Abstract
The impact of mandibular distraction on condyles is poorly understood. To examine how condylar mineralization is affected, we performed distraction in 128 one-month-old rapidly and 126 three-month-old slowly growing rats. The rate of distraction was 0.0 mm (sham), 0.2 mm (slow), 0.4 mm (moderate), or 0.6 mm (rapid). From 7 to 9 rats from each rate (n = 29-32) were killed at 4 time periods (D6, D10, D24, and D38) following osteotomy. Calcein and alizarin were injected 6 and 3 days, respectively, prior to death. Methacrylate-embedded sagittal condylar sections were examined under epifluorescence, and mineral apposition rates were measured. Results indicated that: (1) rapidly growing rats showed higher mineral apposition rates (p < 0.01-0.001) than did slowly growing rats; (2) mineral apposition rates were lower in distracted sides at all times in rapidly growing rats (p < 0.05-0.01), while this side-dependency was seen only at D24 in slowly growing rats (p < 0.05); and (3) distraction rates had little effect on mineral apposition rates. Thus, mandibular distraction decreases condylar mineral apposition rates, but only in rapidly growing rats, which is related to surgery and its functional consequences, not to the distraction rate.
Keywords: distraction osteogenesis, mineral apposition, mandibular condyle, growth, rat
Introduction
Mandibular distraction is done on young patients with craniofacial deformities and young adults with malocclusion. The impact of these procedures on the mandibular condyle is not well-understood, especially with regard to growth status. During mandibular distraction, unusual forces are unavoidably produced by distraction procedures and stretched masticatory muscles. These unusual loads superimpose on the normal mechanics of function, growth, and remodeling of craniofacial structures, leading to various consequences, depending on age, distraction rate/rhythm, and time-course. The TMJ is a highly adaptive structure and therefore may be affected. Many studies of various animal models have indicated that mandibular distraction may lead to degenerative changes in the TMJ, including reduction of cartilage thickness, increase in woven bone, irregularities of articular surface contour, and thinning of the medial aspect of the disc (McCormick et al., 1995a,b; Karaharju-Suvanto et al., 1996; Stelnicki et al., 2001; Thurmuller et al., 2002). Previous studies in a rat model revealed that mandibular distraction led to reduced size, decreased density, and altered orientation of the condyle. These alterations have a negative impact on the desired outcome of mandibular lengthening (Liu et al., 2003, 2004).
In the present investigation, we extended our observations on the morphological effects of mandibular osteodistraction to osseous growth as reflected by condylar mineralization. We hypothesized that rapidly growing rats would have more active condylar mineralization and thus a more negative outcome from distraction. We further hypothesized that these effects would be diminished over time, as consolidation proceeds and loading becomes more normal.
Materials & Methods
Distraction Procedures
Unilateral mandibular ramus osteotomy and device placement were performed in 128 rapidly growing (one-month-old) and 126 slowly growing (three-month-old) rats. After a three-day latency, rats were distracted in the horizontal direction at one of 4 rates per day (sham, 0.0 mm; slow, 0.2 mm; moderate, 0.4 mm; or rapid, 0.6 mm) for 5 days (D3 to D7, n = 29-32). From 7 to 9 rats from each rate were killed at one of 4 time periods following osteotomy (D6, D10, D24, and D38). These time-points represent mid-distraction, early, middle, and late consolidations. Detailed descriptions of the distraction and post-operative care procedures have been published elsewhere (Connolly et al., 2002; King et al., 2003; Liu et al., 2003, 2004, 2005; Shin et al., 2005; Okafuji et al., 2006).
Rats were injected with calcein (20 mg/Kg) and alizarin complexone (30 mg/Kg) (Sigma Chemical, St. Louis, MO, USA) intraperitoneally 6 and 3 days, respectively, prior to death. These fluorescent dyes chelate to calcium irons, resulting in deposition of a double vital label on all actively mineralizing bone surfaces. Body weights were monitored daily throughout the experimental period. The animal protocol was approved by the Institutional Animal Care and Use Committee, University of Washington.
