Abstract
ZnSe-based nanocrystals (ca. 4-5 nm in diameter) emitting in blue region (ca. 445 nm) were incorporated in spherical small silica particles (20–40 nm in diameter) by a reverse micelle method. During the preparation, alkaline solution was used to deposit the hydrolyzed alkoxide on the surface of nanocrystals. It was crucially important for this solution to include Zn2+ ions and surfactant molecules (thioglycolic acid) to preserve the spectral properties of the final silica particles. This is because these substances in the solution prevent the surface of nanocrystals from deterioration by dissolution during processing. The resultant silica particles have an emission efficiency of 16% with maintaining the photoluminescent spectral width and peak wavelength of the initial colloidal solution.
1. INTRODUCTION
Development of bright and stable phosphors is increasingly required in many fields. The II-VI semiconductor nanocrystals (NCs) such as ZnSe and CdTe attract much interest as novel bright phosphors with tunable photoluminescence (PL) wavelength [1] for possible uses including biological labeling [2–6], display, and lighting devices [7–9]. For biological labeling, fluorophores are bound to biological molecules as fluorescent markers. It is possible to monitor the position and movement of virus and various materials by the PL of fluorophores. So far, organic fluorescent dyes were widely used for such biomarkers [2, 10]. However, the emission wavelengths of these dyes are normally close to the excitation wavelengths, and therefore different excitation wavelengths are required for getting multiple colors. Moreover, deterioration and PL quenching of organic dyes occur in a short time of irradiation. For displays and lightings, currently commercialized phosphors are mostly rare earth ion-doped and transition metal ion-doped oxides [11]. These phosphors are highly stable; however, it is not easy to control the emission wavelength. In addition, they show a long decay time of PL (ca. 1 millisecond) because of a forbidden character of the transition. This slow decay causes the saturation of PL intensity when the excitation light intensity is increased.
The techniques to prepare highly luminescent and monodispersed semiconductor NCs have much advanced in this decade. They are organic solution method [12] and aqueous solution method [13]. Bright PL is obtained by capping the NCs with surfactants which deactivate the unpreferably PL-quenching surface defects. Aqueous solution method has several advantages over organic solution method: (1) highly luminescent NCs can be synthesized at lower temperature (~100°C) using a relatively safe and simple system; and that (2) the prepared water-dispersible NCs have higher stability of PL intensity in water and better compatibility with sol-gel fabrication.
We have reported the preparation of highly luminescent CdTe NCs [14] and ZnSe-based NCs [15–17] by the aqueous solution method. The PL wavelengths of these NCs depend on the band gap (Eg*) which can be controlled by the composition and size of the NCs. The PL wavelength and efficiency of the NCs are almost independent of the excitation wavelength. The emission wavelength can be far from the excitation wavelength. Therefore, various PL colors are obtainable from various NCs using a single excitation wavelength. Compared with organic dyes, semiconductor NCs show much slower PL quenching or photobleaching on irradiation [2, 10]. Furthermore, the II-VI semiconductor NCs have a direct transition band gap and fast PL decay in ca. 10 nanoseconds that is faster than those of rare earth ions and transition metal ions by ca. 5 orders of magnitude. This fast decay of PL leads to high brightness when the excitation light intensity is increased.
