Abstract
Green tea and black tea inhibit the formation of carcinogen-DNA adducts and colonic aberrant crypts in rats given 2-amino-3-methylimidazo[4, 5-f]quinoline (IQ), a mutagen from cooked meat. The Salmonella mutagenicity assay was used in the present study to test individual constituents of tea as inhibitors of 2-hydroxyamino-3-methylimidazo[4, 5-f]quinoline (N-hydroxy-IQ), a direct-acting metabolite of IQ. Testing of pure compounds at doses relevant to their levels in tea identified epigallocatechin (EGC) and epigallocatechin-3-gallate (EGCG) as the primary antimutagens. Studies of the inhibitory mechanisms established that the rate of degradation of N-hydroxy-IQ under aqueous conditions was not increased significantly in the presence of tea, in contrast to the results obtained with the complexing agent chlorophyllin (CHL), which rapidly degraded the mutagen. Interaction between N-hydroxy-IQ and several tea constituents was detected in spectro-photometric studies, but the binding constants were only on the order of 1 × 103 M−1, suggesting that mechanisms other than complex formation might prevail under the conditions of the Salmonella assay. Comparison of the results in two different strains of Salmonella typhimurium, TA98 and TA98/1,8-DNP6, indicated that the antimutagenic activity of EGCG was dependent, at least in part, on a functional O-acetyltransferase activity in the bacteria. These studies suggest that tea constituents inhibit the enzyme(s) which generate the aryl nitrenium ion and directly scavenge the reactive electrophile, whereas CHL complexes with heterocyclic amines and facilitates the degradation of active metabolites.
Keywords: Salmonella mutagenicity, inhibition, antimutagenesis
INTRODUCTION
Green tea, black tea, and chlorophyllin (CHL) inhibit carcinogen-induced tumorigenesis in experimental animals [Wang et al., 1992a,b, 1994, 1995; Breinholt et al., 1995; Guo et al., 1995; Hasegawa et al., 1995; Park and Surh, 1996; Singh et al., 1996; Dashwood, 1997], but the precise mechanisms remain to be clarified. We reported recently that green and black tea inhibit the development of colonic aberrant crypt foci (ACF) in rats given 2-amino-3-methylimidazo[4,5-f]quinoline (IQ), a mutagen from cooked meat [Xu et al., 1996]. It was found that tea caused a slight induction of cytochrome P4501A2, the isozyme of cytochrome P450 which activates IQ to form the direct-acting mutagen 2-hydroxyamino-3-methylimidazo[4,5-f]quinoline (N-hydroxy-IQ). The induction of cytochrome P4501A2 would theoretically increase the rate of formation of N-hydroxy-IQ, but several subsequent steps are potential targets for inhibition by antimutagens.
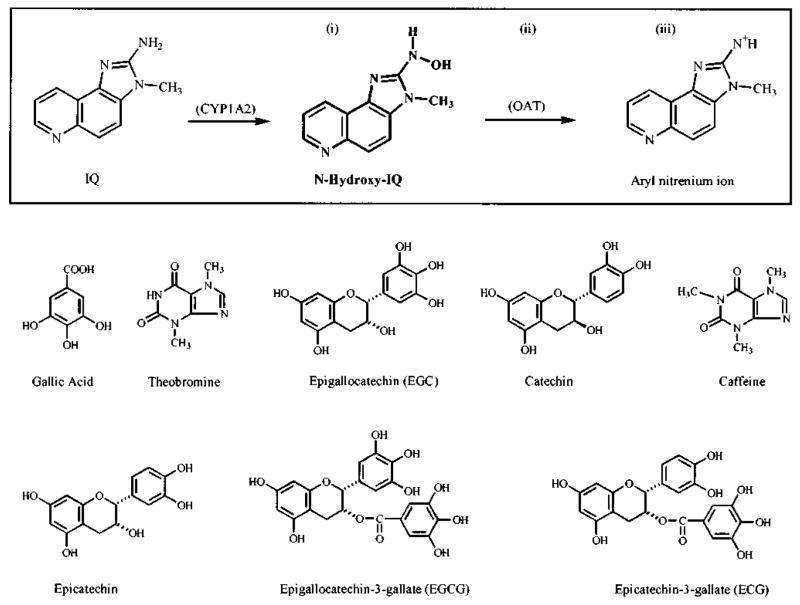
As shown in Figure 1, following the initial activation of IQ by cytochrome P4501A2, N-hydroxy-IQ is converted to an electrophilic aryl nitrenium ion [Snyderwine et al., 1988]. The conversion of N-hydroxy-IQ to the electrophile occurs spontaneously but can be augmented by the presence of sulfotransferases and acetyltransferases, including the O-acetyltransferase (OAT) in Salmonella typhimurium [Saito et al., 1985; Yamazoe et al., 1989]. Inhibitors might act on the pathway shown in Figure 1 by one or more of several mechanisms, including: 1) lowering of the effective concentration of N-hydroxy-IQ via complex formation or enhanced degradation; 2) inhibition of the enzymes, such as OAT, which facilitate the conversion of N-hydroxy-IQ to the aryl nitrenium ion; and/or 3) direct scavenging of the electrophile. This paper describes efforts to clarify these inhibitory mechanisms for the antimutagens in tea. CHL was included in the present investigation as a positive control for some of the end points examined.
Fig. 1.
