Summary
Resistance has emerged to every family of clinically used antibiotics, and there is a pressing need to explore novel antibacterial targets. Wall teichoic acids (WTAs) are anionic polymers that coat the cell walls of many Gram-positive bacteria. Because WTAs play an essential role in Staphylococcus aureus colonization and infection, the enzymes involved in WTA biosynthesis are proposed to be targets for antibiotic development. To facilitate the discovery of WTA inhibitors, we have reconstituted the intracellular steps of S. aureus WTA biosynthesis. We show that two intracellular steps in the biosynthetic pathway are different from what was proposed. The work reported here lays the foundation for the discovery and characterization of inhibitors of wall teichoic acid biosynthetic enzymes to assess their potential for treating bacterial infections.
Introduction
Wall teichoic acids (WTAs) are anionic polymers of Gram-positive bacteria that are covalently attached to the peptidoglycan chains (Figure 1) [1]. In Staphylococcus aureus, WTAs have been shown to play essential roles in host tissue colonization and the spread of infection [2, 3]. They contribute to bacterial resistance to human lysozyme, which is a component of the innate immune response that destroys bacteria by hydrolyzing the peptidoglycan layers [4]. WTAs have also been shown to play a role in biofilm formation [5-7]. Therefore, inhibitors of the WTA biosynthetic pathway may be useful for treating infections caused by S. aureus and other Gram positive pathogens [8]. In order to identify and characterize such inhibitors, it is important to understand the steps involved in wall teichoic acid biosynthesis. Although S. aureus strains are known to make polyribitol phosphate (polyRboP) wall teichoic acids [9-12], the biosynthetic pathway is not well-characterized and the proposed pathway (Figure 2a) is largely based on studies done in B. subtilis [13-15].
Figure 1. Wall teichoic acid structure.

Schematic showing the first intracellular step in polyribitol phosphate WTA biosynthesis and the final WTA product attached to a fragment of nascent S. aureus peptidoglycan. As reported in this manuscript, m=2 GroP units for S. aureus WTAs[10]. X and Y on the ribitol hydroxyls in the schematic indicate S. aureus tailoring modifications such as the attachment of GlcNAc or dalanine [15].
Figure 2. Proposed and reconstituted WTA biosynthetic pathway in S. aureus.
A) The proposed polyribitol-phosphate WTA biosynthetic pathway. The S. aureus NCTC8325 gene encoding each putative protein is shown by its locus tag number in the block arrow next to the enzyme name. B) The revised polyribitol-phosphate WTA pathway in S. aureus based on in vitro reconstitution. R=undecaprenyl (1a-5a) for the natural lipid carrier or farnesyl (1b-5b) for the alternative lipid used in the in vitro experiments. Differences between the proposed and reconstituted pathways are highlighted by larger bold text.
Wall teichoic acids consist of a disaccharide-based linkage unit and a repeating polyol–phosphate polymer such as polyglycerol phosphate (polyGroP) or polyribitol phosphate (polyRboP) [1]. WTAs are synthesized on a diphospholipid carrier anchored in the cytoplasmic membrane and are then translocated through a two-component transporter to the outside of the cell where they are attached by a phosphodiester linkage to the C6 hydroxyl of the N-acetyl muramic acid sugars of peptidoglycan (Figure 1) [1]. The most common linkage unit, found in all B. subtilis and S. aureus strains, is ManNAc-β-(1,4)-GlcNAc-(GroP)n, where n is proposed to be 2 in B. subtilis W23 and 3 in S. aureus [10, 11]. Common repeat units include polyGroP, found in B. subtilis 168, and polyRboP, found in B. subtilis W23 as well as S. aureus [1, 10]. Functions have been established for most of the tag (for teichoic acid glycerol) genes in B. subtilis 168 [14, 16-19], and putative functions were assigned to the tar genes (for teichoic acid ribitol) involved in polyRboP-WTA biosynthesis based on sequence homology to the tag genes [13, 14] (Figure 2a). Thus, it was proposed that polyRboP-WTA biosynthesis starts with the TarO-mediated transfer of GlcNAc to a membrane anchored