Abstract
Acute bolus doses of morphine induce a state of acute opioid dependence as measured by naloxone-precipitated withdrawal. Repeated morphine and precipitated withdrawal experience further enhances naloxone-induced withdrawal severity, in part due to direct neuroadaptation to repeated morphine, and in part due to conditioned associations of context and withdrawal experience. To determine whether a discrete tone/light conditioned stimulus (CS) could elicit conditioned withdrawal responses in acute dependence, rats trained on an FR-15 operant schedule for food reward received morphine (5.6 mg/kg) 4x at daily or weekly intervals, with each morphine injection followed at 4 hr by naloxone (1.0 mg/kg) and an operant session. The CS was presented to a Paired group after each naloxone injection. Separate control groups either experienced the CS at a different time of day or different day of the week than naloxone (Unpaired), received naloxone without any CS exposure (Paired-No CS) or received vehicle instead of naloxone prior to CS presentation (Nal-Naive). On the Test Day, all rats received vehicle prior to CS exposure. The CS alone reliably suppressed responding in Paired groups relative to control conditions with either daily or weekly intervals between conditioning sessions. Administration of morphine 4 hr prior to CS exposure on the Test Day was not necessary to observe conditioned withdrawal. Thus, conditioned withdrawal is reliably established to discrete cues associated with naloxone-precipitated withdrawal from acute infrequent (weekly) opioid exposure.
Keywords: opioid, addiction, dependence, withdrawal, morphine, naloxone, rat, classical conditioning
INTRODUCTION
Opioid dependence as measured by antagonist-precipitated withdrawal symptoms can be observed after just one injection of a moderate dose of an agonist such as morphine (Azorlosa et al., 1994; Bickel et al., 1988; Heishman et al., 1989a, 1989b; Jones, 1980; Martin and Eades, 1964). Considerable recent work in animals has focused upon acute dependence models using suppression of operant responding for food or brain stimulation reward as quantifiable indices of withdrawal severity precipitated by the opioid antagonists naloxone or naltrexone (Adams and Holtzman, 1990; Azar et al., 2003; Easterling and Holtzman, 1997, 2004; Liu and Schulteis, 2004; Meyer and Sparber, 1977; Parker and Joshi, 1998; Schulteis et al., 1997, 1999, 2003, 2004, 2005; Young, 1986).
Acute dependence may reflect adaptive changes in response to opioid exposure that constitute the first step in the transition from initial casual use to addiction (Schulteis and Koob, 1996). In support of this notion, it has been shown that repeated morphine experience at daily or weekly intervals can lead to a potentiation of withdrawal severity produced by opioid antagonists (Adams and Holtzman, 1990; Azorlosa et al., 1994; Schulteis et al., 1999, 2003, 2004). A component of this potentiation is independent of repeated precipitated-withdrawal experience, since exposure to morphine alone at daily or weekly intervals is sufficient to magnify the intensity of withdrawal (Azorlosa et al., 1994; Schulteis et al., 1997, 1999, 2003). This antagonist-independent process might reflect incremental neuroadaptive responses to morphine itself. However, more pronounced potentiation of antagonist-precipitated withdrawal occurs when repeated morphine doses are always followed several hours later by the antagonist (Adams and Holtzman, 1990; Schulteis et al., 2003, 2004, 2005). We recently reported that this antagonist-dependent process apparently reflects the formation of conditioned associations between the naloxone-precipitated withdrawal state and contextual features of the operant environment and withdrawal-testing regimen (Schulteis et al., 2004, 2005).
Neuroadaptive responses to opioids, including tolerance and dependence/withdrawal, are readily influenced by conditioned associations between the drug and environmental cues that reliably predict drug intake (O’Brien et al., 1976; Siegel, 1976; Wikler, 1971, 1973). Within the recreational setting, conditioning processes could be facilitated by the typically habitual behavior of drug addicts, who often repeatedly experience intoxication as well as withdrawal under similar conditions and in specific surroundings (Wikler 1973). Consistent with this notion, several reports by Siegel and colleagues suggest that certain cases of heroin overdose may result from the absence of conditioned tolerance responses when heroin is self-administered in an environment not traditionally associated with heroin intake (Siegel et al., 1982; Siegel and Ellsworth, 1986; Siegel, 1984).
It is widely hypothesized that conditioned drug-like and drug-opposite responses (e.g. conditioned withdrawal) may play a major role in triggering craving and precipitating relapse in addicted individuals (Childress et al., 1987, 1994; Everitt et al., 2001; Koob and Le Moal, 2001; O’Brien, 1996; O’Brien et al., 1976; Schulteis and Koob, 1996; Wikler, 1971, 1973; Wikler and Pescor, 1967). Most prior work on conditioned opioid withdrawal has therefore focused on chronic dependence and relapse after periods of protracted abstinence. However, recent evidence of conditioned withdrawal-like effects following acute morphine exposure (Schulteis et al., 1999, 2003, 2004, 2005) suggest that conditioned associations may play a key role earlier in the development of dependence than was previously thought. To date all of these studies of conditioned withdrawal from acute dependence included both a morphine and a naloxone injection (4 hr post-morphine) on the Test Day, just as on Conditioning Days. Adams and Holtzman (1990) have suggested that interoceptive cues provided by the onset of antagonist effect are sufficient stimuli to support a conditioned potentiation of withdrawal severity in acute dependence models, and recent work by Siegel and colleagues supports the potency of such interoceptive drug cues as potential conditioned stimuli (McDonald and Siegel, 2004; Sokolowska et al., 2002). This calls into question whether discrete stimuli can evoke conditioned withdrawal effects in our acute dependence model without the added benefit of interoceptive drug cues provided by morphine and/or naloxone. The current study sought to evaluate specifically whether conditioned suppression of operant responding could be demonstrated in the absence of naloxone- and morphine-interoceptive cues on the Test Day.
Our conditioning model was based upon prior work in humans (O’Brien et al., 1976), monkeys (Goldberg and Schuster, 1967), and rats (Baldwin and Koob, 1993; Schulteis et al., 2000), wherein repeated pairing of discrete stimuli (tone/light/smell) with an opioid antagonist in chronically dependent subjects led to the expression of withdrawal symptoms in response to the discrete stimuli alone. In the current study, a discrete conditioned stimulus (CS) consisting of an intermittent tone and flashing light was paired with naloxone-precipitated withdrawal from acute bolus doses of morphine administered at daily (Experiment 1) or weekly (Experiment 2) intervals. On the test day, rats were re-exposed to the discrete CS in the absence of naloxone cues (Experiments 1 and 2) and for some groups in the absence of both morphine and naloxone cues (Experiment 2). Precipitated withdrawal was the method of choice in these experiments because it ensures precise temporal contiguity between peak withdrawal intensity (typically 5–30 min post-antagonist) and the conditioned stimuli. It must be recognized that the human opioid user or addict is experiencing spontaneous withdrawal during periods of drug abstinence, not precipitated withdrawal. Care must be taken therefore in extrapolating precise parametric details (e.g. number of conditioning trials, length of conditioning trials, etc.) from the laboratory to the recreational use setting. Nonetheless, a number of thorough parametric comparisons of spontaneous and precipitated opioid withdrawal note striking similarities (Blasig and Herz, 1977; Blasig et al., 1973; Linseman, 1977; Martin et al., 1963; Way et al., 1969; Wei et al., 1973), suggesting that precipitated withdrawal, with its inherent advantages in the laboratory setting, serves as a valid model (at least qualitatively) of withdrawal experienced in the recreational setting.
