duplicate by radioimmunoassay (RIA) (Diagnostic Products Corp, Los Angeles, CA, USA). The lower limit of detection for the estradiol RIA was 10 pg/mL. Samples with estradiol levels < 10 pg/mL were not used in calculating mean estradiol levels per treatment group.
Western Blot Analysis for Akt Activation
For Akt activation determinations, we modified a previously described Western blot protocol (Kako et al, 1988; Ouyang et al, 1999). Twenty-four hours after preconditioning (sham PC, IsoPC), young male and female C57BL/6 mice were euthanized. Cortices were subdissected from the left hemisphere of preconditioned mouse brains (n = 12 per experimental group, total = 48), pooled together from three mice (n = 4 pooled samples per experimental group), and frozen at −80°C. Pooled cortices (approximately 100mg for each pooled sample) were homogenized in cold homogenizing buffer (250 mmol/L sucrose, 60 mmol/L KCl, 15 mmol/L Tris-HCl (pH 7.9), 15 mmol/L NaCl, 5 mmol/L EDTA, 1 mmol/L EGTA), 1 mmol/L DL-Dithiothreitol, 0.5 mmol/L phenylmethanesulfonyl fluoride containing protease (Complete mini EDTA-Free protease inhibitor cocktail; Roche Applied Science Inc., Basel, Switzerland) and phosphatase inhibitors (Sigma-Aldrich, Saint Louis, MO, USA). Cytosolic subcellular fractions were isolated by centrifugation at 2000g, followed by 17,000g at 4°C. Protein concentrations were determined via BCA assay (Pierce Biotechnology, Rockford, IL, USA). Cytosolic proteins were separated out on a 4% to 12% gradient NUPAGE Bis-Tris gel (Invitrogen Corp., Carlsbad, CA, USA) and then transferred to a polyvinylidene diflouride membrane. Membranes were blocked (1 h at room temperature) using a 5% non-fat dry milk/1X phosphate-buffered saline/0.1% Tween-20 solution, then probed with polyclonal anti-phospho-Akt (1:1000 dilution) and anti-Akt (1:2000 dilution) antibodies diluted in blocking solution overnight at 4°C (Cell Signaling Technology, Danvers, MA, USA), followed by horseradish peroxide-linked species-specific secondary antibodies (1:2000 dilution) for 1 h at room temperature (GE Healthcare, Piscataway, NJ, USA). Anti-phospho- Akt antibodies recognize endogenous levels of Akt phosphorylation at the serine-473 site only, while anti-Akt antibodies recognize all Akt endogenous levels present. Chemiluminescent detection reagents (ECL Plus, GE Healthcare Piscataway, NJ, USA) were used along with Kodak X-Omat Blue XB-1 X-ray film (Eastman Kodak Company, Rochester, NY, USA) to visualize protein bands. Blots were quantitatively analyzed using Quantity One image analysis software (Biorad Laboratories, Hercules, CA, USA). All bands were normalized to sham PC male hemisphere, and to total Akt (t-Akt) as a loading control. Akt activation is expressed as the ratio of normalized phosphorylated Akt (p-Akt) to normalized t-Akt.
Quantitative Polymerase Chain Reaction for Neuronal Inducible Cell-Death Putative Kinase Expression
Techniques are as previously described (Stayrook et al, 2005). Twenty-four hours after preconditioning (sham PC, IsoPC), young male and female C57BL/6 mice were euthanized. Cortices were subdissected from the left hemisphere of preconditioned mouse brains (n = 7 per treatment group, total = 28), frozen in 2-methylbutane at −30°C, and stored at −80°C until analyzed. For TaqMan qPCR measurement of NIPK mRNA, total RNA was isolated using the RNeasy Mini kit (Qiagen, Valencia, CA, USA) and further treated with RNase-free DNase set (Qiagen, Valencia, CA, USA) following the manufacturer’s instructions. First-strand cDNA was reverse transcribed from 500 ng total RNA using the high capacity cDNA archive kit (Applied Biosystems, Foster City, CA, USA) and was diluted 1:2 in water for TaqMan analysis. cDNA (50 ng) was used for qPCR in a 96-well plate using a 50 μL total volume. Each TaqMan reaction was performed in triplicate. Specific probe sets for NIPK were obtained from Applied Biosystems as assays on demand (Catalogue no. Mm00454879_m1 for mouse NIPK), and reactions were performed after the manufacturer’s guidelines. NIPK expression is expressed as the ratio of NIPK mRNA to 18S mRNA (18S Genomic Endogenous Control Kit; Eurogentec, North America, San Diego, CA, USA).
Akt1-Deficient Mice
Generation of Akt1-targeted mice is as previously described (Cho et al, 2001). This strain originated on a B6;129 background and has been backcrossed to C57BL/6 for at least 10 generations. Akt1+/− (heterozygote) breeder pairs were obtained from Dr Morris J Birnbaum, Department of Biology, University of Pennsylvania. Breeding colony was maintained utilizing Akt1+/− breeding harems (2 females, 1 male). Akt1−/− (knockout (KO)) mice do not occur with the expected Mendelian frequency because of significant Akt1−/− pup loss in the early neonatal period (Cho et al, 2001). As previously described (Cho et al, 2001), animals were genotyped by PCR using the following primers in a single reaction: 851, 5′-AGATCTTCTTCCACCTGTCTC-3′; 852, 5′-GCTCCATAAGCACACCTTCAGG-3′; and 853, 5′-GTGGATGTGGAATGTGTGCGAG-3′. The expected sizes of the PCR products are 148 base pairs for Akt1+/+ (WT), 260 base pairs for Akt1−/−, and the presence of both PCR products for Akt1+/−. Every set of PCR reactions contained a negative control (no DNA) and a positive control (heterozygous DNA). Akt1−/− mice are distinguishable from WT mice because of their smaller size after weaning and throughout adulthood (Cho et al, 2001). Male and female Akt1+/+ and Akt1−/− mice underwent the same preconditioning (sham PC, IsoPC) and ischemia protocols as described above for young and middle-aged male and female C57BL/6 mice.