undecaprenyl carrier lipid. Consistent with this, an S. aureus SA113 strain in which tarO is disrupted does not express WTAs [2]. WTA biosynthesis was proposed to continue with the TarA-mediated transfer of ManNAc to the C4 hydroxyl of GlcNAc to form ManNAc-β-(1,4)-GlcNAc-pp-undecaprenyl, whereupon TarB adds glycerol-3-phosphate to the C4 hydroxyl of the ManNAc moiety [13, 14, 20]. The proposed functions of TarA and TarB are thus identical to the established functions of TagA and TagB [14, 17]. After the TarB step, the pathways for polyGroP- and polyRboP-WTA biosynthesis diverge. Whereas TagF is a polymerase that adds dozens of GroP units to the disaccharide-based linkage unit in B. subtilis 168 [18, 19], TarF is a putative primase. B. subtilis TarF was proposed to add one GroP to the disaccharide linkage unit, while S. aureus TarF was proposed to add two GroPs [14, 20]. Two additional gene products, a putative ribitol-5-phosphate primase, TarK, and a putative RboP polymerase, TarL, are proposed to build the polyribitol-phosphate polymer [13, 14, 20]. For S. aureus NCTC8325, the source of the enzymes described below, the genes suggested to be responsible for each step in polyRboP-WTA biosynthesis are indicated in Figure 2a.
The pathway proposed in Figure 2a was recently questioned [14] by Qian et al., who carried out a genomic analysis of several fully sequenced S. aureus strains. Their analysis shows that the candidates for TarK and TarL, the two RboP transferases, are very similar in all S. aureus strains. Because of the high sequence homology between TarK and TarL, Qian et al. suggested that both gene products may function as polymerases, and that there may not be a distinct RboP primase and a distinct RboP polymerase [14]. Here we report the in vitro reconstitution of the intracellular steps downstream of TarO in the WTA biosynthetic pathway in S. aureus. In addition to TarD, TarI, and TarJ, which supply the CDP-glycerol and CDP-ribitol substrates [21, 22], we show that TarA, TarB, TarF, and TarL are required to convert the GlcNAc-pp-lipid product of TarO to the full-length polyribitol polymer. Based on our studies, we propose a revised WTA biosynthetic pathway for S. aureus in which the number of GroP units added by TarF is altered and the proposed polyRboP-primase (TarK) is omitted (compare Figures 2a and 2b). This work clarifies the biosynthetic pathway in S. aureus, and may also enable efforts to discover and characterize WTA inhibitors that can be used to assess whether the WTA pathway is a target for therapeutic intervention.
Results
In vitro reconstitution of S. aureus TarA
The SAOUHSC_00640 gene, encoding SA640, a putative N-acetylmannosaminyl transferase with 55% similarity to B. subtilis 168 TagA, was PCR amplified from S. aureus NCTC8325 genomic DNA. The PCR product was cloned into a pET24b(+) vector for expression as a C-terminal hexa-His-tagged protein. The protein was overexpressed in E. coli strain Rosetta2(DE3)pLysS and purified by Ni2+-affinity chromatography. SA640 is proposed to transfer ManNAc from UDP-ManNAc, to a GlcNAc-pp-undecaprenyl carrier lipid. We have previously shown that B. subtilis TagA accepts GlcNAc-pp-lipid substrates containing farnesyl chains and is functional in the absence of membranes [14]. Therefore, we incubated SA640 with UDP-ManNAc and the synthetic GlcNAc-pp-farnesyl acceptor substrate under the same conditions used to characterize B. subtilis TagA [14]. We have previously described the synthesis of these substrates [17]. HPLC analysis (Figure 3a-b) of the reaction showed the disappearance of UDP-ManNAc and the concomitant appearance of a UDP peak. The reaction product was found to have a retention time of 11.7 minutes, identical to an authentic standard generated in the TagA reaction and previously characterized by MS and 1H-NMR (Figure 3c-d) [14, 17]. The reaction was quenched and the lipid-linked product was separated from other reaction components over a Phenomenex Strata C18-E column and analyzed by MS. The product was found to have a mass of 787.3, consistent with compound 2b (calc'd m/z: 787.2819). These experiments verify that SA640 encodes the UDP-ManNAc transferase TarA, which makes compound 2 (Figure 2b).
