Abstract
Human metapneumovirus (hMPV) is a major cause of lower respiratory tract infections (LRTIs) in infants, elderly and immunocompromised patients. In this study, we show that hMPV can infect in a similar manner epithelial cells representative of different tracts of the airways. hMPV-induced expression of chemokines IL-8 and RANTES in primary small alveolar epithelial cells (SAE) and in a human alveolar type II-like epithelial cell line (A549) was similar, suggesting that A549 cells can be used as a model to study lower airway epithelial cell responses to hMPV infection. A549 secreted a variety of CXC and CC chemokines, cytokines and type I interferons, following hMPV infection. hMPV was also a strong inducer of transcription factors belonging to nuclear factor (NF)-κB, interferon regulatory factors (IRFs) and signal transducers and activators of transcription (STATs) families, which are known to orchestrate the expression of inflammatory and immuno-modulatory mediators.
Keywords: hMPV, airway epithelial cells, chemokines, inflammation, transcription factors
Introduction
Human metapneumovirus (hMPV), first identified from children with respiratory symptoms in 2001, is a significant respiratory pathogen worldwide (van den Hoogen, de Jong et al., 2001; Falsey, Erdman et al., 2003;Kahn, 2006). hMPV is a member of the genus Metapneumovirus of the family Paramyxoviridae, based on genomic sequence analysis and organization (van den Hoogen, Bestebroer et al., 2002). Studies have shown that hMPV infects ~ 46% of children <1 year, ~70% of children by the time of age 5 and virtually all children by the ages of 5–10 (Principi, Bosis et al., 2006; Williams, Harris et al., 2004). hMPV accounts for not only 10–15% of pediatrics hospitalization but also for 10% of all hospitalizations of elderly patients with respiratory tract infections (Falsey, Erdman, Anderson, & Walsh, 2003). Furthermore, hMPV is a significant cause of morbidity and mortality in immunocompromised patients and patients with chronic obstructive pulmonary disease (van den Hoogen, van Doornum et al., 2003; Debiaggi, Canducci et al., 2006; Dare, Sanghavi et al., 2007).
hMPV-induced clinical illnesses range from mild upper respiratory tract infections to severe bronchiolitis and pneumonia (van den Hoogen, van Doornum et al., 2003). Investigations done in animal models of infection have shown that hMPV infection induced important pulmonary inflammation, characterized by alveolitis, interstitial inflammation and increased peribronchiolitis (Alvarez, Harrod et al., 2004; Hamelin, Yim et al., 2005; Wyde, Chetty et al., 2005; Williams, Tollefson et al., 2005). Although hMPV shares similar epidemiological and clinical features with RSV (Principi, Bosis, & Esposito, 2006; Williams, Wang et al., 2006), we and others have shown that they induce a different spectrum of cytokines and chemokines both in vitro, using dendritic cells, and in vivo, either in a mouse model of infection, or in children, suggesting a different ability of these two viruses to induce cellular responses (Guerrero-Plata, Casola et al., 2006; Guerrero-Plata, Casola et al., 2005; Jartti, van den Hoogen et al., 2002; Laham, Israele et al., 2004).
Similar to RSV, airway epithelial cells are the primary target of hMPV infection (Biacchesi, Pham et al., 2006; Alvarez, Harrod et al., 2004). However, little is known of their response to hMPV infection. In this study, we show that hMPV could infect and replicate similarly in epithelial cells from various tracts of the airways. hMPV infection of primary small alveolar epithelial cells (SAE) or of an alveolar type II-like epithelial cell line (A549) induced similar expression of the chemokines RANTES and IL-8, suggesting that A549 cell line can be used as a model to study the interaction of hMPV with lower airway epithelial cells, similar to what we have previously shown for RSV infection (Zhang, Luxon et al., 2001). We then determined whether hMPV induced the production of other inflammatory/immunomodulatory mediators. We found that A549 cells secreted a variety of cytokines and chemokines upon hMPV stimulation. Since activation of transcription factors belonging to the NF-κB and IRF families are critical for the expression of many cytokines and chemokines (Siebenlist, Franzoso et al., 1994; Taniguchi, Ogasawara et al., 2001; Barnes, Lubyova et al., 2002), we also investigated the ability of hMPV to induce their nuclear translocation and DNA-binding to IL-8 and RANTES promoter binding sites. Our results show that hMPV was a strong inducer of p50 and p65, which are members of the NF-κB families, as well as of IRF-1, -3 and -7. Finally, we investigated the ability of hMPV to induce type I interferons and to activate IFN-dependent signaling molecules. HMPV-infected A549 cells secreted significant amounts of both IFN-α and IFN-β. In addition, STAT1, STA2 and IRF-9, which form the intracellular ISGF3 complex necessary to induce antiviral genes (Martinez-Moczygemba, Gutch et al., 1997), were strongly activated following infection with hMPV.
Results
hMPV replication in epithelial cells
hMPV is a recently identified respiratory virus and there is limited knowledge of the response of airway epithelial cells, the target of infection, to this virus. Here, we used normal bronchial epithelial cells (NHBE), SAE and A549 cells, which represent cells from bronchial, terminal bronchioli and alveolar type II-like epithelial cells, respectively, to determine whether hMPV replicates similarly in cells from different tract of the airways. Cells were infected with hMPV at MOI of 3 and viral presence was analyzed by immunofluorescence, using an anti-hMPV polyclonal antibody, at 48 h post-infection (p.i.). Infected cells showed typical intracytoplasmic fluorescent granular inclusions when stained with the specific antibody, but not with isotope control (Fig. 1). Similarly, uninfected cells did not show granular staining with either antibody (data not shown). For each cell type, several pictures were taken and the percentage of cells which showed hMPV staining was counted over the total number of cells examined. All three cell type showed a similar percentage of infection, which range from 80 to 90% (data not shown).
Fig. 1. Immunofluorescence of hMPV-infected human airway epithelial cells.
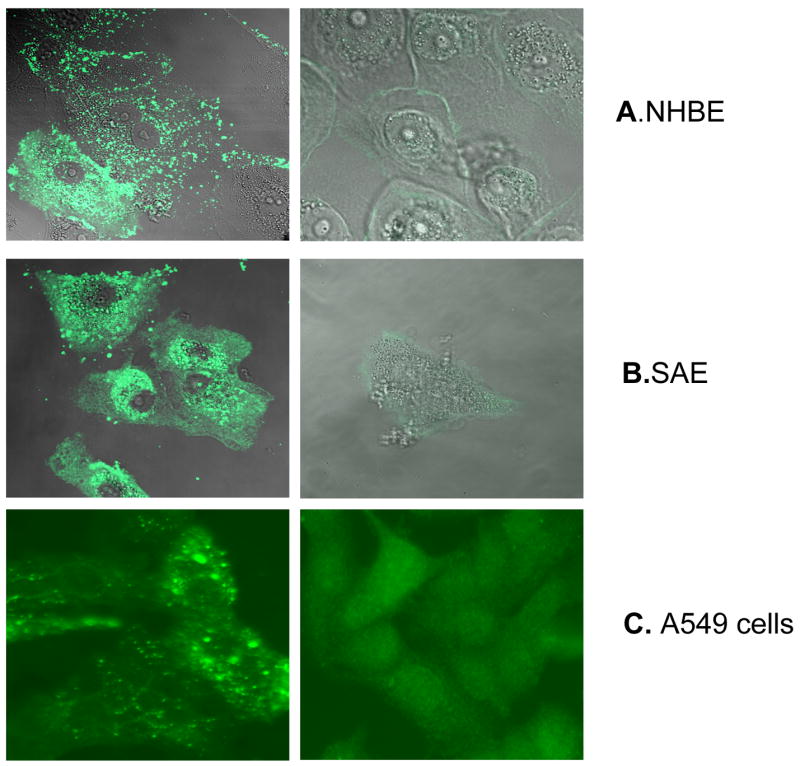
Normal bronchial epithelial cells (NHBE, A), small airway epithelial cell (SAE, B) and human alveolar type II-like epithelial cell line (A549, C) were infected with hMPV at MOI 3 for 48 h and stained with nti-hMPV polyclonal antibody (left panel) or isotype antibody (right panel). Cells were analyzed using a Zeiss LSM 510 meta-ultraviolet laser scan confocal microscope for A and B or fluorescent microcopy for C. No staining was observed in mock-infected cells when similar staining was done (data not shown).
We then investigated hMPV ability to replicate in NHBE, SAE and A549 cells. Cells were infected at MOI of 0.1, viral inoculum was removed after one hour of incubation, and cells were harvested at 72 h p.i. to determine viral titer. LLC-MK2 cells, the cell line used to propagate hMPV, were used for comparison. We could not investigate viral replication at later time points of infections, above 72h p.i., because there was significant cell death in NHBE and SAE, compared to A549 cells, likely due to the absence of serum required for the infection. We found that, although hMPV replication in NHBE, SAE and A549 was a log less than in LLC-MK2 cells, viral titers among the different airway epithelial cells were very similar (Table 1), indicating that they are equally permissive to hMPV infection. On the other hand, there was no detectable replication of hMPV following inactivation with UV light (data not shown).
