Abstract
The intensity of a noise-induced startle response can be reduced by the presentation of an otherwise neutral stimulus immediately before the noise (“prepulse inhibition” or PPI). This effect has been used to study the detection of gaps and other stimuli, but has been applied infrequently to complex stimuli or the ability to discriminate among multiple stimuli. To address both issues and explore the potential of PPI, rats were presented a series of 5 tasks, most contrasting a pair of speech sounds. One of these (the “standard” stimulus) occurred frequently but rarely preceded the startle stimulus. The second occurred infrequently (as an “oddball”) and always preceded a noise. In each such task, startle responses were inhibited more by the oddball than by the standard stimulus, usually within the first test. This suggests that PPI can be adapted to studies of the discrimination of speech and other complex sounds, and that this method can provide useful information on subjects’ ability to discriminate with greater ease and speed than other methods.
I. INTRODUCTION
Studies of “reflex modification” use changes in the latency or magnitude of reflex responses to assess information processing. In one variant, stimuli that precede a loud noise can decrease the size of the subsequent startle response, a change described as “prepulse inhibition” (PPI). The present study was designed to determine if PPI can be adapted to study the processing of speech sounds by animals.
PPI has been studied for more than 40 years (Hoffman and Searle, 1965). Early studies surveyed the impact of parameters including the intensity of the initial stimulus, or “prepulse,” the intensity of the startle stimulus, and the duration of the interstimulus interval (Hoffman and Ison, 1980). The results permitted the use of PPI to explore the mechanisms underlying detection, for instance by assessing the impact of damage to auditory cortex on the inhibition produced by a variety of prepulses (Bowen et al., 2003).
Currently, PPI is used in at least two overlapping contexts. First, it is used to measure the capacity for “sensorimotor gating” (Braff and Geyer, 1990). The concern here is mechanisms that act early in information processing to focus that processing in an automatic or “preattentive” way. Second, PPI is used to study later “attentive” steps in the selection of information for processing. In each of these cases, recent research has used PPI to test for information processing deficits in a variety of populations (e.g., Hawk et al., 2003) and to explore some of the mechanisms that underlie deficits in information processing (e.g., Schell et al., 2000).
Though PPI has been used extensively to study detection, it has been used much less often to study the discrimination of multiple stimuli from the background and each other. Further, of discrimination studies using PPI, most have used an explicit attentional manipulation to stimulate discrimination (e.g., Hawk et al., 2003). If one wanted to use PPI to study discrimination in animals, it would seem necessary to manipulate task structure instead, for instance by creating prepulses that relate to the startle stimulus differently. To our knowledge, the first study to use such an approach exposed rats to tone pairs that ascended or descended in frequency (Clark et al., 2000a). One stimulus helped to define the acoustic background: It was presented very frequently, but rarely preceded a noise. The other stimulus, or “oddball,” was designed to stand out from this background: It was presented infrequently and always preceded a noise. Across a wide range of stimulus parameters, startle responses were inhibited more by the oddball than the background stimulus. These and many subsequent results (Fitch and Peiffer, 2006) suggest that PPI can be adapted to the study of discrimination and represents a more flexible experimental tool than generally recognized.
We hoped to extend this success by refining PPI and applying it to speech sounds, stimuli that are more complex than those emphasized in past work. Speech sounds seem to merit attention both as examples of complex sounds and for any insights they can provide into mechanisms of speech perception. Already, animal models of speech perception seem to be making valuable contributions to our understanding of how acquired or developmental pathologies affect language processing (Fitch and Peiffer, 2006).
Past results show that animals can discriminate a variety of speech sounds (Brown and Sinnott, 2005). At the same time, these studies suggest that operant methods reveal such discriminations only with considerable time and effort (e.g., Kluender et al., 1987). This obviously slows progress in research that uses speech perception in animals to study language processing. To address this issue, we studied speech perception in rats using an adaptation of PPI, a paradigm in which other discriminations can be acquired with impressive speed and that premininary results suggest can be applied to speech sounds (Clark et al., 2000a,b).
II. METHODS
A. Animals and test chamber
The subjects were 12 female Sprague-Dawley rats averaging 139 days of age. Except during tests, each was housed individually in a wire-mesh cage with constant access to food and water. The colony was maintained at constant temperature and humidity, and on a reversed 12:12 h light:dark cycle. All methods and experimental treatments were approved by the University of Texas IACUC.
