Abstract
Oxygen is a critical regulator of placentation. Early placental development occurs in a predominantly low oxygen environment and is, at least partially, under the control of hypoxia signaling pathways. In the present study, in vivo hypobaric hypoxia was used as an experimental tool to delineate hypoxia-sensitive events during placentation. Pregnant rats were exposed to the equivalent of 11% oxygen between days 6.5 and 13.5 of gestation. Pair-fed pregnant animals exposed to ambient conditions were included as a control group. Uterine mesometrial blood vessels in the hypoxia exposed animals were greatly expanded and some contained large cuboidal cells that were positive for cytokeratin and other markers characteristic of invasive trophoblast cells. Unlike later in gestation, the route of trophoblast cell invasion in the hypoxia-exposed animals was restricted to endovascular, with no interstitial invasion observed. Hypoxia-activated endovascular trophoblast invasion required exposure to hypoxia from gestation day 8.5 to day 9.5. Activation of the invasive trophoblast lineage was also associated with an enlargement of the junctional zone of the chorioallantoic placenta, a source of invasive trophoblast cell progenitors. In summary, maternal hypoxia during early stages of placentation activates the invasive endovascular trophoblast cell lineage and promotes uterine vascular remodeling.
Keywords: Hypoxia, trophoblast invasion, metrial gland, chorioallantoic placenta, rat, placentation
INTRODUCTION
Hemochorial placentation is utilized in many mammalian species including rodents and primates (Davies and Glasser, 1968; Enders and Welsh, 1993; Georgiades et al., 2002). It ensures the most intimate contacts between maternal and embryonic compartments. The maternal-embryonic connections include extensive remodeling of the associated maternal uterine vasculature (Enders and Welsh, 1993; Kaufmann et al. 2003; Red-Horse et al., 2004; Pijnenborg et al. 2006). The uterine vascular modifications are fundamental to the delivery of nutrients to the developing embryo/fetus. A key regulator of pregnancy-dependent changes in the uterine vasculature is a specialized lineage of trophoblast cells referred to as invasive trophoblast cells. In the human the invasive trophoblast cell lineage is referred to as extravillous trophoblast.
Intrauterine trophoblast cell invasion is a prominent feature of placentation in the rat (Bridgman, 1949; Correia-da Silva et al., 1999; Ain et al. 2003; Wiemers et al., 2003a; Caluwaerts et al., 2005; Zybina and Zybina, 2005; Vercruysse et al., 2006). Rat invasive trophoblast cells have been characterized by their epithelial nature (expression of cytokeratin), polyploidy, accumulation of glycogen, expression of a unique subset of prolactin (PRL) family cytokines, and their location at the uterine site of placental attachment. The invasive trophoblast cell population first appears at midgestation. It consists of trophoblast cells that penetrate and surround the uterine vasculature present within the developing chorioallantoic placenta. As gestation advances, invasive trophoblast cells exit a region of the chorioallantoic placenta referred to as the junctional zone and enter the uterine mesometrial compartment (site of blood vessel entry into the uterus). Two terms have been used to describe invasive trophoblast cells: endovascular and interstitial (Kaufmann et al., 2003). Endovascular trophoblast cells replace the endothelium and interstitial trophoblast cells are situated between the vasculature. In the rat, invasive trophoblast cells are targeted to a triangular shaped area rich in blood vessels, which is located between the mesometrial decidua and the surface of the uterus, and termed the metrial gland (Selye and McKeown, 1935; Peel, 1989; Ain et al., 2003; Ain and Soares, 2004). In the mouse, intrauterine trophoblast cell invasion is shallow and limited to the mesometrial decidua (Adamson et al., 2002; Ain et al., 2003; Coan et al., 2006).
An assortment of growth factors, proteases, and other modulators has been implicated in the regulation of trophoblast invasion (Lala and Chakraborty, 2003; James et al., 2006a). Of particular interest is a putative role for oxygen in the regulation of the invasive trophoblast lineage. Oxygen tension has an important role in regulating placentation (Genbacev et al., 1996, 1997; Caniggia et al., 2000; Burton and Jaunaiux, 2001; Fryer and Simon, 2006). During early gestation, embryonic development proceeds in an intrauterine environment characterized by low oxygen tension (Rodesch et al., 1992; Zamudio, 2003). Signaling pathways activated by low oxygen trigger morphogenetic events at the placentation site. Disruptions in key regulatory genes controlling responses to hypoxia result in failures in placentation and prenatal lethality (Gnarra et al., 1997; Abbott and Bukalew, 2000; Adelman et al., 2000; Cowden Dahl et al., 2005; Maltepe et al., 2005; Takeda et al., 2006). The impact of oxygen tension on the invasive trophoblast lineages has not been resolved (James et al., 2006a). Some experimental paradigms indicate that low oxygen tension inhibits trophoblast invasion (Genbacev et al., 1996, 1997; Caniggia et al., 2000; Kilburn et al., 2000; Crocker et al., 2005; James et al., 2006b; Lash et al., 2006), whereas others show a stimulatory action of hypoxia on trophoblast invasion or development of the invasive trophoblast lineage (Zhou et al., 1993; Graham et al., 2000; Kadyrov et al., 2003; Hayashi et al., 2005; Robins et al., 2007). The differing results are probably the consequence of the varied experimental designs employed. Many of the experiments were performed in vitro utilizing cell lines, isolated primary trophoblast cells, or trophoblast explants, and various culture conditions. Others were performed in vivo during different stages of gestation, using diverse animal species and types of manipulations or disease states, with variable effects on the duration and magnitude of oxygen tensions.
In this report, we investigate the impact of hypoxia in an in vivo context on placentation using the rat as an experimental model system. We show that exposure of pregnant rats to a hypoxic environment provides a novel model system for exploring pregnancy-dependent adaptations. Maternal hypoxia activates changes in the uterine mesometrial vasculature, which include increases in vascularity and increased endovascular trophoblast cell invasion.
MATERIALS AND METHODS
Animal and tissue preparation
Holtzman Sprague Dawley (HSD) rats were purchased from Harlan Sprague Dawley Inc. (Indianapolis, IN). A colony of transgenic rats expressing the enhanced green fluorescence protein (EGFP) driven by a chicken β-actin promoter (chβA-EGFP; Ikawa et al., 1998; Hasuwa et al., 2002) was established. The animals were housed in an environmentally controlled facility, with lights on from 0600−2000 h, and were allowed free access to food and water. Virgin female HSD rats of 8−10 weeks were cohabited with male HSD or chβA-EGFP rats. Presence of sperm in the vaginal lavage was designated as day 0.5 of pregnancy. Depending on the experimental design animals were sacrificed on days 10.5, 11.5, 12.5, 13.5 or 18.5 of gestation. Placentation sites, including uterus, metrial gland, and placental tissues, were dissected from pregnant animals and frozen in dry ice cooled heptane and stored at −80°C until used for immunohistochemical and in situ hybridization analyses. Tissues were also snap-frozen in liquid nitrogen for RNA analyses and stored at −80°C until used. Protocols for these procedures have been described (Ain et al., 2006). The procedures for handling and experimentation with rodents were approved by the University of Kansas Medical Center Animal Care and Use Committee.
In vivo maternal hypoxia
Pregnant rats were placed in hypobaric chambers on the designated day of pregnancy. Rats were exposed to conditions, in which air is circulated at a barometric pressure of approximately 420 Torr, resulting in an inspired PO2 of approximately 78 Torr, equivalent to breathing 11% oxygen at sea level (Ho-Chen et al., 2006). The chambers were opened daily to replenish food and water (15−20 min). Windows of sensitivity to maternal hypoxia were determined by varying the duration of exposure to hypobaric hypoxia between days 6.5 and 13.5 of gestation. Each treatment group included a minimum of five animals. Pair-fed control pregnant rats were exposed to ambient conditions (barometric pressure of ∼760 Torr and inspired PO2 of ∼149 Torr; equivalent to breathing 21% oxygen at sea level).
Histological and immunohistochemical analyses
Histological analyses were performed on 10-μm tissue sections prepared with the aid of a cryostat and stained with hematoxylin and eosin.