METHODS
Animals
Male Wistar rats (n = 57; Harlan Labs, Indianapolis, Indiana, USA) weighing 300–400 g at the time of testing were used. All rats were group housed (2–3/cage) in a temperature-and humidity-controlled room with a 12-h light/12-h dark cycle. The rats were maintained on 15 g rat chow per day in addition to the food pellets earned in the operant boxes (total food intake was approximately 22 g/rat/day). Rats had ad lib access to water at all times. All training and testing took place from 9:00 AM to 4:00 PM daily, Monday through Friday. On days when rats were not trained in the operant boxes (Saturday and Sunday), an additional 5 g of rat chow was provided to ensure that total food intake remained relatively constant. All rats continued to gain weight at an average of 10–15 g/week throughout training and testing, with no significant differences in weight gain among any experimental groups (data not shown). All procedures employed in the studies described herein were reviewed and approved by the Institutional Animal Care and Use Committee of the VA San Diego Healthcare System, and were carried out in accordance with the guidelines established in the Guide for the Care and Use of Laboratory Animals (National Research Council and National Institutes of Health).
Drugs
Morphine sulfate was obtained from the VA San Diego Pharmacy, and naloxone HCl was purchased from Sigma (St Louis, Mo., USA). Both drugs were prepared for injection in physiological saline, and all injections were made subcutaneously (SC) at a volume of 0.1–ml/100 g body weight. Vehicle injections consisted of physiological saline also at a volume of 0.1–ml/100 g body weight. Morphine was administered at a dose of 5.6 mg/kg, and naloxone was administered at 1.0 mg/kg. All doses are expressed as the salt.
Morphine and naloxone doses were carefully selected based on prior work (Young, 1986; Adams and Holtzman 1990; Schulteis et al., 1997, 1999, 2003, 2004, 2005) which indicated that: 1) a 5.6 mg/kg dose of morphine produces little or no effect on operant responding by itself at the time chosen here for antagonist administration and testing; 2) repeated treatment with this dose of morphine supports potentiation of withdrawal severity, including antagonist-independent and antagonist-dependent components; and 3) the 1.0 mg/kg dose of naloxone has minimal effect on operant responding for food in morphine-naïve rats, but suffices to elicit profound withdrawal symptoms in acutely dependent rats.
Operant Training
Eight operant chambers (Coulbourn Instruments, Columbus, Ohio, USA) were equipped with a food hopper located 4 cm above a grid floor, a lever located to the right of the food hopper, and a cue light located above the lever. The cue light illuminated for 1 s as a food pellet (45 mg) was delivered each time a rat completed a fixed-ratio (FR) component. Rats were trained to lever press for food pellets in 30-min sessions 5 days a week, beginning on an FR-1 schedule and progressing to an FR-15 schedule (1-s timeout). On the first day of FR-1 training, several food pellets were taped to the lever to encourage investigation of the lever, but once the rats exhibited lever-pressing behavior (typically within 1–2 sessions), this training aid was eliminated. Once reliable FR-15 responding was established, rats were acclimated to a split schedule in which a 10-min session was followed by a timeout period of 15 min, for which the rats were returned to their home cages, followed by a 20-min operant session. This schedule permitted an assessment of daily stable baselines (10-min session) prior to any drug administration or CS presentation. Training continued until stable baseline responding were achieved (typically within 3–5 weeks), with stability defined as less than 10% variation from the mean of 3 consecutive test days.
Conditioning and Test Procedures
Experiment 1
Drug pretreatment/treatment regimens of all groups are summarized in Table 1. Over four consecutive days (Conditioning Days) rats were injected each day with morphine (5.6 mg/kg) followed 4 hr later by the precipitation of acute withdrawal through naloxone (1.0 mg/kg). In all experimental groups during both Conditioning and Test Days, a 10 min operant session (Pre-CS Session) preceded the naloxone/vehicle injection, and the 20 min CS session followed 5 min after naloxone/vehicle. Thus, the Pre-CS Session served as an index of daily response rate prior to naloxone-induced withdrawal and/or CS onset on a given day.
Table 1.
Summary of Experimental Design for Experiment 1.
| Experimental Condition (All injections in Home Cage except those labeled CS or NO CSa)
|
||||
|---|---|---|---|---|
| Nal-Naive | Unpaired | Paired-No CS | Paired-CS | |
| Conditioning Sessions (Days 1–4) | ||||
| T = 0 | MOR | VEH + CSa | MOR | MOR |
| T = 4 hr | VEH + CSa | MOR | NAL + NO CSa | NAL + CSa |
| T = 8 hr | VEH | NAL | VEH | VEH |
|
| ||||
| Test Session (Day 5) | ||||
| T = 0 | MOR | MOR | MOR | MOR |
| T = 4 hr | VEH + CSa | VEH + CSa | VEH + NO CSa | VEH + CSa |
Abbreviations: MOR = morphine, NAL = naloxone, VEH = vehicle, CS = tone/light conditioned stimulus present in CS Session, NO CS = tone/light CS omitted during CS Session.
On Conditioning Days and Test Days, a 10-min operant session (Pre-CS Session) preceded the CS session, with the corresponding VEH or NAL injections administered 10 min after the conclusion of the Pre-CS Session and 5 min prior to onset of the CS Session. Note that on Conditioning Days the Unpaired group received VEH prior to the CS session, and morphine and naloxone treatment followed rather than preceded the CS session.
On Conditioning Days, naloxone-precipitated withdrawal in the Paired group took place in the operant chambers during the 20-min session in which the CS was presented throughout (CS Session). The CS consisted of a tone/light compound stimulus (7 kHz, 85 dB tone plus house light on for 5 s, off for 2 s, repeating). An Unpaired control group received vehicle prior to each daily operant session, with morphine administered 4 hr and naloxone 8 hr after the operant session in the home cage. To equalize injection history across experimental groups, all other groups received a vehicle injection at the 8 hr time point (see Table 1). Additional control groups received vehicle in place of naloxone on conditioning days (Nal-Naive), or received naloxone in the absence of the CS in the operant context (Paired-No CS). On the Test Day, which occurred 24 hr after the last Conditioning Day, all groups were again treated with morphine but 4 hr later received only a vehicle injection prior to exposure to the CS. All groups except Paired-No CS were exposed to the CS on the Test Day.
Experiment 2
All subjects in this experiment were assigned initially to either Paired or Unpaired conditions and exposed to morphine once weekly (see Table 2). Rats in the Paired group received a morphine injection (5.6 mg/kg) in their home cages on Friday of each week, followed 4 hr later by naloxone (1 mg/kg). As in Experiment 1, a Pre-CS 10 min operant session preceded the naloxone injection, and a 20 min CS Session in which rats were exposed to the tone/light CS commenced 5 min post-naloxone. This procedure was repeated each Friday of four Conditioning Weeks. The following week (Test Week), the procedure was the same with the exception that a vehicle injection was substituted for naloxone prior to the CS session. In addition to the weekly morphine and naloxone-withdrawal experience on Friday that was contiguous with CS exposure, rats in the Paired group were given several operant sessions each Conditioning and Test week in the absence of any injections whatsoever (Tuesday), or with vehicle injections substituted for morphine and naloxone (Wednesday, Thursday). The CS was omitted during the 20 min CS Session on these days.
Table 2.