Table 1.
hMPV replication in primary airway epithelial cells and cell lines
| Cell type | Viral titer (pfu/ml) |
|---|---|
| NHBE | 1.6 ×104 |
| SAE | 1.6 ×104 |
| A549 | 2.15 ×104 |
| Hep2 | 4.08 ×105 |
| LLC-MK2 | 8.5 ×105 |
A549 and SAE cells secrete similar mediators following hMPV infection
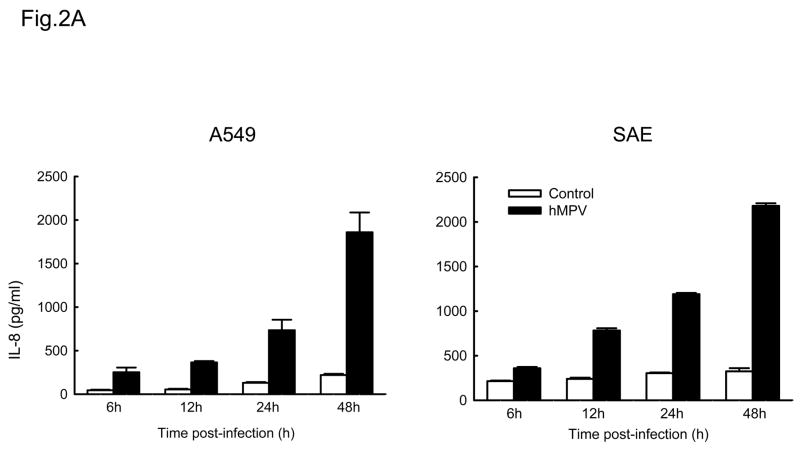
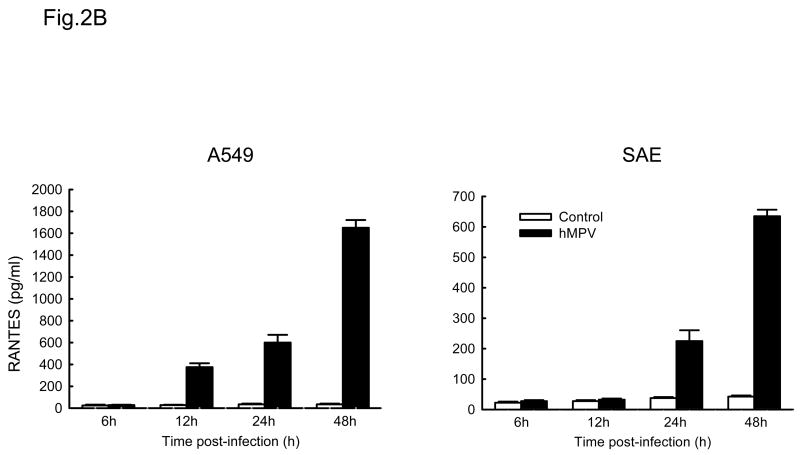
IL-8 and RANTES are prototypes of CXC and CC chemokines, respectively (Miller & Krangel, 1992). To determine whether A549 and SAE cells secreted similar mediators following hMPV infection, supernatants from control or hMPV-infected cells were harvested at different time point of infection to measure IL-8 and RANTES protein by ELISA. As shown in Fig. 2A, hMPV infection induced a similar pattern of IL-8 secretion in both A549 and SAE cells, with induction starting from 6 h p.i. For RANTES, we found that there was a slight delayed kinetic of RANTES production in SAE cells (Fig. 2B), compared to A549 cells, similar to what we have previously shown for RSV infection (Zhang Y, Luxon et al., 2001).
Fig. 2. Chemokines induced by hMPV in A549 and SAE cells.
A549 and SAE cells were infected with hMPV at MOI of 3 for various length of time. Secretion of IL-8 (A) and RANTES (B) by hMPV-infected A549 and SAE cells was assayed by ELISA. Data are expressed as mean ± standard error of two independent experiments performed in triplicates.
To determine whether chemokine induction in epithelial cells in response to hMPV infection was dependent on viral replication, A549 cells were infected with either live or UV-inactivated virus. At 24 h p.i. cell supernatants were harvested to measure RANTES and IL-8 as described above. Only A549 cells infected with live hMPV released significant amounts of both chemokines, while UV-inactivated hMPV failed to do so (data not shown), indicating that chemokine secretion by hMPV requires viral replication.
A549 cells secret multiple pro-inflammatory mediators upon hMPV infection
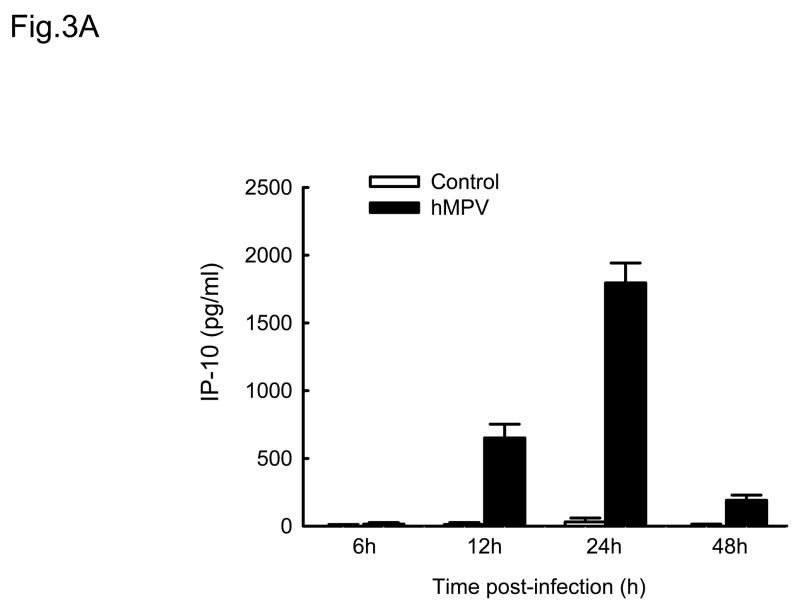
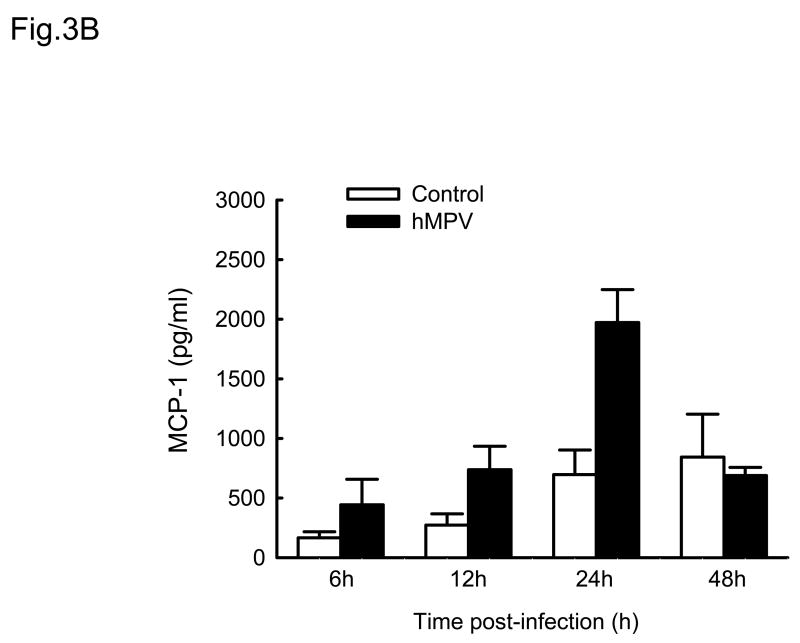
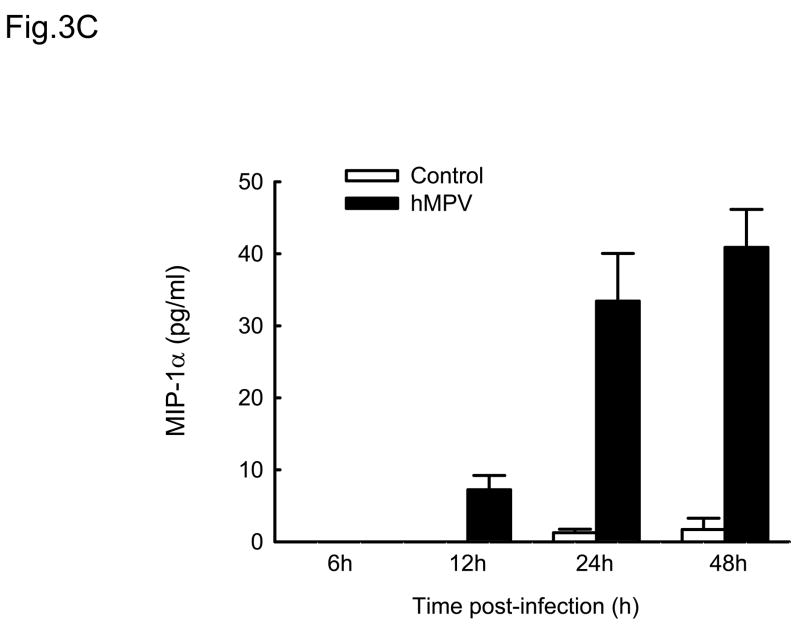
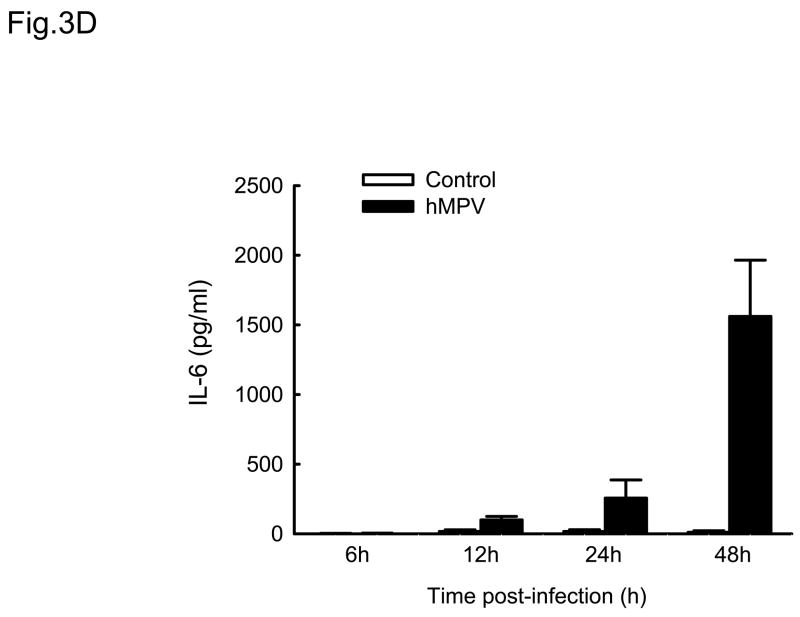
Cytokines and chemokines secreted by airway epithelial cells play a critical role in initiating and modulating pulmonary inflammation upon viral infections. Chemokines are small proteins that control cellular migration at the site of infection and their family in mammals contains nearly 50 members. Depending on the arrangement of the first two N-terminal cysteines, chemokines are divided into four subfamilies: CXC, CC, C and CX3C (Baggiolini, Dewald et al., 1997). The CXC subfamily is further divided in two groups, based on the absence or presence of three specific amino acids (Glu-Leu-Arg), which form the ELR motif (Baggiolini, Dewald et al., 1997). To further investigate the pattern of inflammatory/immune mediators secreted by airway epithelial cells in response to hMPV infection, we used multibead array system, which detects a panel of 24 different cytokines and chemokines. A549 cells were infected with hMPV, MOI of 3, for 6, 12, 24 and 48 h and then harvested to collect cell culture supernatants. We did not detect secretion of IL-1β, IL-2, IL-4, L-5, IL-7, IL-10, IL-12 (p70), IL-13, IL-15, IL-17, TNF-α and IFN-γ by A549 cells following hMPV infection, while we confirmed increased secretion of IL-8 and RANTES, as well as other CXC and CC chemokines (Fig. 3). IL-6 was the only cytokine significantly induced by hMPV infection (Fig. 3D). Among the CXC chemokines, hMPV induced secretion of IP-10 (Fig. 3A), an interferon-inducible protein, which has chemotactic activity for monocytes and T-lymphocytes and has been shown to play a role in limiting viral replication in other infection models (Dufour, Dziejman et al., 2002). IP-10 was secreted in significant amounts from infected epithelial cells starting at 12 h p.i., peaking at 24 h p.i. and declining at 48 h p.i. Among the CC chemokines, hMPV significantly induced the secretion of MCP-1 (Fig. 3B), a monocyte chemoattractant protein (Rollins, Yoshimura et al., 1990). The