Figure 3. HPLC and LC-MS chromatograms of the TarA enzymatic reaction.
(A-B) HPLC chromatograms after incubation of UDP-ManNAc with 1b and (A) heat-treated SA640 or (B) active enzyme. (C-D) Retention times and intensities of the extracted product ions (m/z=787.3) for (C) the authentic product 2b, and (D) the product generated by incubating UDP-ManNAc and 1b with SA640. (E) The in vitro reaction catalyzed by the SA640 (TarA) with the experimental m/z value shown (calculated m/z: 787.2819).
In vitro reconstitution of S. aureus TarB
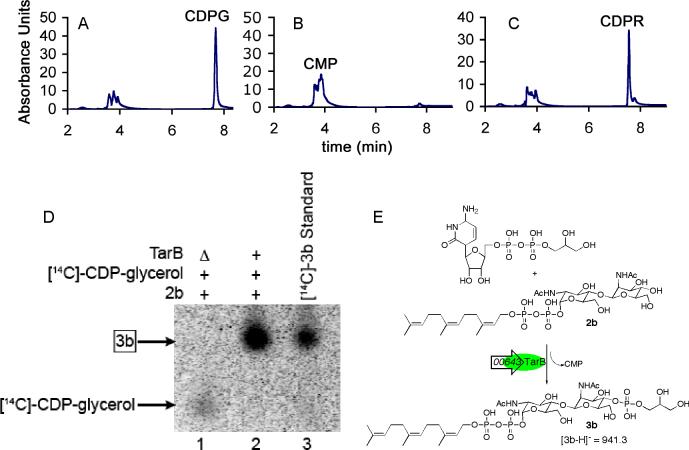
The SAOUHSC_00643 gene, encoding SA643, a putative glycerol phosphotransferase with 53% homology to TagB from B. subtilis 168, was PCR amplified from S. aureus NCTC 8325 genomic DNA. The gene was cloned into a pET24b(+) vector, overexpressed in E. coli strain Rosetta2(DE3)pLysS as a C-terminal hexa-His-tagged protein, and purified via Ni2+-affinity chromatography. SA643 is proposed to transfer glycerol-3-phosphate from CDP-glycerol to the disaccharide-lipid product of TarA. Therefore, we examined its ability to transfer GroP to compound 2b, which we have previously shown is a substrate for B. subtilis 168 TagB [17]. Reactions containing ManNAc-β-(1,4)-GlcNAc-pp-lipid (the TarA product) and CDP-glycerol were monitored by HPLC. The CDP-glycerol peak (retention time of 7.56 minutes) decreased in intensity and a new peak, corresponding to CMP (retention time of 3.85 minutes), appeared. Under the same conditions, SA643 was unable to utilize CDP-ribitol as a substrate, as judged by the stability of the CDP-ribitol peak (retention time of 7.54 minutes) in the HPLC trace (Figure 4a-c). Using radiolabeled [14C]-CDP-glycerol, we could also monitor the SA643 reaction by polyacrylamide gel electrophoresis followed by phosphorimaging analysis. The gel (Figure 4d) shows a new spot in the enzyme reaction that is not present in the heat-treated control. This new spot has the same mobility as the authentic product generated by reacting [14C]-CDP-glycerol and compound 2b with B. subtilis 168 TagB. The lipid-linked product from a TarB reaction utilizing non-radioactive substrates was purified over a C18 column and subjected to MS analysis. The purified product has a mass of 941.3, consistent with structure 3b (calc'd m/z: 941.2851). These results verify that SA643 encodes the glycerol phosphate transferase TarB, which makes compound 3 (Figure 2b).
Figure 4. HPLC chromatograms and radioactive gel analysis of TarB enzymatic reactions.
