Abstract
Background
Alstrom syndrome (AS) is a rare autosomal recessive disease characterized by multiorgan dysfunction. The key features are childhood obesity, blindness due to congenital retinal dystrophy, and sensorineural hearing loss. Associated endocrinologic features include hyperinsulinemia, early-onset type 2 diabetes, and hypertriglyceridemia. Thus, AS shares several features with the common metabolic syndrome, namely obesity, hyperinsulinemia, and hypertriglyceridemia. Mutations in the ALMS1 gene have been found to be causative for AS with a total of 79 disease-causing mutations having been described.
Case presentation
We describe the case of a 27-year old female from an English (Caucasian) kindred. She had been initially referred for hypertriglyceridemia, but demonstrated other features suggestive of AS, including blindness, obesity, type 2 diabetes, renal dysfunction, and hypertension. DNA analysis revealed that she is a compound heterozygote with two novel mutations in the ALMS1 gene – H3882Y and V424I. Examination of her family revealed that her phenotypically unaffected mother and younger sister also had heterozygous mutations in the ALMS1 gene. In addition to presenting these novel molecular findings for AS, we review the clinical and genetic features of AS in the context of our case.
Conclusion
Two novel mutations in the ALMS1 gene causative for AS have been reported here, thereby increasing the number of reported mutations to 81 and providing a wider basis for mutational screening among affected individuals.
Background
Alstrom syndrome (AS; OMIM 203800) was first described in 1959 and has an estimated prevalence of <1:100 000 [1,2]. AS is an autosomal recessive multiorgan disorder, characterized by childhood obesity, adult short stature with initial accelerated childhood linear growth, progressive cone-rod dystrophy leading to blindness, and sensorineural hearing loss [3,4]. Endocrinologic complications include early-onset diabetes mellitus (typically in the 2nd or 3rd decades), hyperinsulinemia (with associated acanthosis nigricans), hypertriglyceridemia, infertility (hypergonadotrophic hypogonadism), and hypothyroidism [4-6]. Systemic fibrosis is commonly observed [3]. The primary cause of mortality among young affected patients is cardiac involvement from dilated cardiomyopathy whereas renal failure is the major cause of death among the older subgroup [2,3].
Mutations in the ALMS1 gene were independently identified as causative for AS by two research groups [7,8]. ALMS1 encodes a protein of 4169 amino acids, which includes a large tandem-repeat domain consisting of 47 amino acids (aa); the exact function of the ALMS1 protein still remains unknown [7]. However, the ALMS1 protein has been shown to be ubiquitously expressed and to localize subcellularly [9]. It has been proposed that ALMS1 is involved in the functioning of centrosomes or basal bodies [9]. Although initial data revealed normal ciliary structure in fibroblasts from affected individuals with ALMS1 mutations, ALMS1 knockout mice demonstrated abnormal ciliary structure that could be rescued with a prematurely truncated fragment of ALMS1 containing the N-terminus [9,10]. Thus, the N-terminus of ALMS1 seems to be crucial to normal ciliary structure [10]. To date, a total of 79 disease-causing ALMS1 mutations have been reported [11]. We report here the clinical and novel molecular findings in a Caucasian kindred with Alstrom syndrome from the United Kingdom and review the current clinical and molecular genetic aspects of this condition.
Case presentation
In 2002, the 27-year old proband was referred to the lipid clinic of a tertiary health care centre for evaluation of an elevated triglyceride (TG) level of 59.1 mmol/L. Her prior history included poor vision since birth, commencing with the development of night blindness, eventually resulting in legal blindness by the age of 17. She had undergone a left nephrectomy at the age of 24 for a perinephric abscess due to chronic pyelonephritis. Ultrasound evaluation revealed a normal-sized right kidney with evidence of cortical scarring. Hypertension and diabetes subsequently developed at the ages of 25 and 26 years, respectively. She experienced learning difficulties in school, but did not have sensorineural deafness. On physical examination, there was evidence of central obesity with her body mass index (BMI) being 34.9 kg/m2. Her blood pressure on antihypertensive treatment was 132/86 with a regular pulse of 80 beats per minute. There was no evidence of poly- or syndactyly suggestive of Bardet-Biedl syndrome. Hirsutism was present on the face, abdomen, and arms. Ophthalmologic examination was notable for retinitis pigmentosa and cataracts bilaterally.
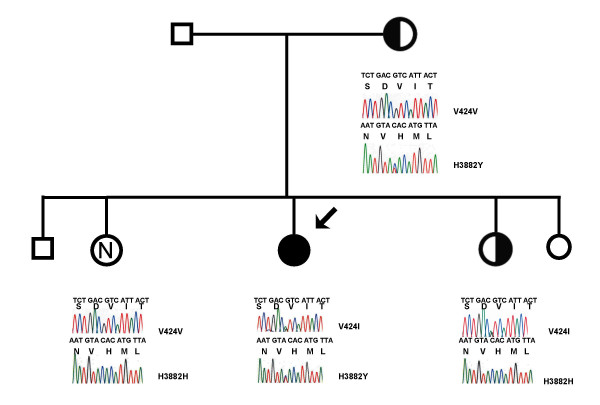
Her family consisted of non-consanguineous parents, both alive and well, as well as four siblings – three sisters (aged 18, 26, 29 years) and one brother (aged 29 years), who were also healthy. The family structure is outlined in Figure 1.
Figure 1.
DNA sequence analysis of Alstrom syndrome mutations. Proband is indicated by the solid circle and arrow. Smaller symbols represent individuals from whom DNA was unavailable. There is no known consanguinity between the parents of this kindred. Electrophoretic tracings for exons 6 and 17 of the ALMS1 gene from the proband and available immediate relatives are shown. The proband is a compound heterozygote for the V424I and H3882 Y mutations while the mother demonstrates heterozygosity only for the H3882Y mutation and the younger sister demonstrates heterozygosity only for the V424I mutation. The older sister has no mutations in the ALMS1 gene.