Pathway for the conversion of IQ to an aryl nitrenium ion, via N-Hydroxy-IQ (box), and the chemical structures of various compounds in tea. Note: formation of the aryl nitrenium ion from N-Hydroxy-IQ occurs spontaneously, but is facilitated by various enzymes, including bacterial O-acetyltransferase (OAT). Sites labeled i–iii are potential targets for inhibition by antimutagens (see text).
MATERIALS AND METHODS
Chemicals
The following tea standards were purchased from Sigma Chemical Co. (St. Louis, MO): (+)catechin, (−)epicatechin (−EC), (+)epicatechin (+EC), (−)epigallocatechin (EGC), (−)epicatechin gallate (ECG), (−)epigallocatechin gallate (EGCG), gallic acid, theobromine, and caffeine. CHL was obtained from Sigma Chemical Co.; the concentrations reported here are corrected for total chlorin content, as described previously [Tachino et al., 1994]. N-Hydroxy-IQ was obtained from the National Cancer Institute Carcinogen Repository (Kansas City, MO). Reagents and media for the mutagenicity assays were from sources described previously [Dashwood and Liew, 1992; Dashwood et al., 1996]. The chemical structure of each tea standard and of N-hydroxy-IQ is shown in Figure 1.
Preparation of Tea
Green tea (Sencha midoriiro) and black tea (English Breakfast tea, Choice Wing Brand) were purchased locally. Teas were brewed fresh immediately before each experiment, as described by Xu et al. [1996], and sterilized by passage through a 0.45 μm filter. On the day of the experiment, individual tea constituents or CHL were dissolved in sterile distilled water and tested immediately for antimutagenic activity in the Salmonella assay.
Mutagenicity Assays
The antimutagenic activities of individual tea standards and CHL were studied using the direct-acting mutagen N-hydroxy-IQ under subdued lighting. Most of the tests were performed using Salmonella typhimurium strain TA98, kindly provided by Dr. Bruce N. Ames. Some experiments used the OAT-deficient strain TA98/1,8-DNP6, generously supplied by Dr. Michael J. Plewa. Each assay involved preincubation of the mutagen and inhibitor at 37°C in the absence of a metabolic activation system, followed by the addition of 2 ml soft agar and pouring onto minimal glucose plates. His+ revertant colonies were scored after 2 days incubation at 37°C in the dark. The dose range typically used for each tea standard, 10–50 nmole/plate, was chosen to reflect the levels present in 0.2 mL green tea brewed for 5 min at a concentration of 2.5% (w/v). This was based on preliminary studies in which the major fractions collected following HPLC separation of tea were quantified and tested for antimutagenic activity [Hernaez et al., 1997]. Unless stated otherwise, none of the inhibitor treatments had an adverse effect on the growth of the bacteria, as judged by the normal appearance of the background lawn of growth and spontaneous counts within the usual range (typically 19 ± 2.5, mean ± SD for triplicate plates).
Degradation of N-Hydroxy-IQ
The rate of degradation of N-hydroxy-IQ in the presence and absence of tea or CHL was studied as described in our previous studies using the direct-acting mutagen benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide (BPDE) [Tachino et al., 1994]. Time-dependent changes in the absorption spectrum of N-hydroxy-IQ in 30 mM Tris-HCl buffer, pH 7.4 were recorded at intervals between 0 min and 60 min. The experiments used matched quartz cuvettes in a double-beam spectrophotometer linked to kinetic and photometric software (Shimadzu UV-2101PC).
Molecular Complex Formation
To investigate the possibility of a direct interaction (molecular complex formation) between N-hydroxy-IQ and CHL or various components in tea, spectrophotometric titrations were performed as described elsewhere [Dashwood and Guo, 1992, 1993; Tachino et al., 1994; Liew et al., 1995; Dashwood et al., 1996]. Absorption spectra were recorded for 1 mL solutions containing 10 nmole N-hydroxy-IQ in 20 mM phosphate buffer, pH 7.4. Binding constants for the complexes were obtained from the x-axis intercept of the Benesi-Hildebrand plot (−1/ΔA vs. 1/inhibitor concentration), using computerized linear regression analysis (SigmaPlot version 3.0, Jandel Scientific).
RESULTS
Antimutagenic Activity of Purified Tea Constituents and CHL
Several purified tea standards were tested for antimutagenic activity toward N-hydroxy-IQ (Fig. 2). Each tea standard was tested in the amount equivalent to that present in 0.2 ml green tea; this was determined in previous studies [Hernaez et al., 1997] which separated tea into several HPLC fractions and generated calibration curves for “integrated area under the peak vs. amount of tea standard injected.” Under these conditions, EGC and EGCG (Figs. 2c and 2h, respectively) produced significant (≥50%), dose-related inhibition. Gallic acid and −EC were less effective, but showed dose-response inhibition at levels ≤30 nmole/plate (Figs. 2a and 2g). All of the other compounds failed to inhibit when tested at doses in the range 10–50 nmole/plate; indeed, theobromine, (+)-catechin, and ECG produced a slight enhancement of the mutagenic activity of N-hydroxy-IQ (Figs. 2b, 2d, and 2i, respectively). In the range 50–250 nmole/plate, (+)-catechin, +EC, and ECG produced dose-response inhibition, but caffeine failed to inhibit at any dose that was non-toxic to the bacteria (results not shown).
Fig. 2.