Specimen Preparation and Image Capture
After death, both hemimandibles were removed and fixed in 10% formalin. Disarticulated hemimandibles were embedded in micro-bed embedding resin blocks according to the protocol provided by the manufacturer (EMS Co., Fort Washington, PA, USA). Successive sagittal sections were taken at 30-μm thicknesses by means of a Leica SP1600 saw microtome (Leica Microsystem, Bannockburn, IL, USA) and mounted on 1% gelatinized slides. The sections from the middle of the condyles were examined unstained under epifluorescence with the aid of a Nikon Eclipse E400 microscope (Nikon Corporation, Tokyo, Japan) with a Spot RT digital camera, and sections showing the entire articular surface of the condyle were selected. Images were captured at 4X magnification with the use of Metavue software (Universal Imaging Corporation, Downingtown, PA, USA). Green (calcein) and red (alizarin complexone) images were captured separately and then merged. After adjustment for brightness/contrast and size calibration, a series of merged images with the double-labeled articular surface was saved for measurements.
Measurements for Mineral Apposition Rate
A series of circles, tangent to the end line of each label, was superimposed over each image between the calcein (green) and the alizarin (red) bands, and these circles were lined up successively to cover the entire band defined by the two lines (Fig. 1). The diameter of each circle was logged directly into an Excel file with the use of Metavue software, and means were calculated. These values represent inter-label distance, and we divided them by the time interval between administration of the two vital markers (3 days) to calculate mineral apposition rate. This nomenclature and calculation are in accordance with the American Society of Bone and Mineral Research Histomorphometry Nomenclature Committee (Parfitt et al., 1987). The observer (ZJL) was blinded to distraction rates and time-points.
Figure 1.
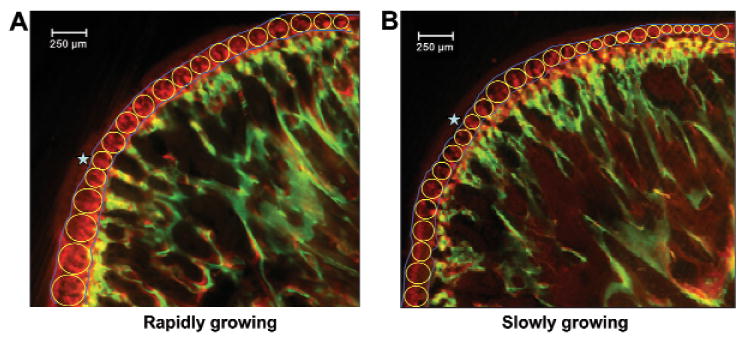
Measurement method for mineral appositional rate. A series of circles, tangent to end lines (blue) of calcein (green) and alizarin (red) labels, was superimposed over the entire band defined by the two labels. The dark red color (stars) over the alizarin band (stars) is autofluorescence and was not measured.
Because of unavoidable variations in condylar orientation, resulting from embedding procedures, some specimens (16.2% of rapidly and 10.5% of slowly growing rats) yielded sections that showed only part of the condylar surface, usually the middle or the posterior 2/3. These specimens were distributed randomly with regard to distraction rates and time-points. To treat these specimens as closely as possible to intact specimens, in terms of having similar numbers of circles measured, we captured and measured at least two successive sections from each specimen, and took the average.
Statistics
We calculated descriptive statistics for each group, to determine means, standard deviations, and ranges. Because the data were normally distributed, ANOVA was used, followed by pairwise comparisons with Tukey post hoc tests. The paired t test was applied for comparisons between the distracted and undistracted sides at the same time-point, and the non-paired t test was used for comparison of values of mineral apposition rates between different time-points. Probability levels of 0.05 or less were considered to indicate statistical significance.