secretion pattern and intensity of MCP-1 from infected cells were similar to those of IP-10, although the basal level of MCP-1 was higher than that of IP-10. Regarding macrophage inflammatory protein 1α (MIP-1α), the secretion of control cells was under the detection limit (4 pg/ml) at all time points. Unlike the secretion of the other chemokines, MIP-1α secretion from hMPV-infected cells was not striking, only around 35 pg/ml at 24 h p.i and 40 pg/ml at 48 h p.i. respectively (Fig. 3C), which is consistent with our previous observation of low MIP-1α secretion in bronchoalveolar lavage of hMPV-infected mice (Guerrero-Plata, Casola, & Garofalo, 2005). The secretion patterns and intensity of MIP-1β was very similar to those of MIP-1α (data not shown). Among the tested cytokines, hMPV infection resulted in significant induction of IL-6 in A549 cells (Fig. 3D). Kinetics and intensity of IL-6 secretion were similar to that of IL-8 and RANTES (Fig. 2).
Fig. 3. Cytokine and chemokine production by A549 cells infected with hMPV.
A549 cells were infected with hMPV at MOI of 3. Cell supernatants were collected at different time points of infection and tested for cytokines and chemokine secretion by multiplex bead assay. Data are expressed as mean ± standard error of two independent experiments performed in triplicates. Open bars represent uninfected and solid bars hMPV-infected cells.
hMPV infection activates transcription factors of the NF-kB and IRF families
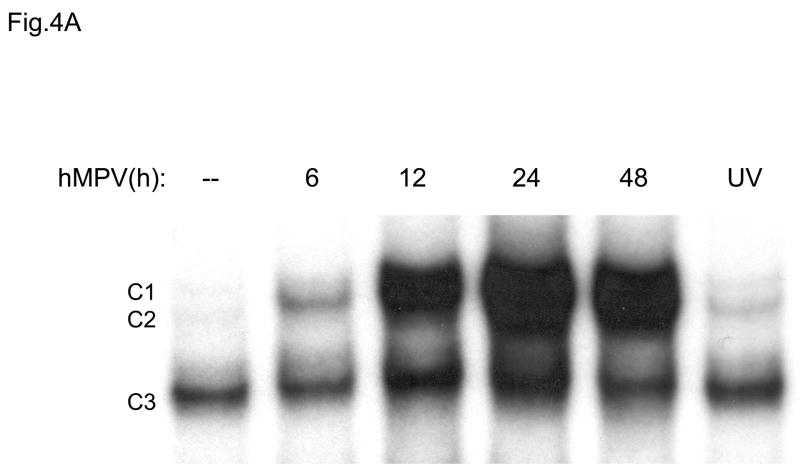
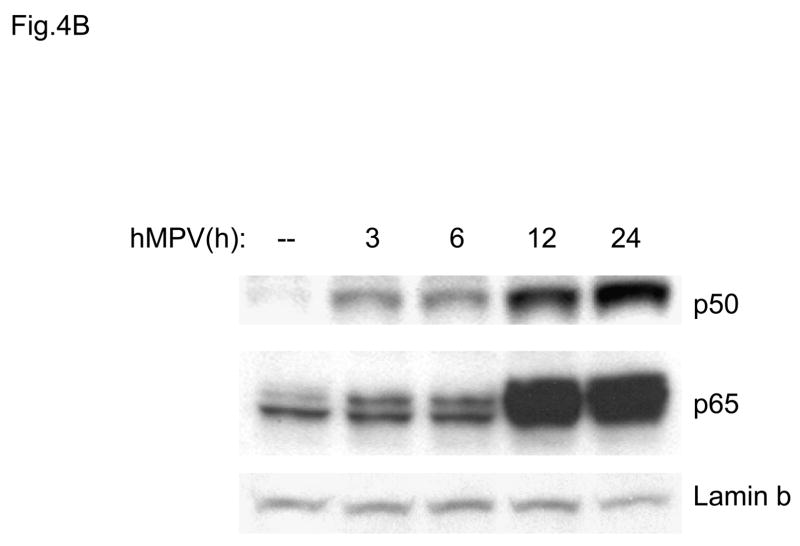
In previous studies we have characterized the mechanisms involved in RSV-induced expression of the chemokines IL-8 and RANTES. Both chemokines are dependent on an intact NF-κB signaling pathway for their transcription, as we and others have previously shown (Garofalo, Sabry et al., 1996; Casola, Garofalo et al., 2001; Tian B, Zhang Y et al., 2002). RSV-induced RANTES expression is also dependent on the activation of IRF proteins which are absolutely necessary for RANTES promoter induction (Garofalo, Sabry, Jamaluddin, Yu, Casola, Ogra, & Brasier, 1996;Casola, Garofalo, Haeberle, Elliott, Lin, Jamaluddin, & Brasier A.R., 2001). NF-κB is a superfamily of ubiquitous transcription factors composed of NF-kB1 or p50, NF-kB2 or p52, Rel A or p65, RelB and c-Rel proteins, which can form homo- and hetero-dimers and produce complexes with various transcriptional activities. Their activation is partly controlled by accessory inhibitory proteins called IκBs (Beg & Baldwin, 1993). NF-κB inducing stimuli cause IκB phosphorylation, through activation of the multicomponent IκB kinase (IKK) complex (Karin & Delhase, 2000), with subsequent IκB proteolytic degradation (Henkel, Machleidt et al., 1993), event that allows NF-κB to enter the nucleus and activate target genes. To determine whether hMPV infection produced changes in abundance of DNA-binding proteins that recognized the NF-κB binding site of the IL-8 gene promoter, we initially used gel mobility shift assays (EMSA). As shown in Fig. 4A, a single nucleoprotein complex (C3) was formed from nuclear extracts of control cells on the NF-κB probe, while two other complexes, C1 and C2, were faintly detected. HMPV infection markedly increased the binding of C1 and C2 starting at 6 h p.i., with maximal binding intensity at 24 h p.i. We have previously shown, in the contest of RSV infection, that C2 is constituted by a p50-p65 heterodimer and C1 represents a p65 homodimer (Garofalo, Sabry, Jamaluddin, Yu, Casola, Ogra, & Brasier, 1996). To determine the mechanism for enhanced NF-κB binding activity, we performed Western blot analysis of p50 and p65 using nuclear extracts from control and hMPV-infected A549 cells (Fig. 4B). Both proteins were detected in the nuclear compartment of uninfected cells. However, upon hMPV infection, a time-dependent increase in p50 and p65 was observed, starting between 3 and 6 h p.i., with maximal nuclear translocation detected at 24 h p.i. The time-dependent increase in nuclear p50 and p65 was parallel to the changes in DNA binding activity observed in EMSA.
