Abstract
The formation of the DNA G-quadruplex is induced by the addition of a novel porphyrin carrying four cationic tethers. Circular dichroism spectroscopy reveals that the porphyrin binds to Tetrahymena telomeric repeat to form G-quadruplex under stabilizing-cation-deficient and no buffer conditions.
1. INTRODUCTION
Guanine-rich tandem repeats can be folded to form quadruplex structures through Hoogsteen hydrogen bonding [1]. These guanine-rich segments are found in biologically important regions such as telomeres [2], c-myc gene promoter regions [3], immunoglobulin switch regions [4], and fragile X-syndrome triplet repeats [5]. Not only because their biological importance but also because the potential applications in supramolecular chemistry and nanotechnology, a G-quadruplex attracted much attention in the field of medicinal chemistry, pharmaceutical biology, and material sciences. For example, telomere sequences have been used for the construction of nanomolecular machines [6–8] and DNA logic gates [9–11].
Because the G-quadruplex polymorphism and the duplex-quadruplex conversion are affected by environmental factors, we [12–14] and others [15, 16] have investigated the structure and thermodynamic stability of DNAs under molecular crowding conditions as a mimic of the cellular conditions. Another important factor for the stabilization and polymorphism of the G-quadruplex structure is the presence of certain metal cations, effectively K+ and Na+ [1, 17, 18]. Most of the previous studies about the structure and stability of G-quadruplex were conducted in cation-containing solutions, and to our knowledge, only a few reports have been published using salt-deficient conditions in buffer solution [19–28]. Since DNA secondary structures such as quadruplex exist only within a narrow range of biophysical conditions or their mimic conditions, chemical probe that traps quadruplex structure under simple conditions is desired. Here, we report that small organic molecule induced a telomeric DNA to G-quadruplex formation under noncrowding, and no buffer conditions in the absence of added cation.
2. EXPERIMENTAL
2.1. Materials and methods
Most of the reagents and solvents were purchased from Wako Pure Chemical Industries, Ltd., Tokyo Kasei Kogyo Co., Japan, and Sigma-Aldrich Co., Mo, USA, and used without further purification. 1H NMR spectra were recorded on a Varian UNITY 300 spectrometer at 299.94 MHz by using CDCl3 or CD3OD as a solvent and tetramethylsilane as an internal standard, and J values are given in Hz. Mass spectra were measured with a PerSeptive Biosystems Voyager DE-Pro. Circular dichroism (CD) spectra of DNA quadruplexes were obtained by using a Jasco J-820 spectropolarimeter.
2.2. Synthesis of a novel cationic porphyrin
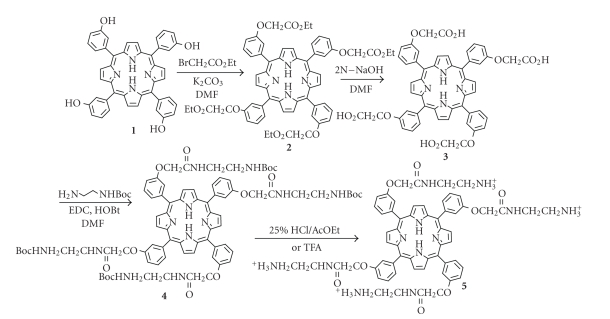
As a G-quadruplex-inducing agent, a novel cationic porphyrin 5 was prepared according to Scheme 1. The molecule 5 was designed to bind to G-quadruplex by a stacking interaction between porphyrin ring and G-quartet plane, together with electrostatic interaction between ammonium cations and DNA backbone. In spite of a postulated low affinity for G-quadruplex that is due to the bulky substituents, porphyrin 5 is expected to grab at the G-quadruplex with the corporation of π-stacking and electrostatic interactions. All the reactions were conducted in the dark.
Scheme 1.
Synthesis of cation tethered porphyrin 5.