Summary of Experimental Design for Experiment 2.
| Experimental Condition
|
|||
|---|---|---|---|
| Conditioning Weeks (each of 4) | Test Week (week 5) | ||
| Paired | Unpaired | Paired and Unpaired | |
| Monday | |||
| Injection at T = 0 (HC) | ------ | ------ | ------ |
| Pre-CS Session (10 min) a | ------ | NO CS | ------ |
| Injection at T = 4 hr | ------ | ------ | ------ |
| CS Session (20 min) | ------b | NO CS b | ------ |
|
| |||
| Tuesday | |||
| Injection at T = 0 (HC) | ------ | ------ | ------ |
| Pre-CS Session (10 min) a | NO CS | NO CS | NO CS |
| Injection at T = 4 hr | ------ | ------ | ------ |
| CS Session (20 min) | NO CS | NO CS | NO CS |
|
| |||
| Wednesday | |||
| Injection at T = 0 (HC) | VEH | VEH | VEH |
| Pre-CS Session (10 min) a | NO CS | NO CS | NO CS |
| Injection at T = 4 hra | VEH | VEH | VEH |
| CS Session (20 min) | NO CS | CS b | NO CS |
|
| |||
| Thursday (BASELINE DAY) | |||
| Injection at T = 0 (HC) | VEH | VEH | VEH |
| Pre-CS Session (10 min) a | NO CS | NO CS | NO CS |
| Injection at T = 4 hra | VEH | VEH | VEH |
| CS Session (20 min) | NO CS | NO CS | NO CS |
|
| |||
| Friday | |||
| Injection at T = 0 (HC) | MOR | MOR | MOR or VEH c |
| Pre-CS Session (10 min) a | NO CS | ------ | NO CS |
| Injection at T = 4 hra | NAL | NAL | VEH |
| CS Session (20 min) | CS | ------b | CS |
Abbreviations: MOR = morphine, NAL = naloxone, VEH = vehicle, HC = home cage injection, CS = conditioned stimulus present in 20-min session, NO CS = no CS in 20-min session.
Each injection at t = 4 hr was preceded by a 10 min operant session (Pre-CS) that started at t= 3:45; the CS was NEVER presented during the Pre-CS Session
During Conditioning Weeks, the Unpaired group received an operant session on Monday to ensure 4 sessions/week, just like Paired group. The Unpaired group required this Monday session since it was not given an operant session on Friday, the day of morphine/naloxone treatment, to preclude any possible association of treatment with operant context or CS. Instead the CS was presented to the Unpaired group on Wednesday, following VEH injections.
On the Test Day, rats from both the Paired and Unpaired groups were further sub-divided into cohorts that received morphine (MOR) or vehicle (VEH) 4 hr prior to the CS session.
During the four Conditioning Weeks, rats in the Unpaired control group received the same number of operant sessions each week as the Paired group, as well as the same number of vehicle injections (see Table 2 for details). However, the operant session with CS exposure occurred on Wednesday following vehicle treatment, and morphine and naloxone were administered on Friday with no operant session on that day.
During the Test Week, rats in both the Paired and the Unpaired groups were further divided into separate cohorts receiving morphine (Paired-MOR, Unpaired-MOR) or vehicle (Paired-VEH, Unpaired-VEH) 4 hr prior to CS exposure on the Test Day (Friday). Vehicle instead of naloxone was administered to all experimental groups after the Pre-CS Session and 5 min prior to the CS Session on the Test Day. The Unpaired groups received exactly the same regimen of injections and operant sessions as their corresponding Paired groups in the Test Week (see Table 2).
Data Analysis
Results of prior studies had shown that conditioned withdrawal responses on the Test Day could wane in strength over the course of a 20 min CS test session, presumably due to extinction of the conditioned response when the unconditioned stimulus (naloxone-induced withdrawal) was absent on the Test Day (Schulteis et al., 2000, 2005). Therefore data from the 20 min CS sessions were further divided into two equal 10-min bins, creating 3 equal 10 min intervals that could be entered as a repeated measure into statistical analyses (Pre-CS, CS 1–10, CS 11–20). For Experiment 1, the mean responses/10 min interval for each rat on the final 3 days prior to initiation of conditioning procedures was taken as the baseline (see Table 3), and data on all Conditioning Days and the Test Day were expressed as percent of baseline responses/10 min in the corresponding operant interval. In Experiment 2, baseline responding (see Figure 2A) was taken from the operant sessions on Thursday of each Conditioning and Test Week (Baseline Day), 24 hr prior to morphine and naloxone administration in both Paired and Unpaired groups.
Table 3.
Baseline Responding: Experiment 1 (Mean ± SEM per 10 min interval)*
| Experimental Condition
|
||||
|---|---|---|---|---|
| Nal-Naive | Unpaired | Paired-No CS | Paired-CS | |
| Pre-CS Interval: | 902 ± 83.5 | 932 ± 54.4 | 899 ± 46.1 | 881 ± 48.7 |
| CS Interval: | ||||
| CS 1–10 | 860 ± 74.7 | 944 ± 56.5 | 803 ± 38.8 | 872 ± 39.6 |
| CS 11–20 | 647 ± 36.3 | 628 ± 37.0 | 556 ± 38.2 | 620 ± 52.4 |
Baseline responding reflected an average of the last 3 days prior to the first Conditioning Session
Data from Experiments 1 and 2 were analyzed using standard 2 or 3-factor mixed design ANOVAs as appropriate, with operant interval (Pre-CS, CS 1–10, CS 11–20) and Conditioning Day (or Week) as a within-subjects factors, and experimental condition (e.g. Paired vs. Unpaired, morphine vs. vehicle pretreatment on Test Day) as between-subjects factors. Follow-up comparisons of relevant simple main effects or interaction contrasts were conducted selectively as suggested by outcome of the overall ANOVAs. Significant simple main effects or interaction contrasts were further dissected through individual means comparisons using the Bonferroni correction to ensure a constant error rate of p < 0.05, regardless of number of comparisons made.
RESULTS
Experiment 1
As shown in Table 3, responding on the baseline days (last 3 days prior to first Conditioning Session) in all groups declined predictably across operant intervals (Pre-CS, CS 1–10, CS 11–20). This time-dependent decline in responding is typical of our studies where continuous operant sessions of 20–30 min duration are employed (Schulteis et al.1997, 1999, 2000, 2005) and presumably reflects fatigue or satiety. In this regard it is noteworthy that a cumulative dosing operant regimen where responding is restricted to 5 min opportunities once every 15 min does not engender this same decrement in responding across consecutive operant intervals (Schulteis et al., 2003, 2004). Importantly, a significant main effect of operant interval (F[2,52] = 89.79, p < 0.0001) with no main effect of treatment condition (F[3,26] = 1.16, p > 0.34) or treatment × operant interval interaction (F[6,52] = 1.11, p > 0.37) indicated that the within-session decline in responding was equivalent among experimental groups.
As shown in Figure 1A, responding in the Pre-CS interval declined markedly across Conditioning Days in both Paired groups, but not in the control groups (Nal-Naive, Unpaired), resulting in a significant treatment group × Conditioning Day interaction (F[9,78] = 9.93, p < 0.0001). Simple main effects analysis of Conditioning Days within each experimental group indicated a significant decline in responding across days in the Paired-No CS group (F[3,21] = 41.36, p < 0.0001) and Paired-CS group (F[3,24] = 4.31, p < 0.02), but not the Nal-Naive or Unpaired control groups (F’s < 1.61, p’s > 0.25). Interestingly, an interaction contrast comparing the Paired-CS and Paired-No CS groups revealed a greater relative degree of response suppression in the Paired-No CS group (Treatment × Conditioning Day Interaction: F[3,45] = 7.52, p < 0.0004).