(A-C) HPLC chromatograms after incubation of CDP-glycerol or CDP-ribitol after incubation with 2b and either heat-treated or untreated SA643. (A) CDP-glycerol reaction with heat-treated enzyme; (B) CDP-glycerol reaction with active enzyme; (C) CDP-ribitol reaction with active enzyme. (D) Autoradiogram of a polyacrylamide gel for reactions of 2b and SA643 in the presence of [14C]-CDP-glycerol. (+) and (−) symbols designate the presence or absence, respectively, of the components indicated on the left; (△) indicates heat treatment. Lane 1: heat-treated enzyme reaction; Lane 2: active enzyme reaction; Lane3: authentic standard of [14C]-3b generated using previously characterized B. subtilis TagB. (E) The in vitro reaction catalyzed by TarB (SA643) with the experimental m/z value shown (calculated m/z: 941.2851).
In vitro reconstitution of S. aureus TarF
SAOUHSC_00223, encoding SA223, which is 67% similar to B. subtilis W23 TarF, was cloned from S. aureus NCTC8325 genomic DNA, overexpressed in E. coli, and purified over a Ni2+ column. S. aureus TarF is proposed to transfer two units of glycerol-3-phosphate from CDP-glycerol to compound 3 . To assess the function of SA223, we incubated the purified enzyme with purified [14C]-glycerol-3-phosphate-ManNAc-β-(1,4)-GlcNAc-pp-lipid (3b) and either [14C]-CDP-glycerol or CDP-ribitol. Reactions were monitored by polyacrylamide gel electrophoresis (Figure 5a). A lower spot appeared in the [14C]-CDP-glycerol reaction that was not present in the heat-treated controls or in the CDP-ribitol reaction. The new spot migrates faster than the starting material but was found to depend on the presence of both [14C]-CDP-glycerol and the TarB product. Therefore, we carried out an enzymatic reaction using non-radiolabeled 3b and CDP-glycerol, purified the lipid-linked material over a C18 column, and subjected it to MS analysis. The product was found to have a m/z of 1095.3 (calc' m/z: 1095.2882), which corresponds to the addition of one glycerol-3-phosphate unit to compound 3b to form compound 4b. No products containing additional GroP units were observed under any conditions. We have concluded that SA223 is TarF, and that TarF in S. aureus NCTC8325 functions to add one unit of glycerol-3-phosphate to compound 3 to produce (glycerol-3-phosphate)2-ManNAc-β-(1,4)-GlcNAc-pp-lipid (compound 4) (Figure 2b). Unless the functions of TarF homologs in other S. aureus strains are different, we propose that S. aureus TarF, like B. subtilis W23 TarF, transfers a single GroP unit.
Figure 5. Radioactive gel analysis of TarF enzymatic reactions.
(A) Autoradiogram of a polyacrylamide gel for TarF-catalyzed reactions of [14C]-3b and either [14C]-CDP-glycerol or CDP-ribitol. (+) and (−) symbols designate the presence or absence, respectively, of the components indicated on the left; (△) indicates heat treatment. Lane 1: heat-treated enzyme reaction containing [14C]-CDP-glycerol; Lane 2: active enzyme reaction containing [14C]-CDP-glycerol; Lane 3: heat-treated enzyme reaction containing CDP-ribitol; Lane4: active enzyme reaction containing CDP-ribitol. (B) The in vitro reaction catalyzed by TarF (SA223) with the experimental m/z values shown (calculated m/z: 1095.2882).