Biochemical attributes and investigations
The proband's initial labwork at the time of consultation revealed a plasma TG level of 20.6 mmol/L with a high-density lipoprotein cholesterol (HDL-C) level of 0.56 mmol/L. Her fasting glucose and insulin levels on oral antidiabetic therapy but not insulin were 14.2 mmol/L and 68.1 mU/L (normal <10 mU/L), respectively, with a HbA1C of 11.2%. Her creatinine was elevated at 255 μmol/L with overt proteinuria of 2.68 g/24 hours. Her total alkaline phosphatase of 416 U/L (normal < 330 U/L) demonstrated increases in both the liver and bone isoenzymes. Abdominal ultrasound revealed fatty infiltration of the liver and splenic enlargement (14 cm in length). Both her electrocardiogram and echocardiogram were normal.
The results of lipoprotein analyses of the proband, proband's mother and two sisters are shown in Table 1. The proband demonstrated significant hypertriglyceridemia (TG = 20.6 mmol/L) and hypoalphalipoproteinemia (HDL-C = 0.56 mmol/L). Both the proband's mother and older sister displayed mildly elevated plasma TG whereas the younger sister tested had relatively normal plasma TG. Plasma low density lipoprotein-cholesterol (LDL-C) levels were higher among the mother and younger sister compared to the others. The proband demonstrated the lowest LDL-C and HDL-C levels of all four members of the kindred examined.
Table 1.
Clinical and biochemical characteristics of the pedigree
| Biochemical parameter | Proband (II-2) | Mother (I-1) | Sister (II-1) | Sister (II-3) |
| Total cholesterol (mmol/L) | 7.40 | 7.70 | 5.78 | 6.79 |
| Triglyceride (mmol/L) | 20.6 | 2.61 | 3.80 | 1.78 |
| VLDL- C (mmol/L) | 4.62 | 0.92 | 1.09 | 0.58 |
| VLDL-TG (mmol/L) | 15.4 | 1.57 | 2.54 | 1.10 |
| VLDL-TG/VLDL-C ratio | 3.33 | 1.71 | 2.33 | 1.90 |
| HDL-C (mmol/L) | 0.56 | 0.94 | 1.04 | 1.72 |
| LDL-C (mmol/L) | 0.83 | 5.32 | 3.29 | 4.19 |
| Apo A-1 (g/L) | 1.25 | 1.20 | 1.45 | 1.59 |
| Apo B (g/L) | 0.91 | 1.59 | 1.16 | 1.22 |
Abbreviations: VLDL-C, very low density lipoprotein – cholesterol; VLDL-TG, very low density lipoprotein- triglyceride; HDL-C, high density lipoprotein – cholesterol; LDL-C, low density lipoprotein – cholesterol
Treatment
Treatment of the proband's hypertriglyceridemia required the use of multiple therapies, including a low-fat diet (< 20 g fat per day), insulin (glargine 14 units/day), pioglitazone (45 mg/day), atorvastatin (40 mg/day), and omega-3-acid ethylesters (Omacor) (2 g bid). As a result of therapy, TG levels decreased by ~84% to 3.30 mmol/L and HDL-C levels improved by ~252% to 1.41 mmol/L.
DNA isolation and sequence analysis
Informed consent was obtained from all available subjects or their guardians, and the study was approved by the institutional review board of the University of Western Ontario (#07920E). DNA and lipoprotein analyses were unavailable for the father, one sister (aged 18 years), and brother (aged 29 years). Genomic DNA from the four available study subjects was isolated from whole blood (Puregene, Gentra Systems, Minneapolis, MN). Exons 1 to 23 of ALMS1 were amplified using the primers in Table 2. The final volume of 50 μL contained 32 pmol of each primer, 0.2 mM each of dATP, dCTP, dGTP, and dTTP, 1.5 mM MgCl2, 50 mM KCl, 20 mM Tris-HCl (pH 8.4), and 2.5 units of Taq platinum DNA polymerase (Life Technologies, Mississauga, Ontario, Canada). DNA amplifications were performed with denaturing at 94°C for 5 minutes, followed by 30 cycles of a denaturing step at 94°C, an annealing step at 60°C, and an extension step at 72°C, each for 30 seconds. A final extension step at 72°C was performed for 10 min. Amplification products were electrophoresed on 1.5% agarose gels and purified with the QIAEX II gel extraction kit (Qiagen, Inc., Mississauga, Ontario, Canada). Purified DNA fragments were sequenced by the chain termination method using the ABI 3730 Automated DNA Sequencer and analyzed using Sequence Navigator Software (both from PE-Applied Biosystems, Mississauga, Ontario, Canada).
Table 2.