Recently, increasing attention has been directed to the semiconductor NCs incorporated in transparent matrices. The incorporation in silica matrices avoids the agglomeration of NCs and improves the long-term stability of PL. Previously, Mulvaney et al. reported the incorporation of NCs in SiO2 [18] and ZrO2-SiO2 [19] matrices by a sol-gel method. They used hydrophobic NCs (CdSe, CdSe/ZnS, and CdSe/CdS) prepared by organic solution method. However, water-dispersible NCs are more compatible with sol-gel method than such hydrophobic NCs. We have developed techniques to incorporate water-dispersible CdTe and ZnSe NCs in three forms of silica matrices (bulk [16, 20, 21], small particles [22, 23], and thin films [24]) and in bulk-form Si-x ZrxO2 matrix [25], by using a sol-gel method. The prepared NC-incorporating phosphors showed bright PL of three primary colors. Among the three forms, small particles are expected to be used as fluorescent markers for biological labeling. Semiconductor NCs have already been reported to be applicable as fluorescent biomarkers [2–6]. There are several advantages in the incorporation of semiconductor NCs in small silica particles, such as protecting the NCs against oxidation and agglomeration, increasing the mechanical stability, and enabling a transfer into various organic and aqueous solvents. Furthermore, the surface of silica particles can be chemically modified to link bioconjugates.
Previously, several groups reported the preparation and PL efficiencies of silica particles incorporating emitting NCs, by chemical growth of silica shell around an NC (PL efficiency up to 18%) [26]; by sol-gel formation of NC-containing layer around a silica particle without containing NCs (PL efficiency up to 13%) [27]; and by reverse micelle method (PL efficiency up to 20%) [22, 23, 28–30]. The reverse micelle method is simpler than the chemical synthetic growth method and has wide controllability of NC concentration in the silica particles. When the NC concentration in a silica particle is high, both the increased emission brightness and decreased blinking of the silica particle can be expected. We have already reported green- and red-emitting silica particles incorporating CdTe NCs which were fabricated by the reverse micelle method [22, 23]. The PL efficiencies reached 27% and 65%, respectively, for green- and red-emitting silica particles. This PL efficiency (65%) is the highest ever reported efficiency for the silica particles incorporating semiconductor NCs. In order to obtain PL of three primary colors for expanding the application range, blue-emitting silica particles are required. However, bright blue-emitting silica particles have not yet been reported to our knowledge. Zinc selenide NCs attract increasing interest because they can be synthesized in organic or aqueous solution and have tunable PL wavelength in blue region [15, 31–33]. Recently, we synthesized highly luminescent ZnSe-based NCs in blue region by an aqueous solution method [16, 17]. The emission color of ZnSe NCs was shifted from blue-violet (~410 nm) to pure blue (~440–485 nm) by doping heavy elements such as Cd or Te, because, generally, the Eg* of II-VI semiconductor NCs becomes narrower when the constituent elements become heavier. Here we report the preparation of silica particle phosphor incorporating ZnSe-based NCs thus prepared by a reverse micelle method.
2. MATERIALS ANDMETHODS
2.1. Chemicals
All chemicals used were of analytical grade or of the highest purity available. Zinc perchlorate, cadmium perchlorate, and thioglycolic acid (TGA) were purchased from Sigma-Aldrich (Miss, USA). Al2Se3 lumps aquired from CERAC (Wiss, USA) were used to produce hydrogen selenide (H2Se) gas. Ammonia water and NaOH solution were purchased from Wako (Osaka, Japan). Deionized water (18.3 MΩ) was obtained from a Milli-Q water system (Millipore (Mass, USA)).
2.2. Preparation of ZnSe-based NCs
We have prepared core-shell ZnSe-based NCs by the previously reported aqueous solution method [15–17]. Briefly, the colloidal solutions of ZnSe-based NCs (core) were prepared using zinc perchlorate, cadmium perchlorate, and H2Se gas. TGA was used as the stabilizing surfactant that caps the NCs. The obtained weakly emitting Cd-doped ZnSe NCs (Zn : Cd = 95 : 5 (molar ratio upon synthesis)) was first dispersed in a solution containing cadmium perchlorate and TGA in the dark, and then irradiated with ultraviolet (UV) light (365 nm) in an aqueous solution containing zinc perchlorate and TGA [17]. The ZnS shell was formed on the Cd-doped ZnSe core by the irradiation. Formation of this shell is effective for decreasing the number of surface defects and for increasing the robustness of NCs. As a result, strongly blue-emitting Cd-doped ZnSe/ZnS NCs with a diameter of ca. 4-5 nm were prepared. The PL peak wavelength was 448 nm and PL efficiency was 49% in aqueous solution.