Fig. 4. NF-kB activation in response to hMPV infection in A549 cells.
(A). Nuclear extracts were prepared from A549 cells control and infected with hMPV, MOI of 3, for 6, 12, 24 and 48 h and used for binding to the IL-8 NF-kB probe in EMSA. Shown are the nucleoprotein complexes formed on the probe in control cells and in response to the infection. C1 and C2 are hMPV-inducible complexes, while C3 is constitutive. UV indicates UV-inactivated virus. (B) Western blot of p50 and p65 in hMPV-infected cells. Nuclear proteins were prepared from control and A549 cells infected for various length of time, fractionated on a 10% SDS-PAGE, transferred to PVDF membranes and probed with the appropriate antibody. Lamin b was used as an internal control to determine equal loading of the samples.
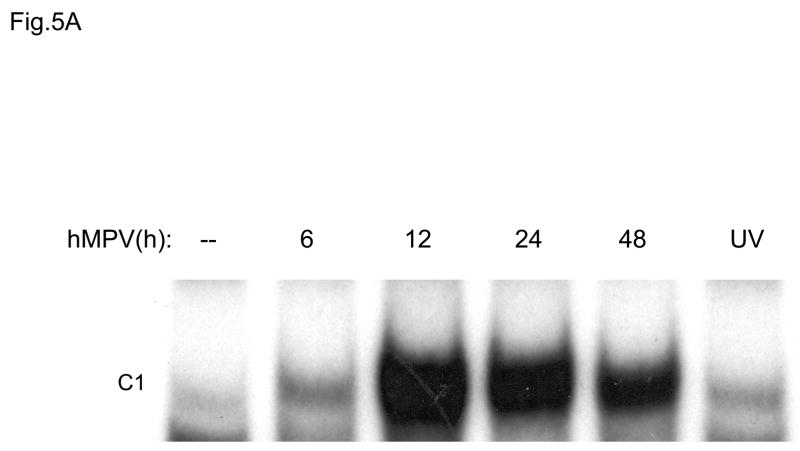
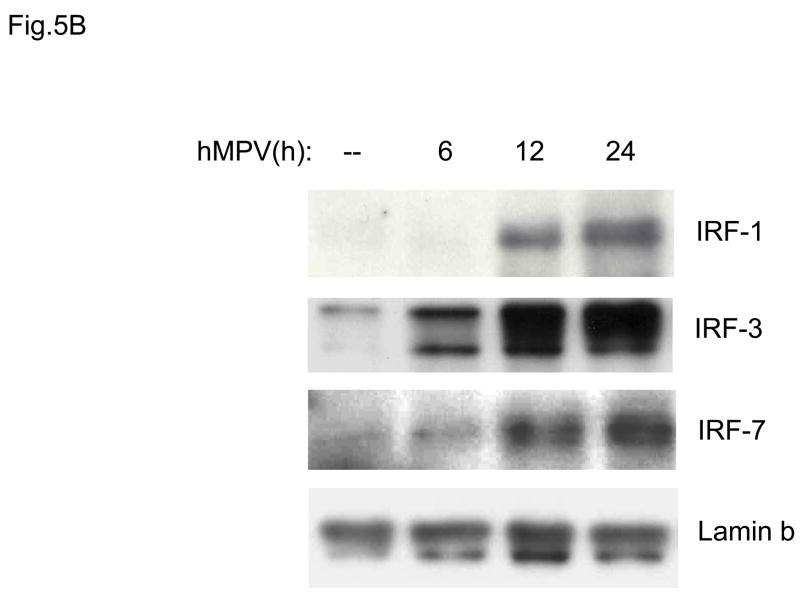
IRF transcription factors have been shown to play a fundamental role in the induction of several genes involved in the immune/inflammatory response to viral infections, including type I IFNs, chemokines and cytokines, like IL-15. Activation of IRFs is stimulus- and cell type-dependent. Among all members of the IRF family, IRF-1, IRF-3 and IRF-7 are the ones best characterized. IRF-1 and IRF-7 are inducible proteins, while IRF-3 is constitutively expressed. IRF-3 and -7 are phosphorylated on several serine/threonine residues, upon viral infection. This event allows dimerization, nuclear localization and DNA-binding (Taniguchi, Ogasawara, Takaoka, & Tanaka, 2001). To determine whether hMPV infection produced changes in the abundance of DNA binding proteins recognizing the ISRE (interferon-stimulated response element) of the RANTES gene promoter, we performed EMSA similar to the one described for NF-κB. As shown in Fig. 5A, one inducible nucleoprotein complex was formed on the ISRE probe, starting at 6 h p.i. We have previously shown that in response to RSV infection, IRF-1, -3 and -7 all bind to the RANTES ISRE (Casola, Garofalo, Haeberle, Elliott, Lin, Jamaluddin, & Brasier A.R., 2001). To determine whether hMPV infection induced a similar activation of the IRF proteins, we performed Western blot analysis of IRF-1, -3 and -7 using nuclear extracts from control and hMPV-infected A549 cells (Fig. 5B). We found increased IRF-3 nuclear translocation starting around 6 h p.i., while IRF-1 and -7 appeared later, around 12 h p.i. Altogether, these data suggest that both NF-κB and IRF proteins are activated in airway epithelial cells in response to hMPV infection, and they are likely to play a key role in regulating pro-inflammatory gene expression.
Fig. 5. IRF activation induced by hMPV infection in A549 cells.
(A). Nuclear extracts were prepared from A549 cells control and infected with hMPV, MOI of 3, for 6, 12, 24 and 48 h and used for binding to the RANTES ISRE probe in EMSA. Shown is the inducible nucleoprotein complex formed on the probe in response to the infection. UV indicates UV-inactivated virus. (B) Western blot of IRF-1,-3, and -7 in A549 cells infected with hMPV. Nuclear proteins were prepared from control and A549 cells infected for various length of time, fractionated on a 10% SDS-PAGE, transferred to PVDF membranes and probed with the appropriate antibody. Lamin b was used as an internal control to determine equal loading of the samples.
hMPV induces type I interferon production and activates interferon-dependent signaling pathways in airway epithelial cells
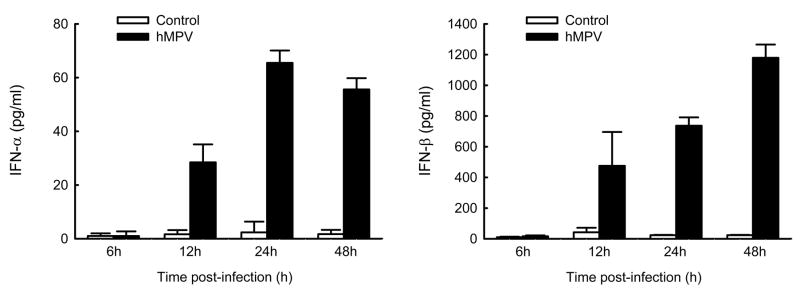
Interferons (IFNs) are a superfamily of cytokines with antiviral, as well as anti-proliferative and immuno-modulatory functions (Smith, Lombardi et al., 2005). Type I IFNs, which include IFN-α,-β and –λ, are the first line of defense against viral infections. As IFNs play an essential role in limiting virus replication, we determined whether hMPV was able to induce type I IFN production in airway epithelial cells. A549 cells were infected with hMPV, MOI of 3, and cell supernatants were harvested at different time points of infection to measure IFN-α and-β by ELISA. We found that both IFN-α and -β were induced by hMPV infection of A549 cells in a time-dependent manner (Fig. 6), with a more significant induction of IFN-β than IFN-α.
Fig. 6. Type I IFN production by airway epithelial cells infected with hMPV.