Immunohistochemical analyses were used to identify trophoblast cells, natural killer (NK) cells, smooth muscle cells, endothelial cells, compartments of the placentation site, and cells expressing EGFP. Analyses were performed with the aid of Histostain-AEC-plus kits (Zymed Laboratories, San Francisco, CA) according to the manufacturer's instructions unless stated otherwise. All histological and immunostained tissue sections and fluorescence images of cryosections from chβA-EGFP positive tissues were examined and captured using a Leica MZFLIII stereomicroscope (Leica Microsystems GmbH, Welzlar, Germany) or a Nikon Eclipse 55i microscope (Nikon Instruments Inc., Melville, NY); both equipped with Leica CCD cameras (Leica).
Trophoblast cells
Rat invasive trophoblast cells were detected using a mouse monoclonal anti-pan cytokeratin antibody (Sigma-Aldrich, St. Louis, MO) at a dilution of 1:400, as previously described (Konno et al., 2007). Normal rat serum (10%; Sigma-Aldrich) was added during the secondary antibody incubation to decrease non-specific reactions.
NK cells
Rabbit polyclonal anti-rat perforin-1 antibodies (PRF1; Torrey Pines Biolabs, Houston, TX) were used to detect NK cells. The PRF1 antibodies were used at a concentration of 2.5 μg/ml.
Endothelial cells
Mouse monoclonal antibodies to platelet/endothelial cell adhesion molecule (PECAM; BD Pharmingen, Franklin Lakes, NJ) and CXCR4 (AbCAM, Cambridge, MA) were used to detect endothelial cells. The PECAM antibody was used at a dilution of 1:50 while the CXCR4 antibody was used at a dilution of 1:75. Cryosections were treated with 0.3% hydrogen peroxide for 45−60 min to decrease non-specific binding.
Smooth muscle cells
Smooth muscle cells were monitored with a mouse monoclonal anti-smooth muscle α-actin (α-actin-2, ACTA2) antibody (Sigma-Aldrich) and used at 1:400 dilution. Normal rat serum (3%; Sigma-Aldrich) was added during the secondary antibody incubation to decrease non-specific reactions.
Placental compartments
Regions of the placentation sites were determined by vimentin immunostaining. A mouse monoclonal antibody to vimentin (Sigma-Aldrich) was used at a dilution of 1:500.
EGFP
EGFP positive trophoblast cells were detected by their intrinsic fluorescence and by using a 1:100 dilution of rabbit anti-green fluorescence protein polyclonal antibody (Chemicon, Temecula, CA).
In situ hybridization
In situ hybridization was performed as described (Ain et al., 2003; Wiemers et al., 2003b). Ten μm cryosections were prepared and stored at −80°C until used. A plasmid containing a cDNA for PRL family 7, subfamily b, member 1 (Prl7b1; also known as PRL-like protein-N, Wiemers et al., 2003a) was used as a template to synthesize sense and antisense digoxigenin-labeled riboprobes according to the manufacturer's instructions (Roche Molecular Biochemicals, Indianapolis, IN). Tissue sections were air dried and fixed in ice-cold 4% paraformaldehyde in phosphate-buffered saline (PBS). Prehybridization, hybridization, and detection of alkaline phosphatase-conjugated anti-digoxigenin were performed as previously reported (Ain et al., 2003; Wiemers et al., 2003b).
Isolation and characterization of EGFP positive invasive trophoblast cells
Female rats were mated with chβA-EGFP transgenic male rats. Pregnant rats were sacrificed on day 18.5 of gestation. Metrial glands were dissected, washed in Hanks balanced salt solution (HBSS, pH 7.4; Cellgro, Mediatech Inc, Herndon,VA), minced into small pieces in Dispase Grade II (Roche Applied Science, Indianapolis, IN) containing 0.002% DNase 1 (Sigma-Aldrich), and incubated at 37°C with shaking for 1 h. Cells were recovered by centrifugation and washed in RPMI 1640 culture medium containing 20% fetal bovine serum, 1 mM sodium pyruvate, 50 μM β-mercaptoethanol, 100 μg/ml penicillin, 100 units/ml of streptomycin, and 20% fetal bovine serum (Sahgal et al., 2006). The EGFP positive cells were sorted in phosphate-buffered saline (PBS) based on fluorescence intensity (FITC fliter) with a BD FACSAria Cell-Sorting System (BD Biosciences, San Jose, CA).
Cytokeratin immunoreactivity
The identity of EGFP positive cell populations were determined from aliquots of cells centrifuged onto glass slides, fixed in 4% paraformaldehyde (Sigma-Aldrich) in PBS for 10 min, treated with Triton X-100 (Sigma-Aldrich) in PBS for 10 min, and immunostained for cytokeratin (Ain et al., 2003).
Ploidy
The DNA content of the isolated invasive trophoblast cells was estimated by flow cytometry following propidium iodide staining. EGFP positive invasive trophoblast cells or spleen cells were resuspended in PBS and fixed with 90% cold ethanol. Cells were pelleted by centrifugation and resuspended in propidium iodide (50 μg/ml in PBS). Cells were analyzed for propidium iodide fluorescence by flow cytometry using BD LSRII and FACS Diva (BD Biosciences).
RT-PCR
EGFP-positive cells were flow sorted into vials containing PicoPure™ RNA extraction buffer (Picopure™ RNA Isolation Kit, Arcturus, Molecular Devices Corporation, CA) and total RNA isolated and treated with DNase 1 (Qiagen Inc., Valencia, CA). Total RNA was also isolated from gestation day 18.5 labryrinth zone, junctional zone, and metrial gland tissues using TRIzol (Invitrogen, Carlsbad, CA) and treated with DNase 1 (Invitrogen). Expression of trophoblast lineage-specific markers was used to characterize the EGFP-positive cells. Prl7b1, achaete-scute complex homolog-like 2 (Ascl2), and glial cell missing 1 (Gcm1) were used as markers for invasive trophoblast cells, spongiotrophoblast, and syncytial trophoblast cells, respectively. Expression of 18sRNA was used as a control for RNA integrity and loading. RT-PCR was conducted using SuperScript III One-Step RT-PCR System with Platinum Taq (Invitrogen) and the respective gene specific primers (Suppl. Table 1) according to the manufacturer's instructions, with some minor modifications (annealing temperatures: Prl7b1, 55°C; Ascl2 and Gcm1, 60°C).
Morphological measurements and statistical analysis
Morphological measurements of the depth of trophoblast invasion, the sizes of placental compartments, and blood vessel and uterine mesometrial compartment cross-sectional areas were performed with National Institutes of Health Image J software.
Definitions of each compartment within the rat placentation site (uterine mesometrial compartment, metrial gland, mesometrial deciduum, junctional zone, and labyrinth zone) have been described (Ain et al., 2006). The uterine mesometrial compartment is the region located between the trophoblast giant cell layer of the chorioallantoic placenta and the outer surface of the uterus. This region includes the uterine mesometrial deciduum (located between the trophoblast giant cell layer of the chorioallantoic placenta and the inner myometrial layer) and the metrial gland. The latter structure is situated between the inner myometrial layer and the outer surface of the uterus. The chorioallantoic placenta consists of the junctional zone and the labyrinth zone. The junctional zone comprises an area bordered by the uterine mesometrial deciduum and the labyrinth zone. It contains trophoblast giant cells, spongiotrophoblast cells, and glycogen cells. The labyrinth zone is defined as the region of the chorioallantoic placenta vascularized by the allantois. This compartment contains syncytial trophoblast, cytotrophoblast, trophoblast giant cells and fetal mesenchyme and its associated vasculature.
An estimate of the uterine mesometrial vasculature was determined from the ratio of blood vessel cross-sectional area to uterine mesometrial compartment cross-sectional area (BV:UMC) The depth of intrauterine trophoblast cell invasion was used to determine an invasion index (distance of endovascular cytokeratin positive cell location relative to the trophoblast giant cell layer of the chorioallantoic placenta ÷ total distance from the trophoblast giant cell layer to the outer mesometrial surface of the uterus). The thickness of the junctional zone was estimated from cross-sectional area measurements of vimentin stained placentation sites. Measurements were expressed as the ratio of junctional zone to labyrinth zone cross-sectional areas. Uterine mesometrial vasculature, depth of intrauterine trophoblast cell invasion, and chorioallantoic zone measurements were all made from a histological plane at the center of each placentation site perpendicular to the flat fetal surface of the placenta. Sample sizes for the analyses were at least five placentation sites from at least five different animals per treatment group. Statistical comparisons between two means were determined with Student's t-test. Comparisons among multiple means were evaluated with analysis of variance. The source of variation from significant F-ratios was determined with Bonferroni's Multiple Comparison test (Heiberger and Holland, 2004).