Statistics
Values expressed as mean±s.e.m. Differences in infarction volumes, plasma hormone levels, Akt activation (p-Akt/t-Akt), and NIPK mRNA expression in IsoPC groups as compared with appropriate sham PC groups were determined with a student t-test. Differences in hormone levels between young and middle-aged male and female mice and between male and female Akt1 WT and KO mice regardless of preconditioning status was also determined with a Student’s t-test. Laser Doppler flowmetry (% baseline), neurologic deficit scores, and rectal temperatures were subjected to two-way ANOVA with post hoc Newman–Keuls test. Statistical significance was P < 0.05. All statistical analyses were performed using SigmaStat Statistical Software, Version 2.0 (SPSS, Inc., Chicago, IL, USA).
Results
Physiologic systemic variables evaluated after 1 and 4 h of IsoPC (Table 1) showed no major perturbations imposed by IsoPC. Mice were mildly hypotensive but glucose, pH, and PaCO2 fell within reported reference ranges for mice (Harkness and Wagner, 1995; Jacoby et al, 2002). PaO2 values were consistent with animals exposed to oxygen-enriched air. Mean laser Doppler flowmetry (% baseline) at induction of MCAO, at end-ischemia, and at initiation of reperfusion was comparable among young and middle-aged male and female mice regardless of preconditioning status (data not shown). Neurologic deficit scores and rectal temperatures during and after MCAO were equivalent between young and middle-aged male and female mice preconditioning groups (Table 2). No mortality occurred in young male, young female, and middle-aged female sham PC or IsoPC mice used for infarct volume, cortical Akt activation, and NIPK expression determinations. One middle-aged female sham PC mouse was excluded because of pregnancy. Mortality in middle-aged male sham PC and IsoPC mice was 21% (three out of 14 mice) and 31% (five out 16 mice), respectively.
Table 1.
Physiological parameters in male mice (n=5) after 1 and 4 h of 1% isoflurane preconditioning (IsoPC)
| Parameter | 1 h IsoPC | 4 h IsoPC |
|---|---|---|
| MABP (mm Hg) | 7±71 | 71±1 |
| Glucose (mg/dL) | 169±14 | 100±23 |
| pH | 7.40±0.01 | 7.32±0.02 |
| PaO2 (mm Hg) | 139±4 | 140±11 |
| PaCO2 (mm Hg) | 32±3 | 36±2 |
MABP, mean arterial blood pressure.
Table 2.
Neurologic deficit scores and rectal temperatures during experimental focal cerebral ischemia in preconditioned young and middle-aged male and female C57BL/6 mice
| Group | Neurologic deficit scores
|
Rectal temperatures (°C)
|
|||||
|---|---|---|---|---|---|---|---|
| End-ischemia | Reperfusion 22 h | Pre-MCAO | 5 mins | MCAO 110 mins | 115 mins | Reperfusion 5 mins | |
| Sham PC YM (n = 7) | 1.7±0.2 | 1.4±0.3 | 37.0±0.2 | 37.2±0.1 | 35.7±0.2* | 36.7±0.2* | 37.1±0.1 |
| IsoPC YM (n = 7) | 1.6±0.3 | 1.7±0.4 | 37.0±0.1 | 37.3±0.1 | 36.0±0.2* | 36.5±0.2* | 37.0±0.1 |
| Sham PC YF (n = 9) | 1.8±0.2 | 1.6±0.2 | 37.1±0.2 | 37.2±0.1 | 35.7±0.2* | 36.6±0.2* | 37.2±0.1 |
| IsoPC YF (n = 9) | 1.6±0.2 | 1.6±0.3 | 37.0±0.1 | 37.1±0.1 | 35.7±0.1* | 36.6±0.2* | 37.1±0.1 |
| Sham PC MM (n = 11) | 1.9±0.2 | 1.7±0.1 | 37.5±0.1 | 37.3±0.1 | 34.9±0.1* | 37.2±0.1 | 37.6±0.1 |
| IsoPC MM (n = 11) | 1.7±0.1 | 1.4±0.3 | 37.5±0.2 | 37.5±0.1 | 34.9±0.1* | 37.2±0.2 | 37.7±0.1 |
| Sham PC MF (n = 13) | 1.8±0.1 | 1.5±0.2 | 37.4±0.1 | 37.5±0.1 | 35.0±0.1* | 37.3±0.1 | 37.6±0.2 |
| IsoPC MF (n = 15) | 1.8±0.1 | 1.3±0.1# | 37.2±0.1 | 37.3±0.1 | 35.0±0.1* | 37.2±0.1 | 37.5±0.1 |
IsoPC, isoflurane preconditioning; MF, middle-aged female; MM, middle-aged male; MCAO, middle cerebral artery occlusion; sham PC, sham preconditioning; YF, young female; YM, young male.
P<0.05 as compared to pre-MCAO.
P<0.05 as compared to end-ischemia.
There were no differences in total testosterone levels between IsoPC and the appropriate corresponding sham PC for young and middle-aged male mice (Table 3). However, regardless of preconditioning status, young male mice (4.8±1.2 ng/mL, n = 28) had significantly higher total testosterone levels than middle-aged male mice (0.33±0.02, n = 22). There were also no differences in estradiol and progesterone levels between IsoPC and the appropriate corresponding sham PC for young and middle-aged female mice (Table 3). However, young female mice (23±1 pg/mL, n = 56) had significantly higher estradiol levels than middle-aged female mice (18±2 pg/mL, n = 23) regardless of preconditioning status. There were two samples from young female mices (IsoPC only) versus five samples from middle-aged female mice (sham PC, n = 3; IsoPC, n = 2) that were below < 10 pg/mL. There were no differences in progesterone levels between young (8±1 ng/mL, n = 58) and middle-aged (8±1 ng/mL, n = 28) female mice regardless of preconditioning status.