A549 cells were infected with hMPV at MOI of 3. Cell supernatants were collected at 6, 12, 24 and 48 h p.i. and tested for type I interferon production by ELISA. Data are expressed as mean ± standard error of two independent experiments performed in triplicates. Open bars represent uninfected and solid bars hMPV-infected cells.
IFN-β gene expression is a primary cellular response to viral infection and depends on activation of the transcription factors NF-κB and IRF-3 (Hiscott, Pitha et al., 1999), constitutively expressed in infected cells. Binding of IFN-β to its receptor activates STAT1 and STAT2, which, together with IRF-9, form the ISGF3 complex, responsible for the expression of a variety of interferon stimulated genes (ISGs), including IRF-7, which is required for IFN-α gene expression (Sato, Suemori et al., 2000). STAT proteins are constitutively expressed and located in the cytoplasm of unstimulated cells. Upon activation, they are phosphorylated on specific tyrosine residues, post-translational modifications necessary for dimerization, nuclear translocation and DNA binding [Reviewed in (Imada & Leonard, 2000)]. To determine whether hMPV infection of A549 cells induced activation of STAT1, STAT2 and IRF-9, we performed Western blot analysis of nuclear proteins extracted from A549 cells uninfected or infected for various lengths of time. As shown in Fig. 7, hMPV infection induced a time-dependent increase in tyrosine phosphorylation and nuclear translocation of STAT1, starting around 6 h p.i. It also induced increased nuclear translocation of STAT2 and IRF-9 between 6 and 12 h p.i., indicating that hMPV can activate the ISGF3 complex necessary to mediate interferon-dependent cellular responses, in particular antiviral gene expression.
Fig. 7. ISGF3 complex activation in response to hMPV infection.
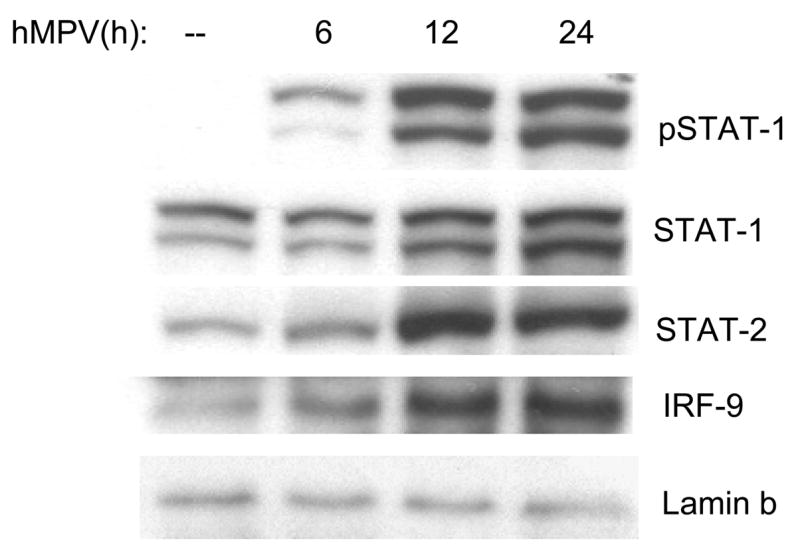
A549 cells were infected with hMPV, MOI of 3, for 6, 12 and 24 h. Cells were harvested to prepare nuclear extracts and equal amounts of protein from control and infected cells were assayed for phosphorylated and total STAT1, total STAT2 and IRF-9 by Western blot. Lamin b was used as internal control to determine equal loading of the samples.
Discussion
Human metapneumovirus has been identified as major viral pathogen, causing both upper and lower respiratory tract infections. Many children with a LRTI have a clinical syndrome consistent with bronchiolitis, with fever, cough, tachypnea, wheezing and hypoxia (Boivin, Abed et al., 2002; Esper, Boucher et al., 2003;Falsey, Erdman, Anderson, & Walsh, 2003). Chest radiographs demonstrate focal infiltrates and peribronchial cuffing (Esper, Boucher, Weibel, Martinello, & Kahn, 2003). Although the mechanisms mediating hMPV-induced lung disease are largely unknown, airway inflammation is likely to play a major role, as suggested by the recently developed small animal models of infection (Hamelin, Prince et al., 2006b;Williams, Tollefson, Johnson, & Crowe, Jr., 2005;Guerrero-Plata, Casola, & Garofalo, 2005). Both in mice and cotton rats, hMPV infection induces significant lung inflammation and treatment with a combination of steroids and ribavirin, which affects hMPV replication, induce a significant reduction in lung pathology and disease (Hamelin, Prince et al., 2006a). Airway epithelial cells represent the major target of respiratory viruses and they are able to synthesize and secrete soluble mediators, upon injury or infection, which are important for the recruitment and activation of immune/inflammatory cells [Reviewed in (Lemanske, Jr., 1992)]. In this study, we show that hMPV can infect and replicate similarly both in normal airway epithelial cells, isolated from different tracts of the airways, as well as in cell lines. Replication of hMPV induces a variety of cytokines and chemokines, whose expression is dependent on viral replication. The finding that hMPV can induces IL-8 and RANTES secretion in SAE and A549 cells in a similar manner suggests that A549 cells can be used as an in vitro model of hMPV lower respiratory tract infection. We have previously shown that SAE cells display a similar profile of chemokine/cytokine gene expression, transcription factor and signaling pathway activation compared to A549 cells, following infection with respiratory syncytial virus (RSV), another member of the paramyxovirus family and the number one cause of viral lower respiratory tract infections in children (Garofalo, Sabry, Jamaluddin, Yu, Casola, Ogra, & Brasier, 1996; Olszewska-Pazdrak, Casola et al., 1998; Casola, Burger et al., 2001;Zhang Y, Luxon, Casola, Garofalo, Jamaluddin, & Brasier A.R., 2001; Pazdrak, Olszewska-Pazdrak et al., 2002).
In this study, we found that hMPV is a strong inducer of both CXC and CC chemokines, as well as of proinflammatory cytokines such as IL-6. While IL-8 secretion was detectable at 6 h p.i., induction of other chemokines, including IP-10, MCP-1 and RANTES was significant starting at 15 h p.i. IL-8 is the prototype of ELR-containing CXC chemokines, which recruit and activate neutrophils. We and others have shown that hMPV induces CXC chemokine production in a mouse model of infection, in which neutrophils are the prominent cell type seen in early airway inflammation (Hamelin, Yim, Kuhn, Cragin, Boukhvalova, Blanco, Prince, & Boivin, 2005;Guerrero-Plata, Casola, & Garofalo, 2005). Furthermore, significant levels of IL-8 have been detected in respiratory secretions of hMPV-infected children with bronchiolitis (Jartti, van den Hoogen, Garofalo, Osterhaus, & Ruuskanen, 2002;Laham, Israele, Casellas, Garcia, Prugent, Hoffman, Hauer, Thumar, Name, Pascual, Taratutto, Ishida, Balduzzi, Maccarone, Jackli, Passarino, Gaivironsky, Karron, Polack, & Polack, 2004).
Mononuclear cells, including macrophages/monocytes and lymphocytes, represent also an important component of the lung inflammatory infiltrate present in animal models of hMPV infection (Hamelin, Yim, Kuhn, Cragin, Boukhvalova, Blanco, Prince, & Boivin, 2005). Among the non-ELR-containing CXC chemokines, hMPV induced the expression of IP-10, which is an interferon-inducible protein and has been shown to play an important role in limiting viral replication in other infection models (Dufour, Dziejman, Liu, Leung, Lane, & Luster, 2002; Hamilton, Mahalingam et al., 2004). Among the CC chemokines, hMPV induced the expression of RANTES, MCP-1 and MIP-1α/β. These molecules have been shown to activate a variety of cell types, including monocytes, T- lymphocytes, eosinophils, basophils and dendritic cells, which are likely to play a significant role in the pathogenesis of hMPV-induced inflammatory lung disease, as well as in the modulation of immune responses, as it has been previously shown in RSV and other models of respiratory viral infections (Haeberle, Kuziel et al., 2001; Bonville, Lau et al., 2004; Domachowske, Bonville et al., 2000). In comparison to RSV, hMPV infection displayed a delayed kinetics and lower induction of chemokines in airway epithelial cells (Olszewska-Pazdrak, Casola, Saito, Alam, Crowe, Mei, Ogra, & Garofalo, 1998)data not shown), in particular for MIP-1α and -1β secretion, which could be due to hMPV slower viral replication and lower viral titers achieved in A549 cells (RSV titer in A549 cells at 24 h is similar to the hMPV titer at 72 h p.i. and it is ten fold higher at later time points, data not shown). A lower chemokine and cytokine induction by hMPV, compared to RSV, was also observed in dendritic cells, as well as in a mouse model of infection and in children (Guerrero-Plata, Casola, Suarez, Yu, Spetch, Peeples, & Garofalo, 2006;Guerrero-Plata, Casola, & Garofalo, 2005;Laham, Israele, Casellas, Garcia, Prugent, Hoffman, Hauer, Thumar, Name, Pascual, Taratutto, Ishida, Balduzzi, Maccarone, Jackli, Passarino, Gaivironsky, Karron, Polack, & Polack, 2004).