2.3. Preparation of silica particles incorporating ZnSe-based NCs by reverse micelle method
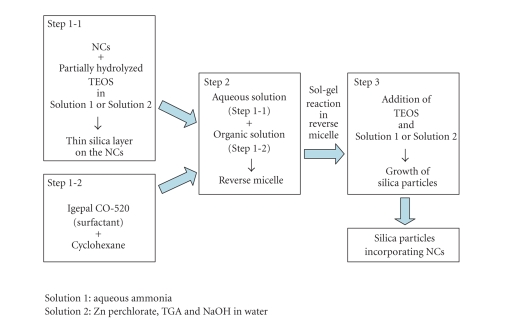
Silica particles incorporating ZnSe-based NCs were prepared by the procedure depicted in Scheme 1. Instead of using Solution 1 (diluted aqueous ammonia (6.25%)) described in a previous report for preparing silica particles incorporating CdTe NCs [23], we used Solution 2 (Zn perchlorate, TGA, and NaOH solution ([Zn2+] = 0.261 mol/L, molar ratio of [Zn2+] : [TGA] = 1 : 2.43, pH~11)) for nucleation of silica component to the surface of the particles in Step 1-1 and Step 3. As described below, the Solution 2 was quite effective to retain the emission efficiency of the NCs.
Scheme 1.
Preparation procedure of silica particles incorporating ZnSe-based NCs.
Step 1-1 —
Aqueous solution of the NCs was mixed with alkaline solution of partially hydrolyzed tetraethyl orthosilicate (TEOS). Typically, 1 mL Cd-doped ZnSe/ZnS colloidal solution (~3 × 10−5 mol particles/L), 1 mL water, 50 μL alkaline solution (Solution 1 or 2), and 0.15 mL TEOS were mixed in a beaker. The pH of the mixed solution was 9-10. Then the solution was stirred for 2-3 hours to form thin silica layer on the surface of NCs [23].
Step 1-2 —
Nonionic surfactant Igepal CO-520 (polyoxyethylene(5)nonylphenyl ether) was dissolved in hydrophobic cyclohexane. Typically, 2.25 g Igepal CO-520 was dissolved in 12.5 g cyclohexane in a beaker.
Step 2 —
The aqueous solution prepared in Step 1-1 was injected dropwise to the cyclohexane solution prepared in Step 1-2. In this step, reverse micelles containing water droplets dispersing Cd-doped ZnSe/ZnS NCs were formed.
Step 3 —
To grow outermost silica shell further, TEOS (typically 0.3 mL) and alkaline aqueous solution (typically 100 μL) were added to the solution prepared in Step 2. Then the solution was stirred for 4 hours.
As a result, silica particles incorporating Cd-doped ZnSe/ZnS NCs were obtained. These silica particles showed blue PL when irradiated with UV light. Transparent cyclohexane solution of silica particles incorporating NCs was obtained as supernatant by centrifuge of the solution at 4000 rpm.
For comparison, nonluminescent silica particles without containing NCs were prepared using Solution 1 in the similar manner. In Step 1-1, 1 mL diluted NaOH solution (pH~11) was used instead of 1 mL Cd-doped ZnSe/ZnS colloidal solution.
Size distribution of the obtained silica particles was measured by using a dynamic light scattering particle size analyzer (Nikkiso Microtrac Nanotrac 150). Transmission electron microscopy (TEM) of the silica particles was performed at an acceleration voltage of 300 kV by using a Hitachi H-9000. For preparing a TEM specimen, the silica particles were extracted from the supernatant by precipitation with acetonitrile followed by drying in air at ~40°C. The PL and absorption spectra of the silica particles were measured in cyclohexane by using conventional fluorescence spectrometer (Hitachi F-4500) and absorption spectrometer (Hitachi U-4000). The PL efficiencies of the solution samples (silica particles in cyclohexane and NCs in water) were estimated by comparison with standard solutions of quinine in aqueous 0.05 M H2SO4 solution (PL efficiency = 54.6% [34]).