Table 3.
Plasma hormone levels for young and middle-aged male and female C57BL/6 mice
| Treatment group | Testosterone (ng/mL) | Estradiol (pg/mL) | Progesterone (ng/mL) |
|---|---|---|---|
| Sham PC YM (n = 14) | 5.93±1.98 | ND | ND |
| IsoPC YM (n = 14) | 3.64±1.59 | ND | ND |
| Sham PC MM (n = 11) | 0.33±0.03 | ND | ND |
| IsoPC MM (n = 11) | 0.33±0.02 | ND | ND |
| Sham PC YF | ND | 24±1 (n = 29) | 9±1 (n = 29) |
| IsoPC YF | ND | 22±1 (n = 27) | 7±1 (n = 29) |
| Sham PC MF | ND | 14±1 (n = 10) | 7±1 (n = 13) |
| IsoPC MF | ND | 21±4 (n = 13) | 8±2 (n = 15) |
IsoPC, isoflurane preconditioning; MF, middle-aged female; MM, middle-aged male; ND, not determined; sham PC, sham preconditioning; YF, young female; YM, young male.
Young IsoPC male mice had significantly reduced cortical (23%±7%) and striatal (58%±7%) infarct volumes when compared with young sham PC male mice (CTX, 57%±5%; CP, 94%±7%) (Figure 1A). However, infarction volumes in young IsoPC female mice (CTX, 48%±5%; CP 86%±3%) were significantly increased as compared with sham PC female mice (CTX, 21%±7%; CP, 61%±4%) (Figure 1A). Significant decreases in infarct volumes were seen in IsoPC middle-aged male mice (CTX, 12%±5%; CP, 59%±8%) as compared with sham PC middle-aged male mice (CTX, 52%±5%; CP, 88%±5%) (Figure 1B). No significant differences in infarct volumes were seen between IsoPC middle-aged female mice (CTX, 28%±8%; CP, 74%±6%) and sham PC middle-aged female mice (CTX, 27%±7%; CP, 64%±8%) (Figure 1B).
Figure 1.

2,3,5-triphenyltetrazolium chloride-determined cortical and caudate-putamen infarction volumes (% contralateral structure) in (A) young male (YM) and female (YF) (8 to 14 weeks of age) and (B) middle-aged male (MM) and female (MF) (35 to 40 weeks of age) C57BL/6 mice preconditioned for 4 h with air (sham PC) or 1% isoflurane (IsoPC). Preconditioning occurred 24 h before 2 h of MCAO followed by 22 h reperfusion. Values are mean±s.e.m. *P<0.05.
At 24 h after the preconditioning period, cortical Akt activation was significantly increased by IsoPC in male mice but unaffected by IsoPC in female mice as compared with corresponding sham PC mice (Figure 2). No significant differences in cortical NIPK expression (NIPK mRNA/18S mRNA) were seen between IsoPC (3.8±0.2) and sham PC (3.8± 0.2) male mice. There were also no differences in cortical NIPK expression between IsoPC (4.8±0.4) and sham PC (4.8±0.2) female mice. However, sham PC and IsoPC female mice had higher levels of cortical NIPK expression as compared with sham PC and IsoPC male mice, respectively (P < 0.05).
Figure 2.
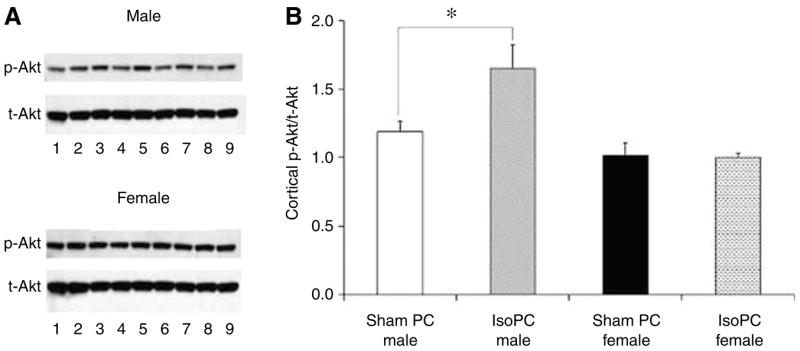
Cortical Akt activation was evaluated in male and female C57BL/6 mice preconditioned for 4 h with air (sham PC) or 1% isoflurane (IsoPC). Preconditioning occurred 24 h before Akt activation determination via Western blot. Cortices were subdissected from the left hemisphere of preconditioned mouse brains (n=12 per experimental group) and pooled together from three mice (n=4 pooled samples per experimental group). (A) Western blots for phosphorylated Akt (p-Akt) and total Akt (t-Akt) from male and female preconditioned C57BL/6 mice. Lane 1, internal control (sham PC male hemisphere); lanes 2, 4, 6, 8, pooled sham PC cortical samples; lanes 3, 5, 7, 9, pooled IsoPC cortical samples. All bands were normalized to sham PC male hemisphere, and to t-Akt as a loading control. (B) Cortical Akt activation expressed as the ratio of normalized p-Akt to t-Akt in male and female preconditioned C57BL/6 mice. Values are mean±s.e.m. *P<0.05.
Mean laser Doppler flowmetry (% baseline) at induction of MCAO, at end-ischemia, and at initiation of reperfusion was comparable between male and female Akt1 WT and Akt1-deficient mice regardless of preconditioning status (data not shown). Neurologic deficit scores and rectal temperatures during MCAO were equivalent between male and female Akt1 WT and Akt1-deficient mice preconditioning groups (Table 4). Pre-ischemic rectal temperatures were lower in female Akt1 WT mice regardless of preconditioning group as compared with female Akt1-deficient sham PC mice (Table 4). Rectal temperature during initial reperfusion was higher in female Akt1-deficient sham PC KO mice as compare to other female Akt1 WT and KO preconditioning groups (Table 4). No mortality occurred in male and female Akt1 WTand Akt1-deficient mice.