Oligonucleotides for genomic amplification and sequencing of ALMS1
| Exon | Forward Primer | Reverse Primer |
| 1 | GCACTGCGCCTAAGCTG | CAGCCTCCACCCCCAAC |
| 2 | ATGTGAAAGGGCTTTATAAACTGG | TTTTTCCATTCTTCATAGCTAAATCA |
| 3 | CAGTTAATGACTTAGCATGTTTTCCT | TCCTTAACTCAAAAAGGGGAAAG |
| 4 | ACGTAAGTAAATAATCAATTTTCAGCA | TCTAAGCCCCACCTCAAAGT |
| 5 | TTTCAGTGACATATGTATTTTTGTGTT | TTCCCTTGGGAATTTTATTTTT |
| 6 | CTTCGTGTGTGGGAGCTGAG | CAATACTGAAAAAGGCCACGTT |
| 7 | TGGGCATTAATGAGTCTTTTTC | TTTTCACAAGGTATCCGTAAGTAGG |
| 8 | GCTTTTTAAAGGCTCAAAGCTG | TCTCTCTATGTGAGTAGGAAGTAGAGG |
| 8 | TGACCAGACAACTGGCATGT | GACTGTCTGCTAAGTCCTGTGG |
| 8 | TTCTTACTCACAAAGAGAAAAGCCTA | GGGCAGCCAATACAGAAACA |
| 8 | TTTCCCTGAAGAAGCTCTGAA | TGGCAAGGTCTGTTGGTAGA |
| 8 | TCACAAAGAGAGAAGCCTGGT | AGCTGGTGTGCCAGTTGTCT |
| 8 | TTCAGTTGCCTCTGAACCAG | TGTGGCAAGACCTGTTGGTA |
| 8 | CACACACAGAGAAGCCTGGT | AAAGGTCCTGCTGGTATGTCA |
| 8 | TCCATTGTTTCTGGACCTACTG | ATCTGGCAACTCTTGCTGGT |
| 8 | ACTGTAACTTCCTCTTTCTATTCACAT | TCTCAGTCTTCCGGTCACCT |
| 8 | AGCAGGAGTTGCCAGATGTT | CTGGTTTTCCAGTATTCACATCA |
| 8 | AAAGATTTCAGCTGTCCCTGA | CTGCATCCTGGATTTCTTCA |
| 8 | CTCAGGCTGATGACAGAGTTG | CCCAATGGTTCCACTACACC |
| 8 | GAGCAAAGTCAGTATGGCATTAGA | TGGCTAAGCTTCCTCAAAACA |
| 9 | TCTTCTGTGTTGCAATTGTTGA | TTCCATCACCCATTCTTTCA |
| 10 | TTGGACTACTTCAAATAAGAACCTG | GACGGCATTTGTGATGAAGA |
| 10 | ACCTGCTTTTGTGCCACCTA | CTTGGTCTGCCCATGCTAAT |
| 10 | CCAGTACCAGGGCAAATTGT | GGAAGGGGAAAATGGTGTTT |
| 10 | ACCTTCCGTCTCCCATTTCT | TCCTGTGCTACAGGTTTACTGG |
| 10 | GCTTCTAAAGCGAGGATGAA | CCCCCAAGAACCGATATCTA |
| 11 | TTCCTTGAAACCACTTTTGGA | GAAAGACACAACCACAAATTTCTAA |
| 12 | GAAGGCATTCCATATTTGTTCA | GCACTGGACTTTTGTCACTCC |
| 13 | TCATAGAATTGGTCTAAGAGGCAAA | AAGATTGGATAGTAATCTCATTTAGGA |
| 14 | ATGGGTTTGGGGTTTTGTTT | GAGCTGAAGACAGCAAGAAGAA |
| 15 | AACAAAGCCTTTCACATAATACG | CACTGACCCTCACATACACAC |
| 16 | GCAGGCAGTGAATTTTCTGAT | TTTTGGATAATCTCTAACTTGACTTTT |
| 16 | CCAGAATAAAGAGCCTCAGCA | TTTTTAAGCTCGCCTGTATTTTT |
| 16 | GCGGTTTAAAAGCCTAGAGAAA | TTTTCACCTGTGTGCAAAGC |
| 17 | TGAATTGGATTAGAAAGAGGACTTG | TCTTACATGTTTAAGAGCCATTTCA |
| 18 | TCCCACACAAAGGGATTGTA | ATCGCAGGGGACTTGAAAT |
| 19 | CTGGGTGGGGCTGTAAAAA | CCAAGTCACAGAGCCAGCTT |
| 20 | GCATATGGAGAGTAGATTGCATCA | TGGGCTGGCCTTTAGCAG |
| 21 | GGTAGGGGCACCAAGTCCTA | CAGAGCTCCCGACCACTTG |
| 22 | GATGAGCTCCTGGAGAGTGG | GGCAACGTGTTTTCTCCATT |
| 23 | GGCATCTGCCTCTGATGG | AAGGATTCTGCTTCTCTAGGTTCA |
Identification of novel disease-causing ALMS1 mutation
Sequencing of genomic DNA from the affected proband demonstrated no mutations in the coding regions of the LPL gene (data not shown), but did reveal 2 novel missense mutations in the ALMS1 gene – a G→A transversion at nucleotide 1381 of exon 6 and a C→T transversion at nucleotide 11755 in exon 17. These mutations resulted in the replacement of valine by isoleucine at codon 424 (V424I, exon 6) and histidine by tyrosine at codon 3882 (H3882Y, exon 17). The proband's mother was heterozygous for the H3882Y mutation while the proband's younger sister was heterozygous for the V424I mutation, proving that the mutations were on opposite chromosomes in the proband. The proband's older sister was unaffected both clinically and molecularly. See Figure 1.
The missense mutation V424I was subsequently genotyped in 200 healthy unaffected Caucasian controls using the primer pair and PCR conditions for exon 6 amplification. The amplified product was digested with restriction enzyme AatII (New England Biolabs Inc., Ipswich, MA, USA). All healthy controls were homozygous for G at nucleotide 1381. Similarly, the missense mutation H3882Y was genotyped in 200 healthy unaffected Caucasian controls using the primer pair and PCR conditions for exon 17 amplification. The amplified product was digested using the restriction enzyme RsaI (New England Biolabs Inc., Ipswich, MA, USA). Among the 200 controls, only 1 control was found to be heterozygous at nucleotide 11755 C/T, giving an allele frequency of 0.9975 for the C allele and 0.0025 for the T allele at this position, based on the Hardy-Weinberg equation. This suggests that the second heterozygote mutation, 11755 C→T, is present in the general population, albeit at a very low frequency.
Literature review and case discussion
The distribution of AS is global without any gender predilection. With an estimated prevalence of < 1:100 000, only ~500 cases of AS have been reported in the literature thus far [2-4,7,11-37]. Yet, a greater proportion of kindreds of English descent have been noted in the literature. Whether this is due to selection bias from kindreds in the United Kingdom or North America having more readily available health care access compared to kindreds from developing nations is uncertain. Certainly, awareness of AS is lacking despite the complexity and potential lethality of this disorder. Moreover, in addition to childhood obesity, affected individuals may develop insulin resistance, type 2 diabetes, and hypertriglyceridemia. Thus, AS can be thought of as a rare genetic disorder with several features similar to the common metabolic syndrome.
General clinical features
Prior to the recent discovery of ALMS1 mutations causative for AS, the diagnosis of AS was made solely based on phenotype. However, AS exhibits a great degree of phenotypic variability, even within families, thereby creating difficulties for a universal definition of AS [17,18]. Recently, Marshall et al defined AS using age-specific criteria [38]. See Table 3.