RESULTS
In these experiments, we have investigated the effects of maternal hypoxia on the placentation site. Maternal hypoxia activated changes in the uterine mesometrial vasculature.
Uterine vascular responses to maternal hypoxia
Exposure of rats to an equivalent of 11% oxygen from gestation day 6.5 to day 13.5 stimulated marked structural changes in the uterine mesometrial vasculature when inspected on gestation day 13.5 (Figs. 1 and 2). Among the structural changes in the uterine mesometrial blood vessels was an expansion of the surface area associated with the uterine vasculature and a modification of the cells lining some of the centrally located mesometrial blood vessels. The increase in vascular surface area was best demonstrated by ACTA2 immunostaining of vascular smooth muscle cells (Fig. 1). The area occupied by smooth muscle associated vessels within the uterine mesometrial compartment significantly increased following exposure to hypoxia (P<0.0006). NK cells have been proposed as potential regulators of the uterine mesometrial vasculature. Consequently, we examined the distribution of PRF1 positive NK cells in control and hypoxia exposed tissues. No apparent hypoxia-induced differences in NK cell distributions were evident (Fig. 1). Virtually all uterine mesometrial blood vessels in pair-fed or ad libitum fed (not shown) controls are lined by endothelial cells (Fig. 2, panels A, C, E, G, and I). In contrast, some centrally located mesometrial vasculature was lined with cells possessing an unusual cuboidal shape, and which did not express PECAM or CXCR4 (Fig. 2, panels B, D, F, H, J, and K). The cuboidal nature of the cells lining the vessels was different from the flattened appearance of cytokeratin immunopositive endovascular invasive trophoblast cells lining gestation day 18.5 uterine mesometrial blood vessels (Fig. 2, panels J and K versus panel L).
Fig. 1. The impact of maternal hypoxia on uterine mesometrial vascularity and natural killer (NK) cell distribution.

Pregnant rats were exposed to hypoxia (equivalent to 11% oxygen) from gestation day 6.5 to day 13.5, panels B and E and sacrificed on day 13.5. Gestationally matched pair-fed rats exposed to ambient conditions were used as controls (panels A and D). Placentation sites were sectioned and stained for ACTA2, identifying smooth muscle-associated blood vessels in the uterine mesometrial compartment (panels A and B) or for perforin (PRF1), a marker of NK cells (panels D and E). Chromogen: AEC; counterstain: hematoxylin; scale bars = 0.5 mm. Panel C, quantification of the ratio of blood vessel cross-sectional area to uterine mesometrial compartment cross-sectional area (BV:UMC) in pair-fed normoxic controls (n=7) and hypoxia exposed (n=7) placentation sites. Values are means ± the standard error of each mean. Please note that hypoxia exposure increased the BV:UMC cross-sectional area (asterisk; P<0.0006). Abbreviations: PF-N, pair-fed normoxia; HYP, maternal hypoxia.
Fig. 2. The impact of maternal hypoxia on the organization of the uterine mesometrial vasculature.

Pregnant rats were placed into hypobaric chambers, in which air was circulated at a barometric pressure of 420 Torr, resulting in an inspired PO2 of approximately 78 Torr, equivalent to breathing 11% oxygen at sea level. Hypoxia exposure was initiated on day 6.5 and continued to day 13.5 of gestation. Immunolocalizations of PECAM (panels A, B, E, and F) and CXCR4 (panels C, D, G, and H) were performed on cryosections of gestation day 13.5 placentation sites exposed to pair-fed normoxia (panels A, C, E, and G) or to maternal hypoxia (panels B, D, F, and H). Panels E-H are high magnifications of areas highlighted in boxes shown in panels A-D, respectively. Please note that the vessels highlighted in panels F and H (maternal hypoxia exposed) do not stain positively for PECAM or CXCR4, respectively. Panels I-K show blood vessels from gestation day 13.5 metrial glands of pair-fed normoxia (panel I) or maternal hypoxia-exposed (panels J and K) rats stained with hematoxylin and eosin. The blood vessel shown in panel I is lined by endothelial cells. The blood vessels shown in panel J are lined by endothelial cells (black arrows) or in part by endothelial cells and in part by a larger cuboidal cell population (black arrowheads). Panel K shows a blood vessel lined by a larger cuboidal cell population (black arrowheads). Panel L shows a blood vessel from an ad libitum-fed gestation day 18.5 metrial gland lined with endovascular trophoblast cells, which were immunostained for cytokeratin. Panels A-H and L: chromogen, 3-amino-9-ethylcarbazole (AEC); counterstain, hematoxylin. Scale bars: panels A-D = 0.5 mm, panels E-H = 0.25 mm, and panels I-L = 40 μm.
Maternal hypoxia activates epithelialization of the uterine mesometrial vasculature
We hypothesized that the cuboidal cells lining the uterine mesometrial vasculature in the hypoxia exposed pregnant rats could be of trophoblast origin. In order to test the hypothesis, we utilized two established markers of the invasive trophoblast cell lineage: cytokeratin and Prl7b1 (Ain et al., 2003; Wiemers et al., 2003a). The cuboidal cells lining the hypoxia-exposed uterine mesometrial blood vessels were cytokeratin immunopositive (Fig. 3) and expressed Prl7b1 mRNA (Fig. 4) and appeared to have continuity with trophoblast cells lining the central placental artery within the junctional zone of the chorioallantoic placenta (Fig. 3E, Fig. 4B). In pair-fed normoxic controls, cytokeratin and Prl7b1 positive endovascular cells were confined to the uterine decidua; whereas, this same cell population had a more distal location relative to the chorioallantoic placenta following hypoxia exposure (Fig. 3J, P<0.0007) and in some cases nearly reached the mesometrial surface of uterus (Fig. 3E). The results were consistent with a hypoxia-induced epithelialization of the uterine mesometrial vasculature that could be of trophoblast origin.
Fig. 3. Hypoxia-activated epithelialization of the uterine mesometrial vasculature.

Maternal hypoxia exposure (equivalent to 11% oxygen) was initiated on gestation day 6.5 and continued to day 13.5 when animals were sacrificed. Gestationally matched pair-fed rats exposed to ambient conditions were used as controls. The epithelial identity of cells in placentation sites was determined by immunostaining for cytokeratin. Panels A, D, and G show representative cytokeratin-immunostained sections from placentation sites of three different pair-fed normoxia control rats. Panels B, E, and H show representative cytokeratin-immunostained sections from placentation sites of three different maternal hypoxia exposed rats. Panels C, F, and I are high magnifications of areas highlighted in the boxes shown in panels B, E, and H, respectively. Chromogen: AEC; counterstain: hematoxylin; scale bars: panels A, B, D, E, G, and H = 0.5 mm; panels C, F, and I = 0.25 mm. Panel J, quantification of the depth of cytokeratin positive cell penetration into the uterine mesometrial vasculature. Values are means ± the standard error of each mean. Invasion index = Distance of endovascular cytokeratin positive cell location relative to the trophoblast giant cell layer of the chorioallantoic placenta ÷ Total distance from the trophoblast giant cell layer to the mesometrial surface of the uterus. The asterisk indicates a significant difference between pair-fed normoxia controls (white bar, n=5) and maternal hypoxia exposed (shaded bar, n=5) placentation sites, P<0.0007. Abbreviations: PF-N, pair-fed normoxia; HYP, maternal hypoxia.
Fig. 4. The epithelial lining of hypoxia-activated uterine mesometrial vasculature expresses a marker of the invasive trophoblast cell lineage, Prl7b1.
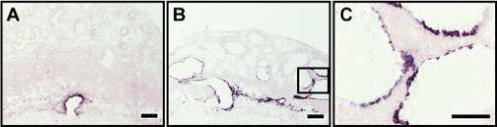
Maternal hypoxia exposure (equivalent to 11% oxygen) was initiated on gestation day 6.5 and continued to day 13.5 when animals were sacrificed. Gestationally matched pair-fed rats exposed to ambient conditions were used as controls. Prl7b1, a marker of the invasive trophoblast lineage, mRNA was localized by in situ hybridization. Panel A, shows representative localization of Prl7b1 in placentation sites from pair-fed normoxia controls. Panel B, shows representative localization of Prl7b1 in placentation sites of maternal hypoxia exposed rats. Panel C, is a high magnification of the area highlighted in the box shown in panel B. Please note the cells lining mesometrial blood vessels express a marker of the invasive trophoblast cell lineage. Chromogen: NBT-BCIP; counterstain: nuclear phospho red. Scale bar: panels A and B = 0.5 mm; panel C = 0.25 mm.