Table 4.
Neurologic deficit scores and rectal temperatures during experimental focal cerebral ischemia in preconditioned male and female Akt1 wild-type and Akt1-deficient mice
| Group | Neurologic deficit scores
|
Rectal temperatures (°C)
|
|||||
|---|---|---|---|---|---|---|---|
| End-ischemia | Reperfusion 22 h | Pre-MCAO | 5 mins | MCAO 110 mins | 115 mins | Reperfusion 5 mins | |
| Akt1 males | |||||||
| Sham PC WT (n = 11) | 1.5±0.2 | 1.5±0.2 | 37.1±0.1 | 37.5±0.1 | 35.5±0.2* | 36.9±0.2 | 37.3±0.1 |
| IsoPC WT (n = 11) | 1.5±0.2 | 1.5±0.2 | 37.2±0.2 | 37.3±0.1 | 35.3±0.2* | 37.0±0.1 | 37.7±0.2 |
| Sham PC KO (n = 10) | 1.9±0.1 | 1.6±0.2 | 37.2±0.1 | 37.3±0.2 | 35.2±0.1* | 37.0±0.2 | 37.7±0.1 |
| IsoPC KO (n = 11) | 1.8±0.1 | 1.6±0.2 | 36.9±0.3 | 37.4±0.2 | 35.5±0.2* | 36.9±0.2 | 37.3±0.2 |
| Akt1 females | |||||||
| Sham PC WT (n = 11) | 1.6±0.2 | 1.6±0.3 | 36.7±0.2$ | 37.1±0.1 | 35.6±0.2* | 36.6±0.1 | 37.0±0.1 |
| IsoPC WT (n = 9) | 1.9±0.2 | 2.2±0.2# | 36.5±0.1$ | 37.0±0.1 | 35.5±0.2* | 36.8±0.1 | 37.1±0.1 |
| Sham PC KO (n = 9) | 1.9±0.2 | 1.4±0.2 | 37.3±0.2 | 37.4±0.2 | 35.1±0.2* | 37.1±0.3 | 37.8±0.2# |
| IsoPC KO (n = 9) | 1.6±0.2 | 1.4±0.2 | 37.0±0.2 | 37.3±0.2 | 35.1±0.1* | 36.8±0.2 | 37.3±0.2 |
IsoPC, isoflurane preconditioning; KO, knockout; MCAO, middle cerebral artery occlusion; sham PC, sham preconditioning; WT, wild-type.
P<0.05 as compared to pre-MCAO.
P<0.05 as compared to other Akt1 female experimental groups.
P<0.05 compared to Akt1 female sham PC KO.
There were no differences in total testosterone levels between IsoPC and the appropriate corresponding sham PC for male Akt1 WT and Akt1-deficient mice (Table 5) as well as between male Akt1 WT (0.30±0.02, n = 22) and Akt1-deficient (0.64±0.31, n = 20) mice regardless of preconditioning group. There were no differences in estradiol and progesterone levels between IsoPC and the appropriate corresponding sham PC for female Akt1 WT and Akt1-deficient mice (Table 5). Regardless of preconditioning group, there were also no differences in estradiol and progesterone levels between female Akt1 WT (estradiol 21±3 pg/mL, n = 16; progesterone 9±1 ng/mL, n = 20) and Akt1-deficient (estradiol 23±5 pg/mL, n = 14; progesterone 7±2 ng/mL, n = 16) mice. There were four samples from female Akt1 WT mice (sham PC, n = 2; IsoPC, n = 2)) versus 2 samples from female Akt1-deficient mice (sham PC, n = 1; IsoPC, n=1) that were below < 10 pg/mL.
Table 5.
Plasma hormone levels for male and female Akt1 wild-type and knockout mice
| Treatment group | Testosterone (ng/mL) | Estradiol (pg/mL) | Progesterone (ng/mL) |
|---|---|---|---|
| Akt1 males | |||
| Sham PC WT (n = 11) | 0.34±0.04 | ND | ND |
| IsoPC WT (n = 11) | 0.26±0.02 | ND | ND |
| Sham PC KO (n = 10) | 1.02±0.64 | ND | ND |
| IsoPC KO (n = 10) | 0.26±0.05 | ND | ND |
| Akt1 females | |||
| Sham PC WT | ND | 21±5 (n = 9) | 9±2 (n = 11) |
| IsoPC WT | ND | 21±6 (n = 7) | 10±3 (n = 9) |
| Sham PC KO | ND | 15±2 (n = 6) | 7±1 (n = 7) |
| IsoPC KO | ND | 29±8 (n = 8) | 7±1 (n = 9) |
IsoPC, isoflurane preconditioning; KO, knockout; ND, not determined; sham PC, sham preconditioning; WT, wild-type.
IsoPC significantly reduced cortical (39%±7%) and striatal (79%±3%) infarct volumes in Akt1 WT male mice as compared with sham PC Akt1 WT male mice (CTX, 55%±3%; CP, 97%±4%) but had no significant effect on infarct volumes in Akt1-deficient male mice (CTX, 33%±5%; CP, 81%±3%) as compared with sham PC Akt1-deficient male mice (CTX, 26%±5%; CP, 78%±7%) (Figure 3A). In both Akt1 WT and Akt1 KO female mice, IsoPC significantly increased cortical (WT, 48%±6%; KO, 37%±5%) and striatal (WT, 96%±5%; KO, 86%±4%) infarct volumes as compared with corresponding sham PC cortical (WT, 30%±6%; KO, 21%±5%) and striatal (WT, 69%±4%, KO, 61%±4%) infarct volumes (Figure 3B).