3. RESULTS AND DISCUSSION
3.1. Appearance of silica particles incorporating ZnSe-based NCs
After centrifuge in the final step of preparation, the supernatant contained small silica particles and precipitate contained larger silica particles. Both supernatant and precipitate showed bright blue PL. The small silica particles were homogeneously dispersed in the supernatant (cyclohexane solution) at room temperature. The supernatant obtained by using Solution 2 showed brighter PL than that obtained by using Solution 1. Figures 1(a) and 1(b) show the appearance of the silica particles incorporating ZnSe-based NCs prepared using Solution 2 under visible light and under UV light, respectively. The cyclohexane solution was almost colorless and transparent under visible light (see Figure 1(a)) and emitted bright blue PL under UV light irradiation (see Figure 1(b)). On the other hand, the powder of small silica particles extracted from the above supernatant by using acetonitrile was white under visible light (see Figure 1(c)) and emitted blue PL under UV light (see Figure 1(d)).
Figure 1.
Appearance of silica particles incorporating ZnSe-based NCs prepared using Solution 2: (a) cyclohexane solution under visible light, (b) cyclohexane solution under UV light (wavelength: 365 nm), (c) powder under visible light, and (d) powder under UV light (wavelength: 365 nm).
3.2. Sizes of silica particles incorporating ZnSe-based NCs
Figure 2 shows the size (diameter) distribution of the small silica particles incorporating ZnSe-based NCs in the supernatant, measured by the dynamic light scattering method. The silica particles prepared using Solution 1 and those prepared using Solution 2 gave similar distribution curve of particle size, however, the latter particle size was a little smaller than the former one. Namely, the mean sizes of the silica particles prepared using Solution 1 and that using Solution 2 were ca. 35 and 31 nm, respectively. These two kinds of silica particles had a spherical shape. Nonluminescent silica particles without containing NCs had similar size. A typical TEM photograph of the small silica particles incorporating ZnSe-based NCs in the supernatant is shown in Figure 3. The shape of these small silica particles is spherical and the particle diameters are 20–40 nm, which are in good agreement with those measured by the dynamic light scattering method (see Figure 2). On the other hand, the precipitate after centrifuge in the final step of preparation contained larger silica particles with diameters from a few hundreds nm to 1-2 μm. Such large silica particles also had a spherical shape. The results show that luminescent silica particles with various diameters were formed in the preparation process, and the size selection of silica particles was possible by centrifuge. It was also possible to select the size of silica particles by using syringe filters.
Figure 2.
Size distribution of silica particles incorporating ZnSe-based NCs measured by dynamic light scattering method: (a) silica particles prepared using Solution 1 and (b) silica particles prepared using Solution 2.
Figure 3.
TEM photograph of silica particles incorporating ZnSe-based NCs prepared using Solution 1.