During testing, an animal was housed in a 20×20 × 20 cm3 wire-mesh cage in a 67×67×67 cm3 chamber lined with 5-cm acoustic foam. The cage was centered on a startle platform (Lafayette Instrument Co.) that uses a piezoelectric transducer to generate a continuous record of activity level. Sounds generated using an RP2.1 (Tucker-Davis Technologies) were delivered by a speaker (Optimus Bullet Horn Tweeter) mounted above the cage, about 20 cm from its center. Stimuli were adjusted for the speaker’s frequency response using SigCal (Tucker-Davis Technologies). Sound intensities were measured using an ACO Pacific microphone (PS9200-7016) placed at a height approximating that of a standing rat’s head.
B. Stimuli and startle response
Startle responses were elicited by 50-ms bursts of white noise at 102.0 dB. The waveform of each response (the peak to peak voltage within 500 ms of the noise) was sampled at 10 kHz using an RP2.1 and processed using MATLAB.
The stimuli also included six speech sounds that served as prepulses. These were derived from syllables (consonant-[æ]-[d])spoken by a female native English speaker in a sound-proof chamber. During recording, these were sampled at 10 μs with 16-bit resolution. To adjust them to a rat’s hearing, frequencies were doubled without changing the amplitude envelope (STRAIGHT vocoder, Kawahara et al., 1998). To shorten prepulses and equate them for elements other than the initial consonant, all were truncated at 100 ms into the vowel. The intensity of the loudest 100 ms of each stimulus then was adjusted to about 60 dB (58.8–60.9).
The resulting stimuli included [bæ], [pæ], [gæ], [∫æ], [d3æ], and [sæ] [Fig. 1]. Initial testing paired [bæ] with silence in a detection task. Later tasks required the discrimination of the sounds in the following pairs, presented in this order: [bæ]/[pæ], [bæ]/[gæ], [∫æ[/[d3æ[, [∫æ]/[sæ]. Order was not counterbalanced because of pilot data suggesting that the later tasks might be the more difficult, along with our initial uncertainty on rats’ ability to master any of these tasks.
FIG. 1.
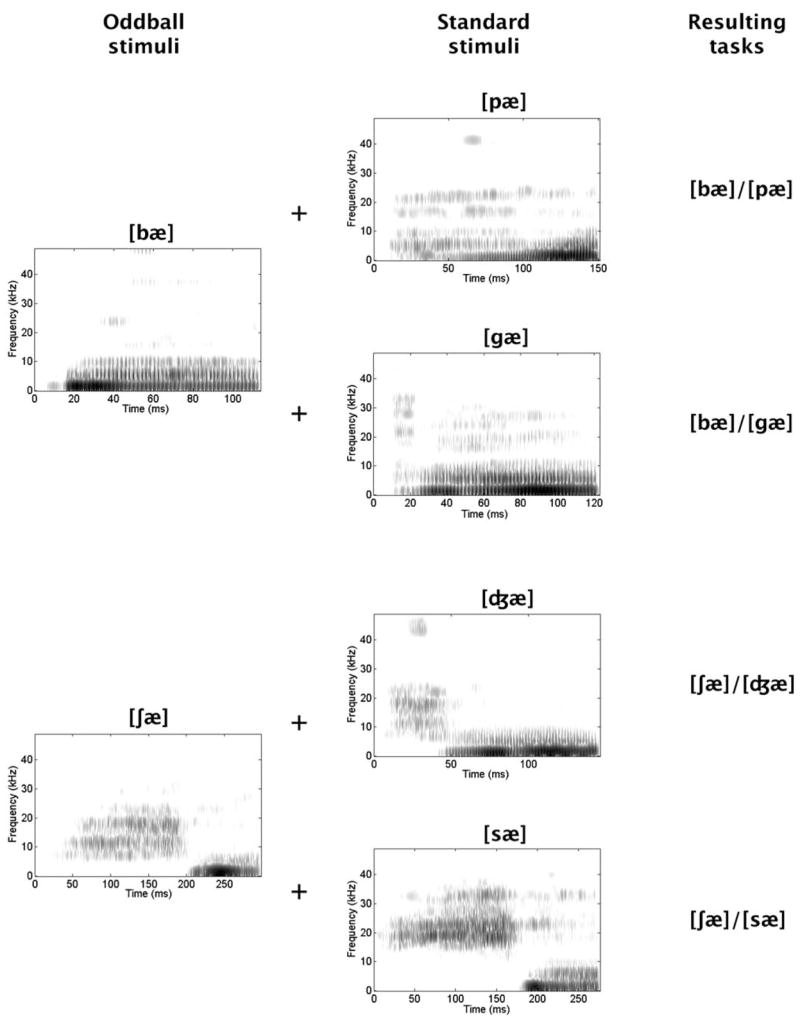
Sound spectrograms of the 6 speech sounds used as stimuli. The 2 sounds that served as oddballs are described on the left. The 4 that served as standard stimuli are described in the middle. The combinations that defined the 4 discrimination tasks are specified on the right.