Table 3.
Diagnostic criteria for Alstrom syndrome [38]
| Age (years) | Major Criteria | Minor Criteria | Other supportive evidence | Diagnosis |
| ≤ 2* | • ALMS 1 mutation in 1 allele and/or family history of AS • Vision (nystagmus, photophobia) |
• Obesity • DCM/CHF |
• Recurrent pulmonary infections • Normal digits • Delayed developmental milestones |
2 major criteria OR 1 major + 2 minor criteria |
| 3–14 | • ALMS 1 mutation in 1 allele and/or family history of AS • Vision (nystagmus, photophobia, decreased acuity, cone dystrophy by ERG**) |
• Obesity and/or insulin resistance • (History of) DCM/CHF • Hearing loss • Advanced bone age • Hepatic dysfunction • Renal failure |
• Recurrent pulmonary infections • Normal digits • Delayed developmental milestones • Hyperlipidemia • Scoliosis • Flat wide feet • Hypothyroidism • Hypertension • GH deficiency • Recurrent UTI |
2 major criteria OR 1 major + 3 minor criteria |
| ≥ 15 | • ALMS 1 mutation in 1 allele and/or family history of AS • Vision (legal blindness, history of nystagmus in infancy/childhood, cone and rod dystrophy by ERG) |
• Obesity and/or insulin resistance and/or DM2 • (History of) DCM/CHF • Hearing loss • Hepatic dysfunction • Renal failure • Short stature • Males – hypogonadism • Females – irregular menses and/or hyperandrogenism |
• Recurrent pulmonary infections • Normal digits • History of developmental delay • Hyperlipidemia • Scoliosis • Flat wide feet • Hypothyroidism • Hypertension • GH deficiency • Alopecia • Recurrent UTI or urinary dysfunction |
2 major + 2 minor criteria OR 1 major + 4 minor criteria |
*These diagnostic criteria should be re-evaluated when the patient becomes older
** ERG to be conducted only if the child is old enough for testing
Abbreviations: AS, Alstrom syndrome; DCM/CHF, dilated cardiomyopathy/congestive heart failure; ERG, electroretinogram; GH, growth hormone; UTI, urinary tract infections; DM2, type 2 diabetes.
Neurosensory and cognitive features
Sensorineural hearing loss and congenital retinal dystrophy are two cardinal features of AS. In fact, pendular or searching nystagmus and photodysphoria are often evident before the age of 1 yr. There is initial loss of cone function followed by rod disintegration, ultimately leading to early blindness. By the age of 16, ~90% of affected individuals are blind [38]. In addition, development of subcapsular cataracts may contribute to vision loss [3,6]. Exudative retinopathy has also been reported in AS [12]. Meanwhile, ~80 % of affected individuals will develop bilateral sensorineural hearing loss [3]. This usually occurs at a later age in childhood and is characterized by the initial loss of high frequency sounds. Progressive deterioration in hearing occurs, and is sometimes accompanied by conductive hearing loss due to chronic otitis media or glue ear [3]. These early changes in neurosensory capabilities have tremendous impact not only on the social development of the child but also on his/her adaptation to the external environment.
Although delay of cognitive development is not a common feature of AS, delay in developmental milestones is seen in ~45% of AS-affected children. Other neurologic manifestations may include absence seizures and general sleep disturbances [3]. The frequency of mood and psychiatric disorders in AS-affected individuals has not been determined.
Anthropometric and growth measures
Individuals with AS often have distinctive facial features, such as round face, deep-set eyes, thick ears, dental anomalies, hyperostosis frontalis interna, and premature frontal balding. Their toes and fingers are typically short and stubby with no polydactyly or syndactyly while their feet are typically noted to be wide and thick [38].
Childhood obesity is present in over 95% of individuals with AS [3]. However, both waist circumference and body fat percentage (as measured using dual-energy X-ray absorptiometry) negatively correlated with age, independent of BMI, indicating the possible recruitment of more metabolically active fat stores [2]. The presence of hyperphagia has been controversial [3,4]. Although childhood is often accompanied by rapid growth with height above the 50th percentile before puberty, most individuals demonstrate a decreased adult height [2,3]. This height discrepancy is supported by evidence for advancement of the bone age by 1–3 years prior to puberty as described by Marshall et al [3]. As well, abnormalities of the insulin growth factor (IGF) system of affected patients have been demonstrated [13]. Yet, the exact reasons for short stature remain to be determined.
Endocrinologic features
Similar to the features of the metabolic syndrome seen in the general population, patients with AS also demonstrate hypertriglyceridemia and type 2 diabetes. Type 2 diabetes is diagnosed in over 80% of individuals above the age of 16 while insulin resistance and hyperinsulinemia have been demonstrated in individuals as young as 1 year old, even prior to the onset of obesity [3,38]. Meanwhile, hypertriglyceridemia is seen in ~50% of affected individuals, with acute pancreatitis secondary to hypertriglyceridemia occurring in ~5% [3]. Other endocrinologic manifestations of AS include hypothyroidism, hypogonadism (particularly among men), alterations in the onset of puberty, ovarian cysts and hirsutism (among females), and short stature with abnormalities in the IGF-growth hormone system [3,13].
Cardiorespiratory features
Dilated cardiomyopathy (DCM), affecting ~60% of individuals, can occur at any age, but most typically during infancy. Although DCM is the most common underlying cause of death in the infantile period, survival with infantile-onset of DCM tends to be better than that for adult-onset DCM. Marshall et al showed that while one-third of adult-onset DCM patients died, ~74% of infantile-onset DCM patients survived [3]. However, children and adults who have survived infantile-onset DCM are still susceptible to sudden recurrences [38]. In addition to cardiac problems, AS-affected patients can have a variety of respiratory problems, including chronic asthma, sinusitis/bronchitis, alveolar hypoventilation and recurrent pneumonia [3].