Figure 3.

2,3,5-triphenyltetrazolium chloride-determined cortical and caudate-putamen infarction volumes (% contralateral structure) in (A) male and (B) female Akt1 WT and Akt1 KO mice preconditioned for 4 h with air (sham PC) or 1% isoflurane (IsoPC). Preconditioning occurred 24 h before 2 h of MCAO followed by 22 h reperfusion. Values are mean±s.e.m. *P<0.05.
Discussion
The present study demonstrates several important findings. First, IsoPC neuroprotection is male-specific with the protective benefits of IsoPC being lost in female mice. Second, the sex-specific responses to IsoPC are mediated through differences in Akt activation and basal NIPK expression. Last, male-specific IsoPC neuroprotection is Akt1-dependent. These findings suggest that there is a divergence in IsoPC-induced neuroprotective mechanisms in male versus female ischemia.
This is the first report of a gender-specific response to anesthetic preconditioning in experimental cerebral ischemia. Limited studies in brain also suggest that the reaction to other forms of preconditioning may not be universal between the sexes. For example, two studies examining hypoxic tolerance of hippocampal slices from mice chemically preconditioned with 3-nitro-propionate concluded that hypoxic tolerance and preconditioning are gender-dependent and modulated during the estrous cycle by gender-specific mechanisms (Kasischke et al, 1999; Von Arnim et al, 2002). These observations challenge the assumption that the general response to preconditioning is similar in brains of either sex.
Outcomes in ischemic brains preconditioned with inhalational anesthetics can be variable (for a review, see Kitano et al, 2006). Such variability may be due to strain- or species-specific effects, choice of inhalational anesthetic agent, and differences amongst ischemic models (e.g. focal versus global ischemia) (Kitano et al, 2006). Therefore, these factors may have also influenced our study results. For example, in diffuse traumatic brain injury, female rats have better outcomes than do male rats but the observation is dependent on the type of anesthesia (O’Connor et al, 2003). Future studies are needed to determine if gender-specific responses to volatile anesthetics as preconditioning agents in ischemic brain are universally applicable under a variety of experimental scenarios.
Sex differences in the pharmacokinetics and pharmacodynamics of anesthetics are well recognized (Pleym et al, 2003). However, sex-based differences in anesthetic requirements and responses could be due to a number of sex-dependent and sex-specific effects. Sex-dependent effects include body weight, height, basal metabolic rate, body fat, and muscle mass whereas sex-specific effects are focused on receptor responses, cyclical variation, neurotransmitter differences, cytochrome enzyme changes, and sex hormone induced events (Ciccone and Holdcroft, 1999). In this study, we focused on sex-specific effects of IsoPC in ischemic brain involving Akt activation and NIPK expression as well as the role of Akt1 in IsoPC neuroprotection.
Because experimental studies examining anesthetic preconditioning protection in brain and its mechanisms have utilized predominantly young male animals (for a review, see Kitano et al, 2006), we examined the effects of gender on IsoPC in ischemic brain injury in young and middle-aged mice. Comparable to observations made by other investigators in young male rodents (for a review, see Kitano et al, 2006), IsoPC significantly reduced infarct size in young male mice when compared with sham PC male mice. We have demonstrated for the first time that IsoPC neuroprotection also occurs in middle-aged male mice. IsoPC in middle-aged female mice had no effect on infarction volume. However, we observed a surprisingly unique response of young female mice to IsoPC in which the infarction volume in IsoPC young female mice was significantly enhanced as compared with sham PC female mice. These results suggest that IsoPC neuroprotection is male-specific in young and middle-aged male mice and that in female mice, IsoPC is not protective and can potentially exacerbate ischemic damage in younger female mice.
Differences in the ischemic response to IsoPC in young versus middle-aged female mice could be explained by differences in age. It is not known whether mechanisms of ischemic injury and neuroprotection are similar in young adult versus middle-aged preconditioned rodents of either gender. Outcome differences may also be a consequence of varying plasma estradiol levels in middle-aged versus young mice. Very few groups report hormone levels for middle-aged rodents. In this study, we did see that young female mice had significantly higher estradiol levels than middle-aged female mice. These observations suggest that ischemic outcome in IsoPC female mice may be linked to estradiol level as well as differential effects linked to age. Future studies from our laboratories will explore the role of estradiol in the gender-specific response to IsoPC.
The results of our study would suggest that female gender does not offer any advantages in IsoPC ischemic brain. This is in contrast to the benefits of female gender and of estrogen in ischemic injury in unconditioned rodent brain (for a review, see Murphy et al, 2004). The overall neuroprotective results described in these experimental animal studies with estrogen in unconditioned ischemic brain have been reflected in some of the earlier clinical trials (for a review, see Langer, 2002), but the results of more recent clinical studies have raised the issue that there are unanticipated and paradoxical effects of estrogen as it is currently administered in women (for a review, see Murphy et al, 2004). Several more recent experimental studies would also suggest that estrogen might not be universally neuroprotective in cerebral ischemia in unconditioned brain (Bingham et al, 2005; Carswell et al, 2004; Gordon et al, 2005; Santizo et al, 2002).