Maternal hypoxia activated endovascular epithelialization is derived from extraembryonic tissues
In order to further investigate the origin of the epithelial cells lining the uterine mesometrial vasculature in hypoxia exposed animals, we utilized a chβA-EGFP transgenic rat model. The assay was based on the exit of EGFP genetically marked trophoblast cells from the chorioallantoic placenta into the uterine mesometrial compartment of a wild-type female rat mated to a chβA-EGFP transgenic male rat (Fig. 5A). EGFP expression within the uterine mesometrial compartment on gestation day 18.5 exhibited a similar distribution to the distribution of cytokeratin immunopositive cells (Fig. 5B). Furthermore, EGFP fluorescence was an effective means of monitoring the gestational time course of trophoblast cell invasion (Fig. 5C). Appearance of the invasive trophoblast lineage within the uterine mesometrial compartment was modest until gestation day 15.5 and then subsequently expanded throughout the metrial gland by term (Fig. 5C). EGFP genetically marked invasive trophoblast cells could be isolated by flow cytometry and cell sorting. The EGFP positive cells were primarily diploid with the presence of some polyploid cells, and expressed Prl7b1 (invasive trophoblast marker) but not Ascl2 (spongiotrophoblast marker) or Gcm1 (syncytial trophoblast marker) (Fig. 6). Since the chβA-EGFP transgenic rat model system was an effective tool for monitoring the invasive trophoblast lineage, we next investigated EGFP marked invasive trophoblast cells from a wild-type female rat mated to a chβA-EGFP transgenic male and then exposed to hypoxia (gestation day 6.5 to day 13.5) or control conditions. Placentation sites were examined on gestation day 13.5. EGFP fluorescently marked invasive trophoblast cells were distributed within the uterine mesometrial vasculature following hypoxia exposure (Fig. 5D, panel d2), in locations similar to that observed for cytokeratin (Fig. 3) and Prl7b1 (Fig. 4). EGFP positive invasive trophoblast cells did not extend beyond the decidua in the pair-fed normoxic controls (Fig. 5D, panel d1).
Fig. 5. Cells that line the uterine vasculature are of extraembryonic origin.

Panel A, Overview of the chβA-EGFP transgenic model for assessing the trophoblast cell lineage. EGFP expression was monitored at the placentation site in wild-type female rats mated to homozygous chβA-EGFP transgenic male rats. Panel B, Validation of EGFP and cytokeratin expression profiles at the gestation day 18.5 placentation sites of wild-type female rats mated to homozygous chβA-EGFP transgenic male rats. Image b1, EGFP fluorescence; Image b2, immunostaining with anti-GFP; Image b3, immunostaining with anti-cytokeratin. Please note the robust EGFP fluorescence throughout the uterine mesometrial compartment, which has a similar distribution to EGFP immunoreactivity, and cytokeratin immunoreactivity. Panel C, Gestational profile of EGFP expression in the placentation sites of wild-type female rats mated to homozygous chβA-EGFP transgenic male rats. Panel D, Effects of maternal hypoxia on of EGFP fluorescence at the placentation site. Wild-type female rats were mated to homozygous chβA-EGFP transgenic male rats. Maternal hypoxia exposure (equivalent to 11% oxygen) was initiated on gestation day 6.5 and continued to day 13.5 when animals were sacrificed (image d2). Gestationally matched pair-fed rats exposed to ambient conditions were used as controls (image d1). Please note that maternal hypoxia stimulated the invasion of extraembryonic-derived EGFP positive cells into the maternal uterine vasculature. Scale bar = 0.5 mm.
Fig. 6. Analysis and characterization of EGFP positive cells isolated from metrial glands of wild-type females mated to chβA-EGFP transgenic males.
Panel A, Fluorescence profile of all cells isolated from the metrial gland. Panel B, Fluorescence profile of an aliquot of GFP negative cells; Panel C, Fluorescence profile of an aliquot of GFP positive cells; Panel D, cytokeratin immunostaining of GFP positive cells. Panel E, RT-PCR analysis of Prl7b1, Ascl2, Gcm1, and 18S ribosomal RNA in EGFP positive cells. Abbreviations; MG, metrial gland; LZ, labryrinth zone; JZ, junctional zone; IT, isolated invasive trophoblast. Panel F, Ploidy analysis of EGFP positive invasive trophoblast cells. Panel f-1, total metrial gland cells sorted by fluorescence; panel f-2, spleen cells stained with propidium iodide and analyzed for DNA content; panel f-3, EGFP positive invasive trophoblast cells stained with propidium iodide and analyzed for DNA content.
In summary, the above experiments demonstrate that maternal hypoxia activates endovascular trophoblast cell invasion.
Maternal hypoxia and organization of the chorioallantoic placenta
The invasive trophoblast lineage arises from the junctional zone region of the chorioallantoic placenta (Simmons and Cross, 2005). Consequently, we next determined whether maternal hypoxia altered the organization of the chorioallantoic placenta and affected junctional zone development. Pregnant rats were exposed to hypoxia (gestation day 6.5 to day 13.5) or control conditions. Animals were sacrificed on gestation day 13.5 and placentation sites prepared for histological analysis. Vimentin immunostaining was used to distinguish uterine mesometrial (positive immunostaining), junctional zone (no immunostaining), and labyrinth zone (positive immunostaining) compartments within the placentation site (Roby and Soares, 1993). The total cross-sectional area of the chorioallantoic placenta and its component parts (junctional and labyrinth zones) were all significantly stimulated by maternal hypoxia (Fig. 7, Suppl. Fig. 1). The junctional and labyrinth zone responses to maternal hypoxia were not equivalent, resulting in an in an increased ratio of junctional zone to labyrinth zone cross-sectional areas (Fig. 7, P<0.04). The results suggest that the influence of maternal hypoxia on the trophoblast lineage includes effects on development of the junctional zone, a repository of invasive trophoblast lineage precursors.
Fig. 7. Effect of maternal hypoxia on the organization of the gestation day 13.5 chorioallantoic placenta.

Maternal hypoxia exposure (equivalent to 11% oxygen) was initiated on gestation day 6.5 and continued to day 13.5 when animals were sacrificed. Gestationally matched pair-fed rats exposed to ambient conditions were used as controls. Vimentin, a marker of mesenchymal cells, was localized within the placentation site by immunocytochemistry. Vimentin immunostaining permits the localization of the labyrinth zone (positive immunostaining), junctional zone (no immunostaining), and uterine mesometrial compartment (positive immunostaining). Panel A, shows representative localization of vimentin in placentation sites from pair-fed normoxia controls. Panel B, shows representative localization of vimentin in placentation sites of maternal hypoxia exposed rats. Chromogen: AEC; counterstain: hematoxylin, scale bar = 1 mm. The dashed black lines on Panels A and B demarcate the junctional zone and underlying labyrinth zone. Panel C, shows the ratio of cross-sectional areas for junctional zone versus labyrinth zone from pair-fed normoxic placentation sites (white bar, n=12) and hypoxia exposed (shaded bar, n=10) placentation sites. Values are means ± the standard error of each mean. Please note the relative enlargement of the JZ from hypoxia exposed placentation sites (asterisk; P<0.004). Abbreviations: PF-N, pair-fed normoxia; HYP, maternal hypoxia; JZ, junctional zone; LZ, labyrinth zone.
Onset of endovascular trophoblast invasion following maternal hypoxia
In the preceding experiments, pregnant rats were exposed to hypoxia for seven days (gestation day 6.5 to day 13.5) prior to examination of the placentation site. In the next series of experiments, we sought to determine the ontogeny of hypoxia-induced trophoblast invasion into the metrial gland. Pregnant rats were exposed to hypoxic or control conditions beginning at gestation day 6.5 for different durations (until days 10.5, 11.5, 12.5, or 13.5 of gestation). Intrauterine trophoblast invasion was monitored by cytokeratin immunostaining. Significant hypoxia-induced increases in trophoblast invasion were first observed at gestation day 13.5 (Fig. 8 panels G, H, and I, P<0.0001). Maternal hypoxia-activated trophoblast invasion into the metrial gland was not initiated until after gestation day 12.5.