2.3. Preparation of 5,10,15,20-tetrakis(3′-hydroxyphenyl)porphyrin 1
To a solution of 3-hydroxybenzaldehyde (3.66 g, 30 mmol) in propionic acid (70 mL), was added pyrrole (2.40 g, 36 mmol) at 110°C under nitrogen atmosphere and stirred for 1 hour. Then, the solution was stirred over night at room temperature under air to afford oxidation of the precursor to porphyrin. The solvent was removed and the residue was purified by silica gel column chromatography using chloroform/methanol = 7/1 as an eluent to obtain compound 1 as a purple crystal (1.06 g, 21% yield).
2.4. Alkylation of phenolic hydroxyl groups of 1
To a solution of 1 (0.34 g, 0.5 mmol) in DMF (10 mL) were added potassium carbonate (5.5 g, 40 mmol) and ethyl α -bromoacetate (3.4 g, 20 mmol). The mixture was stirred at room temperature for 24 hours. The reaction was quenched with water (100 mL) and the mixture was extracted with chloroform (3 × 30 mL). The combined organic phase was washed with water (3 × 30 mL) and brine (1 × 30 mL), then dried over Na2SO4. The solvent was removed and the residue was purified by silica gel column chromatography using chloroform/ethyl acetate = 10/1 mixed solvent as an eluent to give compound 2 as a purple crystal (0.42 g, 82% yield); 1H NMR (CDCl3, 300 MHz) δ-2.84 (s, 2H, NH), 1.25 (t, 12H, J = 7.2, CH3), 4.26 (q, 8H, J = 7.2, CH2CH3), 4.80 (s, 8H, OCH2), 7.34 (dd, 4H, J = 2.9, 8.6, Ar-H), 7.64 (t, 4H, J = 8.1, Ar-H), 7.75 (s, 4H, Ar-H), 7.83 (d, 4H, J = 7.2, Ar-H), 8.84 (s, 8H, pyrrole-β-H); m/z (MALDI-TOF) 1023 (M+ + H, 100%).
2.5. Hydroxylation of 2
To a solution of 2 (0.31 g, 0.3 mmol) in DMF (250 mL) was added 2N NaOHaq (50 mL). The solution was stirred over night at 50°C. The solution was concentrated to a total volume of 150 mL, and the pH of the solution was adjusted to 2.0 with 1N HCl to give a green precipitate. The precipitate was washed with chloroform to give compound 3. This compound was used for the next reaction without further purification.
2.6. Amidation of 3 with monoprotected ethylenediamine
To a solution of 3 (45 mg, 0.05 mmol) in DMF/DMSO = 3/1 (4 mL) were added N-tert-butoxycarbonylaminoethylamine (40 mg, 0.25 mmol), 1-hydroxybenzotriazole (HOBt) (41 mg, 0.3 mmol), and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) (58 mg, 0.3 mmol). After the mixture was stirred over night at room temperature, the reaction was quenched with water (50 mL). The resulting mixture was extracted with dichloromethane (3 × 50 mL) and the combined organic layer was washed with saturated NaHCO3aq (3 × 30 mL) and brine (3 × 30 mL), then dried over Na2SO4. The solvent was removed and the residue was purified by silica gel column chromatography using dichloromethane/ methanol = 15/1 as an eluent to give compound 4 as a purple crystal (0.059 g, 79% two steps overall yield); 1H NMR (CDCl3, 300 MHz) δ-2.83 (s, 2H, pyrrole-NH), 1.31 (s, 36H, tert-Bu), 3.31 (q, 8H, J = 5.3, CH2), 3.48 (q, 8H, J = 5.6, CH2), 4.69 (s, 8H, OCH2), 4.93 (brs, 4H, NH), 7.34 (dd, 4H, J = 2.1, 8.1, Ar-H), 7.40 (brs, 4H, NH), 7.65 (t, 4H, J = 8.1, Ar-H), 7.81 (s, 4H, Ar-H), 7.87 (d, 4H, J = 7.8, Ar-H), 8.83 (s, 8H, pyrrole-β-H).