To our knowledge, we are one of the first laboratories to examine Akt activation in IsoPC female brain and NIPK expression in preconditioned and/or ischemic male and female brain. We hypothesized that increases in Akt activation during the postconditioning period before MCAO reduces an animal’s ischemic sensitivity. Our results would suggest that IsoPC alone enhances Akt activation in male mice but does not alter Akt activation in female mice at 24 h after preconditioning. Current literature suggests that estrogen enhances Akt signaling in brain (Cardona-Gomez et al, 2003; Ivanova et al, 2002; Singh, 2001; Znamensky et al, 2003) but its effects on Akt activation may be different in injured brain (Silasi et al, 2004). As mentioned previously, NIPK is thought to be a negative modulator of Akt (Du et al, 2003; Koo et al, 2004). Recently, NIPK was identified as an estrogen responsive gene by micro-array analysis (Ise et al, 2005, Terasaka et al, 2004), suggesting that the presence or absence of estrogen could alter NIPK levels, thus providing a novel transcriptional mechanism through which estrogen could alter Akt activation in IsoPC female brain. Although IsoPC had no effect on NIPK expression 24 h after preconditioning as compared with sham PC mice regardless of gender, we did see overall higher levels of NIPK expression in female cortex as compared with males. Our findings would imply that IsoPC-induced Akt activation is blocked in female mice, possibly because of higher baseline NIPK levels.
Several laboratories have shown that other forms of preconditioning enhance Akt activation in male animals, leading to neuroprotection from stroke (Garcia et al, 2004; Hashiguchi et al, 2004; Nakajima et al, 2004; Yano et al, 2001). However, the majority of these studies have focused on the period after ischemia rather than the postconditioning period preceding ischemia. This would suggest along with our results that in male mice, preconditioning induces Akt activation before as well as after ischemia. Conversely, in female mice, this protective pathway may not be recruited in response to preconditioning at least before an ischemic insult and therefore may not be relevant to female ischemic outcome.
After observing a gender difference in cortical Akt activation to IsoPC, we were interested in examining the role of Akt1 in the response to IsoPC in ischemic brain. Previous work has shown that Akt1 activation is suppressed after transient focal cerebral ischemia, suggesting that Akt1 may be important in attenuating neuronal cell death after an ischemic insult (Janelidze et al, 2001). We were able to utilize Akt1 WT and KO male and female mice to determine what role Akt1 plays in the gender-specific response to IsoPC in ischemic brain. Our results suggest that male-specific IsoPC neuroprotection is dependent on the presence of Akt1, but its absence does not change the exacerbated ischemic injury seen in IsoPC female mice.
While the current literature suggests that Akt activation is neuroprotective in experimental cerebral ischemia in unconditioned brain (Friguls et al, 2001; Kitagawa et al, 1999; Ouyang et al, 1999; Noshita et al, 2001; Shibata et al, 2002), we unexpectedly observed that sham PC Akt1 WT male mice had significantly larger cortical (55%±3%) and striatal (97%±4%) infarct volumes than sham PC Akt1 KO male mice (CTX, 26%±5%; CP, 78%± 7%). We also saw no significant change in infarct volumes between sham PC Akt1 WT (CTX, 30%± 6%; CP, 69%±4%) and KO (CTX, 21%±5%; CP, 61%±4%) female mice. As mentioned previously, there are three Akt isoforms (Yang et al, 2004), but isoform-specific Akt activation in cerebral ischemia alone has been understudied. Only one study in male Wistar rats has suggested that Akt1 may be important to neuronal survival after transient focal cerebral ischemia (Janelidze et al, 2001). It may be that the other Akt isoforms play a more critical role in neuroprotection from ischemia than Akt1.
The observation that IsoPC is effective only in ischemic male brain and actually exacerbates ischemic injury in young female brain would suggest that there is a deviation in neuroprotective mechanisms involving Akt and NIPK in male versus female ischemia in IsoPC brain. Future studies are needed to examine the role of sex steroids like estrogen and androgens in the response to IsoPC in ischemic brain and their possible influence on Akt and NIPK. One potential clinical implication of our findings is that the anesthesia chosen during an ‘at-risk’ cardiovascular surgical procedure may alter perioperative stroke outcome in a gender-specific manner.
Acknowledgments
The authors thank Dr Morris J Birnbaum, Department of Biology, University of Pennsylvania, for providing Akt1+/− (heterozygous) breeder pairs. The authors also thank Ms Robin Feidelson for her technical expertise and assistance with all manuscript figures. They would also like to acknowledge Dr Wang’s parent affiliation of the Department of Neurology at the Affiliated Drum Tower Hospital of Nanjing University, PR China.
This work was supported by NS49210, NS33668, NS20020, NR03521, and OHSU Medical Research Foundation Seed Grant.