Fig. 8. Determination of the onset of trophoblast cell invasion following hypoxia-activation.

Maternal hypoxia exposure (equivalent to 11% oxygen) was initiated on gestation day 6.5 and continued to days 10.5, 11.5, 12.5, or 13.5 of gestation when animals were sacrificed. Gestationally matched pair-fed rats exposed to ambient conditions were used as controls. Invasive trophoblast cells were monitored by cytokeratin immunostaining. Panels A-H, show representative cytokeratin-immunostained sections from placentation sites from gestation day 10.5 (panels A and B), day 11.5 (panels C and D), day 12.5 (panels E and F), and day 13.5 (panels G and H). Panels A, C, E, and G show representative cytokeratin-immunostained sections from placentation sites of pair-fed controls. Panels B, D, F, and H show representative cytokeratin-immunostained sections from hypoxia exposed placentation sites. Chromogen: AEC; counterstain: hematoxylin; scale bar = 0.5 mm. Panel I, quantification of the depth of cytokeratin positive cell penetration into the uterine mesometrial vasculature. Values are means ± the standard error of each mean (n=5 for each group). The asterisk indicates a significant difference between pair-fed normoxia controls and maternal hypoxia exposed placentation sites, P<0.0001. Abbreviations: PF-N, pair-fed normoxia; HYP, maternal hypoxia; Invasion Index, see Materials and Methods or legend to Fig. 3. Please note that hypoxia stimulated trophoblast invasion into the uterine mesometrial compartment is initiated between days 12.5 and 13.5 of gestation.
Initiation of responsiveness to maternal hypoxia
Next, we determined the gestational time-frame associated with the initiation of trophoblast cell responsiveness to maternal hypoxia. We used trophoblast invasion as a measure of responsiveness to maternal hypoxia. Maternal hypoxia was initiated on gestation days 6.5, 8.5, 10.5, or 11.5 and continued until day 13.5 when animals were sacrificed. Gestationally matched pair-fed rats exposed to ambient conditions were used as controls. The invasive trophoblast lineage was monitored by cytokeratin immunostaining of gestation day 13.5 placentation sites. Activation of endovascular trophoblast invasion was observed following initiation of maternal hypoxia on gestation day 6.5 and day 8.5 (Fig. 9, panels A-F and M; day 6.5 to day 13.5, P<0.0001; day 8.5 to day 13.5, P<0.04) but not following initiation of hypoxia exposure on gestation day 10.5 or day 11.5 (Fig. 9, panels G-L and M). Based on the results generated from experiments presented in Figs. 8 and 9, it appeared that there may be a developmental window of placental sensitivity to maternal hypoxia that spanned a gestational time interval between day 6.5 and day 10.5 of gestation.
Fig. 9. Gestational ontogeny of hypoxia activation of the invasive endovascular trophoblast cell lineage.

Maternal hypoxia exposure (equivalent to 11% oxygen) was initiated on gestation day 6.5 (panels B and C), day 8.5 (panels E and F), day 10.5 (panels H and I), or day 11.5 (panels K and L) and continued until day 13.5 of gestation when animals were sacrificed. Gestationally matched pair-fed rats exposed to ambient conditions were used as controls (panels A, D, G, and J). Invasive trophoblast cells were monitored by cytokeratin immunostaining. Panels C, F, I, and L are high magnifications of areas highlighted in the boxes shown in panels B, E, H, and K, respectively. Chromogen: AEC; counterstain: hematoxylin; scale bars: panels A, B, D, E, G, H, J and K = 0.5 mm; panels C, F, I, and L = 0.25 mm. Panel M, quantification of the depth of cytokeratin positive cell penetration into the uterine mesometrial vasculature. Values are means ± the standard error of each mean (n=5 for all groups except day 8.5 to day 13.5, normoxia, n=7 and day 8.5 to day 13.5, hypoxia, n=6). The asterisks indicate a significant difference between pair-fed normoxia controls and maternal hypoxia exposed placentation sites (day 6.5 to day 13.5, P<0.0001; day 8.5 to day 13.5, P<0.04). Abbreviations: PF-N, pair-fed normoxia; HYP, maternal hypoxia; Invasion Index, see Materials and Methods or legend to Fig. 3. Please note that exposure to hypoxia from gestation day 6.5 to day 10.5 was critical to activation of endovascular trophoblast invasion.
Identification of a developmental window of sensitivity to hypoxia-induced activation of the invasive trophoblast cell lineage
In order to further define a putative developmental window for activation of endovascular trophoblast lineage in response to hypoxia, pregnant rats were exposed to hypoxia for different time intervals (gestation day 6.5 to day 8.5; day 6.5 to day 9.5; day 7.5 to day 9.5; day 8.5 to day 9.5) and then returned to ambient conditions until gestation day 13.5 when animals were sacrificed. Gestationally matched pair-fed rats exposed to ambient conditions were used as controls. Cytokeratin immunostaining was used to assess trophoblast invasion in gestation day 13.5 placentation sites. Hypoxia exposure durations including gestation day 8.5 to day 9.5 significantly increased the depth of intrauterine endovascular trophoblast invasion beyond that observed in pair-fed normoxia control placentation sites (Fig. 10, day 6.5 to day 9.5, P<0.0003; day 7.5 to day 9.5, P<0.006; day 8.5 to day 9.5, P<0.003). Thus, an appropriately timed hypoxia exposure of 24 h is sufficient to activate the endovascular trophoblast cell lineage. Existence of a developmental window for hypoxia activation of endovascular trophoblast cells prompted us to investigate whether a similar developmental window exists for expansion of the surface area associated with the uterine mesometrial vasculature (see Fig. 1, panels A-C). Three day exposures to hypoxia between day 6.5 to day 9.5 or day 10.5 to day 13.5 failed to increase surface area associated with the uterine mesometrial vasculature (Fig. 11, panels A-D and G). Expansion of the uterine mesometrial vasculature required hypoxia exposure from gestation day 6.5 to day 13.5 (Fig. 11, panels E-G, P<0.005). Therefore hypoxia-activated endovascular trophoblast cell invasion and hypoxia-activated expansion of uterine mesometrial blood vessel surface area are differentially regulated.
Fig. 10. Identification of a developmental window of sensitivity to hypoxia activation of the invasive endovascular trophoblast cell lineage.

Pregnant rats were exposed to hypoxia (equivalent to 11% oxygen) for defined intervals during gestation (day 6.5 to day 8.5, panels B and C; day 6.5 to day 9.5, panels E and F; day 7.5 to day 9.5, panels H and I; day 8.5 to day 9.5, panels K and L) and then returned to normoxia until gestation day 13.5 when all animals were sacrificed. Gestationally matched pair-fed rats exposed to ambient conditions were used as controls (panels A, D, G, and J). Invasive trophoblast cells were monitored by cytokeratin immunostaining. Panels C, F, I, and L are high magnifications of areas highlighted in the boxes shown in panels B, E, H, and K, respectively. Chromogen: AEC; counterstain: hematoxylin; scale bars: panels A, B, D, E, G, H, J and K = 0.5 mm; panels C, F, I, and L = 0.25 mm. Panel M, quantification of the depth of cytokeratin positive cell penetration into the uterine mesometrial vasculature. Values are means ± the standard error of each mean (n=5 for each group). Asterisks indicate a significant difference between pair-fed normoxia controls and maternal hypoxia exposed placentation sites (day 6.5 to day 9.5, P<0.0003; day 7.5 to day 9.5, P<0.006; day 8.5 to day 9.5, P<0.003). Abbreviations: PF-N, pair-fed normoxia; HYP, maternal hypoxia; Invasion Index, see Materials and Methods or legend to Fig. 3. Please note that exposure to hypoxia from day 8.5 to day 9.5 of gestation is critical to activate the invasive trophoblast lineage.
Fig. 11. Impact of gestational windows of maternal hypoxia exposure on uterine mesometrial vascularity.