Pro-inflammatory cytokines, such as IL-1, IL-6 and TNF, have been implicated in the pathogenesis of paramyxovirus-induced lung disease [Reviewed in (Graham, Johnson et al., 2000;Garofalo & Haeberle, 2000)]. We did not detect significant production of IL-1 and TNF, but there was significant secretion of IL-6 in response to hMPV infection. IL-6 production has been previously observed both in an animal model of hMPV infection and in infected children (Guerrero-Plata, Casola, & Garofalo, 2005;Laham, Israele, Casellas, Garcia, Prugent, Hoffman, Hauer, Thumar, Name, Pascual, Taratutto, Ishida, Balduzzi, Maccarone, Jackli, Passarino, Gaivironsky, Karron, Polack, & Polack, 2004). Whether pro-inflammatory cytokines play a role in hMPV-induced lung inflammation and disease pathogenesis will require further investigations.
Different patterns of inflammatory mediator secretion in response to RSV and hMPV infection may be also caused by different activation of intracellular signaling pathways, and subsequent different induction of transcription factors by the two viruses. To start to address this question, we investigated two of the key transcription factors involved in proinflammatory gene expression, NF-κB and IRF proteins. Our results show that hMPV is a strong inducer of both p50 and p65, belonging to the NF-κB family, as well as of IRF-1, -3 and -7. HMPV infection of A549 cells induced nuclear translocation of these proteins and increased binding to their specific DNA binding sites present in the IL-8 and RANTES gene promoters. We have previously shown that RSV infection of A549 cells is a potent activator of p65/Rel A, which is absolutely required for RSV-inducible IL-8 gene transcription (Garofalo, Sabry, Jamaluddin, Yu, Casola, Ogra, & Brasier, 1996). We have also shown that RSV infection of A549 cells induces IRF-1 synthesis, nuclear translocation and binding to a newly identified responsive element of IL-8 promoter involved in RSV-induced IL-8 transcription (Casola, Garofalo et al., 2000). IRF-7 synthesis is also induced in RSV-infected A549 cells, while IRF-3 is constitutive, and in alveolar epithelial cells IRF-1, -3 and -7 are the IRF proteins necessary for RSV-induced RANTES promoter activation (Casola, Burger, Liu, Jamaluddin, Brasier A.R., & Garofal, 2001). Therefore, it is likely that both NF-κB and IRF play a critical role in cytokine and chemokine gene expression in response also to hMPV infection.
Different from RSV, we found that hMPV induced IFN-α secretion in infected A549 cells. This result is consistent with our previous observation in monocyte-derived dendritic cells, as well as in a mouse model of infection, in which hMPV, but not RSV infection, induces significant production of IFN-α (Guerrero-Plata, Casola, Suarez, Yu, Spetch, Peeples, & Garofalo, 2006;Guerrero-Plata, Casola, & Garofalo, 2005). RSV failure to induce IFN-α production in A549 cells has been previously reported (Spann, Tran et al., 2004). Two RSV non-structural proteins, NS1 and NS2, have been shown to partially inhibit activation of NF-κB and IRF-3 and disrupt STAT-2-mediated signaling pathway, which is important for type I IFN-mediated signaling, including IFN-α production and expression of antiviral genes (Spann, Tran et al., 2005; Ramaswamy, Shi et al., 2006; Wright, Karron et al., 2006). Compared to RSV, hMPV does not have NS1 and NS2, and this could explain hMPV ability to induce higher levels of both IFN-α and -β in epithelial, as well as non-epithelial cells. However, we have also reported that hMPV, similar to RSV, can inhibit Toll-like receptor (TLR)-dependent type I IFN production (Guerrero-Plata, Baron et al., 2005). This observation, together with the finding that the amount of IFN-α secreted by hMPV-infected A549 cells is much less than that secreted by monocyte-derived dendritic cells, would suggest that hMPV also might have evolved strategies to modulate interferon production and interferon-dependent signaling pathways. Which viral protein(s) is responsible for this activity remains to be investigated.
In summary, in this study we have started to characterize the airway epithelial cell responses to hMPV, an important respiratory pathogen. As the airway epithelium is the primary target of hMPV infection, the identification of genes and pathways activated by this respiratory virus is highly relevant for a better understanding of the pathogenesis of hMPV-induced lung disease.
Material and Methods
Materials
The following reagents were purchased from the indicated suppliers: Eagle’s minimal essential medium, F12K medium, L-glutamine, penicillin-streptomycin-amphotericin B, (Gibcol, Carlsbad, CA); LLC-MK2 cells (Rhesus monkey kidney ATCC CCL-7) and human alveolar type II-like epithelial cells (A549 cells) (ATCC; Manassas, VA); small alveolar epithelial cells (SAE) and normal bronchial epithelial cells (NHBE) (Clonetics, San Diego, CA); double antibody ELISA kit (Duo-Set, R&D Systems, Minneapolis, MN); antibodies for IRFs, p50 and lamin B (Santa Cruz, Santa Cruz, CA); antibody for p65 (Upstate, Charlottesville, VA); RiboQuant kit (Pharmingen, San Diego, CA); radioactive probe (Du Pont NEN Research Products, Boston, MA); human interferon-α and -β ELISA kit (PBL Biomedical Laboratories, Piscataway, NJ); Bio-Plex Human Cytokine panel (Bio-Rad Laboratories, Hercules, CA).
Cell culture and viral preparation
LLC-MK2 cells were maintained in MEM supplemented with 10% Fetal Bovine Serum (FBS), Penicillin and Streptomycin (100U/ml). A549 cells were maintained in F12K medium containing 10% (v/v) FBS, 10 mM glutamine, 100 IU/ml penicillin and 100 μg/ml streptomycin. Primary cultured SAE and NHBE cells were grown according to the company instructions. For most experiments, confluent cells were infected with hMPV in serum-free media with 1.0 μg trypsin/ml at MOI 3 to 5. Viral stocks were obtained by infecting LLC-MK2 cells at MOI 0.1. Around day 5 post-infection, cells were disrupted using glass beads, and then scraped completely from the flasks. Total medium was collected and sonicated on ice for 5 min. The preparation was then vortexed and centrifuged at 2900g for 15 min on ice. Crude virus was collected and then purified on a sucrose cushion. Viral titers were determined by immunostaining, as previously described (Guerrero-Plata, Casola, Suarez, Yu, Spetch, Peeples, & Garofalo, 2006).
Immunofluorescence
Replication of hMPV in NHBE, SAE and A549 cells were verified by immunochemistry staining using anti-hMPV polyclonal antibody (a gift from MedImmune Inc., Gaithersburg, MD). Cells were plated in a slide chamber and infected with hMPV using MOI of 3 for 24 h. Cells were washed with PBS and fixed with 1% paraformaldehyde at room temperature for 15 min. Cells were then washed with PBS twice, followed by incubation with 0.2% Triton 100-X. Following PBS washes, slides were incubated with a primary guinea pig isotype control Ab or anti-hMPV antibody (1:200) for one hour. Cells were washed again and incubated with a secondary FITC-conjugated anti-guinea pig antibody. Picture was taken using Zeiss LSM Image Browser software v. 3.0 (Zeiss, Thornwood, NY).
Bio-Plex
Chemokines and cytokines (IL-1RA, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12 p70, IL-13, IL-17, G-CSF, GM-CSF, IFN-γ, IP-10,EOTAXIN, MIP-1α, MIP-1β, GCSF, FGFB, PDGF, VEGF and TNF-α) were quantified by the Luminex-based Bio-Plex system (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s instructions. The lower limit of detection for all cytokines measured by Bio-Plex is 3 pg/ml.
ELISA
Human IL-8 and RANTES concentrations in cell culture supernatants were determined using DuoSet ELISA kits (R&D, Systems Minneapolis, MN). IFN-α and -β were determined by Human interferon-α and- β ELISA kit ((lower limit of detection, 12.5 pg/ml, PBL Biomedical Laboratories, Piscataway, NJ) according to manufactory’s instructions.
EMSA
Nuclear extracts of uninfected and infected cells were prepared using hypotonic/nonionic detergent lysis, according to Schaffner protocol (Schreiber, Matthias et al., 1989). To prevent contamination with cytoplasmic proteins, isolated nuclei were purified by centrifugation through 1.7 M sucrose buffer A for 30 minutes, at 12,000 rpm, before nuclear protein extraction, as previously described (Brasier, Spratt et al., 2004). After normalization, nuclear proteins were used to bind to duplex oligonucleotides corresponding to either the RANTES ISRE or the IL-8 NF-κB binding sites, as previously described (Casola, Garofalo, Haeberle, Elliott, Lin, Jamaluddin, & Brasier A.R., 2001;Casola, Garofalo, Jamaluddin, Vlahopoulos, & Brasier, 2000). DNA-binding reactions contained 10- 15 μg nuclear proteins, 5% glycerol, 12 mM HEPES, 80 mM NaCl, 5 mM DTT, 5 mM Mg2Cl, 0.5 mM EDTA, 1 μg of poly dI-dC and 40,000 cpm of 32P- labeled double-stranded oligonucleotide in a total volume of 20 μl. The nuclear proteins were incubated with the probe for 15 min at room temperature and then fractionated by 6% nondenaturing polyacrylamide gels (PAGE) in TBE buffer (22 mM Tris-HCl, 22 mM boric acid, 0.25 mM EDTA, pH 8). After electrophoretic separation, gels were dried and exposed for autoradiography using Kodak XAR film at −70 °C using intensifying screens.