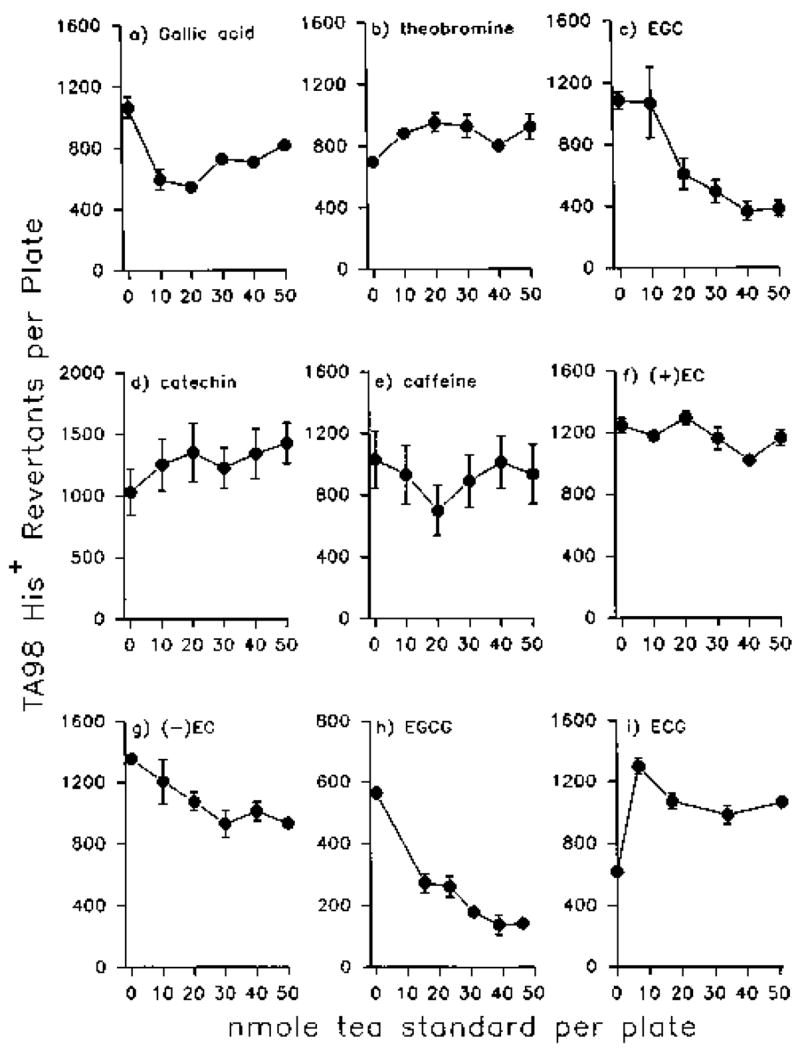
Antimutagenic activity of individual pure tea constituents toward N-Hydroxy-IQ. Salmonella typhimurium strain TA98 was incubated with mutagen (0.015 nmole) plus inhibitor for 15 min, in the absence of a metabolic activation system, prior to the addition of soft agar. Data are given as means ± SD from the testing of triplicate plates, and are representative of results from two or more such assays.
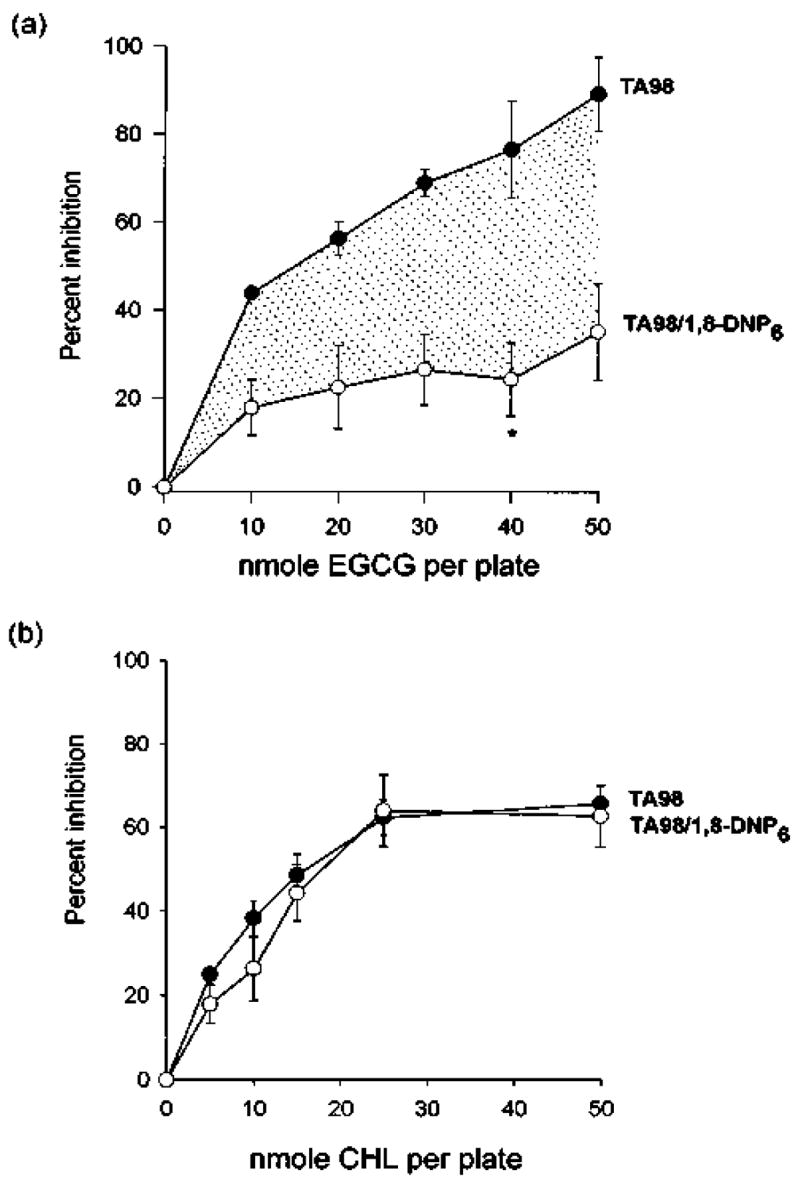
In subsequent studies, the inhibitory activity of EGCG was compared in Salmonella TA98 and the OAT-deficient strain TA98/1,8-DNP6. Under otherwise identical assay conditions, an inhibitory mechanism totally independent of OAT would in theory give overlapping curves in the two strains, whereas a mechanism based entirely on inhibition of OAT would predict the complete absence of antimutagenic activity in strain TA98/1,8-DNP6. As shown in Figure 3a, the antimutagenic activity of EGCG was partially, but not completely, dependent on OAT. The area under the curve for strain TA98/1,8-DNP6 indicates that part of the inhibitory mechanism related to inhibition of OAT, the remainder (shaded) being associated with alternative mechanisms, such as redox scavenging of the aryl nitrenium ion. By way of contrast, Figure 3b shows that CHL was antimutagenic in both strains of Salmonella, most likely due to the ability of this inhibitor to enhance the degradation of N-hydroxy-IQ (next section).