Results
On average, body weight increased 181% (from 112 to 315 g) for rapidly and 15.3% (from 365 to 421 g) for slowly growing rats throughout the experimental period.
Appositional bands for both calcein and alizarin labeling periods were apparent in all condyles (Fig. 2). Rapidly growing rats had much wider bands than slowly growing rats in both calcein and alizarin labeling (Figs. 2C, 2D), and their distracted condyles showed narrower bands than those of the undistracted condyles (Figs. 2A, 2C).
Figure 2.
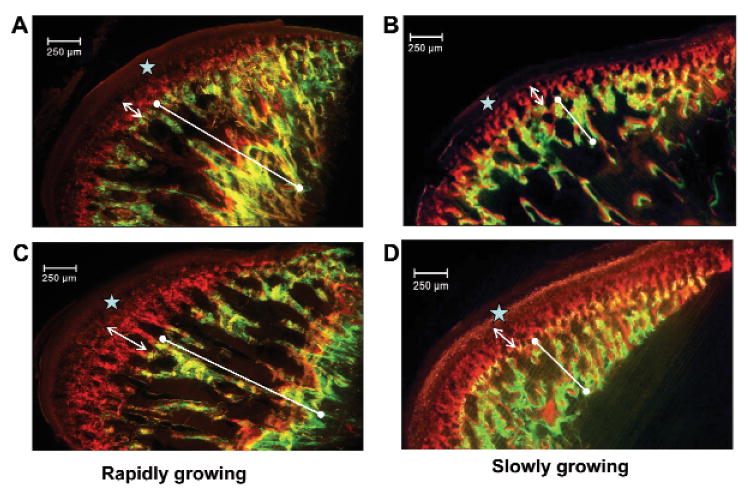
Examples of double-labeled condyles from rapidly (A,C) and slowly (B,D) growing animals at D24. The distracted side (A,B) is compared with the undistracted side (C,D). Note that both calcein (line with end dots) and alizarin (double-headed arrow) bands are narrower in the distracted than the undistracted sides. This side difference occurred at all time-points in rapidly growing rats, but only at D24 in slowly growing rats. The stars denote autofluorescence of unmineralized tissue.
The time-course curves (Figs. 3A, 3B) showed that mineral apposition rates were significantly lower in the distracted sides at all time-points in rapidly growing rats (p < 0.05-0.01), while this side-dependency was seen only at D24 in the slowly growing rats (p < 0.05). Furthermore, mineral apposition rates decreased significantly after D10 and were lowest at D38 on both sides in rapidly growing rats (p < 0.001). This time-course change was seen only in the distracted side in slowly growing rats (p < 0.001), and mineral apposition rates remained at approximately the same level at the two late time-points (D24 and D38). Conversely, in slowly growing rats, a significantly higher mineral apposition rate (p < 0.05) at D6 was seen in the distracted side, rather than in the undistracted side as was the case for rapidly growing rats.
Figure 3.