3.3. PL spectra, absorption spectra, and PL efficiencies of silica particles incorporating ZnSe-based NCs
Figures 4 and 5 depict the PL spectra and absorption spectra of the small silica particles incorporating ZnSe-based NCs in the supernatant, respectively. The NC-incorporating silica particles prepared using Solution 2 showed an excitonic absorption of the NCs [16, 17] around 420–440 nm. This absorption band was less clearly observed in the NC-incorporating silica particles using Solution 1 and was not seen in the silica particles without incorporating NCs. This suggests that the deterioration of the NCs during incorporation in the silica particles was suppressed by using Solution 2. The PL peak of the NC-incorporating silica particles appeared in the absorption edge region. When the absorbance of the supernatant at the excitation wavelength (350 nm) was adjusted to the same value, the PL intensity of the silica particles prepared using Solution 2 was 2.3 times larger than that of the silica particles prepared using Solution 1 (see Figure 4). As shown in Figure 4, these two PL spectra and that of the initial colloidal solution of NCs had almost the same shape. The widths of the PL spectra were also very close (Table 1). Compared with the initial colloidal solution of NCs, the silica particles showed small blue shift (1–5 nm) of the PL peak wavelength (Table 1). However, the blue shift in the silica particles prepared using Solution 2 was significantly smaller than that in the silica particles prepared using Solution 1. From the PL and absorption spectra, the PL efficiencies of the blue-emitting small silica particles using Solutions 1 and 2 were estimated to be 7 and 16%, respectively (Table 1). As exemplified by the preparation of silica phosphor dispersing CdTe NCs [20], the Zn2+ ions and TGA in Solution 2 prevent the surface of NCs from the deterioration during incorporation into silica particles. This leads to the almost unchanged PL wavelength and high PL efficiency. By contrast, when using the conventional aqueous ammonia (Solution 1), the observed larger blue shift of PL wavelength and lower PL efficiency were derived by deterioration of the NCs such as partial dissolution of the surface part into the surrounding media during incorporation into silica particles.
Figure 4.
PL spectra of silica particles incorporating ZnSe-based NCs in solution: (a) silica particles prepared using Solution 1, (b) silica particles prepared using Solution 2. Excitation wavelength = 350 nm.
Table 1.
PL peak wavelength (, spectral width (FWHM), and PL efficiency ( of silica particles incorporating the NCs together with the initial colloidal NCs.
| No. | (i) | (ii) | (iii) |
|---|---|---|---|
| Sample | Silica particles | Silica particles | Initial NCs |
| Solution for Steps 1-1 and 3 | Solution 1 | Solution 2 | — |
| λ/nm | 442.8 | 446.8 | 448.0 |
| FWHa/nm | 35.2 | 35.6 | 35.0 |
| η (%) | 7 | 16 | 49 |
aFull width at half maximum.
4. CONCLUSION
We have prepared bright blue-emitting silica particles incorporating core-shell Cd-doped ZnSe/ZnS NCs by a reverse micelle method. When using an alkaline solution containing zinc perchlorate and TGA during silica formation on the surface of NCs, the PL efficiency of the silica particles after preparation reached 16%, which was 2.3 times larger than the PL efficiency of the silica particles prepared by using conventional aqueous ammonia. This is because the TGA-capped ZnS shell of the NCs is retained by the presence of zinc ions and TGA molecules during incorporation in silica particles. As we have already reported the preparation of green- and red-emitting silica particles, three primary emission colors are now obtainable from silica particles incorporating semiconductor NCs. These emitting silica particles are expected to be applicable as fluorescent biomarkers.
Figure 5.
Absorption spectra of silica particles with and without ZnSe-based NCs in solution: (a) NC-incorporating silica particles prepared using Solution 1, (b) NC-incorporating silica particles prepared using Solution 2, and (c) silica particles without incorporating the NCs prepared using Solution 1.
ACKNOWLEDGMENT
This study was supported in part by the Core Research for Evolutional Science and Technology (CREST) (research area: novel measuring and analytical technology contributions to the elucidation and application of life phenomena), sponsored by the Japan Science and Technology Agency (JST), Japan.