Fig. 3.
Degradation of N-Hydroxy-IQ
Figure 4a shows the time-dependent degradation of N-hydroxy-IQ under aqueous conditions. The Soret band at 260 nm decreased and the peaks at 230 nm, 295 nm, and 350–360 nm increased with time. These changes were most rapid during the first 15 min, but reached completion within ~ 1 hr. In the presence of green tea, no significant increase in the rate of degradation of N-hydroxy-IQ was detected (Fig. 4b). A quenching effect was observed at 260 nm due to partial interference by the tea (λmax ~ 260–270 nm), but the spectral changes at wavelengths > 280 nm occurred at the same rate in the presence and absence of tea. In contrast, CHL enhanced significantly the degradation of N-hydroxy-IQ at all wavelengths, and the spectral changes reached completion within 25–30 min (Fig. 4c). These results are similar to those from previous studies in which CHL and related pigments increased the rate of degradation of the direct-acting mutagens BPDE and 3-hydroxyamino-1-methyl-5H-pyrido[4,3-b]indole (N-hydroxy-Trp-P-2) [Arimoto and Hayatsu, 1989; Tachino et al., 1994; Arimoto et al., 1995].
Fig. 4.
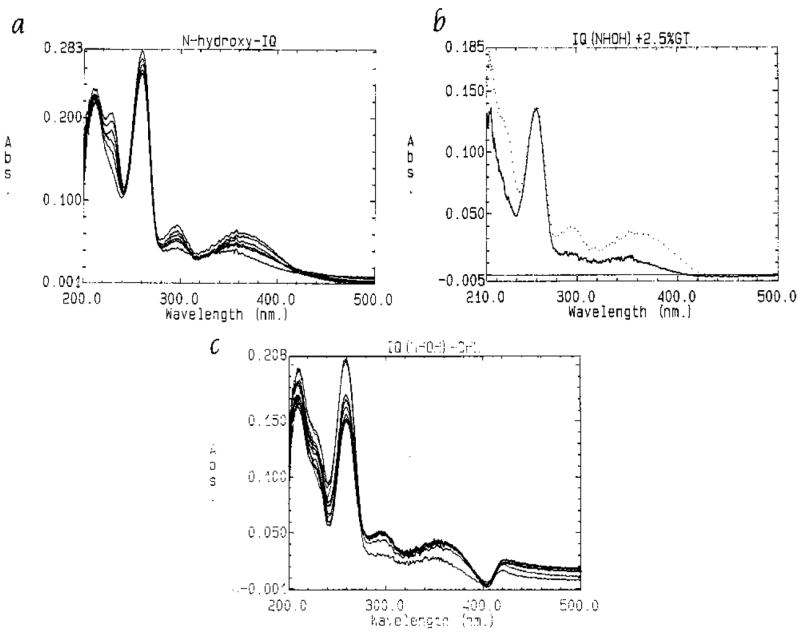
Degradation of N-Hydroxy-IQ under aqueous conditions. (a) Time-dependent changes in the absorption spectrum of mutagen alone in 30 mM Tris-HCl buffer, pH 7.4; 25°C, b = 1 cm. The scan was taken at 3-min intervals. (b) Repeat of the experiment in (a) but with the addition of 10 μl of 2.5% (w/v) green tea to both cuvettes; for clarity, only the spectra at 0 min (solid line) and 15 min (dotted line) are shown. (c) Repeat of (a) in the presence of 10 μM CHL.