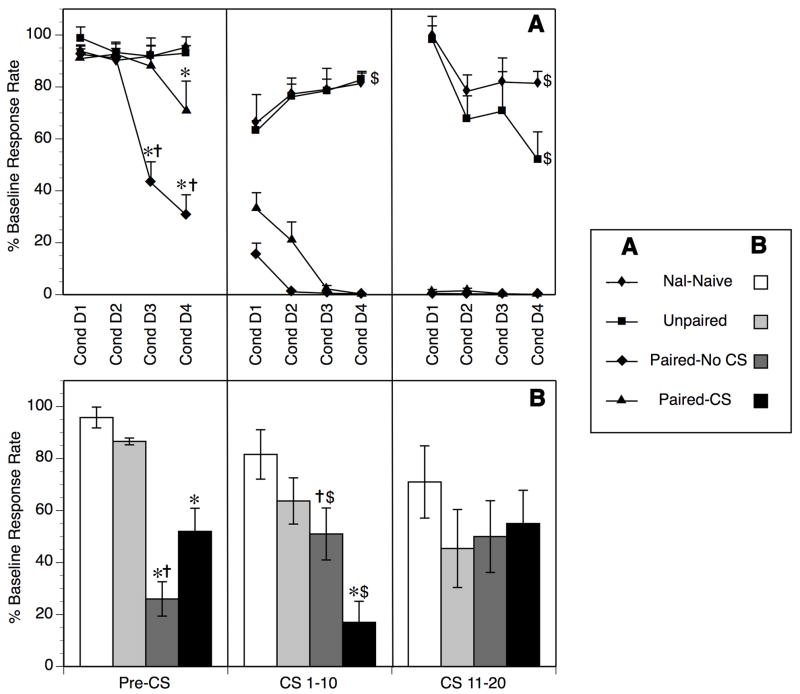
Fig. 1.
Operant responding in Paired (Paired-CS, Paired-No CS) and control (Nal-Naive, Unpaired) groups from Experiment 1 as a function of operant interval (Pre-CS, CS 1–10, CS 11–20). A) On Conditioning Days, responding during Pre-CS session (left panel) were suppressed in both the Paired groups as conditioning proceeded (*p < 0.05 vs. Unpaired and Nal-Naive controls), but the effect in the Paired-No CS group was significantly greater than in the Paired-CS group (†p < 0.05, Paired CS vs. Paired-No CS). In the CS 1–10 interval (center panel), responding was predictably suppressed totally within 2 sessions in the Paired groups. A transient decrease in operant responding due to CS novelty followed by habituation was noted in both control groups during the CS 1–10 interval ($p < 0.05, main effect of Conditioning Day). In the CS 11–20 interval (right panel), responding again was predictably suppressed in the Paired groups experiencing naloxone-precipitated withdrawal during operant testing on Conditioning Days. Somewhat unexpected was a modest suppression in responding that emerged over Conditioning Days in both the Nal-Naive and Unpaired control groups ($p< 0.05, main effect of Conditioning Day); this may reflect the emergence of a mild spontaneous withdrawal from repeated daily morphine treatments (see text for further details). B) On Test Day, responding in the Pre-CS session (left panel) was again suppressed in both Paired groups (*p < 0.05 vs. Unpaired and Nal-Naive controls), with the Paired-No CS groups showing greater suppression in this interval than the Paired-CS group (†p < 0.05). Operant responding during the first 10 minutes of CS session (center panel) were suppressed in the Paired-CS group relative to the both control groups(*p < 0.05) and relative to Paired-CS responding in the Pre-CS interval ($p < 0.05 vs. Pre-CS), but responding in the Paired-No CS group actually increased significantly from the Pre-CS to the CS 1-10 interval ($p<0.05 vs. Pre-CS). There was no difference in responding during final 10 minutes of CS session (right panel) between any treatment groups.
Naloxone-precipitated withdrawal during CS exposure resulted in a predictable dramatic suppression of responding in both the CS 1–10 and CS 11–20 intervals (Figure 1A), with near 100% suppression within 1–2 days. A slight suppression in responding during the CS 1–10 interval relative to the Pre-CS interval was noted in the Nal-Naive and Unpaired groups on initial Conditioning Days (Figure 1A). A main effect of Conditioning Day (F[3,33] = 3.13, p < 0.05) with no main effect of treatment (Nal-Naive, Unpaired) or treatment × Conditioning Day Interaction (F’s < 0.03, p’s > 0.86) indicated that responding increases significantly across Conditioning Days in both control groups (Unpaired, Nal-Naive) in the CS 1–10 interval, presumably due to a modest suppression when the CS was novel (days 1–2) followed by habituation to the CS.
Somewhat unexpected was the significant decline in responding during the final component of the CS session (CS 11–20) in both Nal-Naive and Unpaired groups (Figure 1A) across Conditioning Days. A significant main effect of Conditioning Day in the CS 11–20 interval (F[3,33] = 5.56, p < 0.0005) confirmed this effect; lack of any main effect of treatment or treatment × Conditioning Day interaction (F’s < 2.7, p’s > 0.13) indicated that the Nal-Naive and Unpaired groups did not differ reliably from each other across Conditioning Days. The cause of this suppression in responding is not clear, but may reflect the emergence of a mild spontaneous withdrawal from repeated morphine, as has been reported previously (Liu and Schulteis, 2004).
Two-factor mixed design ANOVA of the Test Day data (Figure 1B) revealed a significant treatment group × operant interval interaction (F[6,52] = 5.13, p < 0.0005). Follow-up comparisons at each separate operant interval revealed a significant simple main effect of treatment during the Pre-CS interval (F[3,26] = 2.24, p < 0.0001) and the CS 1–10 interval (F[3,26] = 8.82, p < 0.005), but not the CS 11–20 interval (F[3,26] = 0.55, N.S.). Individual means comparisons (Bonferroni-corrected) confirmed that within the Pre-CS interval responding was significantly lower in the Paired-No CS and Paired-CS groups than in either control group (Nal-Naive, Unpaired), but significantly higher in the Paired-CS group than in the Paired No-CS group (p < 0.05 corrected by Bonferroni). A different pattern of results was evident during the CS 1–10 interval, where responding was significantly lower in the Paired-CS group than in the Paired-No CS group or in either control group (p < 0.05), and the Paired-No CS group did not differ significantly from either of the control groups (Nal-Naive, Unpaired). In addition, within-subjects comparisons of operant responding across test intervals in the Paired-CS group indicated that responding during the CS 1–10 interval was significantly lower than responding during the Pre-CS session (p < 0.05), indicating a significant incremental effect of CS exposure beyond the effects produced by reintroduction to the operant context alone. In contrast, responding in the Paired-No CS group increased significantly from the Pre-CS to the CS 1–10 interval, presumably reflecting extinction of conditioned suppression to context. Finally, operant responding during the last 10 minutes of the CS session (CS 11–20) did not differ significantly across groups.