In vitro reconstitution of the ribitolphosphotransferase polymerase (TarL)
All sequenced S. aureus strains contain two genes that share high homology to one another and to the B. subtilis W23 tarL and tarK genes [14]. In B. subtilis W23, TarK is proposed to be a primase that adds one or a few units of ribitol-5-phosphate, while TarL is proposed to be a ribitol-5-phosphate polymerase that adds many RboP units [13, 14, 20]. Qian et al. have suggested that the two candidate genes for TarK and TarL in S. aureus may actually be duplicates of one another and have similar functions [14]. To assess the functions of the two putative RboP transferases in S. aureus NCTC8325 (SA222 and SA227), we cloned the genes for these proteins from genomic DNA into pET24b(+), expressed them in E. coli and purified the expressed proteins by Ni2+ column chromatagraphy. SA222 was incubated with [14C]-4b and CDP-ribitol and the reaction mixture was analyzed by polyacrylamide gel electrophoresis, but no change in the mobility of the radiolabeled starting material was observed. Similarly, no change was observed when CDP-glycerol was included in the reaction. Concerned that a product involving the addition of a single RboP unit might not separate from the starting material under the gel electrophoresis conditions used, we analyzed a set of non-radioactive reactions by HPLC. SA222 was separately incubated with compound 3b and compound 4b and either CDP-ribitol or CDP-glycerol, but we did not observe under any conditions a decrease in peaks corresponding to starting material or the appearance of a peak for CMP (data not shown).
Unable to reconstitute any activity for SA222, we subjected SA227 to a similar series of experiments. SA227 was incubated with [14C]-4b and either CDP-ribitol or CDP-glycerol and the reactions were analyzed by polyacrylamide gel electrophoresis (Figure 6a). In this case, [14C]-4b reacted to produce a higher band in the presence of CDP-ribitol but not in the presence of CDP-glycerol. No slower migrating band was visible in the heat-treated control or in reactions containing [14C]-3b rather than [14C]-4b. We also used [2-3H]-CDP-ribitol as a substrate along with [14C]-4b in the SA227 reactions. The reaction mixtures were spotted onto Whatman 3MM paper strips and the strips were developed by TLC. Non-polymeric starting materials migrated up the strips while polymeric product remained at the origin. Scintillation counting showed that the material at the origin was labeled with both 14C and 3H in a ratio of >1:17 under the reaction conditions used, which confirms the attachment of multiple [2-3H]-ribitol-5-phosphate units to the [14C]-TarF product. Taken together, these results show that SA227 transfers multiple RboP units to the disaccharide 4b, which contains two GroP units, but not to the disaccharide 3b, which contains one GroP unit.
Figure 6. Analysis of TarL (SA227) enzymatic reactions.
(A) Autoradiogram of a polyacrylamide gel for TarL-catalyzed reactions of [14C]-4b or [14C]-3b and either CDP-glycerol or CDP-ribitol. (+) and (−) symbols designate the presence or absence, respectively, of the components indicated on the left; (△) indicates heat treatment. Only Lane 3, which contains [14C]-4b and CDP-ribitol in addition to active enzyme, shows the disappearance of radiolabeled starting material and the formation of a radiolabeled higher product. (B) Polyacrylamide gel of WTAs stained with silver and Alcian Blue according to an established method for detecting wall teichoic acid polymers. Lane 1: WTAs synthesized in vitro from UDP-ManNAc, 1b, CDP-glycerol, and CDP-ribitol by the tandem action of TarA, TarB, TarF, and TarL. Lane 2: WTAs extracted from S. aureus NCTC8325 cells. (C) The in vitro reaction catalyzed by TarL (SA227).
We incubated the product of the SA227 reaction with SA222 and CDP-ribitol but did not detect any change in the length of the polyRboP-WTA polymer, suggesting that SA222 does not further extend this product.
The results reported above establish that SA227 is a ribitol-5-phosphate polymerase, which transfers ribitol-5-phosphate from CDP-ribitol to compound 4 to form the (ribitol-5-phosphate)n-(glycerol-3-phosphate)2-ManNAc-β-(1,4)-GlcNAc-pp-undecaprenyl product 5 (Figure 2b). We have hence identified SA227 as the polymerase TarL. We have also concluded that a ribitol-5-phosphate primase is not required for the synthesis of polyRboP-WTA polymers in S. aureus, as one apparently is in B. subtilis W23 [13]. The function of SA222, suggested first to be a primase and later to be another polymerase, remains to be established.