References
- 1.Alivisatos AP. Perspectives on the physical chemistry of semiconductor nanocrystals. Journal of Physical Chemistry. 1996;100(31):13226–13239. [Google Scholar]
- 2.Bruchez M, Jr, Moronne M, Gin P, Weiss S, Alivisatos AP. Semiconductor nanocrystals as fluorescent biological labels. Science. 1998;281(5385):2013–2016. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- 3.Han M, Gao X, Su JZ, Nie S. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nature Biotechnology. 2001;19(7):631–635. doi: 10.1038/90228. [DOI] [PubMed] [Google Scholar]
- 4.Chan WCW, Maxwell DJ, Gao X, Bailey RE, Han M, Nie S. Luminescent quantum dots for multiplexed biological detection and imaging. Current Opinion in Biotechnology. 2002;13(1):40–46. doi: 10.1016/s0958-1669(02)00282-3. [DOI] [PubMed] [Google Scholar]
- 5.Parak WJ, Gerion D, Pellegrino T, et al. Biological applications of colloidal nanocrystals. Nanotechnology. 2003;14(7):R15–R27. [Google Scholar]
- 6.Michalet X, Pinaud FF, Bentolila LA, et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307(5709):538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaponik NP, Talapin DV, Rogach AL, Eychmüller A. Electrochemical synthesis of CdTe nanocrystal/polypyrrole composites for optoelectronic applications. Journal of Materials Chemistry. 2000;10(9):2163–2166. [Google Scholar]
- 8.Coe S, Woo W-K, Bawendi M, Bulović V. Electroluminescence from single monolayers of nanocrystals in molecular organic devices. Nature. 2002;420(6917):800–803. doi: 10.1038/nature01217. [DOI] [PubMed] [Google Scholar]
- 9.Mori Y, Arao Y, Tsuchiya K, Shimamura K. Photoluminescence properties of ZnS nanoparticles prepared in clay suspension. In: Proceedings of the 58th Divisional Meeting on Colloid and Interface Chemistry; 2005; Utsunomiya, Japan. Sep, p. 300. [Google Scholar]
- 10.Toomre D, Manstein DJ. Lighting up the cell surface with evanescent wave microscopy. Trends in Cell Biology. 2001;11(7):298–303. doi: 10.1016/s0962-8924(01)02027-x. [DOI] [PubMed] [Google Scholar]
- 11.Phosphor Research Society, editor. Handbook of Phosphors. Tokyo, Japan: Ohmsha; 1987. [Google Scholar]
- 12.Dabbousi BO, Rodriguez-Viejo J, Mikulec FV, et al. (CdSe)ZnS core-shell quantum dots: synthesis and characterization of a size series of highly luminescent nanocrystallites. Journal of Physical Chemistry B. 1997;101(46):9463–9475. [Google Scholar]
- 13.Rogach AL, Katsikas L, Kornowski A, Su DS, Eychmüller A, Weller H. Synthesis and characterization of thiol-stabilized CdTe nanocrystals. Berichte der Bunsen-Gesellschaft-Physical Chemistry. 1996;100:1772–1778. [Google Scholar]
- 14.Li C, Murase N. Surfactant-dependent photoluminescence of CdTe nanocrystals in aqueous solution. Chemistry Letters. 2005;34(1):92–93. [Google Scholar]
- 15.Murase N, Gao M. Preparation and photoluminescence of water-dispersible ZnSe nanocrystals. Materials Letters. 2004;58(30):3898–3902. [Google Scholar]
- 16.Li C, Nishikawa K, Ando M, Enomoto H, Murase N. Highly luminescent water-soluble ZnSe nanocrystals and their incorporation in a glass matrix. Colloids and Surfaces A. 2007;294(1–3):33–39. [Google Scholar]
- 17.Li C, Nishikawa K, Ando M, Enomoto H, Murase N. Blue-emitting type-II semiconductor nanocrystals with high efficiency prepared by aqueous method. Chemistry Letters. 2007;36(3):438–439. [Google Scholar]