2.7. Preparation of cationic porphyrin 5
A solution of 4 (29 mg, 0.02 mmol) in trifluoroacetic acid (5 mL) was stirred at room temperature for 2 hours. After the solvent was evaporated off, diethyl ether (50 mL) was added to give a green precipitate. To the solution of the precipitate in water was added excess NaOHaq. and the resulting solid was dissolved in chloroform. 1 M HCl was added to this solution to give green precipitate of porphyrin tetra hydrochloric acid salt 5 (22 mg, 92% yield); 1H NMR (CD3OD, 300 MHz) δ 3.20 (t, 8H, J = 5.3, CH2), 3.70 (t, 8H, J = 5.6, CH2), 4.94 (s, 8H, OCH2), 7.69 (dd, 4H, J = 2.6, 8.3, Ar-H), 7.99 (t, 4H, J = 8.1, Ar-H), 8.20 (s, 4H, Ar-H), 8.23 (brs, 4H, Ar-H), 8.85 (s, 8H, pyrrole-β-H); m/z (MALDI-TOF) 1079 (M+ + H, 100%).
3. RESULTS AND DISCUSSION
Tetrahymena telomeric sequence d(T2G4)4 was chosen as a model molecule and the cationic porphyrin 5 was used as a G-quadruplex inducing agent. Besides the effect of monovalent cation, the difference between intrastrand and interstrand G-quadruplexes was also examined to compare sequences d(T2G4)4 and d(TG4T2G4T).
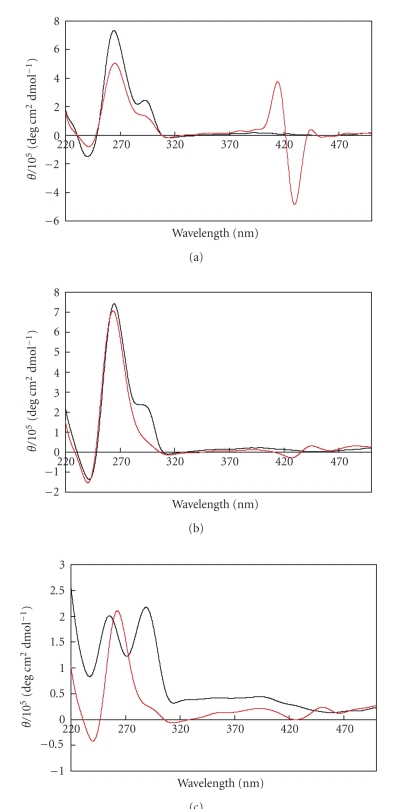
The structure of d(T2G4)4 in the presence and absence of cationic porphyrin 5 was verified by circular dichroism spectroscopy in a buffer containing 100 mM KCl and 50 mM Tris-HCl (see Figure 1(a)), in 100 mM KCl solution (see Figure 1(b))), and in water (see Figure 1(c)). CD spectrum of (T2G4)4 without porphyrin 5 had shoulder around 295 nm (see Figures 1(a) and Figure 1(b)) or positive peak around 295 nm (as shown in Figure 1(c)). These CD spectra indicate a hybrid G-quadruplex structure of (T2G4)4 without porphyrin 5, whose CD spectrum has positive intensities both at 260 and 295 nm. On the other hand, the positive peak or shoulder around 295 nm disappeared by the addition of the porphyrin. This indicates that a structural transition of (T2G4)4 from a hybrid to a parallel G-quadruplex was induced by the porphyrin. Therefore, these results lead us to conclude that porphyrin 5 induces a parallel G-quadruplex structure of (T2G4)4 under various conditions. Bisignate signals of porphyrin were strongly induced in a buffer solution, suggesting that the porphyrin was stacked on the G-quadruplex surface. Relatively, weak signals for the porphyrin were observed in solutions without Tris-HCl. The self-aggregation of porphyrin in such solutions seems to be the reason for these weak interactions.