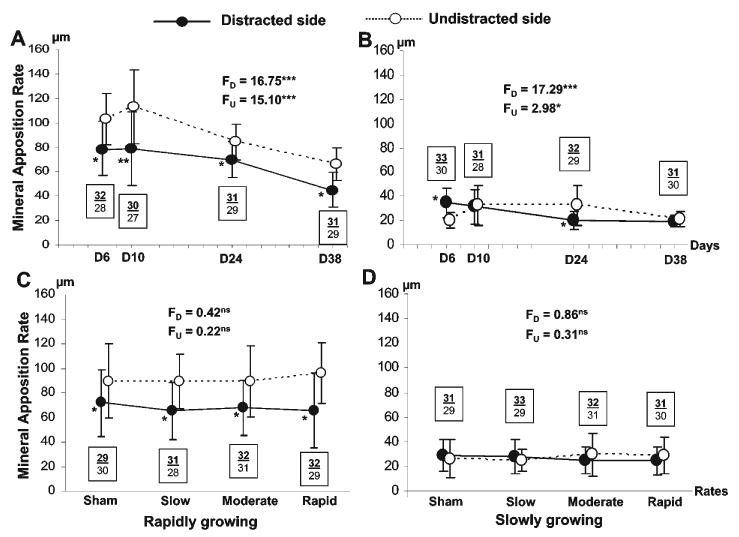
The overall time-course of mineral apposition rates in rapidly (A) and slowly (B) growing rats (distraction rates combined), and the overall effects of distraction rate on mineral apposition rate in rapidly (C) and slowly (D) growing rats (time-points combined). Solid and empty circles indicate the mean values of mineral apposition rates in distracted and undistracted sides of condyles, respectively, and vertical lines indicate one standard deviation for each mean value. F values and superscripted asterisks indicate the one-way ANOVA results for distracted (FD) and undistracted (FU) sides. Asterisks by the solid circles indicate significant differences between the distracted and undistracted sides at each time-point or distraction rate, by paired t tests. *p < 0.05, **p < 0.01, ***p < 0.001. ns: not significant. Bold/underlined and plain numbers indicate sample sizes in the distracted and undistracted sides, respectively. Note that in B, the mineral apposition rate of the distracted side at D6 was significantly higher than that of the undistracted side. In all other significant differences, the mineral apposition rate of the distracted side was lower than that of the undistracted side.
When time-points were grouped (Figs. 3C, 3D), mineral apposition rates did not show significant differences across different distraction rates, indicating that the rate of distraction was not an important influence on mineral apposition rate. Furthermore, mineral apposition rates of the distracted side were significantly lower than those of the undistracted side across all distraction rates in rapidly, but not in slowly, growing rats (Figs. 3C, 3D).
Two-way comparisons of the 4 distraction rates at each time-point (Fig. 4) further demonstrated that rate had no significant impact on mineral apposition rates of the distracted-side condyle at any time-point for either age. Rapidly growing rats maintained significantly higher values of mineral apposition rate at all time-points, but mineral apposition rates decreased at D38 (p < 0.05). For the slowly growing rats, mineral apposition rates decreased from the two early (D6 and D10) to the two late (D24 and D38) time-points (p < 0.05).
Figure 4.
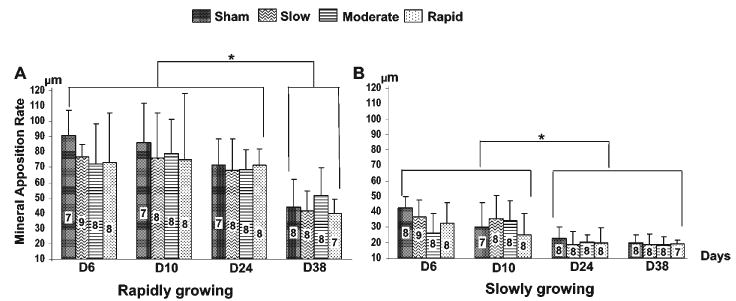
Comparisons of the effects of distraction rate at each time-point on the mineral apposition rate of the distracted condyle in rapidly (A) and slowly (B) growing rats. Bars indicate the mean values of mineral apposition rate in the condyle of the distracted side at 4 different distraction rates, and vertical lines above the bars indicate one standard deviation. Numbers within each bar indicate sample sizes. Asterisks above the horizontal lines indicate significant differences (p < 0.05) between various time-points for each distraction rate, by non-paired t tests.