Gastrointestinal and genitourinary involvement
Hepatic involvement in AS was first described in 1991 [39]. Approximately 80% of patients affected with AS may have hepatic involvement, ranging from mild elevation in liver transaminases to hepatic steatosis to overt cirrhosis with portal hypertension [3,30,31]. Other gastrointestinal effects include upper gastrointestinal pain, chronic diarrhea, constipation, and gastroesophageal reflux [3].
Renal insufficiency occurs in ~50% of AS-affected individuals. Whether hypertension is a consequence of renal insufficiency or contributes to renal insufficiency is uncertain, but it is present in ~30% of individuals [3]. Urologic dysfunction is common in both men and women and can be manifest as urge incontinence, poor flow, urinary retention, or difficulty initiating voiding [3]. Urologic anatomical abnormalities can also occur in AS, including calyceal deformities, narrowed ureteropelvic angles, dilated ureters, and misalignment of the kidneys [6].
Differential diagnosis
It remains important to distinguish AS from other disorders characterized by childhood obesity and retinal dystrophy, such as the Laurence-Moon and Bardet-Biedl syndromes. Normal mentation and lack of poly/syndactyly help to distinguish AS from Bardet-Biedl, while deafness and the absence of spastic paraparesis help to differentiate AS from Laurence-Moon. Refsum disease is a rare disorder characterized by hearing loss, visual loss, and hepatic involvement, but also includes several features not associated with AS [40]. See Table 4 for clinical features of these disorders.
Table 4.
Differential diagnosis for Alstrom syndrome [41, 42]
| Alstrom syndrome | Laurence- Moon syndrome | Bardet- Biedl syndrome | Refsum disease | |
| Inheritance | AR | AR | AR | AR |
| Childhood Obesity | + | + | + | - |
| Visual Impairment | + | + | + | + |
| Sensorineural Deafness | + | - | - | + |
| Short Stature | + | - | -/+ | - |
| Diabetes Mellitus | + | - | + | - |
| Renal Disease | + | - | + | - |
| Polydactyly/syndactyly | - | - | + | + |
| Mental Delay | - | + | + | +/- |
| Hypogonadism | + | + | + | - |
| Dilated Cardiomyopathy | + | - | -/+ | -/+ |
| Other | Hepatic Involvement | Spastic paraplegia | - | Anosmia Dysmorphic features Cerebellar ataxia Polyneuropathy |
Abbreviations: AR, autosomal recessive
Genetic features of AS
AS is an autosomal-recessive disease that demonstrates intrafamilial and interfamilial variation. To date, a total of 81 disease-causing ALMS1 mutations have been reported, including the 2 reported from our group [11]. A schematic representation of the normal ALMS1 gene and the ALMS1 mutations causative of AS are shown in Figures 2, 3, 4.
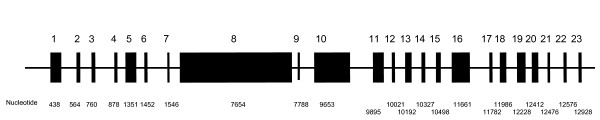
Figure 2.
Normal ALMS1 gene structure*. * Figure 2 is not drawn to scale.
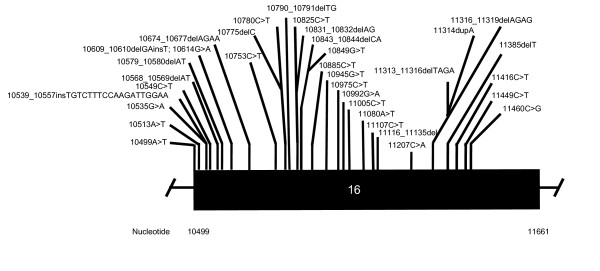
Figure 3.
Reported ALMS1 Mutations for Exon 16*. * Figure 3 is not drawn to scale.
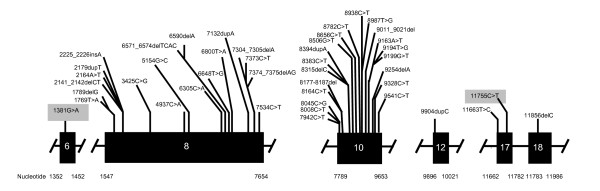
Figure 4.
Reported ALMS1 Mutations for Exons 6, 8, 10, 12, 17, 18*. * Figure 4 is not drawn to scale. The two novel mutations found in this study are shaded in gray. Other mutations not included in the figure are AC074008.5:g.124455_125899del and 10483C>T.
Affected individuals of most kindreds demonstrate compound heterozygosity with the majority of ALMS1 mutations reported being nonsense. Approximately 40% of all known ALMS1 mutations occur in exon 16 while other ALMS1 mutational hotspots include exon 10 (23%) and exon 8 (21%) [11]. Mutations in exon 16 have correlated with a more severe disease phenotype, manifest as early onset of retinal degeneration before the age of 1 year (p = 0.02), presence of urologic dysfunction (p = 0.02), occurrence of dilated cardiomyopathy (p = 0.03), and development of diabetes (p = 0.03). Meanwhile, the severity of renal disease in AS has correlated with mutations in exon 8 [11].
Among 250 AS-affected individuals examined, 15 synonymous single nucleotide polymorphisms (SNPs), 51 nonsynonymous SNPs, and a deletion of 3 nucleotides in exon 8 have also been described. Using prediction modeling, Marshall et al have determined that 8 of these variants are disease-causing [11]. The most common ALMS1 mutation is c.10775delC, which is observed in 50% of reported English kindreds. Similarly, haplotype sharing has been demonstrated amongst members of Turkish kindreds, indicating the possibility of separate founder effects in both the English and the Turkish kindreds [11].
Discussion of our case
Our results indicate that ALMS1 mutations of V424I and H3882Y are the molecular basis for the Alstrom phenotype in the proband presented here. The H3882Y mutation was present in only 1 of 200 normal healthy controls while the V424I mutation was absent in all 200 controls. These two disease-causing mutations have not been reported before and represent the first mutation reported for exon 6 and the second reported for exon 17 [11]. Interestingly, the predicted frequency of homozygotes for the H3882Y mutation would be 1 in 160 000, signifying that, if homozygotes are affected, AS may indeed be more prevalent than previously thought.