These pairings were designed to create tasks that could be solved by exploiting differences on particular dimensions (but see caveat below). Specifically, the first pair combines bilabial stop consonants differing in voicing ([b] voiced, [p] voiceless). These should be discriminable on the basis of voice onset time, a temporal difference. The second includes consonants differing in place of articulation, permitting a spectral discrimination revolving around the origins and trajectories of the second formant (low initially then ascending in [b], high initially then descending in [g]). The third contrasts a voiceless fricative with a voiced affricative. These should be discriminable using the duration of each consonant’s initial noise burst (longer in [∫] than [d3]). This is a temporal difference, but of a different type from that distinguishing [bæ] and [pæ]. The fourth combines voiceless fricatives differing in place of articulation. These could be discriminated on the basis of the frequency range of the initial noise burst (relatively narrow and low for [∫], relatively broad and high for [s]), potentially representing a second type of spectral distinction. It is important to note, however, that the availability of a dimension does not guarantee the exploitation of that dimension. Natural speech sounds vary in multiple ways (Handel, 1993) and we cannot be sure how discriminations here were achieved.
C. Test procedures
Following 1 test on the detection task, each subject experienced 5 tests presenting [bæ] and [pæ], followed by 4 tests on each of the remaining 3 tasks. Each test included 3 phases. The first, in the detection task, consisted of 5 min of silence. In the other tasks, it involved the presentation at 1/s of a standard stimulus, which always was the second stimulus in a pair. Our goal here was to habituate any responses to this stimulus, causing it to recede into the background.
The second phase introduced the remaining speech sound (the first in each pair) and the startle stimulus. Ten of each were presented, with an interstimulus interval of 5 ms and spaced an average of 30 s (range=15–45 s) apart. Since the standard stimulus continued to appear at 1/s, it seems reasonable to refer to the newly introduced speech sound as an oddball (Clark et al., 2000a). This phase was designed to highlight the relationship between the oddball and startle stimulus. The interstimulus interval was selected on the basis of pilot testing. It is shorter than generally optimal for PPI (e.g., Hoffman and Searle, 1965). This disparity may relate to the fact that much of the information available to support discriminations was concentrated prior to the vowel, and thus 105 ms or more before the startle stimulus.
Each test concluded with a 20-min “test phase” in which animals were exposed to a mixture of “cued” and “uncued” trials, each involving the presentation of the startle stimulus 5 ms after a prepulse. Based on prior usage (Clark et al., 2000a), cued trials refer to those on which the oddball served as the prepulse. Uncued trials were those on which no stimulus (detection) or the standard stimulus (discriminations) preceded the startle stimulus. Each test included 10 blocks of 4 trials, each including 3 cued trials and one uncued, in random order and at intervals that again averaged 30 s. Except during these trials, “unreinforced” presentations of the standard stimulus continued at 1/s. Considering this, uncued trials are those on which there should be no prepulse that stands out from the background. Conversely, cued trials are those on which a prepulse should stand out, as long as the subject can distinguish the standard and oddball prepulses. Accordingly, one would expect greater PPI on cued than uncued trials, again as long as discrimination is possible.