Discussion
It is well-known that the ramus, the alveolar ridge, and the condyle are the major sites of mandibular growth, and that growth of the condyle in the superior and posterior directions contributes greatly to mandibular height and length (Tuominen et al., 1994; Buschang and Gandini Junior, 2002). Our previous studies have revealed that mandibular distraction retarded condylar growth in rapidly growing rats, and that the restrained growth could be detected as early as mid-distraction (D6). This retardation produced a significant reduction in condylar size (length, width, and area) compared with the condyle of the undistracted side. However, the reduced size of the distracted condyle in slowly growing rats appeared to be an actual loss of bony dimension, rather than a growth effect. Furthermore, condylar angulation relative to the occlusal plane became more upright in rapidly growing rats and less upright in slowly growing rats (Liu et al., 2003, 2004). In the present study, we found that a decrease in condylar mineral apposition rate of the distracted side was mainly a feature of rapidly growing rats. Compared with the undistracted side, which grows relatively normally (Liu et al., 2003), the mineral apposition rate was at least 30% less at all time-points for the rapidly growing rats, whereas a decrease was detectable only at middle consolidation (D24) in slowly growing rats (Figs. 3A, 3B). The overall rate effects further revealed that the reduction of mineral apposition rates was large and significant for all rates in growing rats, but this reduction was absent or trivial in slowly growing rats (Figs. 3C, 3D). Therefore, we can conclude that, in rapidly growing rats, the mechanism by which mandibular distraction procedures retard condylar growth on the distracted side includes slowing its mineral apposition. This mechanism, however, is not involved in producing the reduced dimensions of the condyle in slowly growing rats.
A finding of reduced growth on the distraction-side condyle is not universal. Several studies dealing with the effects of distraction found more growth in the distracted condyle, at least at early time-points after distraction. A sheep model indicated that, compared with controls, the bone of the distracted condyle became more dense, the hypertrophic zone became thicker, and endochondral ossification increased between the cartilaginous zone and bone tissue (Karaharju-Suvanto et al., 1996). With Fluorine-18-labeled fluoride as a tracer to show new bone formation in positron emission tomographic (PET) images in a rabbit model, osteoblastic activity was shown to be higher on the distracted sides, and these effects were greater with greater distraction rates (Kim et al., 2004).
Three aspects of the current study explain the conflicting literature. First, the effects on the condyle are clearly age-specific. Rather than showing a decrease in mineral apposition rate, as did rapidly growing rats, slowly growing rats showed an increase of mineral apposition rate at D6 (Fig. 3B). Second, the cited studies did not distinguish between condylar growth specifically and osteoblastic activity generally. Even though the external dimensions of the distracted condyles are smaller than those of the undistracted condyle at early time-points (up to D10), the condyles are more dense for both ages (Liu et al., 2004). Third, the increase in microdensity diminishes over time from early to late consolidation, and eventually becomes lower than that of the undistracted condyle (Liu et al., 2003, 2004). These changes in microdensity may reflect loading from the distraction procedures (Muhonen et al., 2004). Therefore, even though the distraction may transiently increase bone appositional activity in the distracted condyle at the early time-points, the longer-term effects of these procedures on condylar growth and remodeling are negative.
Distraction rate is a critical factor in the determination of a desirable outcome. Several studies on large- or middle-sized animal models have reported that faster distraction rates (> 1 mm/day) may induce various degenerative alterations in the condylar cartilage and disc (Harper et al., 1997; Kruse-Losler t al., 2001; Thurmuller et al., 2002; Zou et al., 2001). A human study further found that the amount of condylar displacement in the upward and backward directions correlates with the amount of mandibular lengthening by distraction (Azumi et al., 2004). Our previous studies indicated that, while moderate and rapid distraction rates caused greater size reduction and re-orientation in the condyles of slowly growing rats, these rate effects were not prominent in rapidly growing rats (Liu et al., 2003, 2004). The present study further revealed that the mineral apposition rate in the condyle was not affected by the distraction rate. Given the fact that rapidly growing animals gained 10 times more body weight than the slowly growing animals, it is clear that the condylar mineral apposition rate was mostly affected by growth rate. In fact, for rapidly growing animals, the decrease of the mineral apposition rate was related to the overall surgery, and not to the distraction, because even sham treatment resulted in a significant reduction of the mineral apposition rate (Figs. 3C, 4). Therefore, the detachments of the masseter and parts of the medial pterygoid muscles by device placement and the destruction of the integrity of the mandible by the osteotomy may be the primary contributors to these negative changes. Furthermore, the size of the animal model may be relevant. For small-sized animals, such as the rats in the current study, distraction surgery per se might provide a significant insult overriding other distraction-dependent factors, such as latency period, distraction rate, rhythm, and amount. These distraction-dependent features could have a relatively larger influence on larger animals. This possibility should be taken into account when the current data are applied in larger animal or human studies.