To date, with the 2 missense mutations of our kindred, there are a total of 10 missense AS-causing mutations. Marshall et al showed that the majority of kindreds affected by AS, as in our kindred, demonstrate compound heterozygote mutations [11]. The V424I mutation localizes to the N-terminus of the ALMS1 protein while the H3882Y mutation localizes to the C-terminus of the protein [7]. Since even fragments of the N-terminus have been shown to be able to normalize the abnormal ciliary structure of ALMS1 knockout mice, it would be expected that isolated heterozygous mutations still including the relatively intact N-terminus would not necessarily result in significant clinical findings, as evidenced in our proband's mother and younger sister [10]. The functional importance of the C-terminus remains to be determined. Importantly, no correlations could be drawn between the presence of a single ALMS1 mutation, as seen in the mother and younger sister, and abnormalities in any of the lipid parameters examined.
Hypertriglyceridemia was evident in our proband and is a common finding among individuals affected with AS. Our proband had no documented episodes of pancreatitis, but was at high risk for pancreatitis given her significant hypertriglyceridemia. There can be multiple factors contributing to hypertriglyceridemia among AS-affected patients, including insulin resistance or diabetes, liver dysfunction, renal dysfunction, dietary factors, and alcohol intake. However, examination of a group of AS-affected individuals demonstrated no significant correlations between hepatic or renal function, BMI, or degree of glucose intolerance and high TG levels. Instead, there was indeed a major correlation between hyperinsulinemia and hypertriglyceridemia in that study [5]. Our proband demonstrated significant hypertriglyceridemia, accompanied by insulin resistance (type 2 diabetes), elevated BMI, and renal dysfunction. A multifaceted treatment approach resulted in improvements in TG and HDL-C to 3.30 mmol/L and 1.41 mmol/L, respectively, demonstrating that it is possible to effectively treat lipid abnormalities among AS-affected individuals.
The correlation of mutations in exons 6 or 17 to phenotype remains to be determined. Interestingly, our proband did not have sensorineural deafness, which typically occurs in ~84% of affected individuals [11]. No genotype-phenotype correlations have been made for sensorineural deafness [11]. However, it is possible that mutations in exon 6 or 17 may not be associated with sensorineural deafness although further kindreds with these mutations are required to substantiate this possibility. Yet, the mutations in this proband seem to have resulted in an "intermediate-severity" phenotype as judged by an early onset of retinal degeneration, severe renal dysfunction, and significant elevation of TG levels, but a later development of diabetes and a lack of dilated cardiomyopathy.
Conclusion
We have reported here two novel missense mutations in the ALMS1 gene causative for Alstrom syndrome in an English kindred. These extend the mutational spectrum in AS and provide a resource for mutational screening. Furthermore, we hope that these mutations may eventually add insight into the function of the ALMS1 protein and contribute to the understanding of the phenotypic variety observed among AS-affected individuals.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
TJ wrote the manuscript. HC participated in the design of the study and carried out all the molecular genetic studies for the family. GB examined the proband and suggested the diagnosis of AS in the proband, which resulted in molecular genetic testing. RM played a crucial role in the management of the proband. VC-M and PND conducted the examination of the proband and her family, obtained consent for molecular genetic testing, and drafted the manuscript. RAH conceived the design of the study and provided critical revisions to the manuscript. All authors read and approved the final manuscript.
Contributor Information
Tisha Joy, Email: tjoy@uwo.ca.
Henian Cao, Email: hcao@robarts.ca.
Graeme Black, Email: graeme.black@manchester.ac.uk.
Rayaz Malik, Email: rayaz.a.malik@manchester.ac.uk.
Valentine Charlton-Menys, Email: valentine.menys@manchester.ac.uk.
Robert A Hegele, Email: hegele@robarts.ca.
Paul N Durrington, Email: Pdurrington@manchester.ac.uk.
References
- Alstrom CH, Hallgren B, Nilsson LB, Asander H. Retinal degeneration combined with obesity, diabetes mellitus and neurogenous deafness: a specific syndrome (not hitherto described) distinct from the Laurence-Moon-Bardet-Biedl syndrome: a clinical, endocrinological and genetic examination based on a large pedigree. Acta psychiatrica et neurologica Scandinavica. 1959;129:1–35. [PubMed] [Google Scholar]
- Minton JA, Owen KR, Ricketts CJ, Crabtree N, Shaikh G, Ehtisham S, Porter JR, Carey C, Hodge D, Paisey R, Walker M, Barrett TG. Syndromic obesity and diabetes: changes in body composition with age and mutation analysis of ALMS1 in 12 United Kingdom kindreds with Alstrom syndrome. The Journal of clinical endocrinology and metabolism. 2006;91:3110–3116. doi: 10.1210/jc.2005-2633. [DOI] [PubMed] [Google Scholar]