Molecular Complex Formation
To avoid the possible complication associated with the degradation of N-hydroxy-IQ under aqueous conditions, spectra were recorded 5 sec after green tea or purified tea standard was added to the mutagen. With increasing concentrations of green tea, the peak at 260 nm was quenched in a manner indicative of complex formation (Fig. 5a). Because tea represents a mixture of potential complexing agents, we sought to determine the binding constants for individual purified tea components. As shown in Figure 5b, EGCG quenched the peak at 260 nm and, in contrast to the complete tea, an apparent isosbestic point indicative of 1:1 complex formation was observed at 272 nm (see Fig. 5b, inset). Similar results were obtained with EGC, ECG, (+)-catechin, and caffeine titrated against N-hydroxy-IQ (spectra not presented). Data obtained from the spectra in Figure 5b were plotted as “−1/ΔA vs. 1/EGCG concentration” in order to determine the binding constant for the complex. As shown in Figure 6, the Kb for the EGCG/N-hydroxy-IQ complex was 1 × 103 M−1. Binding constants for several other tea constituents were determined in a similar manner, and these were on the order of ≤1 × 103 M−1 (Table I). By comparison, the Kb for the complexes between various chlorophylls, chlorins, and porphyrins with heterocyclic amine promutagens were in the range 10–100 × 103 M−1 [Dashwood et al., 1996]. Efforts to measure the binding constant for the interaction between N-hydroxy-IQ and CHL were complicated by CHL-mediated degradation of the mutagen, and for this reason a reliable Kb could not be determined.
Fig. 5.
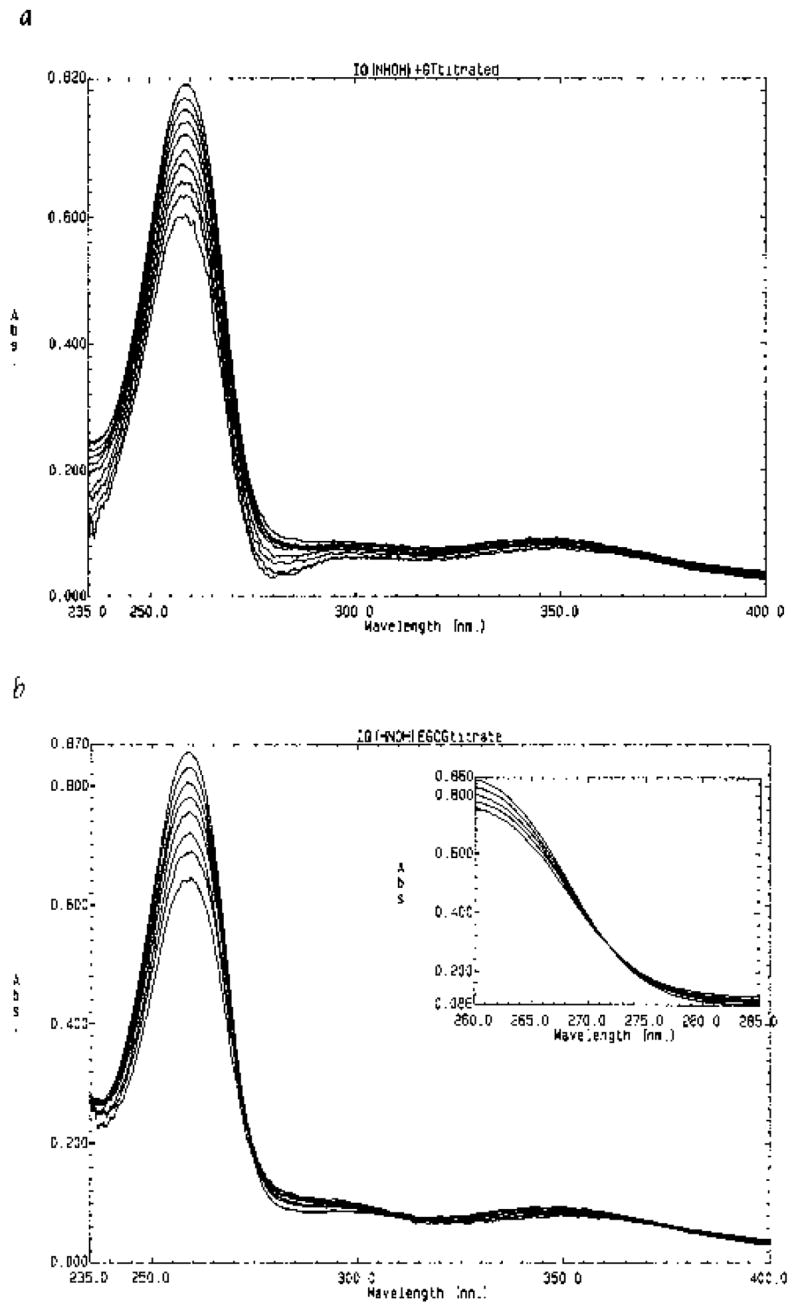
Spectrophotometric titration of N-Hydroxy-IQ with (a) green tea or (b) EGCG. Experiments were conducted in 30 mM Tris-HCl buffer, pH 7.4; b = 25°C. (a) Quenching due to the sequential additions of 1 μL 2.5% (w/v) green tea; (b) titration of mutagen with 25 μM EGCG, each addition; inset: expanded view of the data in the region 260–285 nm, showing an apparent isosbestic point at 272 nm.
Fig. 6.