In vitro reconstitution of WTA synthesis
We carried out a tandem reaction of TarA, TarB, TarF, and TarL to produce wall teichoic acid polymers in vitro from the GlcNAc-pp-lipid substrate 1b. The synthetic WTA polymers formed in vitro were analyzed on a polyacrylamide gel that also contained WTAs extracted from S. aureus NCTC8325 cells (Figure 6b). The synthetic polymers could be stained with alcian blue, an established method for staining WTAs. The most abundant polymers synthesized in vitro are approximately 10 units smaller than the most abundant extracted WTAs, which have been estimated to contain about 40 RboP units [15]. We were unable to increase significantly the lengths of the WTA polymers produced in vitro by manipulating substrate ratios or enzyme concentrations. The molecular basis for the control of polymer length is not known, but it is evident from the distribution of polymeric products that TarL is a processive enzyme. Additional factors may control polymer lengths and product distributions in bacterial cells, explaining the longer lengths of the natural WTA polymers. Whether WTA polymer length is of any biological significance is not known.
Discussion
Bacterial resistance has developed to all main classes of antibiotics. Most current antibiotics target a few essential bacterial pathways, including DNA replication, cell wall biosynthesis, bacterial protein biosynthesis, and folate biosynthesis [23]. It remains to be seen how many new inhibitors can be developed to these well-studied pathways. There exists a need to explore new targets. Virulence factors, which are involved in the progression of disease in the host, but are not critical for the survival of the bacterium in vitro, are intriguing targets. Virulence factors include toxins as well as factors important for colonization and infection of host tissue [24]. Recent studies support the promise of small-molecule inhibitors of virulence factors for treating bacterial infection [25].
S. aureus, one of the major nosocomial pathogens, is covered in wall teichoic acids, anionic polymers that are attached to peptidoglycan and that play a variety of important but poorly understood roles in the biology of these and many other Gram positive organisms [26]. Recent experiments showed that deleting the first gene in the WTA biosynthetic pathway, tarO, produces mutants that are viable in vitro but are unable to colonize epithelial or endothelial tissue [2, 3]. These studies highlighted the essential role that WTAs play in S. aureus infection and have prompted interest in understanding the WTA biosynthetic pathway in more detail since it may be a target for antibacterial agents. Brown and coworkers recently reported that several genes downstream of tarO in the proposed S. aureus biosynthetic pathway cannot be deleted [20], and it was suggested that deletions of these genes may be lethal because toxic intermediates accumulate in the cell and/or because a blockade in the WTA biosynthetic pathway diverts building blocks, such as undecaprenyl phosphate, that would otherwise be recycled and used in essential metabolic pathways such as peptidoglycan biosynthesis [20]. That disruption of tar genes leads to a lethal phenotype increases interest in the WTA biosynthetic pathway as a target for intervention, but it also makes it challenging to characterize the pathway in detail using a genetic approach. Therefore, we decided to establish the functions of several of the genes proposed to be involved in WTA biosynthesis via in vitro reconstitution of key intracellular steps.
We cloned, overexpressed, and purified eight S. aureus NCTC8325 enzymes, tentatively identified as TarA, B, D, F, I, J, K, and L. Five of these enzymes were proposed to be involved in the conversion of compound 1 to compound 5 via the linear sequence of steps shown in Figure 2a. The other three, TarD, I and J [21, 22], were cloned to enable the synthesis of CDP-glycerol and CDP-ribitol in both radiolabeled and non-radiolabeled forms. In addition, we synthesized via chemical methods both UDP-ManNAc and compound 1b, which are the donor and acceptor substrates, respectively, for TarA. The acceptor substrate we prepared, 1b, differs from the natural substrate in containing a farnesyl chain rather than the undecaprenyl carrier lipid, but we have previously established that other WTA biosynthetic enzymes are not very sensitive to the structure of the attached lipid chain [14, 17]. Therefore, there was precedent for the use of synthetic alternative substrates in reconstituting the WTA biosynthetic pathway of S. aureus.
We were able to reconstitute the activities of four of the five Tar pathway enzymes proposed to be involved in the conversion of compound 1 to compound 5. Although the four enzymes, TarA, TarB, TarF, and TarL, operate on a membrane-anchored undecaprenyl-containing substrate in cells, none of them are predicted to have membrane-spanning domains, and none require the undecaprenyl substrate or a membrane interface for activity. Kinetic studies have not been done, but the enzymes have reasonable activity with the alternative substrate since all the reactions largely went to completion in two hours. We were not able to reconstitute the activity of the fifth putative Tar pathway enzyme, the TarK/TarL homolog SA222; however, the work reported here clearly shows that it is not required to form polyRboP-WTA polymers.