Western blot analysis
Nuclear extracts were prepared as described above. Proteins (10 to 30 μg per sample) were then boiled in 2X Laemmli buffer for 2 min and resolved on SDS-PAGE. Proteins were transferred to polyvinylidene difluoride membranes (Bio-Rad). Nonspecific binding sites were blocked by immersing the membrane in TBST blocking solution [10mM Tris-HCl, pH 7.6, 150 mM NaCl, 0.05% Tween-20 (v/v)] containing 5% skim milk powder or 5% bovine serum albumin for 30 min. After a short wash in TBST, the membranes were incubated with the primary antibody for 1 h, followed by an anti-rabbit peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology), diluted 1:10,000 in TBST for 30 min. After washing, the proteins were detected by autoradiography using ECL regular or plus (Amersham Pharmacia Biotech) according to manufacturer’s protocol. Equal loading of proteins was evaluated by stripping and reprobing the membranes with anti-lamin B antibody.
Statistical Analysis
Statistical significance was analyzed by using analysis of variance (ANOVA). P value of less than 0.05 was considered significant. Mean ± SEM is shown.
Acknowledgments
This work was supported by grants NIEHS 06676 and NIAID P01 062885, a pilot project from the Sealy center for Vaccine Development at UTMB and the VRPRU N01 AI30039. X. B. was supported by the NIAID training grant T32 AI07536.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez R, Harrod KS, Shieh WJ, Zaki S, Tripp RA. Human metapneumovirus persists in BALB/c mice despite the presence of neutralizing antibodies. J Virol. 2004;78:14003–14011. doi: 10.1128/JVI.78.24.14003-14011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annual Review Immunology. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- Barnes B, Lubyova B, Pitha PM. On the role of IRF in host defense. J Interferon Cytokine Res. 2002;22:59–71. doi: 10.1089/107999002753452665. [DOI] [PubMed] [Google Scholar]
- Beg AA, Baldwin ASJ. The I kappa B proteins: multifunctional regulators of Rel/NF- kappa B transcription factors. Genes and Development. 1993;7:2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- Biacchesi S, Pham QN, Skiadopoulos MH, Murphy BR, Collins PL, Buchholz UJ. Modification of the trypsin-dependent cleavage activation site of the human metapneumovirus fusion protein to be trypsin independent does not increase replication or spread in rodents or nonhuman primates. J Virol. 2006;80:5798–5806. doi: 10.1128/JVI.00294-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin G, Abed Y, Pelletier G, Ruel L, Moisan D, Cote S, Peret TC, Erdman DD, Anderson LJ. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. Journal of Infectious Diseases. 2002;186:1330–1334. doi: 10.1086/344319. [DOI] [PubMed] [Google Scholar]
- Bonville CA, Lau VK, DeLeon JM, Gao JL, Easton AJ, Rosenberg HF, Domachowske JB. Functional antagonism of chemokine receptor CCR1 reduces mortality in acute pneumovirus infection in vivo. J Virol. 2004;78:7984–7989. doi: 10.1128/JVI.78.15.7984-7989.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier AR, Spratt H, Wu Z, Boldogh I, Zhang Y, Garofal RP, Casola A, Pashmi J, Haag A, Luxon B, Kurosky A. Nuclear Heat Shock Response and Novel Nuclear Domain 10 Reogranization in Respiratory Syncytial Virus-Infected A549 Cells identified By High Resolution 2D Gel Electrophoresis. Journal of Virology. 2004;78:11461–11476. doi: 10.1128/JVI.78.21.11461-11476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casola A, Burger N, Liu T, Jamaluddin M, Brasier AR, Garofal RP. Oxidant tone regulates RANTES gene transcription in airway epithelial cells infected with Respiratory Syncytial Virus: role in viral-induced Interferon Regulatory Factor activation. J Biol Chem. 2001;276:19715–19722. doi: 10.1074/jbc.M101526200. [DOI] [PubMed] [Google Scholar]
- Casola A, Garofalo RP, Haeberle H, Elliott TF, Lin A, Jamaluddin M, Brasier AR. Multiple cis regulatory elements control RANTES promoter activity in alveolar epithelial cells infected with respiratory syncytial virus. Journal of Virology. 2001;75:6428–6439. doi: 10.1128/JVI.75.14.6428-6439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casola A, Garofalo RP, Jamaluddin M, Vlahopoulos S, Brasier AR. Requirement of a novel upstream response element in RSV induction of interleukin-8 gene expression: stimulus-specific differences with cytokine activation. Journal of Immunology. 2000;164:5944–5951. doi: 10.4049/jimmunol.164.11.5944. [DOI] [PubMed] [Google Scholar]
- Dare R, Sanghavi S, Bullotta A, Keightley MC, George KS, Wadowsky RM, Paterson DL, McCurry KR, Reinhart TA, Husain S, Rinaldo CR. Diagnosis of human metapneumovirus infection in immunosuppressed lung transplant recipients and children evaluated for pertussis. Journal of Clinical Microbiology. 2007;45:548–552. doi: 10.1128/JCM.01621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiaggi M, Canducci F, Sampaolo M, Marinozzi MC, Parea M, Terulla C, Colombo AA, Alessandrino EP, Bragotti LZ, Arghittu M, Goglio A, Migliavacca R, Romero E, Clementi M. Persistent symptomless human metapneumovirus infection in hematopoietic stem cell transplant recipients. J Infect Dis. 2006;194:474–478. doi: 10.1086/505881. [DOI] [PubMed] [Google Scholar]
- Domachowske JB, Bonville CA, Gao JL, Murphy PM, Easton AJ, Rosenberg HF. The chemokine macrophage-inflammatory protein-1alpha and its receptor CCR1 control pulmonary inflammation and antiviral host defense in paramyxovirus infection. Journal of Immunology (Baltimore MD) 2000;165:2677–2682. doi: 10.4049/jimmunol.165.5.2677. [DOI] [PubMed] [Google Scholar]
- Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. Journal of Immunology. 2002;168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- Esper F, Boucher D, Weibel C, Martinello RA, Kahn JS. Human metapneumovirus infection in the United States: clinical manifestations associated with a newly emerging respiratory infection in children. Pediatrics (Evanston IL) 2003;111:1407–1410. doi: 10.1542/peds.111.6.1407. [DOI] [PubMed] [Google Scholar]
- Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. Journal of Infectious Diseases. 2003;187:785–790. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- Garofalo RP, Haeberle H. Epithelial regulation of innate immunity to respiratory syncytial virus. American Journal of Respiratory Cell and Molecular Biology. 2000;23:581–585. doi: 10.1165/ajrcmb.23.5.f204. [DOI] [PubMed] [Google Scholar]
- Garofalo RP, Sabry M, Jamaluddin M, Yu RK, Casola A, Ogra PL, Brasier AR. Transcriptional activation of the interleukin-8 gene by respiratory syncytial virus infection in alveolar epithelial cells: Nuclear translocation of the RelA transcription factor as a mechanism producing airway mucosal inflammation. Journal of Virology. 1996;70:8773–8781. doi: 10.1128/jvi.70.12.8773-8781.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BS, Johnson TR, Peebles RS. Immune-mediated disease pathogenesis in respiratory syncytial virus infection. Immunopharmacology. 2000;48:237–247. doi: 10.1016/s0162-3109(00)00233-2. [DOI] [PubMed] [Google Scholar]
- Guerrero-Plata A, Baron S, Poast JS, Adegboyega PA, Casola A, Garofalo RP. Activity and regulation of alpha interferon in respiratory syncytial virus and human metapneumovirus experimental infections. Journal of Virology. 2005;79:10190–10199. doi: 10.1128/JVI.79.16.10190-10199.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Plata A, Casola A, Garofalo RP. Human metapneumovirus induces a profile of lung cytokines distinct from that of respiratory syncytial virus. Journal of Virology. 2005;79:14992–14997. doi: 10.1128/JVI.79.23.14992-14997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Plata A, Casola A, Suarez G, Yu X, Spetch L, Peeples ME, Garofalo RP. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am J Respir Cell Mol Biol. 2006;34:320–329. doi: 10.1165/rcmb.2005-0287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeberle HA, Kuziel WA, Dieterich HJ, Casola A, Gatalica Z, Garofalo RP. Inducible expression of inflammatory chemokines in respiratory syncytial virus-infected mice: role of MIP-1alpha in lung pathology. Journal of Virology. 2001;75:878–890. doi: 10.1128/JVI.75.2.878-890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin ME, Prince GA, Boivin G. Effect of ribavirin and glucocorticoid treatment in a mouse model of human metapneumovirus infection. Antimicrob Agents Chemother. 2006a;50:774–777. doi: 10.1128/AAC.50.2.774-777.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin ME, Prince GA, Gomez AM, Kinkead R, Boivin G. Human metapneumovirus infection induces long-term pulmonary inflammation associated with airway obstruction and hyperresponsiveness in mice. J Infect Dis. 2006b;193:1634–1642. doi: 10.1086/504262. [DOI] [PubMed] [Google Scholar]