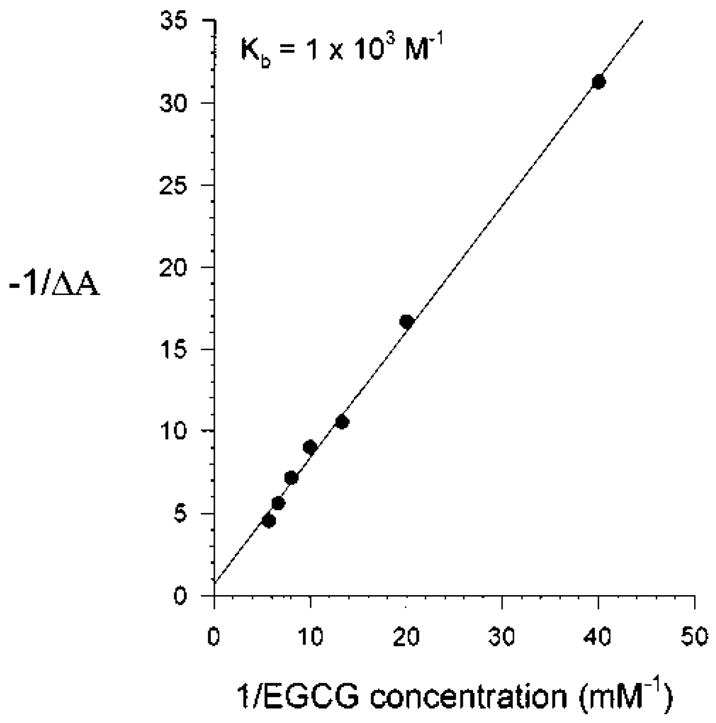
Benesi-Hildebrand plot of the data obtained from Figure 5b for the interaction between EGCG/N-hydroxy-IQ. The binding constant (Kb) was obtained from the x-axis intercept, as described previously [Dashwood et al., 1996]. Similar experiments were conducted with other compounds from tea, and the Kb are presented in Table I.
TABLE I.
Binding Constants for the Complexes Between N-Hydroxy-IQ and Various Compounds in Tea*
| Tea constituent | Kb (× 103 M−1) |
|---|---|
| EGC | 1.1 |
| EGCG | 1.0 |
| (+)-catechin | 0.95 |
| ECG | 0.88 |
| caffeine | <0.5 |
DISCUSSION
The present study has shown that individual tea constituents, in particular EGC and EGCG, inhibit the direct- acting mutagenicity of N-hydroxy-IQ in the Salmonella assay. This extends earlier work which described the anti-mutagenic action of EGCG toward the N-hydroxylated forms of 3-amino-1-methyl-5H-pyrido[4,3-b]indole (Trp- P-2) and 2-amino-6-methyldipyrido[1,2-a:3′,2′-d]imidazole (Glu-P-1) [Hayatsu et al., 1992]. Hayatsu et al. [1992] concluded that complex formation probably does not account for the antimutagenic action of EGCG because spectral changes were not detected upon mixing equimolar concentrations of mutagen and inhibitor. We provide evidence here that complex formation can occur between the mutagen and EGCG, but the interaction is weak and does not alter the rate of degradation of N-hydroxy-IQ. Under the conditions of the Salmonella assay, where the mole ratio of inhibitor to mutagen can be as high as 3000:1, complex formation cannot be totally excluded, but it is unlikely to account for the major anti-mutagenic activity of tea vs. N-hydroxy-IQ.
Other mechanisms considered in the present studies were scavenging of the aryl nitrenium ion and inhibition of the enzyme(s) involved in converting N-hydroxylated metabolites of heterocyclic amines to the reactive electrophile. Results for EGCG in Salmonella strains TA98 and TA98/1,8-DNP6 are consistent with both mechanisms, that is, electrophile scavenging plus inhibition of OAT. Enzyme inhibition has been suggested previously as a mechanism of antimutagenesis by tea. Bu-Abbas et al. [1994] reported that the antimutagenic action of tea toward the promutagens IQ and Glu-P-1 involved two mechanisms, namely, complex formation and inhibition of the enzymes in S9. Rather than having a direct action on cytochromes P450, constituents of tea competitively inhibit the enzyme NADPH-cytochrome P450 reductase and thereby nonspecifically interfere in the metabolic activation of mutagens in vitro [Steele et al., 1985; Hasaniya et al., 1997]. In the case of EGCG, the inhibitor constant (Ki) toward NADPH-cytochrome P450 reductase was found to be on the order of 10 μM [Hasaniya et al., 1997]. Similar results were obtained in studies with CHL and BPDE; however, the Ki for inhibition of NADPH-cytochrome P450 reductase (120 μM CHL) was significantly higher than the 5–10 μM concentrations of CHL, which rapidly degrade BPDE [Tachino et al., 1994] or N-hydroxy-IQ (this study).
In addition to enzyme inhibition, the induction of various detoxification enzymes has been studied in rats given green tea or black tea [Bu-Abbas et al., 1995]. No induction was observed of the enzymes which detoxify reactive oxygen species, namely catalase, glutathione peroxidase, and superoxide dismutase [Bu-Abbas et al., 1995]; thus, the antioxidant properties of tea most likely result from direct effects on the reactive oxygen species, that is, free-radical scavenging [Yen and Chen, 1995]. However, the induction of UDP-glucuronosyl transferase has been reported [Bu-Abbas et al., 1995] and this might account for the higher levels of glucuronides excreted in the urine of rats given IQ plus green or black tea [Xu et al., 1996].