References
- Ardelt AA, McCullough LD, Korach KS, Wang MM, Munzemaier DH, Hurn PD. Estradiol regulates angiopoietin-1 mRNA expression through estrogen receptor-α in a rodent experimental stroke model. Stroke. 2005;36:337–41. doi: 10.1161/01.STR.0000153795.38388.72. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–8. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- Bingham D, Macrae IM, Carswell HV. Detrimental effects of 17β-oestradiol after permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2005;25:414–20. doi: 10.1038/sj.jcbfm.9600031. [DOI] [PubMed] [Google Scholar]
- Bond R, Rerkasem K, Cuffe R, Rothwell PM. A systematic review of the associations between age and sex and the operative risks of carotid endarterectomy. Cerebrovasc Dis. 2005;20:69–77. doi: 10.1159/000086509. [DOI] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K–Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. Interactions of estrogen and insulin-like growth factor-I in the brain: molecular mechanisms and functional implications. J Steroid Biochem Mol Biol. 2003;83:211–7. doi: 10.1016/s0960-0760(02)00261-3. [DOI] [PubMed] [Google Scholar]
- Carswell HV, Bingham D, Wallace K, Nilsen M, Graham DI, Dominiczak AF, Macrae IM. Differential effects of 17β-estradiol upon stroke damage in stroke prone and normotensive rats. J Cereb Blood Flow Metab. 2004;24:298–304. doi: 10.1097/01.WCB.0000112322.75217.FD. [DOI] [PubMed] [Google Scholar]
- Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBa is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–52. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- Ciccone GK, Holdcroft A. Drugs and sex differences: a review of drugs relating to anaesthesia. Br J Anes. 1999;82:255–65. doi: 10.1093/bja/82.2.255. [DOI] [PubMed] [Google Scholar]
- Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300:1574–7. doi: 10.1126/science.1079817. [DOI] [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine–threonine protein kinase Akt. Science. 1997;275:661–5. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- Friguls B, Justicia C, Pallas M, Planas AM. Focal cerebral ischemia causes two temporal waves of Akt activation. NeuroReport. 2001;12:3381–4. doi: 10.1097/00001756-200110290-00046. [DOI] [PubMed] [Google Scholar]
- Garcia L, Burda J, Hrehorovska M, Burda R, Martin ME, Salinas M. Ischaemic preconditioning in the rat brain: effect on the activity of several initiation factors, Akt and extracellular signal-regulated protein kinase phosphorylation, and GRP78 and GADD34 expression. J Neurochem. 2004;88:136–47. doi: 10.1111/j.1471-4159.2004.02188.x. [DOI] [PubMed] [Google Scholar]
- Gordon KB, Macrae IM, Carswell HVO. Effects of 17β-oestradiol on cerebral ischaemic damage and lipid peroxidation. Brain Res. 2005;1036:155–62. doi: 10.1016/j.brainres.2004.12.052. [DOI] [PubMed] [Google Scholar]
- Harkness JE, Wagner JE. Clinical procedures. In: Harkness JE, Wagner JE, editors. The biology and medicine of rabbits and rodents. 4. Philadelphia, PA: Williams & Wilkins; 1995. pp. 75–142. [Google Scholar]
- Hashiguchi A, Yano S, Morioka M, Hamada J, Ushio Y, Takeuchi Y, Fukugnaga K. Up-regulation of endothelial nitric oxide synthase via phosphatidylinositol 3-kinase pathway contributes to ischemic tolerance in the CA1 subfield of gerbil hippocampus. J Cereb Blood Flow Metab. 2004;24:271–9. doi: 10.1097/01.WCB.0000110539.96047.FC. [DOI] [PubMed] [Google Scholar]
- Ise R, Han D, Takahashi Y, Terasaka S, Inoue A, Tanji M, Kiyama R. Expression profiling of the estrogen responsive genes in response to phytoestrogens using a customized DNA microarray. FEBS Lett. 2005;579:1732–40. doi: 10.1016/j.febslet.2005.02.033. [DOI] [PubMed] [Google Scholar]
- Ivanova T, Mendez P, Garcia-Segura LM, Beyer C. Rapid stimulation of the PI3-kinase/Akt signaling pathway in developing midbrain neurones by oestrogen. J Neuroendocrinol. 2002;14:73–9. doi: 10.1046/j.0007-1331.2001.00742.x. [DOI] [PubMed] [Google Scholar]
- Jacoby RO, Fox JG, Davisson M. Biology and diseases of mice. In: Fox JG, Anderson LC, Loew FM, Quimby FW, editors. Laboratory animal medicine. 2. San Diego, CA: Academic Press; 2002. pp. 35–120. [Google Scholar]
- Janelidze S, Hu BR, Siesjo P, Siesjo BK. Alterations of Akt1 (PKBα) and p70S6K in transient focal ischemia. Neurobiol Dis. 2001;8:147–54. doi: 10.1006/nbdi.2000.0325. [DOI] [PubMed] [Google Scholar]
- Kako K, Wakamatsu H, Hamada T, Banasik M, Ohata K, Niki-Kuroiwa T, Suzuk S, Takeuchi J, Ishida N. Examination of DNA binding activity of neuronal transcription factors by electrophoretical mobility shift assay. Brain Res Protocols. 1988;2:243–9. doi: 10.1016/s1385-299x(97)00040-8. [DOI] [PubMed] [Google Scholar]
- Kasischke K, Huber R, Li H, Timmler M, Riepe MW. Primary hypoxic tolerance and chemical preconditioning during estrus cycle in mice. Stroke. 1999;30:1256–62. doi: 10.1161/01.str.30.6.1256. [DOI] [PubMed] [Google Scholar]
- Kelley RE. Stroke in the postoperative period. Med Clin North Am. 2001;85:1263–76. doi: 10.1016/s0025-7125(05)70377-1. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Warita H, Sasaki C, Zhang WR, Sakai K, Shiro Y, Mitsumoto Y, Mori T, Abe K. Immunoreactive Akt, PI3-K and ERK protein kinase expression in ischemic rat brain. Neurosci Lett. 1999;274:45–8. doi: 10.1016/s0304-3940(99)00676-x. [DOI] [PubMed] [Google Scholar]