- 18.Selvan ST, Bullen C, Ashokkumar M, Mulvaney P. Synthesis of tunable, highly luminescent QD-glasses through sol-gel processing. Advanced Materials. 2001;13(12-13):985–988. [Google Scholar]
- 19.Bullen C, Mulvaney P, Sada C, Ferrari M, Chiasera A, Martucci A. Incorporation of a highly luminescent semiconductor quantum dot in ZrO2 – SiO2 hybrid sol-gel glass film. Journal of Materials Chemistry. 2004;14(7):1112–1116. [Google Scholar]
- 20.Li C, Murase N. Synthesis of highly luminescent glasses incorporating CdTe nanocrystals through sol-gel processing. Langmuir. 2004;20(1):1–4. doi: 10.1021/la035546o. [DOI] [PubMed] [Google Scholar]
- 21.Li C, Ando M, Murase N. Preparation and characterization of glass embedding photoluminescent CdTe nanocrystals. Journal of Non-Crystalline Solids. 2004;342(1-3):32–38. [Google Scholar]
- 22.Selvan ST, Li C, Ando M, Murase N. Formation of luminescent CdTe-silica nanoparticles through an inverse microemulsion technique. Chemistry Letters. 2004;33(4):434–435. [Google Scholar]
- 23.Yang P, Ando M, Murase N. Encapsulation of emitting CdTe QDs within silica beads to retain initial photoluminescence efficiency. Journal of Colloid and Interface Science. 2007;316(2):420–427. doi: 10.1016/j.jcis.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 24.Yang P, Li C, Murase N. Highly photoluminescent multilayer QD-glass films prepared by LbL self-assembly. Langmuir. 2005;21(19):8913–8917. doi: 10.1021/la050397q. [DOI] [PubMed] [Google Scholar]
- 25.Yang P, Murase N. Intensely emitting CdTe nanocrystals retained initial photoluminescence efficiency in sol-gel derived Si1–xZrxO2 glass,”. Applied Physics A. 2007;89(1):189–193. [Google Scholar]
- 26.Gerion D, Pinaud F, Williams SC, et al. Synthesis and properties of biocompatible water-soluble silica-coated CdSe/ZnS semiconductor quantum dots. Journal of Physical Chemistry B. 2001;105(37):8861–8871. [Google Scholar]
- 27.Chan Y, Zimmer JP, Stroh M, Steckel JS, Jain RK, Bawendi MG. Incorporation of luminescent nanocrystals into monodisperse core-shell silica microspheres. Advanced Materials. 2004;16(23-24):2092–2097. [Google Scholar]
- 28.Selvan ST, Tan TT, Ying JY. Robust, non-cytotoxic, silica-coated CdSe quantum dots with efficient photoluminescence. Advanced Materials. 2005;17(13):1620–1625. [Google Scholar]
- 29.Yang Y, Gao M. Preparation of fluorescent SiO2 particles with single CdTe nanocrystal cores by the reverse microemulsion method. Advanced Materials. 2005;17(19):2354–2357. [Google Scholar]
- 30.Darbandi M, Thomann R, Nann T. Single quantum dots in silica spheres by microemulsion synthesis. Chemistry of Materials. 2005;17(23):5720–5725. [Google Scholar]
- 31.Zhong X, Han M, Dong Z, White TJ, Knoll W. Composition-tunable ZnxCd1–xSe nanocrystals with high luminescence and stability. Journal of the American Chemical Society. 2003;125(28):8589–8594. doi: 10.1021/ja035096m. [DOI] [PubMed] [Google Scholar]
- 32.Li R, Lee J, Kang D, Luo Z, Aindow M, Papadimitrakopoulos F. Band-edge photoluminescence recovery from zinc-blende CdSe nanocrystals synthesized at room temperature. Advanced Functional Materials. 2006;16(3):345–350. [Google Scholar]
- 33.Zhao Q, Xu W. One-step preparation of ZnSe nanorod aggregates. Chemistry Letters. 2006;35(10):1186–1187. [Google Scholar]
- 34.Eaton DF. Reference materials for fluorescence measurement. Pure and Applied Chemistry. 1988;60(7):1107–1114. doi: 10.1016/1011-1344(88)85081-4. [DOI] [PubMed] [Google Scholar]