Pregnant rats were exposed to hypoxia (equivalent to 11% oxygen) for defined intervals during gestation (day 6.5 to day 9.5, panel B; day 10.5 to day 13.5, panel D; day 6.5 to day 13.5, panels F) and then returned to normoxia until day 13.5 of gestation when all animals were sacrificed. Gestationally matched pair-fed rats exposed to ambient conditions were used as controls (panels A, C, and E). Placentation sites were sectioned and stained for ACTA2, identifying smooth muscle-associated blood vessels in the uterine mesometrial compartment. Chromogen: AEC; counterstain: hematoxylin; scale bars = 0.5 mm. Panel G, quantification of the ratio of blood vessel cross-sectional area to uterine mesometrial compartment cross-sectional area (BV:UMC) in pair-fed normoxic controls and hypoxia exposed placentation sites. Values are means ± the standard error of each mean (n=5 per group). The asterisk indicates a significant increase in BV:UMC cross-sectional area following hypoxia exposure (day 6.5 to day 13.5, P<0.005). Abbreviations: PF-N, pair-fed normoxia; HYP, maternal hypoxia.
In summary, pregnant rats exposed to hypobaric hypoxia during critical phases of pregnancy activate precise adaptations involving uterine mesometrial vasculature and the invasive trophoblast cell lineage.
DISCUSSION
Reproductive adaptations to environmental challenges are a key to the success of a species. Pregnancy is a physiological state that is characterized by an active dialog between maternal and extraembryonic tissues. Conditions within the maternal uterus signal developmental instructions to the primordial placenta, which lead to the construction of a delivery route for nutrients to the embryo/fetus. In this report, we demonstrate that maternal hypoxia is an effective stimulus eliciting adaptations at the maternal-fetal interface. The adaptations include activation of the invasive endovascular trophoblast cell lineage and modifications of uterine blood vessels supplying the developing chorioallantoic placenta. We have observed similar results using hypobaric hypoxia (present study) or by creating a hypoxic environment by gas mixtures (data not shown).
At least two populations of invasive trophoblast cells can be identified in the rat: endovascular and interstitial (Ain et al., 2003; Caluwaerts et al., 2005; Vercruysse et al., 2006). Each subpopulation is identified by its location relative to the uterine spiral arteries (Pijnenborg et al., 2006). Endovascular invasive trophoblast cells replace the endothelium of the uterine spiral arteries, whereas interstitial invasive trophoblast cells surround the uterine spiral arteries. There is evidence for two separate routes of intrauterine trophoblast cell migration: 1) using the endothelium as a guidance system or 2) alternatively moving through the parenchyma of the mesometrial decidua and metrial gland. Invasive trophoblast cells arising from either migratory route may ultimately contribute to the endovascular and interstitial subpopulations (Pijnenborg et al., 2006). In the present study, maternal hypoxia activated an endovascular course of trophoblast cell migration and a restricted endovascular destination. These endovascular invasive trophoblast cells were cuboidal in shape and expressed cytokeratin and Prl7b1 (present study). In support of our observations, maternal anemia and chronic constriction of the lower aorta also appear to be triggers for increasing the depth of intrauterine trophoblast cell invasion (Zhou et al., 1993; Kadyrov et al., 2003). Decreased oxygen concentrations are likely common determining factors in stimulating the depth of trophoblast cell invasion. In contrast to our report in the rat, where hypoxia activated endovascular trophoblast invasion, the in vivo manipulations in primates led to enhanced interstitial trophoblast invasion. The observed differences in the present report with the rat versus those reported in primates (Zhou et al. 1993; Kadyrov et al. 2003) may reflect the nature of the stressors, the timing of exposure to the stressors, or to the species used in the analyses.
Activation of the invasive endovascular trophoblast lineage can be separated from initiation of trophoblast cell invasion. Exposure to maternal hypoxia from gestation day 8.5 to day 9.5 affected development of the invasive trophoblast cell lineage (present study). The timing of the activation coincides with a pivotal developmental stage in rat placentation. During this interval, the chorion and allantois fuse and populations of cells within the ectoplacental cone are allocated to each specialized trophoblast cell lineage of the junctional zone, including the invasive trophoblast cell lineage. The invasive trophoblast cell lineage is best recognized by its exit from the junctional zone of the chorioallantoic placenta (Ain et al. 2003; Caluwaerts et al., 2005; Vercruysse et al., 2006). Thus, there is a latent period between activation of the invasive trophoblast cell lineage and when the maternal hypoxia-activated invasive trophoblast cells physically move into the metrial gland. Prior to gestation day 13.5, trophoblast cell invasion is limited to endovascular trophoblast cells and is confined to the mesometrial deciduum. After gestation day 14.5, endovascular and interstitial invasive trophoblast can be identified within the metrial gland (Ain et al., 2003). Maternal hypoxia hastens the appearance of endovascular invasive trophoblast cells, which become readily detectable within the metrial gland by gestation day 13.5. Thus there is either a maturational process for the hypoxia-activated invasive trophoblast cells that must transpire or there is a barrier within the maternal uterine vasculature that must removed. Mechanisms underlying activation of the invasive trophoblast lineage and subsequent trophoblast cell invasion are yet to be determined.
Oxygen tension is an effective regulator of the trophoblast lineage and may be a candidate activator of endovascular invasive trophoblast cell differentiation or a factor initiating trophoblast cell invasion. Simon, Fisher, and their colleagues have shown that that low oxygen directs the differentiation of trophoblast stem cells towards a cellular phenotype associated with the junctional zone (Adelman et al., 2000; Cowden Dahl et al., 2005; Maltepe et al., 2005). This developmental process is dependent upon hypoxia inducible factor signaling pathways. Furthermore, in vitro exposure of primary human cytotrophoblast to low oxygen tension stimulates differentiation along the homologous extravillous trophoblast phenotype (Robins et al., 2007). Consistent with these findings, we observed that exposure to maternal hypoxia resulted in chorioallantoic placental development that favored the junctional zone (present study). Although oxygen tension may represent a mediator in redirecting placentation following in vivo exposure to hypoxia, such a conclusion is not possible and will need to await future analyses. The actions of maternal hypoxia may be direct and the target may be a trophoblast stem cell population or alternatively the effects on trophoblast cell lineage determination may be indirect and mediated by other cell types present at the maternal-placenta interface (endothelial cells, uterine stromal cells, decidual cells, NK cells, etc.), or the result of other physiological stimuli elicited by maternal hypoxia. A caveat to our experimental procedure for creating a hypoxic environment is that the chambers were opened daily for the purpose of replenishing food and water. The brief daily exposure to ambient conditions may have simulated an oxygen reperfusion state, which may have also contributed to the observed adaptive response. Burton and coworkers have provided experimental evidence that hypoxia-reoxygenation contributes to placental pathologies (Hung et al., 2001, 2002; Jauniaux et al. 2006).
The uterine mesometrial vasculature changes dramatically following exposure to maternal hypoxia. Vessels increase in diameter and the endothelium is replaced by trophoblast cells (present study). In contrast to the acute responsiveness of the invasive trophoblast lineage to maternal hypoxia, the increase in mesometrial vascular size required continuous hypoxia exposure from gestation day 6.5 to day 13.5. Replacement of the endothelium of uterine spiral arteries with invasive trophoblast cells is also observed in human placentation sites (Zhou et al., 1997; Damsky and Fisher, 1998; Pijnenborg et al., 2006). Mechanisms leading to the replacement of the endothelium by trophoblast cells may be mediated by vascular-endothelial cadherin and Fas ligand/Fas signaling (Ashton et al., 2005; Bulla et al., 2005) and involve trophoblast phagocytosis of endothelial cells (Chen et al., 2005). Invasive trophoblast cells also impact endothelial cells and the uterine vasculature in other ways, including effects on vascular permeability (Wang et al., 2004) and through their production of angiogenic factors (Zhou et al., 2002, 2003; Hemberger et al., 2003; Yuan et al., 2005; Red-Horse et al., 2005, 2006). Substituting endothelium with endovascular trophoblast cells has the potential to impact intravascular dynamics, including permeability and tone.
It is evident that there is plasticity in the process of placentation. Organization of the maternal-fetal interface is influenced by internal and external environmental challenges. Maternal and extraembryonic compensatory responses constitute an adaptive reflex that protects the pregnancy and allows fetal development to proceed for the duration of pregnancy. Failure of adaptation results in disease. In summary, we have identified a new tool for investigating the regulation of placentation, activation of the invasive trophoblast cell lineage, invasion of trophoblast cells, and modification of the uterine spiral arteries.