We investigated the CD spectral changes of d(T2G4)4 by the addition of porphyrin 5 in the absence of added cation (see Figure 1(c)). All DNA samples with or without porphyrin were annealed by heating to 90°C followed by slow cooling to 0°C over 8 hours. Both spectra shown in Figure 1(c) were measured using a d(T2G4)4 solution in Milli-Q water with no addition of external cation. In the absence of cationic porphyrin 5, d(T2G4)4 did not form a G-quadruplex. However, when the sample was prepared in the presence of porphyrin 5, the CD spectrum exhibited both a positive signal around 260 nm and a negative signal around 240 nm, indicating the formation of the d(T2G4)4 G-quadruplex. A weak induced CD signal for the porphyrin 5 was also observed between 420 and 460 nm. The induced CD signal is additional evidence for the interaction between d(T2G4)4 and 5, since its appearance in the CD spectra is indicative of the change in the chirality of the proximal chemical environment of 5.
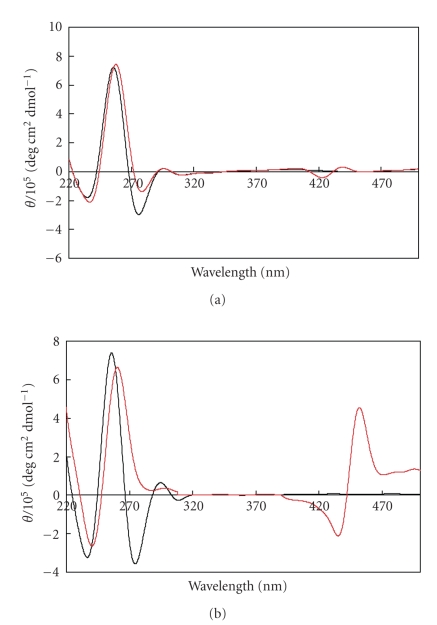
The structure of a half-length of Tetrahymena telomere sequence, d(TG4T2G4T), was studied by CD spectroscopy. CD spectra of 50 μM of d(TG4T2G4T) (G-quadruplex concentration: 25 μM) in a 100 mM KCl solution had positive and negative peaks near 260 and 240 nm, respectively, both in the absence and presence of porphyrin 5, indicating parallel G-quadruplex (see Figure 2(a)). These spectral features were similar to those of d(T2G4)4. On the other hand, in the absence of added cation, CD spectrum of d(TG4T2G4T) was completely different from that of d(T2G4)4 without porphyrin 5; while in the presence of 5, the locations of positive and negative CD signals of d(TG4T2G4T) and d(T2G4)4 were almost identical (as in Figure 2(b)). These results indicate that, while these two sequences have different structure in water, both are induced to form parallel G-quadruplex by the addition of cationic porphyrin 5.
Figure 1.
CD spectra of d(T2G4)4 (25 μM) with (red) or without (black) porphyrin 5 (75 μM) in buffer containing 100 mM KCl and 50 mM Tris-HCl (pH 7.5) (a), in 100 mM KCl (b), and in water (c) at 0°C.
In conclusion, the novel porphyrin carrying four cationic tethers 5 induced the formation of parallel G-quadruplex with Tetrahymena telomeric sequence. The effect of G-quadruplex induction was strong enough and made both sequences d(T2G4)4 and d(TG4T2G4T) folded into G-quadruplex without the addition of cation and buffer. The formation of the G-quadruplex under in vitro environment is mandatory for recent applications of G-quadruplex to nano-materials. Thus, the simple conditions for the formation of G-quadruplex are desired. The system described here must be useful for the development of nanobiomaterials. In more general, it will be important to control the structure of biomolecules with small organic compounds for nanobiotechnology.
Figure 2.