D. Analysis
The critical data emerged from the test phase and included the average magnitudes of responses on cued and uncued trials. Average responses on the 1 day of detection testing were compared using a t test for dependent samples. The initial analysis of the discrimination data applied to each of these tasks an analysis of variance (ANOVA) using stimulus (standard, oddball) and day of testing (5 days for [bæ]/[pæ], 4 days for others) as within-subject factors. Ratios of the responses on cued and uncued trials (cued/uncued) were used to describe performance on each task and to compare levels of performance on the 4 discrimination tasks. Each data set was tested for heterogeneity of variance. Where found, significant heterogeneity was eliminated by logarithmic transformation prior to ANOVA. Throughout, a probability of 0.05 was used to define significance.
III. RESULTS
On the only day of detection testing, mean levels of startle on cued and uncued trials were 1.60 (SEM=0.28) and 3.52 (0.40), respectively. A t test for dependent samples confirmed a reliable reduction in the magnitude of startle on cued trials [t(11) =8.25, p<0.001, two-tailed].
The first discrimination task included 5 days of training using [bæ] and [pæ] as prepulses. ANOVA on these data revealed a reliable main effect of stimulus [F(1,11)=26.19, p<0.001], reflecting reduced levels of startle on cued trials [Fig. 2(a)]. This analysis also revealed a reliable main effect of days [F(4,44) =10.20, p<0.001]. Together with the absence of a reliable interaction, this reflects similar declines in cued and uncued responses over the 5 days of testing. These results are consistent with cued/uncued ratios that were stable and averaged significantly below 1 [mean=0.66, SEM=0.06, t(11)=5.59, p<0.001, one-sample t test, two-tailed].
FIG. 2.
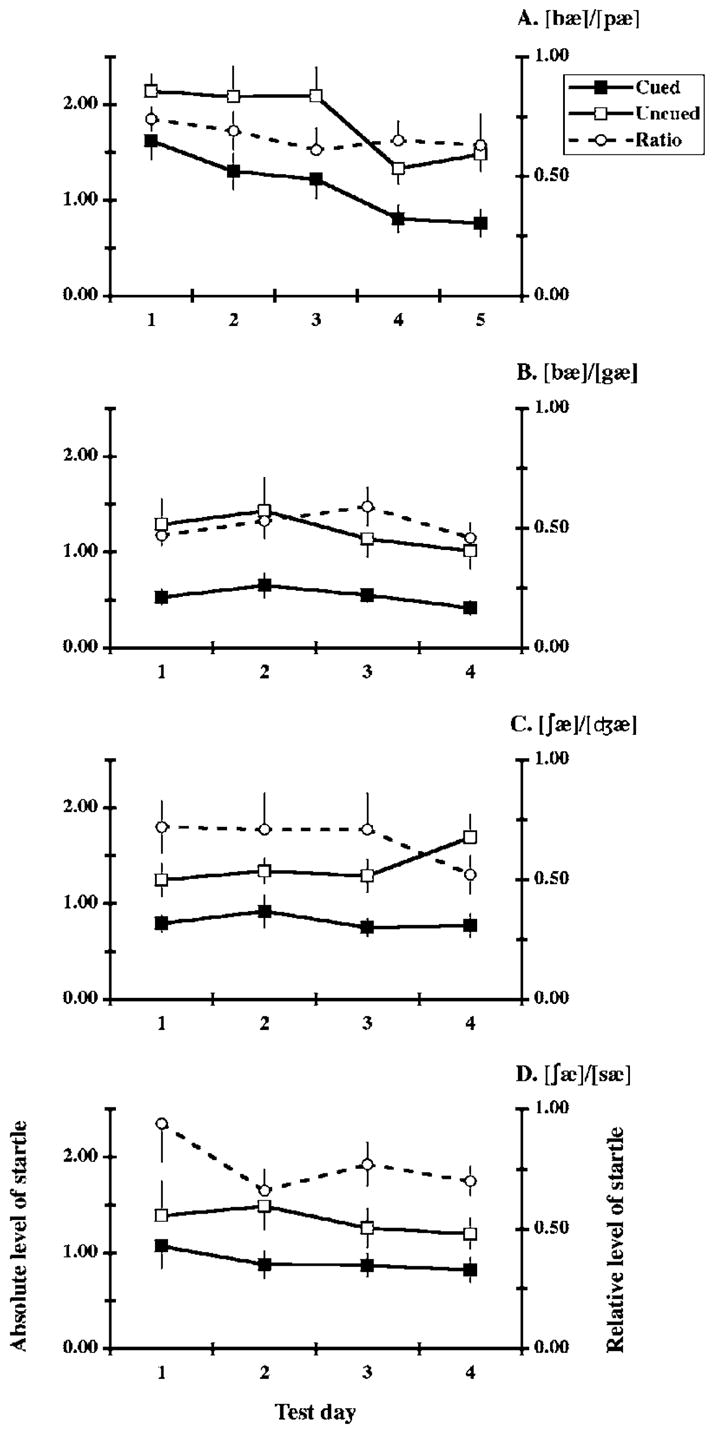
Solid lines depict daily mean (±SEM) levels of startle (represented in volts on left axis) on cued and uncued trials (filled and open squares, respectively). Dashed lines depict mean cued/uncued ratios (scale on the right). Successive test days are represented on the horizontal axis. As indicated, panels [a]–[d] describe performance on the four tasks requiring the discrimination of speech sounds.