ACKNOWLEDGEMENTS
The authors thank Dr. Norberto C. Gonzalez for providing helpful advice and guidance in the use of hypobaric hypoxia as an experimental tool. The chβA-EGFP transgenic rats were provided by Dr. Masaru Okabe of Osaka University, Osaka, Japan. We also acknowledge the assistance of Dr. Joyce Slusser with the flow cytometry analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported by grants from the National Institutes of Health (HD020676 and HD048861) and the Hall Family Foundation.
Supplementary Material
REFERENCES
- Abbott BD, Buckalew AR. Placental defects in ARNT-knockout conceptus correlate with localized decreases in VEGF-R2, Ang-1, and Tie-2. Dev. Dyn. 2000;219:526–538. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1080>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev. Biol. 2002;250:358–373. doi: 10.1016/s0012-1606(02)90773-6. [DOI] [PubMed] [Google Scholar]
- Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000;14:3191–3203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ain R, Canham LN, Soares MJ. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev. Biol. 2003;260:176–190. doi: 10.1016/s0012-1606(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Ain R, Konno T, Canham LN, Soares MJ. Phenotypic analysis of the placenta in the rat. In: Soares MJ, Hunt JS, editors. Placenta and Trophoblast: Methods and Protocols. Vol. 1. Humana Press; Totowa, New Jersey: 2006. pp. 295–313. [Google Scholar]
- Ain R, Soares MJ. Is the metrial gland really a gland? J. Reprod. Immunol. 2004;61:129–131. doi: 10.1016/j.jri.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Ashton SV, Whitley GS, Dash PR, Wareing M, Crocker IP, Baker PN, Cartwright JE. Uterine spiral artery remodeling involves endothelial apoptosis induced by extravillous trophoblasts through Fas/FasL interactions. Arterioscler. Thromb. Vasc. Biol. 2005;25:102–108. doi: 10.1161/01.ATV.0000148547.70187.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman J. A morphological study of the development of the placenta of the rat. II. An histological and cytological study of the development of the chorioallantoic placenta of the white rat. J. Morphol. 1949;83:195–224. doi: 10.1002/jmor.1050830204. [DOI] [PubMed] [Google Scholar]
- Bulla R, Villa A, Bossi F, Cassetti A, Radillo O, Spessotto P, De Seta F, Guaschino S, Tedesco F. VE-cadherin is a critical molecule for trophoblastendothelial cell interaction in decidual spiral arteries. Exp. Cell Res. 2005;303:101–113. doi: 10.1016/j.yexcr.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jaunaiux E. Maternal vascularization of the human placenta: does the embryo develop in a hypoxic environment? Gynecol. Obstet. Fertil. 2001;29:503–508. doi: 10.1016/s1297-9589(01)00179-5. [DOI] [PubMed] [Google Scholar]
- Caluwaerts S, Vercruysse L, Luyten C, Pijnenborg R. Endovascular trophoblast invasion and associated structural changes in uterine spiral arteries of the pregnant rat. Placenta. 2005;26:574–584. doi: 10.1016/j.placenta.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, Post M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFβ3. J. Clin. Invest. 2000;105:577–587. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Stone PR, McCowan LM, Chamley LW. Interaction of Jar choriocarcinoma cells with endothelial cell monolayers. Placenta. 2005;26:617–625. doi: 10.1016/j.placenta.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Coan PM, Conroy N, Burton GJ, Ferguson-Smith AC. Origin and characteristics of glycogen cells in the developing murine placenta. Dev. Dyn. 2006;235:3280–3294. doi: 10.1002/dvdy.20981. [DOI] [PubMed] [Google Scholar]
- Correia-da-Silva G, Bell SC, Pringel JH, Teixeira N. Expression of mRNA encoding insulin-like growth factors I and II by uterine tissues and placenta during pregnancy in the rat. Mol. Reprod. Dev. 1999;53:294–305. doi: 10.1002/(SICI)1098-2795(199907)53:3<294::AID-MRD5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Cowden Dahl KD, Fryer BH, Mack FA, Compernolle V, Maltepe E, Adelman DM, Carmeliet P, Simon MC. Hypoxia-inducible factors 1α and 2α regulate trophoblast differentiation. Mol. Cell. Biol. 2005;25:10479–10491. doi: 10.1128/MCB.25.23.10479-10491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker IP, Wareing M, Ferris GR, Jones CJ, Cartwright J, Baker PN, Aplin JD. The effect of vascular origin, oxygen, and tumour necrosis factor alpha on trophoblast invasion of maternal arteries in vitro. J. Pathol. 2005;206:476–485. doi: 10.1002/path.1801. [DOI] [PubMed] [Google Scholar]
- Damsky CH, Fisher SJ. Trophoblast pseudo-vasculogenesis: faking it with endothelial adhesion receptors. Curr. Opin. Cell Biol. 1998;10:660–666. doi: 10.1016/s0955-0674(98)80043-4. [DOI] [PubMed] [Google Scholar]
- Davies J, Glasser SR. Histological and fine structural observations on the placenta of the rat. Acta Anat. (Basel) 1968;69:542–608. doi: 10.1159/000143100. [DOI] [PubMed] [Google Scholar]
- Enders AC, Welsh AO. Structural interactions of trophoblast and uterus during hemochorial placenta formation. J. Exp. Zool. 1993;266:578–587. doi: 10.1002/jez.1402660608. [DOI] [PubMed] [Google Scholar]
- Fryer BH, Simon MC. Hypoxia, HIF, and the placenta. Cell Cycle. 2006;5:495–498. doi: 10.4161/cc.5.5.2497. [DOI] [PubMed] [Google Scholar]
- Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J. Clin. Invest. 1996;97:540–550. doi: 10.1172/JCI118447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placenta. Placenta. 2002;23:3–19. doi: 10.1053/plac.2001.0738. [DOI] [PubMed] [Google Scholar]
- Gnarra JR, Ward JM, Porter FD, Wagner JR, Devor DE, Grinberg A, Emmert-Buck MR, Westphal H, Klausner RD, Linehan WM. Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc. Natl. Acad. Sci. USA. 1997;94:9102–9107. doi: 10.1073/pnas.94.17.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CH, Postovit LM, Park H, Canning MT, Fitzpatrick TE. Role of oxygen in the regulation of trophoblast gene expression and invasion. Placenta. 2000;21:443–450. doi: 10.1053/plac.2000.0543. [DOI] [PubMed] [Google Scholar]
- Hasuwa H, Kaseda K, Einarsdottir T, Okabe M. Small interfering RNA and gene silencing in transgenic mice and rats. FEBS Letters. 2002;532:227–230. doi: 10.1016/s0014-5793(02)03680-3. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Sakata M, Takeda T, Tahara M, Yamamoto T, Okamoto Y, Minekawa R, Isobe A, Ohmichi M, Tasaka K, Murata Y. Up-regulation of c-met protooncogene product expression through hypoxia-inducible factor-1alpha is involved in trophoblast invasion under low-oxygen tension. Endocrinology. 2005;146:4682–4689. doi: 10.1210/en.2005-0416. [DOI] [PubMed] [Google Scholar]
- Heiberger RM, Holland B. Springer Texts in Statistics. Springer; New York: 2004. Statistical Analysis and Data Display: An Intermediate Course with Examples in S-Plus, R, and SAS. [Google Scholar]
- Hemberger M, Nozaki T, Masutani M, Cross JC. Differential expression of angiogenic and vasodilatory factors by invasive trophoblast giant cells depending on depth of invasion. Dev. Dyn. 2003;227:185–191. doi: 10.1002/dvdy.10291. [DOI] [PubMed] [Google Scholar]
- Ho-Chen JK, Ain R, Alt A, Wood JG, Gonzalez NC, Soares MJ. Hypobaric-hypoxia as a tool to study pregnancy-dependent responses at the maternal-fetal interface. In: Soares MJ, Hunt JS, editors. Placenta and Trophoblast: Methods and Protocols. Vol. 2. Humana Press; Totowa, New Jersey: 2006. pp. 427–434. [DOI] [PubMed] [Google Scholar]
- Hung TH, Skepper JN, Burton GJ. In vitro ischemia-reperfusion injury in term human placenta as a model for oxidative stress in pathological pregnancies. Am. J. Pathol. 2001;159:1031–1043. doi: 10.1016/S0002-9440(10)61778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung TH, Skepper JN, Charnock-Jones DS, Burton GJ. Hypoxia-reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ. Res. 2002;90:1274–1281. doi: 10.1161/01.res.0000024411.22110.aa. [DOI] [PubMed] [Google Scholar]