- Hamelin ME, Yim K, Kuhn KH, Cragin RP, Boukhvalova M, Blanco JC, Prince GA, Boivin G. Pathogenesis of human metapneumovirus lung infection in BALB/c mice and cotton rats. Journal of Virology. 2005;79:8894–8903. doi: 10.1128/JVI.79.14.8894-8903.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NH, Mahalingam S, Banyer JL, Ramshaw IA, Thomson SA. A recombinant vaccinia virus encoding the interferon-inducible T-cell alpha chemoattractant is attenuated in vivo. Scand J Immunol. 2004;59:246–254. doi: 10.1111/j.0300-9475.2004.01391.x. [DOI] [PubMed] [Google Scholar]
- Henkel T, Machleidt T, Alkalay I, Kronke M, Ben-Neriah Y, Baeuerle PA. Rapid proteolysis of I kappa B-alpha is necessary for activation of transcription factor NF-kappa B. Nature (London) 1993;365:182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- Hiscott J, Pitha P, Genin P, Nguyen H, Heylbroeck C, Mamane Y, Algarte M, Lin R. Triggering the interferon response: The role of IRF-3 transcription factor. J Interferon Cytokine Res. 1999;19:1–13. doi: 10.1089/107999099314360. [DOI] [PubMed] [Google Scholar]
- Imada K, Leonard WJ. The Jak-STAT pathway. Mol Immunol. 2000;37:1–11. doi: 10.1016/s0161-5890(00)00018-3. [DOI] [PubMed] [Google Scholar]
- Jartti T, van den Hoogen BG, Garofalo RP, Osterhaus AD, Ruuskanen O. Metapneumovirus and acute wheezing in children. Lancet. 2002;360:1393–1394. doi: 10.1016/S0140-6736(02)11391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn JS. Epidemiology of human metapneumovirus. Clin Microbiol Rev. 2006;19:546–557. doi: 10.1128/CMR.00014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- Laham FR, Israele V, Casellas JM, Garcia AM, Prugent CML, Hoffman SJ, Hauer D, Thumar B, Name MI, Pascual A, Taratutto N, Ishida MT, Balduzzi M, Maccarone M, Jackli S, Passarino R, Gaivironsky RA, Karron RA, Polack NR, Polack FP. Differential production of inflammatory cytokines in primary infection with human metapneumovirus and with other common respiratory viruses of infancy. Journal of Infectious Diseases. 2004;189:2047–2056. doi: 10.1086/383350. [DOI] [PubMed] [Google Scholar]
- Lemanske RF., Jr Mechanisms of airway inflammation. Chest. 1992;101:372S–377S. doi: 10.1378/chest.101.6_supplement.372s. [DOI] [PubMed] [Google Scholar]
- Martinez-Moczygemba M, Gutch MJ, French DL, Reich NC. Distinct STAT structure promotes interaction of STAT2 with the p48 subunit of the interferon-alpha-stimulated transcription factor ISGF3. Journal of Biological Chemistry. 1997;272:20070–20076. doi: 10.1074/jbc.272.32.20070. [DOI] [PubMed] [Google Scholar]
- Miller MD, Krangel MS. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. [Review] [183 refs] Critical Reviews in Immunology. 1992;12:17–46. [PubMed] [Google Scholar]
- Olszewska-Pazdrak B, Casola A, Saito T, Alam R, Crowe SE, Mei F, Ogra PL, Garofalo RP. Cell-specific expression of RANTES, MCP-1, and MIP-1alpha by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. Journal of Virology. 1998;72:4756–4764. doi: 10.1128/jvi.72.6.4756-4764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazdrak K, Olszewska-Pazdrak B, Liu B, Takizawa R, Brasier AR, Garofalo RP, Casola A. MAP-kinase activation is involved in post-transcriptional regulation of RSV-induced RANTES gene expression. Am J Physiol. 2002 doi: 10.1152/ajplung.00331.2001. 000. [DOI] [PubMed] [Google Scholar]
- Principi N, Bosis S, Esposito S. Human metapneumovirus in paediatric patients. Clin Microbiol Infect. 2006;12:301–308. doi: 10.1111/j.1469-0691.2005.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy M, Shi L, Varga SM, Barik S, Behlke MA, Look DC. Respiratory syncytial virus nonstructural protein 2 specifically inhibits type I interferon signal transduction. Virology (New York NY) 2006;344:328–339. doi: 10.1016/j.virol.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Rollins BJ, Yoshimura T, Leonard EJ, Pober J. Cytokine-activated human endothelial cells synthesize and secrete monocyte chemoattractant protein, MCP-1. American Journal of Pathology. 1990;136:1229–1233. [PMC free article] [PubMed] [Google Scholar]
- Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, Taniguchi T. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000;13:539–548. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kB. Annual Review in Cell Biology. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- Smith PL, Lombardi G, Foster GR. Type I interferons and the innate immune response--more than just antiviral cytokines. Mol Immunol. 2005;42:869–877. doi: 10.1016/j.molimm.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Spann KM, Tran KC, Chi B, Rabin RL, Collins PL. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages [corrected] Journal of Virology. 2004;78:4363–4369. doi: 10.1128/JVI.78.8.4363-4369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spann KM, Tran KC, Collins PL. Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-kappaB, and proinflammatory cytokines. J Virol. 2005;79:5353–5362. doi: 10.1128/JVI.79.9.5353-5362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF family of transcription factors as regulators of host defense. Annu Rev Immunol. 2001;19:623–655. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]
- Tian B, Zhang Y, Luxon B, Garofalo RP, Casola A, Sinha M, Brasier AR. Identification of NF-kB dependent gene networks in respiratory syncytial virus-infected cells. Journal of Virology. 2002;76:6800–6814. doi: 10.1128/JVI.76.13.6800-6814.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoogen BG, Bestebroer TM, Osterhaus AD, Fouchier RA. Analysis of the genomic sequence of a human metapneumovirus. Virology (New York NY) 2002;295:119–132. doi: 10.1006/viro.2001.1355. [DOI] [PubMed] [Google Scholar]
- van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, Osterhaus AD. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoogen BG, van Doornum GJ, Fockens JC, Cornelissen JJ, Beyer WE, de Groot R, Osterhaus AD, Fouchier RA. Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J Infect Dis. 2003;188:1571–1577. doi: 10.1086/379200. [DOI] [PubMed] [Google Scholar]
- Williams JV, Harris PA, Tollefson SJ, Halburnt-Rush LL, Pingsterhaus JM, Edwards KM, Wright PF, Crowe JE., Jr Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JV, Tollefson SJ, Johnson JE, Crowe JE., Jr The cotton rat (Sigmodon hispidus) is a permissive small animal model of human metapneumovirus infection, pathogenesis, and protective immunity. J Virol. 2005;79:10944–10951. doi: 10.1128/JVI.79.17.10944-10951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JV, Wang CK, Yang CF, Tollefson SJ, House FS, Heck JM, Chu M, Brown JB, Lintao LD, Quinto JD, Chu D, Spaete RR, Edwards KM, Wright PF, Crowe JE., Jr The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. J Infect Dis. 2006;193:387–395. doi: 10.1086/499274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright PF, Karron RA, Madhi SA, Treanor JJ, King JC, O’Shea A, Ikizler MR, Zhu Y, Collins PL, Cutland C, Randolph VB, Deatly AM, Hackell JG, Gruber WC, Murphy BR. The interferon antagonist NS2 protein of respiratory syncytial virus is an important virulence determinant for humans. Journal of Infectious Diseases. 2006;193:573–581. doi: 10.1086/499600. [DOI] [PubMed] [Google Scholar]
- Wyde PR, Chetty SN, Jewell AM, Schoonover SL, Piedra PA. Development of a cotton rat-human metapneumovirus (hMPV) model for identifying and evaluating potential hMPV antivirals and vaccines. Antiviral Res. 2005;66:57–66. doi: 10.1016/j.antiviral.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Luxon B, Casola A, Garofalo RP, Jamaluddin M, Brasier AR. Expression of RSV-induced chemokine gene networks in lower airway epithelial cells revealed by cDNA microarrays. Journal of Virology. 2001;75:9044–9058. doi: 10.1128/JVI.75.19.9044-9058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Luxon BA, Casola A, Garofalo RP, Jamaluddin M, Brasier AR. Expression of respiratory syncytial virus-induced chemokine gene networks in lower airway epithelial cells revealed by cDNA microarrays. J Virol. 2001;75:9044–9058. doi: 10.1128/JVI.75.19.9044-9058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]