CD spectra of d(TG4T2G4T) (25 μM) with (red) or without (black) porphyrin 5 (75 μM) in buffer containing 100 mM KCl and 50 mM Tris-HCl (pH 7.5) (a) and in water (b) at 0°C.
ACKNOWLEDGMENTS
This work was supported in part by Grants-in-Aid for Scientific Research and the “Academic Frontier” Project (2004–2009) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1.Williamson JR, Raghuraman MK, Cech TR. Monovalent cation-induced structure of telomeric DNA: the G-quartet model. Cell. 1989;59(5):871–880. doi: 10.1016/0092-8674(89)90610-7. [DOI] [PubMed] [Google Scholar]
- 2.Blackburn EH. Telomeres: no end in sight. Cell. 1994;77(5):621–623. doi: 10.1016/0092-8674(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 3.Evans T, Schon E, Gora-Maslak G, Patterson J, Efstratiadis A. S1-hypersensitive sites in eukaryotic promoter regions. Nucleic Acids Research. 1984;12(21):8043–8058. doi: 10.1093/nar/12.21.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sen D, Gilbert W. Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implications for meiosis. Nature. 1988;334(6180):364–366. doi: 10.1038/334364a0. [DOI] [PubMed] [Google Scholar]
- 5.Fry M, Loeb LA. The fragile X syndrome d(CGGn) nucleotide repeats form a stable tetrahelical structure. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(11):4950–4954. doi: 10.1073/pnas.91.11.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alberti P, Mergny J-L. DNA duplex-quadruplex exchange as the basis for a nanomolecular machine. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(4):1569–1573. doi: 10.1073/pnas.0335459100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makita N, Inoue S, Akaike T, Maruyama A. Improved performance of a DNA nanomachine by cationic copolymers. Nucleic Acids Symposium Series. 2004;48(1):173–174. doi: 10.1093/nass/48.1.173. [DOI] [PubMed] [Google Scholar]
- 8.Alberti P, Bourdoncle A, Saccá B, Lacroix L, Mergny J-L. DNA nanomachines and nanostructures involving quadruplexes. Organic & Biomolecular Chemistry. 2006;4(18):3383–3391. doi: 10.1039/b605739j. [DOI] [PubMed] [Google Scholar]
- 9.Miyoshi D, Inoue M, Sugimoto N. DNA logic gates based on structural polymorphism of telomere DNA molecules responding to chemical input signals. Angewandte Chemie International Edition. 2006;45(46):7716–7719. doi: 10.1002/anie.200602404. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto A. Synthesis of highly functional nucleic acids and their application to DNA technology. Bulletin of the Chemical Society of Japan. 2005;78(12):2083–2097. [Google Scholar]
- 11.Cheah IK, Langford SJ, Latter MJ. Concept transfer-from genetic instruction to molecular logic. Supramolecular Chemistry. 2005;17(1-2):121–128. [Google Scholar]
- 12.Miyoshi D, Nakao A, Sugimoto N. Molecular crowding regulates the structural switch of the DNA G-quadruplex. Biochemistry. 2002;41(50):15017–15024. doi: 10.1021/bi020412f. [DOI] [PubMed] [Google Scholar]
- 13.Miyoshi D, Matsumura S, Nakano S, Sugimoto N. Duplex dissociation of telomere DNAs induced by molecular crowding. Journal of the American Chemical Society. 2004;126(1):165–169. doi: 10.1021/ja036721q. [DOI] [PubMed] [Google Scholar]
- 14.Miyoshi D, Karimata H, Sugimoto N. Drastic effect of a single base difference between human and Tetrahymena telomere sequences on their structures under molecular crowding conditions. Angewandte Chemie International Edition. 2005;44(24):3740–3744. doi: 10.1002/anie.200462667. [DOI] [PubMed] [Google Scholar]
- 15.Minton AP. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. Journal of Biological Chemistry. 2001;276(14):10577–10580. doi: 10.1074/jbc.R100005200. [DOI] [PubMed] [Google Scholar]