- Marshall JD, Bronson RT, Collin GB, Nordstrom AD, Maffei P, Paisey RB, Carey C, Macdermott S, Russell-Eggitt I, Shea SE, Davis J, Beck S, Shatirishvili G, Mihai CM, Hoeltzenbein M, Pozzan GB, Hopkinson I, Sicolo N, Naggert JK, Nishina PM. New Alstrom syndrome phenotypes based on the evaluation of 182 cases. Archives of internal medicine. 2005;165:675–683. doi: 10.1001/archinte.165.6.675. [DOI] [PubMed] [Google Scholar]
- Marshall JD, Ludman MD, Shea SE, Salisbury SR, Willi SM, LaRoche RG, Nishina PM. Genealogy, natural history, and phenotype of Alstrom syndrome in a large Acadian kindred and three additional families. American journal of medical genetics. 1997;73:150–161. doi: 10.1002/(SICI)1096-8628(19971212)73:2<150::AID-AJMG9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Paisey RB, Carey CM, Bower L, Marshall J, Taylor P, Maffei P, Mansell P. Hypertriglyceridaemia in Alstrom's syndrome: causes and associations in 37 cases. Clinical endocrinology. 2004;60:228–231. doi: 10.1111/j.1365-2265.2004.01952.x. [DOI] [PubMed] [Google Scholar]
- Satman I, Yilmaz MT, Gursoy N, Karsidag K, Dinccag N, Ovali T, Karadeniz S, Uysal V, Bugra Z, Okten A, Devrim S. Evaluation of insulin resistant diabetes mellitus in Alstrom syndrome: a long-term prospective follow-up of three siblings. Diabetes research and clinical practice. 2002;56:189–196. doi: 10.1016/S0168-8227(02)00004-9. [DOI] [PubMed] [Google Scholar]
- Hearn T, Renforth GL, Spalluto C, Hanley NA, Piper K, Brickwood S, White C, Connolly V, Taylor JF, Russell-Eggitt I, Bonneau D, Walker M, Wilson DI. Mutation of ALMS1, a large gene with a tandem repeat encoding 47 amino acids, causes Alstrom syndrome. Nature genetics. 2002;31:79–83. doi: 10.1038/ng874. [DOI] [PubMed] [Google Scholar]
- Collin GB, Marshall JD, Ikeda A, So WV, Russell-Eggitt I, Maffei P, Beck S, Boerkoel CF, Sicolo N, Martin M, Nishina PM, Naggert JK. Mutations in ALMS1 cause obesity, type 2 diabetes and neurosensory degeneration in Alstrom syndrome. Nature genetics. 2002;31:74–78. doi: 10.1038/ng867. [DOI] [PubMed] [Google Scholar]
- Hearn T, Spalluto C, Phillips VJ, Renforth GL, Copin N, Hanley NA, Wilson DI. Subcellular localization of ALMS1 supports involvement of centrosome and basal body dysfunction in the pathogenesis of obesity, insulin resistance, and type 2 diabetes. Diabetes. 2005;54:1581–1587. doi: 10.2337/diabetes.54.5.1581. [DOI] [PubMed] [Google Scholar]
- Li G, Vega R, Nelms K, Gekakis N, Goodnow C, McNamara P, Wu H, Hong NA, Glynne R. A role for Alstrom syndrome protein, alms1, in kidney ciliogenesis and cellular quiescence. PLoS genetics. 2007;3:e8. doi: 10.1371/journal.pgen.0030008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JD, Hinman EG, Collin GB, Beck S, Cerqueira R, Maffei P, Milan G, Zhang W, Wilson DI, Hearn T, Tavares P, Vettor R, Veronese C, Martin M, So WV, Nishina PM, Naggert JK. Spectrum of ALMS1 variants and evaluation of genotype-phenotype correlations in Alstrom syndrome. Hum Mutat. 2007 doi: 10.1002/humu.20577. [DOI] [PubMed] [Google Scholar]
- Gogi D, Bond J, Long V, Sheridan E, Woods CG. Exudative retinopathy in a girl with Alstrom syndrome due to a novel mutation. The British journal of ophthalmology. 2007;91:983–984. doi: 10.1136/bjo.2005.088781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei P, Boschetti M, Marshall JD, Paisey RB, Beck S, Resmini E, Collin GB, Naggert JK, Milan G, Vettor R, Minuto F, Sicolo N, Barreca A. Characterization of the IGF system in 15 patients with Alstrom syndrome. Clinical endocrinology. 2007;66:269–275. doi: 10.1111/j.1365-2265.2007.02721.x. [DOI] [PubMed] [Google Scholar]
- Welsh LW. Alstrom syndrome: progressive deafness and blindness. The Annals of otology, rhinology, and laryngology. 2007;116:281–285. doi: 10.1177/000348940711600411. [DOI] [PubMed] [Google Scholar]
- Hamamy H, Barham M, Alkhawaldeh AE, Cockburn D, Snowden H, Ajlouni K. Alstrom syndrome in four sibs from northern Jordan. Annals of Saudi medicine. 2006;26:480–483. doi: 10.5144/0256-4947.2006.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koc E, Bayrak G, Suher M, Ensari C, Aktas D, Ensari A. Rare case of Alstrom syndrome without obesity and with short stature, diagnosed in adulthood. Nephrology (Carlton, Vic) 2006;11:81–84. doi: 10.1111/j.1440-1797.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- Hoffman JD, Jacobson Z, Young TL, Marshall JD, Kaplan P. Familial variable expression of dilated cardiomyopathy in Alstrom syndrome: a report of four sibs. Am J Med Genet A. 2005;135:96–98. doi: 10.1002/ajmg.a.30688. [DOI] [PubMed] [Google Scholar]
- Titomanlio L, De Brasi D, Buoninconti A, Sperandeo MP, Pepe A, Andria G, Sebastio G. Alstrom syndrome: intrafamilial phenotypic variability in sibs with a novel nonsense mutation of the ALMS1 gene. Clinical genetics. 2004;65:156–157. doi: 10.1111/j.0009-9163.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- Makaryus AN, Popowski B, Kort S, Paris Y, Mangion J. A rare case of Alstrom syndrome presenting with rapidly progressive severe dilated cardiomyopathy diagnosed by echocardiography. J Am Soc Echocardiogr. 2003;16:194–196. doi: 10.1067/mje.2003.1. [DOI] [PubMed] [Google Scholar]
- Wu WC, Chen SC, Dia CY, Yu ML, Hsieh MY, Lin ZY, Wang LY, Tsai JF, Chang WY, Chuang WL. Alstrom syndrome with acute pancreatitis: a case report. The Kaohsiung journal of medical sciences. 2003;19:358–361. doi: 10.1016/S1607-551X(09)70438-3. [DOI] [PubMed] [Google Scholar]