- Kitano H, Kirsch JR, Hurn PD, Murphy SJ. Inhalational anesthetics as neuroprotectants or chemical preconditioning agents in ischemic brain. J Cereb Blood Flow Metab. 2006 doi: 10.1038/sj.jcbfm.9600410. advance online publication, 18 October 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch CG, Khandwala F, Cywinski JB, Ishwaran H, Estafanous FG, Loop FD, Blackstone EH. Health-related quality of life after coronary artery bypass grafting: a gender analysis using the Duke Activity Status Index. J Thorac Cardiovasc Surg. 2004;128:284–95. doi: 10.1016/j.jtcvs.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Koo SH, Satoh H, Herzig S, Lee CH, Hedrick S, Kulkarni R, Evans RM, Olefsky J, Montminy M. PGC-1 promotes insulin resistance in liver through PPAR-α-dependent induction of TRB-3. Nat Med. 2004;10:530–734. doi: 10.1038/nm1044. [DOI] [PubMed] [Google Scholar]
- Langer RD. Hormone replacement and the prevention of cardiovascular disease. Am J Cardiol. 2002;89(Suppl 12):36E–46E. doi: 10.1016/s0002-9149(02)02411-6. [DOI] [PubMed] [Google Scholar]
- Murphy SJ, McCullough LD, Smith JM. Stroke in the female: role of biological sex and estrogen. ILAR J. 2004;45:147–59. doi: 10.1093/ilar.45.2.147. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Iwabuchi S, Miyazaki H, Okuma Y, Kuwabara M, Nomura Y, Kawahara K. Preconditioning prevents ischemia-induced neuronal death through persistent Akt activation in the penumbra region of the rat brain. J Vet Med Sci. 2004;66:521–7. doi: 10.1292/jvms.66.521. [DOI] [PubMed] [Google Scholar]
- Noshita N, Lewen A, Sugawara T, Chan PH. Evidence of phosphorylation of Akt and neuronal survival after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2001;21:1442–50. doi: 10.1097/00004647-200112000-00009. [DOI] [PubMed] [Google Scholar]
- O’Connor CA, Cernak I, Vink R. Interaction between anesthesia, gender, and functional outcome task following diffuse traumatic brain injury in rats. J Neurotrauma. 2003;20:533–41. doi: 10.1089/089771503767168465. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Tan Y, Comb M, Liu CL, Martone ME, Siesjo BK, Hu BR. Survival- and death-promoting events after transient cerebral ischemia: phosphorylation of Akt, release of cytochrome C and activation of caspase-like proteases. J Cereb Blood Flow Metab. 1999;19:1126–35. doi: 10.1097/00004647-199910000-00009. [DOI] [PubMed] [Google Scholar]
- Ozatik MA, Gol MK, Fansa I, Uncu H, Kucuker SA, Kucukaksu S, Bayazit M, Sener E, Tasdemir O. Risk factors for stroke following coronary artery bypass operations. J Card Surg. 2005;20:52–7. doi: 10.1111/j.0886-0440.2005.200384.x. [DOI] [PubMed] [Google Scholar]
- Pleym H, Spigset O, Kharasch ED, Dale O. Gender differences in drug effects: implications for anesthesiologists. Act Anaesthesiol Scand. 2003;47:241–59. doi: 10.1034/j.1399-6576.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- Santizo RA, Xu HL, Ye S, Baughman VL, Pellegrino DA. Loss of benefit form estrogen replacement therapy in diabetic ovariectomized female rats subjected to transient forebrain ischemia. Brain Res. 2002;956:86–95. doi: 10.1016/s0006-8993(02)03484-4. [DOI] [PubMed] [Google Scholar]
- Shibata M, Yamawaki T, Sasaki T, Hattori H, Hamada J, Fukuuchi Y, Okano H, Miura M. Upregulation of Akt phosphorylation at the early stage of middle cerebral artery occlusion in mice. Brain Res. 2002;942:1–10. doi: 10.1016/s0006-8993(02)02474-5. [DOI] [PubMed] [Google Scholar]
- Silasi G, Diaz-Heijtz R, Besplug J, Rodriguez-Juarez R, Titov V, Kolb B, Kovalchuk O. Selective brain responses to acute and chronic low-dose X-ray irradiation in males and females. Biochem Biophys Res Comm. 2004;325:1223–35. doi: 10.1016/j.bbrc.2004.10.166. [DOI] [PubMed] [Google Scholar]
- Singh M. Ovarian hormones elicit phosphorylation of Akt and extracellular-signal regulated kinase in explants of the cerebral cortex. Endocrine. 2001;14:407–15. doi: 10.1385/ENDO:14:3:407. [DOI] [PubMed] [Google Scholar]
- Stayrook KR, Bramlett KS, Savkur RS, Ficorilli J, Cook T, Christe ME, Michael LF, Burris TP. Regulation of carbohydrate metabolism by the farnesoid X receptor. Endocrinology. 2005;146:984–91. doi: 10.1210/en.2004-0965. [DOI] [PubMed] [Google Scholar]
- Terasaka S, Aita Y, Inoue A, Hayashi S, Nishigaki M, Aoyagi K, Sasaki H, Wada-Kiyama Y, Sakuma Y, Akaba S, Tanaka J, Sone H, Yonemoto J, Tanji M, Kiyama R. Using a customized DNA microarray for expression profiling of the estrogen-responsive genes to evaluate estrogen activity among natural estrogens and industrial chemicals. Environ Health Perspect. 2004;112:773–81. doi: 10.1289/ehp.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Arnim CA, Etrich SM, Timmler M, Riepe MW. Gender-dependent hypoxic tolerance mediated via gender-specific mechanisms. J Neurosci Res. 2002;68:84–8. doi: 10.1002/jnr.10195. [DOI] [PubMed] [Google Scholar]
- Weise J, Kuschke S, Bahr M. Gender-specific risk of perioperative complications in carotid endarterectomy patients with contralateral carotid artery stenosis or occlusion. J Neurol. 2004;251:838–44. doi: 10.1007/s00415-004-0438-8. [DOI] [PubMed] [Google Scholar]
- Yang ZZ, Tschopp O, Baudry A, Dummler B, Hynx D, Hemmings BA. Physiological functions of protein kinase B/Akt. Biochem Soc Trans. 2004;32:350–4. doi: 10.1042/bst0320350. [DOI] [PubMed] [Google Scholar]
- Yano S, Morioka M, Fukunaga K, Kawano T, Hara T, Kai Y, Harmada J, Miyamoto E, Ushio Y. Activation of Akt/protein kinase B contributes to induction of ischemic tolerance in the CA1 subfield of gerbil hippocampus. J Cereb Blood Flow Metab. 2001;21:351–60. doi: 10.1097/00004647-200104000-00004. [DOI] [PubMed] [Google Scholar]
- Znamensky V, Akama KT, McEwen BS, Milner TA. Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal CA1 dendrites. J Neurosci. 2003;23:2340–7. doi: 10.1523/JNEUROSCI.23-06-02340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