As consolidation proceeded and loading from the distraction lessened, the adverse effect on condylar mineral apposition rate by distraction was not diminished as hypothesized. On the contrary, the reduction of mineral apposition rate became worse at the two late time-points (Fig. 3A). This phenomenon suggests that the mechanical load on the condyle from the distraction per se is not a primary cause of the reduction of mineral apposition rate. Instead, the procedure influences growth by another mechanism, possibly involving the muscle detachment, atrophy or fibrosis by the surgery, muscle overstretching by elongation of the mandible, local mechanical environmental change by mandibular ramus osteotomy, and chewing/grinding pattern change by altered occlusion.
Acknowledgments
We thank Ms. Noralyn Altares and Ms. Xian-Qin Bai for their help with histology and lab assistance. Thanks also go to Ms. Alice Kim and Mr. Eric Tso for their help with image capturing. This study was supported by NIH grant PHS P60 13061. A part of the present study was presented in 2005 at the 83rd General Session & Exhibition of the International Association for Dental Research (IADR), Baltimore, MD, USA.
References
- Azumi Y, Sugawara J, Takahashi I, Mitani H, Nagasaka H, Kawamura H. Positional and morphologic changes of the mandibular condyle after mandibular distraction osteogenesis in skeletal class II patients. World J Orthod. 2004;5(1):32–39. [PubMed] [Google Scholar]
- Buschang PH, Gandini LG., Junior Mandibular skeletal growth and modelling between 10 and 15 years of age. Eur J Orthod. 2002;24(1):69–79. doi: 10.1093/ejo/24.1.69. [DOI] [PubMed] [Google Scholar]
- Connolly JP, Liu ZJ, Wang L, Whelan MF, Huang GJ, Williams JK, et al. A custom mandibular distraction device for the rat. J Craniofac Surg. 2002;13:445–450. 450–452. doi: 10.1097/00001665-200205000-00015. discussion. [DOI] [PubMed] [Google Scholar]
- Harper RP, Bell WH, Hinton RJ, Browne R, Cherkashin AM, Samchukov ML. Reactive changes in the temporomandibular joint after mandibular midline osteodistraction. Br J Oral Maxillofac Surg. 1997;35:20–25. doi: 10.1016/s0266-4356(97)90004-8. [DOI] [PubMed] [Google Scholar]
- Karaharju-Suvanto T, Peltonen J, Laitinen O, Kahri A. The effect of gradual distraction of the mandible on the sheep temporomandibular joint. Int J Oral Maxillofac Surg. 1996;25:152–156. doi: 10.1016/s0901-5027(96)80063-4. [DOI] [PubMed] [Google Scholar]
- Kim SG, Ha JW, Park JC. Histological changes in the temporomandibular joint in rabbits depending on the extent of mandibular lengthening by osteodistraction. Br J Oral Maxillofac Surg. 2004;42:559–565. doi: 10.1016/j.bjoms.2004.06.018. [DOI] [PubMed] [Google Scholar]
- King GJ, Liu ZJ, Wang LL, Chiu IY, Whelan MF, Huang GJ. Effect of distraction rate and consolidation period on bone density following mandibular osteodistraction in rats. Arch Oral Biol. 2003;48:299–308. doi: 10.1016/s0003-9969(03)00004-9. [DOI] [PubMed] [Google Scholar]