- 16.Ellis RJ. Macromolecular crowding: obvious but underappreciated. Trends in Biochemical Sciences. 2001;26(10):597–604. doi: 10.1016/s0968-0004(01)01938-7. [DOI] [PubMed] [Google Scholar]
- 17.Simonsson T. G-quadruplex DNA structures variations on a theme. Biological Chemistry. 2001;382(4):621–628. doi: 10.1515/BC.2001.073. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Patel DJ. Solution structure of the Tetrahymena telomeric repeat d (T2G4)4 G-tetraplex. Structure. 1994;2(12):1141–1156. doi: 10.1016/s0969-2126(94)00117-0. [DOI] [PubMed] [Google Scholar]
- 19.Izbicka E, Wheelhouse RT, Raymond E, et al. Effects of cationic porphyrins as G-quadruplex interactive agents in human tumor cells. Cancer Research. 1999;59(3):639–644. [PubMed] [Google Scholar]
- 20.Siddiqui-Jain A, Grand CL, Bearss DJ, Hurley LH. Direct evidence for a G-quadruplex in a promoter region and its targeting with a small molecule to repress c-MYC transcription. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(18):11593–11598. doi: 10.1073/pnas.182256799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herzig MCS, Rodriguez KA, Trevino AV, et al. The genome factor in region-specific DNA damage: the DNA-reactive drug U-78779 prefers mixed A/T-G/C sequences at the nucleotide level but is region-specific for long pure AT islands at the genomic level. Biochemistry. 2002;41(5):1545–1555. doi: 10.1021/bi011907s. [DOI] [PubMed] [Google Scholar]
- 22.Kim M-Y, Gleason-Guzman M, Izbicka E, Nishioka D, Hurley LH. The different biological effects of Telomestatin and TMPyP4 can be attributed to their selectivity for interaction with intramolecular or intermolecular G-quadruplex structures. Cancer Research. 2003;63(12):3247–3256. [PubMed] [Google Scholar]
- 23.Rezler EM, Seenisamy J, Bashyam S, et al. Telomestatin and diseleno sapphyrin bind selectively to two different forms of the human telomeric G-quadruplex structure. Journal of the American Chemical Society. 2005;127(26):9439–9447. doi: 10.1021/ja0505088. [DOI] [PubMed] [Google Scholar]
- 24.Gonçalves DPN, Rodriguez R, Balasubramanian S, Sanders JKM. Tetramethylpyridiniumporphyrazines—a new class of G-quadruplex inducing and stabilising ligands. Chemical Communications. 2006;(45):4685–4687. doi: 10.1039/b611731g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonçalves DPN, Ladame S, Balasubramanian S, Sanders JKM. Synthesis and G-quadruplex binding studies of new 4- N-methylpyridinium porphyrins. Organic & Biomolecular Chemistry. 2006;4(17):3337–3342. doi: 10.1039/b608494j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kan Z-Y, Yao Y, Wang P, Li X-H, Hao Y-H, Tan Z. Molecular crowding induces telomere G-quadruplex formation under salt-deficient conditions and enhances its competition with duplex formation. Angewandte Chemie International Edition. 2006;45(10):1629–1632. doi: 10.1002/anie.200502960. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez R, Pantoş GD, Gonçalves DPN, Sanders JKM, Balasubramanian S. Ligand-driven G-quadruplex conformational switching by using an unusual mode of interaction. Angewandte Chemie International Edition. 2007;46(28):5405–5407. doi: 10.1002/anie.200605075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagatoshi S, Tanaka Y, Tsumoto K. Circular dichromism spectra demonstrate formation of the thrombin-binding DNA aptamar G-quadruplex under stabilizing-cation-deficient conditions. Biochemical and Biophysical Research Communications. 2007;352(3):812–817. doi: 10.1016/j.bbrc.2006.11.088. [DOI] [PubMed] [Google Scholar]