Experiment 2
Results from Experiment 1 suggested that a novel tone/light CS could elicit a marked but transient conditioned suppression of responding to the CS alone following repeated daily pairing with naloxone-precipitated withdrawal, and that cues provided by low-dose naloxone on the Test Day (Schulteis et al., 2005) were not essential to the conditioned effect. However, the emergence across Conditioning Days of a significant suppression of responding in both Paired groups in the operant interval prior to CS onset (Pre-CS), as well as suppression of responding in the control groups in the latter components of the CS session (CS 11–20), suggested that further refinements to the model were needed to more clearly separate the influence of tone/light CS from other contextual features. Therefore, Experiment 2 was completed in an effort to minimize the predictive salience of operant environment and injection regimen (see Methods and Table 2 for details). In addition, we sought to determine whether the morphine cue was essential to effective conditioning to the CS in our paradigm, and therefore Paired and Unpaired groups were further sub-divided during the Test Week into sub-groups receiving morphine (Paired-Mor, Unpaired-Mor) or vehicle (Paired-Veh, Unpaired-Veh) 4 hr prior to the Test Session. However, because these sub-groups were treated identically up to the Test Day in the final week of testing, analysis of all baseline data and Conditioning Session data were conducted on the combined Paired and Unpaired groups to increase statistical power and minimize the number of comparisons required.
As detailed in Table 2, baseline performance was measured on the Thursday of each week, one day prior to morphine treatment for the given week. Consistent with baseline performance from Experiment 1 (see Table 3), responses/10 min declined sequentially across operant intervals (Pre-CS, CS 1–10, CS 11–20) in both Paired and Unpaired groups (Figure 2A). Three-way ANOVA indicated no main effect of treatment group (Paired vs. Unpaired) or treatment × operant interval (Pre-CS, CS 1–10, CS 11–20) interaction (F’s < 1.70, p’s > 0.25), indicating a comparable decline in responding across CS intervals regardless of treatment group. However, the overall ANOVA did reveal a significant treatment × Conditioning Week interaction (F[4,100] = 8.46, p < 0.0001). As shown in Figure 2A, responding on Baseline Days in the Unpaired group appeared to increase slightly over the first 2–3 weeks of testing, but remained unchanged across weeks in the Paired group. A significant simple main effect of Conditioning Week in the Unpaired (F[4,52] = 14.83, p < 0.0001) but not Paired (F[4,48] = 0.21, p > 0.85) group confirmed this subtle difference in baseline responding across weeks, which cannot be attributed to differing morphine exposure or withdrawal experience (both groups received morphine and naloxone on Friday of Weeks 1–4). The groups also did not differ in average weight at any time during training or testing (data not shown). It is possible that a slight increase in responding across weeks as seen in the Unpaired group was masked in the Paired group by a modest conditioning to the operant context. As a result of the modest change in operant responding over time in one group, performance of each group within a given Conditioning or Test Week was always expressed as a percentage of its baseline (Thursday) responses/10 min for the same week.
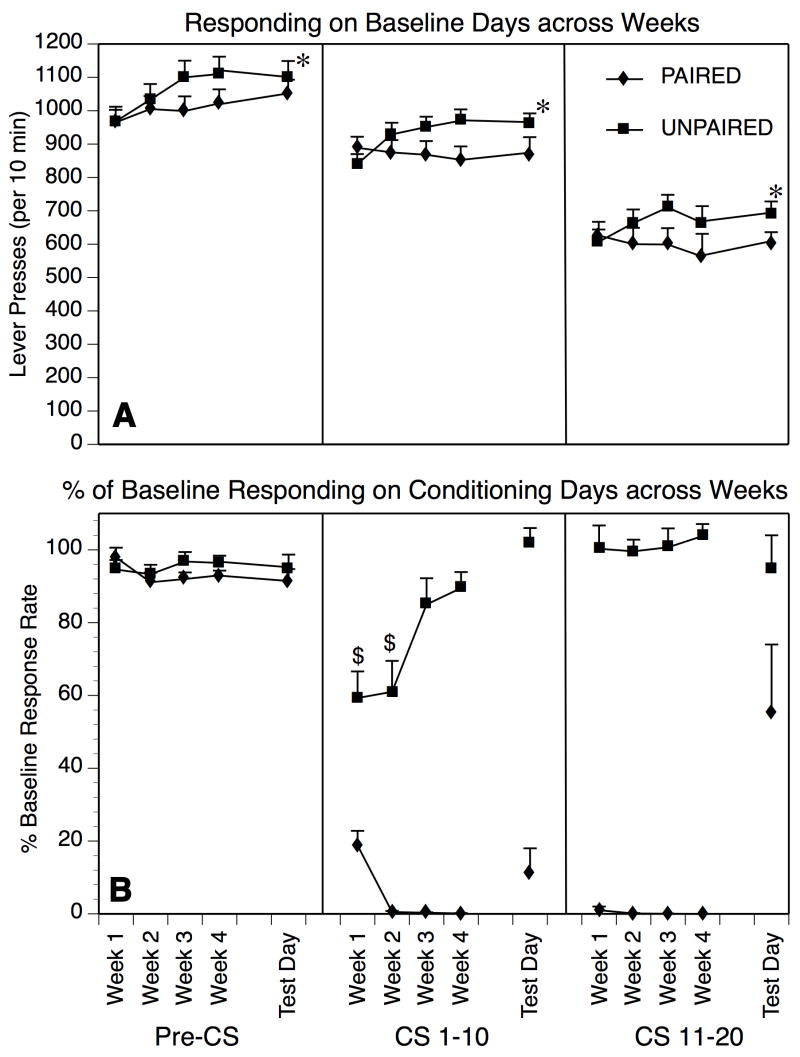
Fig. 2.
A) Baseline responding across Conditioning and Test weeks for Experiment 2. Operant responding declined in both Paired and Unpaired groups as a function of CS interval (Pre-CS, CS 1–10, CS 11–20). Rats in the Unpaired group showed a modest increase in responding across conditioning weeks, and this increase was not seen in the Paired group (*p < 0.05, treatment × conditioning week interaction). B) Responding on Conditioning Days across weeks. There was no significant change in responding in the Pre-CS interval across Conditioning Weeks. A transient decrease in responding was noted in the CS 1–10 interval when the CS was first introduced to the Unpaired group ($p < 0.05 vs. Pre-CS interval in the corresponding Conditioning Week. This effect was masked in the Paired groups that also received naloxone-precipitated withdrawal (1.0 mg/kg) immediately prior to CS exposure. The decline in responding in the Unpaired group during Conditioning Weeks 1–2 was not present in the CS 11–20 interval; the Paired group showed essentially 100% suppression of responding in the CS 11–20 interval in all Conditioning Weeks.
As shown in Figure 2B, responding during Conditioning Days remained constant across consecutive Conditioning sessions in the Pre-CS interval in both Paired and Unpaired conditions, as confirmed by lack of any significant main effect of treatment, Conditioning Week, or treatment × Conditioning Week interaction (F’s,< 2.54, p’s > 0.10). In both CS intervals (CS 1–10, CS 11–20), responding in the Paired group on Conditioning Days was suppressed almost entirely within 2 sessions (Figure 2B). In contrast, responding in the Unpaired group was modestly suppressed in the CS 1–10 interval below Pre-CS responding during the first two conditioning sessions, but this suppression dissipated across Conditioning Weeks as subjects habituated to the CS (simple main effect of Conditioning Week: F[3,39] = 13.45, p < 0.0001). Notably, the Unpaired control group showed no significant decline in responding in the CS 11–20 interval (Figure 2B; simple main effect of Conditioning Week: F[3,39] = 0.14, p > 0.90).