Analysis of the data from the remaining 3 tasks revealed similar results. In each, ANOVA revealed a reliable stimulus effect [F(1,11)⩾13.99, p≤0.003], reflecting lower levels of startle on cued trials [Figs. 2(b)–2(d)]. In no case was the main effect of day or the interaction significant. Again, these results are consistent with cued/uncued ratios that were stable over days, with averages consistently below 1 [t(11)⩾2.93, p≤0.014].
Though task order was not counterbalanced, we compared performance across the 4 discrimination tasks. This analysis subjected the cued/uncued ratios to a task × day ANOVA, including 4 days of testing on each task (the last 4 of those on [bæ]/[pæ]). This revealed a reliable main effect of task [F(3,33) =4.04, p=0.015]. This was clarified by the comparison of ratios averaged over days using the Tukey test. This revealed just one reliable difference, between the tasks with the highest and lowest average ratios ([∫æ]/[sæ] and [bæ]/[gæ], respectively; p<0.05, Fig. 2).
IV. DISCUSSION AND CONCLUSIONS
Our results are consistent with evidence documenting the value of PPI in studies of sensory function (e.g., Wecker et al., 1985). They also add to a more recent and smaller literature suggesting the utility of discriminative forms of PPI in studies of stimulus discrimination, sensorimotor gating, and attentional processes (e.g., Clark et al., 2000a; Hazlett et al., 2001). They extend these studies by demonstrating how easily PPI can be applied to the discrimination of complex sounds in animals. The paradigm described here elicited reliable detection within a single 30-min test. Further, it revealed reliable discriminations in each of 4 tasks presenting different pairs of speech sounds. As in the case of detection, discrimination on each of these was achieved rapidly, most or all within the first test.
The rapidity with which these discriminations were displayed is consistent with previous descriptions of PPI as reflexive and unlearned (e.g., Hoffman and Ison, 1980). On the other hand, some of our data are equivocal on this point, suggesting that stable discrimination on our most difficult task may not have been achieved until the second test day (data not shown). This suggests that perceptual or other learning may be required, at least when PPI is used to monitor some, possibly difficult, discriminations. This is consistent with the changes over tests and conditions seen in some previous studies (Crofton et al., 1990).
These results confirm the ability of nonhuman animals to discriminate human speech sounds. In itself, this finding is not novel (e.g., Brown and Sinnott, 2005). However, the ease with which discriminations were achieved here contrasts with the results of most past studies of speech processing by animals (e.g., Sinnott and Mosteller, 2001). Some of this contrast probably reflects our use of natural speech sounds, which incorporate more potential cues for discrimination than the synthetic stimuli used in much recent operant work (e.g., Sinnott and Mosteller, 2001). But even operant studies using natural speech sounds have required many trials for the emergence of reliable discriminations (e.g., Kluender et al., 1987). This suggests that stimulus features account for only part of the difference in efficiency between operant methods and PPI. Consequently, our results support the application of PPI to tests of complex sound discrimination in animals, suggesting that at least some tasks could be studied more efficiently with some variant of reflex modification than with the operant methods that currently dominate the field.
Acknowledgments
This work was made possible by Grant No. R15DC006624 (Cortical Plasticity and Processing of Speech Sounds) from the National Institute for Deafness and Other Communicative Disorders. We thank H. Chen, C. Engineer, C. Heydrick, D. Listhrop, and K. Chang for their help.