In summary, the results from this investigation and from several published studies suggest that tea and CHL protect via multiple inhibitory mechanisms. With specific reference to IQ and related heterocyclic amine mutagens, the inhibitory mechanisms of tea and CHL differ. Whereas CHL acts mainly by (a) complexing with promutagens and (b) augmenting the degradation of activated metabolites, tea constituents operate predominantly via: (c) inhibition of NADPH-cytochrome P450 reductase and acetyltransferase, enzymes involved (respectively) in converting the promutagen to the N-hydroxylated species and the aryl nitrenium ion; (d) induction of phase II detoxification pathways involving glucuronosyl transferase; and (e) direct scavenging of free radicals and electrophiles (including the aryl nitrenium ion) generated by a suite of enzymes, including NADPH-cytochrome P450 reductase and acetyltransferase. The fact that mechanisms a–e apply to a broad range of compounds, not just the heterocyclic amines, suggests that tea and CHL might complement each other and protect against many of the carcinogens and mutagens which are present in the human diet.
Acknowledgments
We thank Dr. Bruce N. Ames for providing Salmonella typhimurium strain TA98 and Dr. Michael J. Plewa for the generous gift of Salmonella strain TA98/1,8-DNP6. The assistance of Rongliang Chen with Figure 1 is greatly appreciated.
Contract grant sponsor: NIH; Contract grant number: CA65525; Contract grant sponsor: USDA CSRS; Contract grant numbers: 95-34135-1775, managed by PBAG, HAW 626H/628H.
References
- Arimoto S, Hayatsu H. Role of hemin in the inhibition of mutagenic activity of 3-amino-1-methyl-5H-pyrido[4,3-b]indole (Trp- P-2) and other aminoazaarenes. Mutat Res. 1989;213:217–226. doi: 10.1016/0027-5107(89)90153-x. [DOI] [PubMed] [Google Scholar]
- Arimoto S, Kanyama K, Rai H, Hayatsu H. Inhibitory effect of hemin, chlorophyllin and related pyrrole pigments on the mutagenicity of benzo[a]pyrene and its metabolites. Mutat Res. 1995;345:127–135. doi: 10.1016/0165-1218(95)90048-9. [DOI] [PubMed] [Google Scholar]
- Breinholt V, Hendricks J, Pereira C, Arbogast D, Bailey G. Dietary chlorophyllin is a potent inhibitor of aflatoxin B1 hepatocarcinogenesis in rainbow trout. Cancer Res. 1995;55:57–62. [PubMed] [Google Scholar]
- Bu-Abbas A, Clifford MN, Walker R, Ioannides C. Marked antimutagenic potential of aqueous green tea extracts: Mechanism of action. Mutagenesis. 1994;9:325–331. doi: 10.1093/mutage/9.4.325. [DOI] [PubMed] [Google Scholar]
- Bu-Abbas A, Clifford MN, Ioannides C, Walker R. Stimulation of rat hepatic UDP-glucuronosyl transferase activity following treatment with green tea. Food Chem Toxic. 1995;33:27–30. doi: 10.1016/0278-6915(95)80244-4. [DOI] [PubMed] [Google Scholar]
- Dashwood R. Chlorophylls as anticarcinogens (Review) Int J Oncol. 1997;10:721–727. doi: 10.3892/ijo.10.4.721. [DOI] [PubMed] [Google Scholar]
- Dashwood RH, Guo D. Inhibition of 2-amino-3-methylimidazo[4,5-f]quinoline (IQ)-DNA binding by chlorophyllin: Studies of enzyme inhibition and molecular complex formation. Carcinogenesis. 1992;13:1121–1126. doi: 10.1093/carcin/13.7.1121. [DOI] [PubMed] [Google Scholar]
- Dashwood RH, Guo D. Antimutagenic potency of chlorophyllin in the Salmonella assay and its correlation with binding constants of mutagen-inhibitor complexes. Environ Mol Mutagen. 1993;22:164–171. doi: 10.1002/em.2850220309. [DOI] [PubMed] [Google Scholar]
- Dashwood RH, Liew C. Chlorophyllin-enhanced excretion of urinary and fecal mutagens in rats given 2-amino-3-methylimidazo[4,5-f]quinoline. Environ Mol Mutagen. 1992;20:199–205. doi: 10.1002/em.2850200308. [DOI] [PubMed] [Google Scholar]
- Dashwood RH, Yamane S, Larsen R. Study of the forces stabilizing complexes between chlorophylls and heterocyclic amine mutagens. Environ Mol Mutagen. 1996;27:211–218. doi: 10.1002/(SICI)1098-2280(1996)27:3<211::AID-EM6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Guo D, Horio DT, Grove JS, Dashwood RH. Inhibition by chlorophyllin of 2-amino-3-methylimidazo[4,5-f]quinoline-induced tumorigenesis in the male F344 rat. Cancer Lett. 1995;95:161–165. doi: 10.1016/0304-3835(95)03882-w. [DOI] [PubMed] [Google Scholar]
- Hasaniya N, Youn K, Xu M, Hernaez J, Dashwood RH. Inhibitory activity of green and black tea in a free radical-generating system using 2-amino-3-methylimidazo[4,5-f]quinoline as substrate. Jpn J Cancer Res. 1997;88:553–558. doi: 10.1111/j.1349-7006.1997.tb00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa R, Hirose M, Kato T, Hagiwara A, Boonyaphiphat P, Nagao M, Ito N, Shirai T. Inhibitory effect of chlorophyllin on PhIP-induced mammary carcinogenesis in female F344 rats. Carcinogenesis. 1995;16:2243–2246. doi: 10.1093/carcin/16.9.2243. [DOI] [PubMed] [Google Scholar]