On the Test Day rats from the Paired and Unpaired groups were further subdivided into cohorts receiving morphine (Paired-Mor, Unpaired-Mor) or vehicle (Paired-Veh, Unpaired-Veh) 4 hr prior to the test session as described above. The CS dramatically suppressed responding in both Paired groups relative to their corresponding Unpaired groups during the CS session (Figure 3). A three-factor ANOVA with pairing condition (Paired, Unpaired) and pretreatment on Test Day (Veh, Mor) as between-subjects factors and operant interval as a within-subjects factor revealed a significant pairing condition × operant interval interaction (F[2,46] = 27.86, p < 0.0001), as well as significant main effects of pairing condition (F[1,23] = 44.93, p < 0.0001) and operant interval (F[2,46] = 18.17, p < 0.0001). There was no main effect of pretreatment on Test Day, nor was any interaction involving this factor significant (all F’s < 0.79, p’s > 0.46), suggesting that the presence of morphine on the Test Day did not substantially alter the conditioned withdrawal response. Follow-up analyses indicated no significant main effect of pairing condition in the Pre-CS Interval (F[1,23] = 1.08, p > 0.31), but a highly reliable main effect of pairing condition in both the CS 1–10 (F[1,23] = 217, p < 0.0001) and CS 11–20 intervals (F[1,23] = 7.25, p < 0.02). Moreover, repeated measures ANOVA within each experimental sub-group revealed highly significant effects of operant interval in both the Paired-Mor (F[2,12] = 17.58, p < 0.0001) and Paired-Veh (F[2,10] = 12.79, p < 0.005) groups, but not in either Unpaired group (F’s < 1.95, p’s > 0.15). Finally, individual means comparisons in the Paired-Mor group revealed that responding in both the CS 1–10 and CS 11–20 intervals was significantly lower than responding in the Pre-CS interval. In the Paired-Veh group, only the CS 1–10 interval differed significantly from the Pre-CS interval. However, the slight difference in responding during CS 11–20 between Paired-Mor and Paired-Veh groups was not statistically reliable, and both groups showed a significant increase in responding from the CS 1–10 to the CS 11–20 interval, again suggesting a degree of within-session extinction of the conditioned suppression.
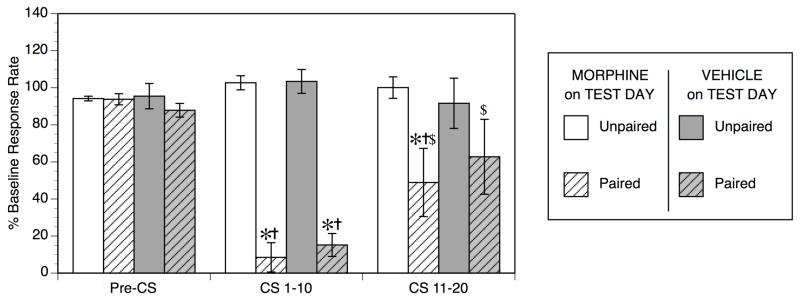
Fig. 3.
Operant responding is suppressed only by cue exposure on the Test Day following 4 weekly Conditioning Sessions in Experiment 2. Operant responding during the Pre-CS interval did not differ between Unpaired and Paired groups. Responding during the CS 1–10 interval were suppressed equally in both Paired groups relative to their corresponding Unpaired control groups (*p < 0.05 vs. Unpaired control), and relative to Pre-CS responding in the same Paired group (†p < 0.05 vs. Pre-CS in same group). Responding during the CS 11–20 interval session were still suppressed in the Paired-Mor group relative to Pre-CS responding in the same group (†p < 0.05), and relative to the Unpaired-Mor group (*p < 0.05 vs. Unpaired control in same CS interval). Both Paired-Mor and Paired-Veh groups showed significant extinction of the conditioned suppression response in the CS 11–20 interval ($p < 0.05 vs. same group in CS 1–10 interval).
DISCUSSION
Previously we reported that the potency of the opioid antagonist naloxone to precipitate withdrawal from acute bolus doses of morphine is potentiated by re-exposure to a context (Schulteis et al., 2003,2004) or a discrete tone/light stimulus (Schulteis et al., 2005) that was repeatedly paired with antagonist-induced withdrawal. Herein we demonstrate for the first time that repeated experience of acute withdrawal at daily (Experiment 1) or weekly (Experiment 2) intervals in the presence of the discrete CS subsequently elicits symptoms of withdrawal in response to the CS alone (i.e. the antagonist is not required on Test Day). This effect was not observed in control groups that had not experienced acute withdrawal during exposure to the CS (Nal-Naive group), or had experienced repeated naloxone-induced withdrawal in the absence of the CS (Unpaired controls). This reinforces the assumption that the antagonist-dependent component of potentiation of withdrawal severity observed in our prior studies (Schulteis et al., 1999, 2003, 2004, 2005) was the result of conditioning processes.
As in these prior studies, the effects of operant context could not be entirely eliminated when rats were repeatedly exposed to naloxone-induced withdrawal at daily intervals within the operant environment (Experiment 1). This was evident in the fact that suppression of responding emerged across Conditioning Days in both the Paired-CS and Paired-No CS groups during the Pre-CS session (Figure 1), prior to onset of the tone/light CS. Although the subjects had been training in the operant chambers for a number of weeks prior to the initial acute withdrawal experience, recent theories of contextual conditioning recognize that a “context” consists not merely of fixed geometric and multimodal sensory (visual, tactile, olfactory, etc.) features of the environment but also includes temporal or episodic context (Anagnostaras et al., 2001; Anderson and Jeffery, 2003; Moser and Paulsen, 2001; Sharp, 1999). As argued previously (Schulteis et al., 2004, 2005), the predictive context in our daily acute morphine withdrawal regimen likely includes features such as the drug injection regimen and perhaps even the interoceptive cues provided by the drugs themselves (Adams and Holtzman, 1990; McDonald and Siegel, 2004; Sokolowska et al., 2002).
In the Paired-No CS group of Experiment 1, suppression of responding on the Test Day was greatest during the Pre-CS interval, and responding recovered significantly during subsequent components of the operant session, whereas responding in the Paired-CS group declined further from the Pre-CS to the CS 1–10 interval (Figure 1B). This suggests that the addition of a more predictive stimulus (tone/light CS) that had not been experienced prior to the first precipitated-withdrawal episode can overshadow to a degree the more familiar components of the operant context. However, this effect was not complete, and a significant suppression in the Pre-CS interval on Conditioning and Test Days in the Paired groups was still evident, suggesting the influence of other contextual features had not been eliminated entirely (Figure 1A and 1B).
In addition, a modest suppression of responding unexpectedly emerged across Conditioning Days during the latter component of the CS interval (CS 11–20) in the Unpaired and Nal-Naive control groups (Figure 1A). It is possible that this effect may reflect the development of a spontaneous withdrawal-induced suppression of responding in these groups who still received morphine at daily intervals; the modest influence of spontaneous withdrawal would be masked in the Paired groups by the profound suppression precipitated by 1 mg/kg of naloxone. We have previously noted such modest spontaneous withdrawal effects in acute dependence studies where morphine was administered 4 or more times at daily intervals (e.g. Liu and Schulteis, 2004). Thus, a modification of our daily withdrawal conditioning procedure (Experiment 1) seemed necessary to further narrow the range of factors capable of altering operant responding on the Test Day.