- Ikawa M, Yamada S, Nakanishi T, Okabe M. Green mice’ and their potential usage in biological research. FEBS Letters. 1998;430:83–87. doi: 10.1016/s0014-5793(98)00593-6. [DOI] [PubMed] [Google Scholar]
- James JL, Stone PR, Chamley LW. The regulation of trophoblast differentiation by oxygen in the first trimester of pregnancy. Hum. Reprod. Update. 2006a;12:137–144. doi: 10.1093/humupd/dmi043. [DOI] [PubMed] [Google Scholar]
- James JL, Stone PR, Chamley LW. The effects of oxygen concentration and gestational age on extravillous trophoblast outgrowth in a human first trimester explant model. Hum. Reprod. 2006b;21:2699–2705. doi: 10.1093/humrep/del212. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum. Reprod. Update. 2006;12:747–755. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadyrov M, Schmitz C, Black S, Kaufmann P, Huppertz B. Pre-eclampsia and maternal anaemia display reduced apoptosis and opposite invasive phenotypes of extravillous trophoblast. Placenta. 2003;24:540–548. doi: 10.1053/plac.2002.0946. [DOI] [PubMed] [Google Scholar]
- Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol. Reprod. 2003;69:1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- Kilburn BA, Wang J, Duniec-Dmuchkowski ZM, Leach RE, Romero R, Armant DR. Extracellular matrix composition and hypoxia regulate the expression of HLA-G and integrins in a human trophoblast cell line. Biol. Reprod. 2000;62:739–747. doi: 10.1095/biolreprod62.3.739. [DOI] [PubMed] [Google Scholar]
- Konno T, Rempel LA, Arroyo JA, Soares MJ. Pregnancy in the Brown Norway rat: a model for investigating the genetics of placentation. Biol. Reprod. 2007;76:709–718. doi: 10.1095/biolreprod.106.056481. [DOI] [PubMed] [Google Scholar]
- Lala PK, Chakraborty C. Factors regulating trophoblast migration and invasiveness: possible derangements contributing to pre-eclampsia and fetal injury. Placenta. 2003;24:575–587. doi: 10.1016/s0143-4004(03)00063-8. [DOI] [PubMed] [Google Scholar]
- Lash GE, Otun HA, Innes BA, Bulmer JN, Searle RF, Robson SC. Low oxygen concentrations inhibit trophoblast cell invasion from early gestation placental explants via alterations in levels of the urokinase plasminogen activator system. Biol. Reprod. 2006;74:403–409. doi: 10.1095/biolreprod.105.047332. [DOI] [PubMed] [Google Scholar]
- Maltepe E, Krampitz GW, Okazaki KM, Red-Horse K, Mak W, Simon MC, Fisher SJ. Hypoxia-inducible factor-dependent histone deacetylase activity determines stem cell fate in the placenta. Development. 2005;132:3393–3403. doi: 10.1242/dev.01923. [DOI] [PubMed] [Google Scholar]
- Peel S. Granulated metrial gland cells. Adv. Anat. Embryol. Cell Biol. 1989;115:1–112. doi: 10.1007/978-3-642-74170-8. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Red-Horse K, Rivera J, Schanz A, Zhou Y, Winn V, Kapidzic M, Maltepe E, Okazaki K, Kochman R, Vo KC, Giudice L, Erlebacher A, McCune JM, Stoddart CA, Fisher SJ. Cytotrophoblast induction of arterial apoptosis and lymphangiogenesis in an in vivo model of human placentation. J. Clin. Invest. 2006;116:2643–2652. doi: 10.1172/JCI27306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Red-Horse K, Kapidzic M, Zhou Y, Feng KT, Singh H, Fisher SJ. EPHB4 regulates chemokine-evoked trophoblast responses: a mechanism for incorporating the human placenta into the maternal circulation. Development. 2005;132:4097–4106. doi: 10.1242/dev.01971. [DOI] [PubMed] [Google Scholar]
- Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, Fisher SJ. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J. Clin. Invest. 2004;114:744–754. doi: 10.1172/JCI22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins JC, Heizer A, Hardiman A, Hubert M, Handwerger S. Oxygen tension directs the differentiation pathway of human cytotrophoblast cells. Placenta. 2007;28:1141–1146. doi: 10.1016/j.placenta.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Roby KF, Soares MJ. Trophoblast cell differentiation and organization: role of fetal and ovarian signals. Placenta. 1993;14:529–545. doi: 10.1016/s0143-4004(05)80206-1. [DOI] [PubMed] [Google Scholar]
- Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet. Gynecol. 1992;80:283–285. [PubMed] [Google Scholar]
- Sahgal N, Canham LN, Canham B, Soares MJ. Rcho-1 trophoblast cells: a model for studying trophoblast differentiation. In: Soares MJ, Hunt JS, editors. Placenta and Trophoblast: Methods and Protocols. Vol. 1. Humana Press; Totowa, New Jersey: 2006. pp. 159–178. [PubMed] [Google Scholar]
- Selye H, McKeown T. Studies on the physiology of the maternal placenta in the rat. Proc. R. Soc. Lond. Biol. 1935;119:1–31. [Google Scholar]
- Simmons DG, Cross JC. Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev. Biol. 2005;284:12–24. doi: 10.1016/j.ydbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Takeda K, Ho VC, Takeda H, Duan LJ, Nagy A, Fong GH. Placental but not heart defects are associated with elevated hypoxia-inducible factor alpha levels in mice lacking prolyl hydroxylase domain protein 2. Mol. Cell. Biol. 2006;26:8336–8346. doi: 10.1128/MCB.00425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercruysse L, Caluwaerts S, Luyten C, Pijnenborg R. Interstitial trophoblast invasion in the decidua and mesometrial triangle during the last third of pregnancy in the rat. Placenta. 2006;27:22–33. doi: 10.1016/j.placenta.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lewis DF, Gu Y, Zhang Y, Alexander JS, Granger DN. Placental trophoblast-derived factors diminish endothelial barrier function. J. Clin. Endocrinol. Metab. 2004;89:2421–2428. doi: 10.1210/jc.2003-031707. [DOI] [PubMed] [Google Scholar]
- Wiemers DO, Ain R, Ohboshi S, Soares MJ. Migratory trophoblast cells express a newly identified member of the prolactin gene family. J. Endocrinol. 2003a;179:335–346. doi: 10.1677/joe.0.1790335. [DOI] [PubMed] [Google Scholar]
- Wiemers DO, Shao L-J, Ain R, Dai G, Soares MJ. The mouse prolactin gene family locus. Endocrinology. 2003b;144:313–325. doi: 10.1210/en.2002-220724. [DOI] [PubMed] [Google Scholar]
- Yuan H-T, Haig D, Karumanchi SA. Angiogenic factors in the pathogenesis of preeclampsia. Curr. Topics Dev. Biol. 2005;71:297–312. doi: 10.1016/S0070-2153(05)71009-7. [DOI] [PubMed] [Google Scholar]
- Zamudio S. The placenta at high altitude. High Altitude Med. Biol. 2003;4:171–191. doi: 10.1089/152702903322022785. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Bellingard V, Feng KT, McMaster M, Fisher SJ. Human cytotrophoblasts promote endothelial survival and vascular remodeling through secretion of Ang2, PlGF, and VEGF-C. Dev. Biol. 2003;263:114–125. doi: 10.1016/s0012-1606(03)00449-4. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Chiu K, Brescia RJ, Combs CA, Katz MA, Kitzmiller JL, Heilbron DC, Fisher SJ. Increased depth of trophoblast invasion after chronic constriction of the lower aorta in rhesus monkeys. Am. J. Obstet. Gynecol. 1993;169:224–229. doi: 10.1016/0002-9378(93)90172-f. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J. Clin. Invest. 1997;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, Alitalo K, Damsky C, Fisher SJ. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am. J. Pathol. 2002;160:1405–1423. doi: 10.1016/S0002-9440(10)62567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zybina TG, Zybina EV. Cell reproduction and genome multiplication in the proliferative and invasive trophoblast cell populations of mammalian placenta. Cell Biol. Int. 2005;29:1071–1083. doi: 10.1016/j.cellbi.2005.10.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