Based on the results of the reconstitution experiments, we have revised the S. aureus tar pathway in two ways. First, we have altered the proposed function of TarF. This enzyme had been thought to add two GroP units [10, 11, 20], but we have shown here that it adds only one. There may be several explanations for the discrepancy between earlier results and the results reported here. For example, the S. aureus strain that was originally analyzed to determine the structure of the linkage unit may have been unusual in having a TarF that adds two GroP units. Alternatively, the S. aureus strain we used as a source of genomic DNA may be unusual in having a TarF that adds only one unit. There may be an as yet undiscovered enzyme in this or other S. aureus strains that adds a third GroP unit; if so, however, the third GroP unit is not required for extension of the polyRboP chain. Finally, it is possible that the original structural analyses were incorrect. Analysis of polymers fractionated from complex biological mixtures is more complicated than analysis of the products of an enzymatic reaction in vitro. At this point, we cannot distinguish among the possibilities. Therefore, we have revised the function of S. aureus TarF in Figure 2b with the caveat that generality has not yet been established for all S. aureus TarF homologs.
The other revision is more substantive. We have shown that TarL, the poly(RboP) polymerase, can act directly on the TarF product (compound 4), indicating that there is no need in S. aureus for an RboP primase. The putative primase was thus removed from the proposed pathway (compare Figure 2a and 2b). The function of SA222, a candidate for the primase encoding a gene product that shows 88% end-to-end homology to SA227, which we have identified as TarL, could not be established from these in vitro experiments. However, all sequenced S. aureus strains, which now number 12, contain a pair of genes encoding polypeptides homologous to SA222 and SA227. Given that the 222 gene is present in all 12 sequenced S. aureus strains, it seems likely that it plays a role in WTA biosynthesis, although that role does not appear to be essential [27]. We are currently trying to determine the function of SA222. In the meantime, the reconstitution experiments described here lay the groundwork for the discovery and characterization of WTA inhibitors directed against key intracellular enzymes.
Significance
Wall teichoic acids are virulence factors in gram-positive bacteria [2]. Their involvement in bacterial colonization and infection suggest that WTA biosynthesis may be a target for therapeutic intervention. Molecules that inhibit WTA biosynthesis could be used to determine whether targeting virulence factors would have efficacy as antibiotics. Using in vitro reconstitution of enzymatic activity, we have delineated several intracellular steps in the WTA biosynthetic pathway in S. aureus. To our knowledge this is the first biochemical study assigning the function of each gene product involved in the synthesis of WTAs from a polyribitol-forming strain, and the work has led to two corrections in the proposed biosynthetic pathway in S. aureus. The reconstitution of the intracellular steps of the WTA biosynthetic pathway in a clinically relevant pathogen paves the way for the discovery inhibitors.
Materials and Methods
Reagents
Vectors, expression hosts, and His-Bind resin were obtained from Novagen. Acrylamide solutions were purchased from National Diagnostics, TEMED from American Bioanalytical and TBE from Bio-Rad. All other enzymes, reagents, and buffers were obtained from Sigma-Aldrich.