- Benso C, Hadjadj E, Conrath J, Denis D. Three new cases of Alstrom syndrome. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2002;240:622–627. doi: 10.1007/s00417-002-0479-6. [DOI] [PubMed] [Google Scholar]
- Koray F, Dorter C, Benderli Y, Satman I, Yilmaz T, Dinccag N, Karsidag K. Alstrom syndrome: a case report. Journal of oral science. 2001;43:221–224. doi: 10.2334/josnusd.43.221. [DOI] [PubMed] [Google Scholar]
- Van den Abeele K, Craen M, Schuil J, Meire FM. Ophthalmologic and systemic features of the Alstrom syndrome: report of 9 cases. Bulletin de la Societe belge d'ophtalmologie. 2001:67–72. [PubMed] [Google Scholar]
- Worthley MI, Zeitz CJ. Case of Alstrom syndrome with late presentation dilated cardiomyopathy. Internal medicine journal. 2001;31:569–570. doi: 10.1046/j.1445-5994.2001.00140.x. [DOI] [PubMed] [Google Scholar]
- Chang KW, Hou JW, Lin SJ, Kong MS. Alstrom syndrome with hepatic dysfunction: report of one case. Acta paediatrica Taiwanica = Taiwan er ke yi xue hui za zhi. 2000;41:270–272. [PubMed] [Google Scholar]
- Chen BH, Chiou SS, Tsai RK, Lin YF, Wu JR. Acute lymphoblastic leukemia in one of two siblings with Alstrom syndrome. Journal of the Formosan Medical Association = Taiwan yi zhi. 2000;99:792–795. [PubMed] [Google Scholar]
- Hung YJ, Jeng C, Pei D, Chou PI, Wu DA. Alstrom syndrome in two siblings. Journal of the Formosan Medical Association = Taiwan yi zhi. 2001;100:45–49. [PubMed] [Google Scholar]
- Quiros-Tejeira RE, Vargas J, Ament ME. Early-onset liver disease complicated with acute liver failure in Alstrom syndrome. American journal of medical genetics. 2001;101:9–11. doi: 10.1002/ajmg.1292. [DOI] [PubMed] [Google Scholar]
- Russell-Eggitt IM, Clayton PT, Coffey R, Kriss A, Taylor DS, Taylor JF. Alstrom syndrome. Report of 22 cases and literature review. Ophthalmology. 1998;105:1274–1280. doi: 10.1016/S0161-6420(98)97033-6. [DOI] [PubMed] [Google Scholar]
- Awazu M, Tanaka T, Sato S, Anzo M, Higuchi M, Yamazaki K, Matsuo N. Hepatic dysfunction in two sibs with Alstrom syndrome: case report and review of the literature. American journal of medical genetics. 1997;69:13–16. doi: 10.1002/(SICI)1096-8628(19970303)69:1<13::AID-AJMG3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Awazu M, Tanaka T, Yamazaki K, Kato S, Higuchi M, Matsuo N. A 27-year-old woman with Alstrom syndrome who had liver cirrhosis. The Keio journal of medicine. 1995;44:67–73. [PubMed] [Google Scholar]
- Aynaci FM, Okten A, Mocan H, Gedik Y, Sarpkaya AO. A case of Alstrom syndrome associated with diabetes insipidus. Clinical genetics. 1995;48:164–166. doi: 10.1111/j.1399-0004.1995.tb04080.x. [DOI] [PubMed] [Google Scholar]
- Dyer DS, Wilson ME, Small KW, Pai GS. Alstrom syndrome: a case misdiagnosed as Bardet-Biedl syndrome. Journal of pediatric ophthalmology and strabismus. 1994;31:272–274. doi: 10.3928/0191-3913-19940701-19. [DOI] [PubMed] [Google Scholar]
- Holder M, Hecker W, Gilli G. Impaired glucose tolerance leads to delayed diagnosis of Alstrom syndrome. Diabetes care. 1995;18:698–700. doi: 10.2337/diacare.18.5.698. [DOI] [PubMed] [Google Scholar]
- Michaud JL, Heon E, Guilbert F, Weill J, Puech B, Benson L, Smallhorn JF, Shuman CT, Buncic JR, Levin AV, Weksberg R, Breviere GM. Natural history of Alstrom syndrome in early childhood: onset with dilated cardiomyopathy. The Journal of pediatrics. 1996;128:225–229. doi: 10.1016/S0022-3476(96)70394-3. [DOI] [PubMed] [Google Scholar]
- Alter CA, Moshang T., Jr Growth hormone deficiency in two siblings with Alstrom syndrome. American journal of diseases of children (1960) 1993;147:97–99. doi: 10.1001/archpedi.1993.02160250099030. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Fialkow PJ. The Alstrom syndrome. Report of three cases with further delineation of the clinical, pathophysiological, and genetic aspects of the disorder. Medicine. 1973;52:53–71. doi: 10.1097/00005792-197301000-00003. [DOI] [PubMed] [Google Scholar]
- Marshall JD, Beck S, Maffei P, Naggert JK. Alstrom syndrome. Eur J Hum Genet. 2007;15:1193–1202. doi: 10.1038/sj.ejhg.5201933. [DOI] [PubMed] [Google Scholar]
- Connolly MB, Jan JE, Couch RM, Wong LT, Dimmick JE, Rigg JM. Hepatic dysfunction in Alstrom disease. American journal of medical genetics. 1991;40:421–424. doi: 10.1002/ajmg.1320400408. [DOI] [PubMed] [Google Scholar]
- Budden SS, Kennaway NG, Buist NR, Poulos A, Weleber RG. Dysmorphic syndrome with phytanic acid oxidase deficiency, abnormal very long chain fatty acids, and pipecolic acidemia: studies in four children. The Journal of pediatrics. 1986;108:33–39. doi: 10.1016/S0022-3476(86)80765-X. [DOI] [PubMed] [Google Scholar]
- Iannello S, Bosco P, Cavaleri A, Camuto M, Milazzo P, Belfiore F. A review of the literature of Bardet-Biedl disease and report of three cases associated with metabolic syndrome and diagnosed after the age of fifty. Obes Rev. 2002;3:123–135. doi: 10.1046/j.1467-789X.2002.00055.x. [DOI] [PubMed] [Google Scholar]
- Wanders RJ, Jansen GA, Skjeldal OH. Refsum disease, peroxisomes and phytanic acid oxidation: a review. J Neuropathol Exp Neurol. 2001;60:1021–1031. doi: 10.1093/jnen/60.11.1021. [DOI] [PubMed] [Google Scholar]