- Kruse-Losler B, Meyer U, Floren C, Joos U. Influence of distraction rates on the temporomandibular joint position and cartilage morphology in a rabbit model of mandibular lengthening. J Oral Maxillofac Surg. 2001;59:1452–1459. 1460–1461. doi: 10.1053/joms.2001.28281. discussion. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, King GJ, Herring SW. Alterations of morphology and microdensity in the condyle after mandibular osteodistraction in the rat. J Oral Maxillofac Surg. 2003;61:918–927. doi: 10.1016/s0278-2391(03)00294-5. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, King GJ, Herring SW. Why do we fail to achieve predicted lengthening in mandibular osteodistraction? Observations on condylar morphology and microdensity in growing and maturing rat. In: Davidovitch Z, Mah J, editors. Biological mechanisms of tooth movement and craniofacial adaption. Bangkok: Harvard Soc. Adv. Orthodontics; 2004. pp. 39–52. [Google Scholar]
- Liu ZJ, Anderson MW, Gu GM, King GJ. Apoptosis in the regenerate produced by mandibular osteodistraction in the mature rat. Orthod Craniofac Res. 2005;8:41–51. doi: 10.1111/j.1601-6343.2004.00310.x. [DOI] [PubMed] [Google Scholar]
- McCormick SU, McCarthy JG, Grayson BH, Staffenberg D, McCormick SA. Effect of mandibular distraction on the temporomandibular joint: Part 1, Canine study. J Craniofac Surg. 1995a;6:358–363. doi: 10.1097/00001665-199509000-00005. [DOI] [PubMed] [Google Scholar]
- McCormick SU, Grayson BH, McCarthy JG, Staffenberg D. Effect of mandibular distraction on the temporomandibular joint: Part 2, Clinical study. J Craniofac Surg. 1995b;6:364–367. doi: 10.1097/00001665-199509000-00006. [DOI] [PubMed] [Google Scholar]
- Muhonen A, Haaparanta M, Gronroos T, Bergman J, Knuuti J, Hinkka S, Happonen RP. Osteoblastic activity and neoangiogenesis in distracted bone of irradiated rabbit mandible with or without hyperbaric oxygen treatment. Int J Oral Maxillofac Surg. 2004;33:173–178. doi: 10.1054/ijom.2003.0489. [DOI] [PubMed] [Google Scholar]
- Okafuji N, Liu ZJ, King GJ. Assessment of cell proliferation during mandibular osteodistraction in the mature rat. Am J Orthod Dentofac Orthop. 2006 doi: 10.1016/j.ajodo.2005.06.023. in press. [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Shin JY, Liu ZJ, King GJ. Trabecular organization in mandibular osteodistraction in growing and maturing rats. J Oral Maxillofac Surg. 2005;63:77–86. doi: 10.1016/j.joms.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Stelnicki EJ, Stucki-McCormick SU, Rowe N, McCarthy JG. Remodeling of the temporomandibular joint following mandibular distraction osteogenesis in the transverse dimension. Plast Reconstr Surg. 2001;107:647–658. doi: 10.1097/00006534-200103000-00002. [DOI] [PubMed] [Google Scholar]
- Thurmuller P, Troulis MJ, Rosenberg A, Kaban LB. Changes in the condyle and disc in response to distraction osteogenesis of the minipig mandible. J Oral Maxillofac Surg. 2002;60:1327–1333. doi: 10.1053/joms.2002.35733. [DOI] [PubMed] [Google Scholar]
- Tuominen M, Kantomaa T, Pirttiniemi P. Effect of altered loading on condylar growth in the rat. Acta Odontol Scand. 1994;52:129–134. doi: 10.3109/00016359409027586. [DOI] [PubMed] [Google Scholar]
- Zou S, Hu J, Wang D, Li J, Tang Z. Changes in the temporomandibular joint after mandibular lengthening with different rates of distraction. Int J Adult Orthodon Orthognath Surg. 2001;16:221–225. [PubMed] [Google Scholar]