In Experiment 2 a shift to a weekly conditioning procedure enabled the interspersion of several withdrawal-free experiences in the operant environment between each successive Conditioning Session (see Table 2). Administration of vehicle infusions prior to some of these withdrawal-free operant sessions helped to reduce the salience of the drug injection regimen as predictive of withdrawal. Notably, there was no significant suppression of responding in any of the groups in Experiment 2 in the absence of the CS (i.e. during the Pre-CS session), but the CS itself elicited a potent suppression of operant responding during the CS interval on the Test Day in Paired but not Unpaired groups (Figure 3). The Paired-Mor and Paired-Veh sub-groups demonstrated an equivalent level of response suppression in the CS 1–10 interval, with partial extinction of the conditioned suppression evident in the CS 11–20 interval in both groups. Only the Paired-Mor group was significantly different from its Unpaired control group during the CS 11–20 interval, but the Paired-Mor and Paired-Veh groups did not differ from each other. This pattern of results indicates that while it is possible that the presence of a morphine cue on Test Day may contribute modestly to the stimulus complex that elicits conditioned suppression, the morphine cue is by no means necessary to demonstrate reliable conditioning.
Partial extinction of the response on the Test Day within a single 20 min test session is not unexpected, since the operant session on that day involves re-exposure to the CS in absence of the potent naloxone withdrawal stimulus. Consistent with this interpretation, the loss of conditioned suppression over the course of a 20 min test session was not seen in our earlier study where low doses of naloxone (0.003–0.03 mg/kg) were administered 5 min prior to the onset of the CS Session on the Test Day (Schulteis et al., 2005). Moreover, partial extinction of the conditioned withdrawal response is also observed in rats withdrawn from chronic morphine exposure (Baldwin and Koob, 1993; Schulteis et al., 2000). In fact, the magnitude and duration of the conditioned withdrawal response reported herein is comparable to that reported in these prior studies with chronically dependent subjects, although substantially lower doses of naloxone (0.025–0.03 mg/kg) are required during conditioning in the chronic dependence model.
It is self-evident that frequency of morphine administration varied dramatically from Experiment 1 (daily) to Experiment 2 (weekly). Earlier work indicated that while the magnitude of increase in naloxone-induced suppression of operant responding was similar whether subjects received repeated morphine and naloxone at daily or weekly intervals, somatic signs of withdrawal showed progressive increases in severity only with daily intervals between successive morphine/naloxone treatments (Schulteis et al., 1999). The most prominent somatic signs of withdrawal from acute or repeated daily injections of 5.6 mg/kg of morphine are teeth chattering, swallowing movements, eye blinks, and mild irritability upon touch (Schulteis et al., 1997, 1999). Profound signs such as body weight loss, diarrhea, and wet dog shakes (Blasig and Herz, 1977; Blasig et al., 1973; Gellert and Holtzman, 1978; Linseman, 1977; Martin et al., 1963; Schulteis et al., 1994; Wei et al., 1973) are rarely observed under such conditions of low-dose morphine treatment, even after 3–4 repeated daily treatments. Nonetheless, these results suggest that subjects in Experiment 1 receiving morphine and naloxone daily may have experienced more significant somatic symptoms of withdrawal as conditioning proceeded than subjects in Experiment 2. It is possible therefore that this factor in addition to our systematic attempts to lessen the predictive value of operant contextual elements (aside from the tone/light CS) and drug injection regimen may have contributed to the marked differences in results between experiments.
Regardless, the results of Experiment 2 clearly indicate that acute morphine withdrawal experienced at weekly intervals can be rapidly and selectively associated with a discrete CS. This further supports our hypothesis based upon earlier work (Schulteis et al., 2003, 2004, 2005) that conditioning mechanisms can play a significant role at the very onset of opioid dependence. However the current study also represents a significant refinement over these earlier studies with its definitive demonstration that the conditioned response can be evoked selectively by a discrete CS, with minimal influence of extraneous contextual elements (operant environment, injection regimen, interoceptive morphine and/or naloxone cues). It has been argued that a thorough understanding of the transition from occasional use of drugs of abuse to loss of control and habitual use (i.e. addiction) will require a thorough understanding of the neuroanatomical, neurochemical, and cellular/molecular substrates of both direct (unconditioned) as well as conditioned neuroadaptive responses (Everitt et al., 2001; Koob and Le Moal, 2001). Our model as developed and refined herein should prove useful in the delineation of the neural circuits underlying unconditioned and conditioned components of the withdrawal response from acute opioid dependence, at a time when intervention is likely to be of greatest promise.
Acknowledgments
Work Supported by: NIH grant DA010475 and VA Merit Award to GS
References
- Adams JU, Holtzman SG. Pharmacologic characterization of the sensitization to the rate-decreasing effects of naltrexone induced by acute opioid pretreatment in rats. J Pharmacol Exp Ther. 1990;253:483–9. [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Anderson MI, Jeffery KJ. Heterogeneous modulation of place cell firing by changes in context. J Neurosci. 2003;23:8827–35. doi: 10.1523/JNEUROSCI.23-26-08827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar MR, Jones BC, Schulteis G. Conditioned place aversion is a highly sensitive index of acute opioid dependence and withdrawal. Psychopharmacology (Berl) 2003;170:42–50. doi: 10.1007/s00213-003-1514-y. [DOI] [PubMed] [Google Scholar]
- Azorlosa JL, Stitzer ML, Greenwald MK. Opioid physical dependence development: effect of single versus repeated morphine pretreatments and of subjects’ opioid exposure history. Psychopharmacology (Berl) 1994;114:71–80. doi: 10.1007/BF02245446. [DOI] [PubMed] [Google Scholar]
- Baldwin HA, Koob GF. Rapid induction of conditioned opiate withdrawal in the rat. Neuropsychopharmacology. 1993;8:15–21. doi: 10.1038/npp.1993.3. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Stitzer ML, Liebson IA, Bigelow GE. Acute physical dependence in man: effects of naloxone after brief morphine exposure. J Pharmacol Exp Ther. 1988;244:126–32. [PubMed] [Google Scholar]
- Blasig J, Herz A. Precipitated morphine withdrawal as a tool in opiate research. Curr Dev Psychopharmacol. 1977;4:129–49. [PubMed] [Google Scholar]
- Blasig J, Herz A, Reinhold K, Zieglgansberger S. Development of physical dependence on morphine in respect to time and dosage and quantification of the precipitated withdrawal syndrome in rats. Psychopharmacologia. 1973;33:19–38. doi: 10.1007/BF00428791. [DOI] [PubMed] [Google Scholar]
- Childress AR, Ehrman R, McLellan AT, MacRae J, Natale M, O’Brien CP. Can induced moods trigger drug-related responses in opiate abuse patients? J Subst Abuse Treat. 1994;11:17–23. doi: 10.1016/0740-5472(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, Natale M, O’Brien CP. Mood states can elicit conditioned withdrawal and craving in opiate abuse patients. NIDA Res Monogr. 1987;76:137–44. [PubMed] [Google Scholar]