- Hayatsu H, Inada N, Kakutani T, Arimoto S, Negishi T, Mori K, Okuda T, Sakata I. Suppression of genotoxicity of carcinogens by (−)-epigallocatechin gallate. Prev Med. 1992;21:370–376. doi: 10.1016/0091-7435(92)90044-i. [DOI] [PubMed] [Google Scholar]
- Hernaez J, Xu M, Dashwood RH. Antimutagenic activity of tea towards 2-hydroxyamino-3-methylimidazo[4,5-f]quinoline: Effect of tea concentration and brew time on electrophile-scavenging. Mutat Res. 1997 doi: 10.1016/s0027-5107(97)00309-6. in press. [DOI] [PubMed] [Google Scholar]
- Liew C, Schut H, Ching S, Pariza MW, Dashwood RH. Protection of conjugated linoleic acids against 2-amino-3-methylimidazo[4,5-f]quinoline (IQ)-induced colon carcinogenesis in the F344 rat: A study of inhibitory mechanisms. Carcinogenesis. 1995;16:3037–3043. doi: 10.1093/carcin/16.12.3037. [DOI] [PubMed] [Google Scholar]
- Park K-K, Surh Y-S. Chemopreventive activity of chlorophyllin against mouse skin carcinogenesis by benzo[a]pyrene and benzo-[a]pyrene-7,8-dihydrodiol-9,10-epoxide. Cancer Lett. 1996;102:143–149. doi: 10.1016/0304-3835(96)04173-0. [DOI] [PubMed] [Google Scholar]
- Saito K, Shinohara A, Kamataki T, Kato R. Metabolic activation of mutagenic N-hydroxyarylamines by O-acetyltransferase in Salmonella typhimurium TA98. Arch Biochem Biophys. 1985;239:286–295. doi: 10.1016/0003-9861(85)90838-0. [DOI] [PubMed] [Google Scholar]
- Singh A, Singh S, Bamezai R. Modulatory influence of chlorophyllin on the mouse skin papillomagenesis and xenobiotic detoxication system. Carcinogenesis. 1996;17:1459–1463. doi: 10.1093/carcin/17.7.1459. [DOI] [PubMed] [Google Scholar]
- Snyderwine EG, Wirth PJ, Roller PP, Adamson RH, Sato S, Thorgeirsson SS. Mutagenicity and in vitro covalent DNA binding of 2-hydroxyamino-3-methylimidazo[4,5-f]quinoline. Carcinogenesis. 1988;9:411–418. doi: 10.1093/carcin/9.3.411. [DOI] [PubMed] [Google Scholar]
- Steele CM, Lalies M, Ioannides C. Inhibition of the mutagenicity of aromatic amines by the plant flavonoid (+)-catechin. Cancer Res. 1985;45:3573–3577. [PubMed] [Google Scholar]
- Tachino N, Guo D, Dashwood WM, Yamane S, Larsen R, Dashwood RH. Mechanisms of the in vitro antimutagenic action of chlorophyllin against benzo(a)pyrene: Studies of enzyme inhibition, molecular complex formation and degradation of the ultimate carcinogen. Mutat Res. 1994;308:191–203. doi: 10.1016/0027-5107(94)90154-6. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Agarwal R, Khan WA, Mukhtar H. Protection against benzo[a]pyrene- and N-nitrosodiethylamine-induced lung and forestomach tumorigenesis in A/J mice by water extracts of green tea and licorice. Carcinogenesis. 1992a;8:1491–1494. doi: 10.1093/carcin/13.8.1491. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Huang M-T, Ferraro T, Wong C-Q, Lou Y-R, Reuhl K, Iatropoulos M, Yang C, Conney AH. Inhibitory effect of green tea in the drinking water on tumorigenesis by ultraviolet light and 12-O-tetradecanoyl-phorbol-13-acetate in the skin of SKH-1 mice. Cancer Res. 1992b;52:1162–1170. [PubMed] [Google Scholar]
- Wang ZY, Huang M-T, Lou Y-R, Xie J-G, Reuhl KR, Newmark HL, Ho C-T, Yang CS, Conney AH. Inhibitory effects of black tea, green tea, decaffeinated black tea and decaffeinated green tea on ultraviolet B light-induced skin carcinogenesis in 7,12-dimethylbenz[a]anthracene-initiated SKH-1 mice. Cancer Res. 1994;54:3428–3435. [PubMed] [Google Scholar]
- Wang ZY, Wang L-D, Lee M-J, Ho C-T, Huang M-T, Conney A, Yang CS. Inhibition of N-nitrosomethylbenzylamine-induced esophageal tumorigenesis in rats by green and black tea. Carcinogenesis. 1995;16:2143–2148. doi: 10.1093/carcin/16.9.2143. [DOI] [PubMed] [Google Scholar]
- Xu M, Bailey AC, Hernaez JF, Taoka CR, Schut HAJ, Dashwood RH. Protection by green tea, black tea and indole-3-carbinol against 2-amino-3-methylimidazo[4,5-f]quinoline-induced DNA adducts and colonic aberrant crypts in the F344 rat. Carcinogenesis. 1996;17:1429–1434. doi: 10.1093/carcin/17.7.1429. [DOI] [PubMed] [Google Scholar]
- Yamazoe Y, Abu-Zeid M, Gong D, Staiano N, Kato R. Enzymatic acetylation and sulfation of N-hydroxyarylamines in bacteria and rat livers. Carcinogenesis. 1989;10:1675–1679. doi: 10.1093/carcin/10.9.1675. [DOI] [PubMed] [Google Scholar]
- Yen G-C, Chen H-Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem. 1995;43:27–32. [Google Scholar]