Footnotes
PACS number(s): 43.66.Gf, 43.80.Lb, 43.71.Es [JES]
Contributor Information
Owen R. Floody, Department of Psychology, Bucknell University, Lewisburg, Pennsylvania 17837.
Michael P. Kilgard, Neuroscience Program, School of Behavioral and Brain Sciences, P.O. Box 830688, GR 41, University of Texas at Dallas, Richardson, Texas 75083-0688
References
- Bowen GP, Lin D, Taylor MK, Ison JR. Auditory cortex lesions in the rat impair both temporal acuity and noise increment thresholds, revealing a common neural substrate. Cereb Cortex. 2003;13:815–822. doi: 10.1093/cercor/13.8.815. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Brown CH, Sinnott JM. Cross-species comparisons of vocal perception. In: Greenberg S, Ainsworth W, editors. Listening to Speech: An Auditory Perspective. Oxford U.P., New York: 2005. pp. 183–201. Chap. 12. [Google Scholar]
- Clark MG, Rosen GD, Tallal P, Fitch RH. Impaired processing of complex auditory stimuli in rats with induced cerebrocortical microgyria: An animal model of developmental language disabilities. J Cogn Neurosci. 2000a;12:828–839. doi: 10.1162/089892900562435. [DOI] [PubMed] [Google Scholar]
- Clark MG, Tallal P, Rosen GD, Peiffer AM, Fitch RH. Impaired perception of speech stimuli in rats with cerebrocortical microgyria. Abstr Soc Neurosci. 2000b;26:74. doi: 10.1162/089892900562435. [DOI] [PubMed] [Google Scholar]
- Croften KM, Dean KF, Sheets LP, Peele DB. Evidence for an involvement of associative conditioning in reflex modification of the acoustic startle response with gaps in background noise. Psychobiol. 1990;18:467–474. [Google Scholar]
- Fitch RH, Peiffer AM. Behavioral consequences of focal anomalies in the cerebral cortex. In: Rosen GD, editor. The Dyslexic Brain: New Pathways in Neuroscience Discovery. Erlbaum; Mahwah, NJ: 2006. pp. 259–288. [Google Scholar]
- Handel S. Listening: An Introduction to the Perception of Auditory Events. MIT; Cambridge, MA: 1993. [Google Scholar]
- Hawk LW, Jr, Yartz AR, Pelham WE, Jr, Lock TM. The effects of methylphenidate on prepulse inhibition during attended and ignored prestimuli among boys with attention-deficit hyperactivity disorder. Psychopharmacol. 2003;165:118–127. doi: 10.1007/s00213-002-1235-7. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Dawson ME, Schell AM, Nuechterlein KH. Attentional stages of information processing during a continuous performance test: A startle modification analysis. Psychophysiology. 2001;38:669–677. [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev. 1980;87:175–189. [PubMed] [Google Scholar]
- Hoffman HS, Searle JL. Acoustic variables in the modification of startle reaction in the rat. J Comp Physiol Psychol. 1965;60:53–58. doi: 10.1037/h0022325. [DOI] [PubMed] [Google Scholar]
- Kawahara H, de Cheveigne A, Patterson RD. An instantaneous-frequency-based pitch extraction method for high-quality speech transformation: revised TEMPO in the STRAIGHT-suite. Proc 5th Int Conf on Spoken Language Processing (ICSLP’96); Sydney. 1998. [Google Scholar]
- Kluender KR, Diehl RL, Killeen PR. Japanese quail can learn phonetic categories. Science. 1987;237:1195–1197. doi: 10.1126/science.3629235. [DOI] [PubMed] [Google Scholar]
- Schell AM, Wynn JK, Dawson ME, Sinaii N, Niebala CB. Automatic and controlled attentional processes in startle eyeblink modification: Effects of habituation of the prepulse. Psychophysiology. 2000;37:409–417. [PubMed] [Google Scholar]
- Sinnott JM, Mosteller KW. A comparative assessment of speech sound discrimination in the Mongolian gerbil. J Acoust Soc Am. 2001;110:1729–1732. doi: 10.1121/1.1398055. [DOI] [PubMed] [Google Scholar]
- Wecker JR, Ison JR, Foss JA. Reflex modification as a test for sensory function. Neurobehav Toxicol Teratol. 1985;7:733–738. [PubMed] [Google Scholar]