- Easterling KW, Holtzman SG. Intracranial self-stimulation in rats: sensitization to an opioid antagonist following acute or chronic treatment with mu opioid agonists. J Pharmacol Exp Ther. 1997;281:188–99. [PubMed] [Google Scholar]
- Easterling KW, Holtzman SG. In rats, acute morphine dependence results in antagonist-induced response suppression of intracranial self-stimulation. Psychopharmacology (Berl) 2004;175:287–95. doi: 10.1007/s00213-004-1829-3. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Rev. 2001;36:129–38. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Gellert VF, Holtzman SG. Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. J Pharmacol Exp Ther. 1978;205:536–46. [PubMed] [Google Scholar]
- Goldberg SR, Schuster CR. Conditioned suppression by a stimulus associated with nalorphine in morphine-dependent monkeys. J Exp Anal Behav. 1967;10:235–42. doi: 10.1901/jeab.1967.10-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Stitzer ML, Bigelow GE, Liebson IA. Acute opioid physical dependence in humans: effect of varying the morphine-naloxone interval. I. J Pharmacol Exp Ther. 1989a;250:485–91. [PubMed] [Google Scholar]
- Heishman SJ, Stitzer ML, Bigelow GE, Liebson IA. Acute opioid physical dependence in postaddict humans: naloxone dose effects after brief morphine exposure. J Pharmacol Exp Ther. 1989b;248:127–34. [PubMed] [Google Scholar]
- Jones R. Dependence in non-addict humans after a single dose of morphine. In: Way E, editor. Endogenous and exogenous opiate agonists and antagonists. Pergamon Press; New York: 1980. pp. 557–560. [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Linseman MA. Naloxone-precipitated withdrawal as a function of the morphine-naloxone interval. Psychopharmacology (Berl) 1977;54:159–64. doi: 10.1007/BF00426773. [DOI] [PubMed] [Google Scholar]
- Liu J, Schulteis G. Brain reward deficits accompany naloxone-precipitated withdrawal from acute opioid dependence. Pharmacol Biochem Behav. 2004;79:101–8. doi: 10.1016/j.pbb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Martin WR, Eades CG. A Comparison between Acute and Chronic Physical Dependence in the Chronic Spinal Dog. J Pharmacol Exp Ther. 1964;146:385–94. [PubMed] [Google Scholar]
- Martin WR, Wikler A, Eades CG, Pescor FT. Tolerance to and physical dependence on morphine in rats. Psychopharmacologia. 1963;4:247–60. doi: 10.1007/BF00408180. [DOI] [PubMed] [Google Scholar]
- McDonald RV, Siegel S. Intra-administration associations and withdrawal symptoms: Morphine-elicited morphine withdrawal. Exp Clin Psychopharmacol. 2004;12:3–11. doi: 10.1037/1064-1297.12.1.3. [DOI] [PubMed] [Google Scholar]
- Meyer DR, Sparber SB. Evidence of possible opiate dependence during the behavioral depressant action of a single dose of morphine. Life Sci. 1977;21:1087–94. doi: 10.1016/0024-3205(77)90106-0. [DOI] [PubMed] [Google Scholar]
- Moser EI, Paulsen O. New excitement in cognitive space: between place cells and spatial memory. Curr Opin Neurobiol. 2001;11:745–51. doi: 10.1016/s0959-4388(01)00279-3. [DOI] [PubMed] [Google Scholar]
- O’Brien CP. In: Drug addiction and drug abuse. Hardman J PhD, Limbird LE PhD, Molinoff PB MD, Ruddon RW MD, PhD, Gilman AG MD, PhD, DSc(Hon), editors. McGraw-Hill; 1996. pp. 557–577. [Google Scholar]
- O’Brien CP, Testa T, O’Brien TJ, Greenstein R. Conditioning in human opiate addicts. Pavlov J Biol Sci. 1976;11:195–202. doi: 10.1007/BF03000314. [DOI] [PubMed] [Google Scholar]
- Parker LA, Joshi A. Naloxone-precipitated morphine withdrawal induced place aversions: effect of naloxone at 24 hours postmorphine. Pharmacol Biochem Behav. 1998;61:331–3. doi: 10.1016/s0091-3057(98)00104-x. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Ahmed SH, Morse AC, Koob GF, Everitt BJ. Conditioning and opiate withdrawal. Nature. 2000;405:1013–4. doi: 10.1038/35016630. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Heyser CJ, Koob GF. Opiate withdrawal signs precipitated by naloxone following a single exposure to morphine: potentiation with a second morphine exposure. Psychopharmacology (Berl) 1997;129:56–65. doi: 10.1007/s002130050162. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Heyser CJ, Koob GF. Differential expression of response-disruptive and somatic indices of opiate withdrawal during the initiation and development of opiate dependence. Behav Pharmacol. 1999;10:235–42. doi: 10.1097/00008877-199905000-00001. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Koob GF. Reinforcement processes in opiate addiction: a homeostatic model. Neurochem Res. 1996;21:1437–54. doi: 10.1007/BF02532385. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Liu J, Amitai N, Tzeng S. Context- and cue-conditioned potentiation of acute morphine dependence and withdrawal. Pharmacol Biochem Behav. 2005;82:82–9. doi: 10.1016/j.pbb.2005.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Morse AC, Liu J. Repeated experience with naloxone facilitates acute morphine withdrawal: potential role for conditioning processes in acute opioid dependence. Pharmacol Biochem Behav. 2003;76:493–503. doi: 10.1016/j.pbb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Morse AC, Liu J. Conditioning processes contribute to severity of naloxone-precipitated withdrawal from acute opioid dependence. Psychopharmacology (Berl) 2004;175:463–72. doi: 10.1007/s00213-004-1843-5. [DOI] [PubMed] [Google Scholar]
- Sharp PE. Complimentary roles for hippocampal versus subicular/entorhinal place cells in coding place, context, and events. Hippocampus. 1999;9:432–43. doi: 10.1002/(SICI)1098-1063(1999)9:4<432::AID-HIPO9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Siegel S. Morphine analgesic tolerance: Its situation specificity supports a Pavlovian conditioning model. Science. 1976;193:323–25. doi: 10.1126/science.935870. [DOI] [PubMed] [Google Scholar]
- Siegel S. Pavlovian conditioning and heroin overdose: Reports by overdose victims. Bull Psychonomic Soc. 1984;22:428–30. [Google Scholar]
- Siegel S, Ellsworth DW. Pavlovian conditioning and death from apparent overdose of medically prescribed morphine: A case report. Bull Psychonomic Soc. 1986;24:278–80. [Google Scholar]
- Siegel S, Hinson RE, Krank MD, McCully J. Heroin “overdose” death: Contribution of drug-associated environmental cues. Science. 1982;216:436–37. doi: 10.1126/science.7200260. [DOI] [PubMed] [Google Scholar]
- Sokolowska M, Siegel S, Kim JA. Intraadministration associations: conditional hyperalgesia elicited by morphine onset cues. J Exp Psychol Anim Behav Process. 2002;28:309–20. [PubMed] [Google Scholar]
- Way EL, Loh HH, Shen FH. Simultaneous quantitative assessment of morphine tolerance and physical dependence. J Pharmacol Exp Ther. 1969;167:1–8. [PubMed] [Google Scholar]
- Wei E, Loh HH, Way EL. Quantitative aspects of precipitated abstinence in morphine-dependent rats. J Pharmacol Exp Ther. 1973;184:398–403. [PubMed] [Google Scholar]
- Wikler A. Some implications of conditioning theory for problems of drug abuse. Behav Sci. 1971;16:92–7. doi: 10.1002/bs.3830160108. [DOI] [PubMed] [Google Scholar]
- Wikler A. Dynamics of drug dependence. Implications of a conditioning theory for research and treatment. Arch Gen Psychiatry. 1973;28:611–6. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- Wikler A, Pescor FT. Classical conditioning of a morphine abstinence phenomenon, reinforcement of opioid-drinking behavior and “relapse” in morphine-addicted rats. Psychopharmacologia. 1967;10:255–84. doi: 10.1007/BF00401386. [DOI] [PubMed] [Google Scholar]
- Young AM. Effects of acute morphine pretreatment on the rate-decreasing and antagonist activity of naloxone. Psychopharmacology (Berl) 1986;88:201–8. doi: 10.1007/BF00652241. [DOI] [PubMed] [Google Scholar]