Cloning, Expression, and Purification of Putative Enzymes
The SAOUHSC_00640, SAOUHSC_00643, SAOUHSC_00223, SAOUHSC_00222, SAOUHSC_00227 and tarD, tarI, and tarJ genes were PCR amplified from Staphylococcus aureus NCTC8325 genomic DNA. The primer pairs used for amplification are included in Supplemental Table 1. The PCR products were subcloned into pET24b(+) (Novagen) at the XhoI and BamHI restriction sites for expression in E. coli Rosetta2(DE3)pLysS (Novagen) as C-terminal hexa-His-tagged proteins. The proteins were expressed in mid-log phase cultures after induction with 0.5 mM or 1mM IPTG for 4 h at 37 °C, except for SA222 and SA227, which were induced at 16 °C for overnight growth. Cells were lysed by freeze-thaw, sonication, and French pressure cell at 16,000 lb/in2 in buffer supplemented with rLysozyme and Benzonase (Novagen) and protease inhibitor cocktail (CalBioChem). SA222 and SA227 lysis buffer was 50mM HEPES, pH 7.0, 200mM NaCl, 0.5% CHAPS, 5% glycerol buffer. The remaining proteins were lysed in 100mM Tris-HCl, pH 7.5, 500mM NaCl, 0.6% CHAPS, 0.5% Triton X-100. Clarified lysate was purified by nickel affinity chromatography (Novagen His-Bind resin) yielding 5 mg/L TarA, 5 mg/L TarD, 1 mg/L TarB, 8 mg/L TarF, 7 mg/L TarI, 3 mg/L TarJ, 0.5 mg/L TarL, 0.25 mg/L TarK as determined by Lowry Assay. The proteins were stored as 20% and 50% glycerol stocks at −80 °C or −20 °C.
Preparation of UDP-ManNAc, [14C]-CDP-glycerol, Ribitol-5-phosphate and Compounds 1b, 2b, 3b
UDP-ManNAc, compound 1b and compound 2b were prepared as we previously described [17]. [14C]-CDP-glycerol was prepared as previously described using purified TarD [21]. Ribitol-5-phosphate was prepared as described previously [28, 29]. Briefly, 0.1g ribulose-5-phosphate was dissolved in water. After the solution pH was confirmed to be above 7, sodium borohydride was added to give a 1:1 molar ratio. The mixture was incubated at RT overnight, quenched with acetic acid (to pH 5), and NaOH was added to adjust the pH to 8. The addition and removal by rotary evaporator of MeOH was used to rid of the resulting borate salts. The reaction products were analyzed by Benedict's Reagent to confirm the disappearance of ribulose-5-phosphate. The reaction was purified using Bio-Rad P2 gel. [14C]-radiolabeled compound 3b was prepared by incubating TarB (500nM) overnight with 10μM 2 and 30μM [14C]-CDP-glycerol at RT in 20mM Tris-HCl, pH 7.5, 100mM NaCl, and 10mM MgCl2 buffer. The product, [14C]-labeled-3b, was purified by C18 column (Accubond SPE ODS-C18) by washing with 100% water and eluting product with 100% ethanol.
Preparation of CDP-ribitol and [2-3H]-CDP-Ribitol
Non-radiolabeled CDP-ribitol was prepared by incubating TarI overnight with 1 mM ribitol-5-phosphate and 2 mM CTP in 50mM HEPES pH 8, 10mM MgCl2, 1mM DTT, and 1 unit inorganic pyrophosphatase [22]. The reaction was monitored by HPLC using an anion exchange column, Phenosphere 5μ SAX 250 × 4.6 mm, 5 μM (Phenomenex), to record the disappearance of the CTP peak and production of a new peak (Buffer A: 5mM NH4H2PO4, pH 2.8, Buffer B: 750mM NH4H2PO4, pH 3.7, linear gradient of 0−20% B over 60 minutes, UV monitored at 271nm). The identity of CDP-ribitol was confirmed by mass spectroscopy. CDP-ribitol was used unpurified in subsequent enzymatic reactions after spinning the reaction in a 3000 MWCO filter to remove the enzyme. [2-3H]-radiolabeled CDP-ribitol was prepared by extension of a previously outlined protocol to make [2-3H]-ribitol-5-phosphate and CDP-ribitol [22, 30]. A duplicate non-radiolabeled reaction was used to monitor the reaction progress by analytical anion exchange HPLC (Phenosphere 5μ SAX 250 × 4.6 mm, 5 μM, Phenomenex). A 1:200 mixture of [2-3H]-D-glucose:D-glucose (2mM total), 3mM ATP, 4mM NADP, 1.5 units glucose-6-phosphate dehydrogenase, 1 unit of hexokinase, 1 unit of 6-phosphogluconate dehydrogenase was incubated at 30 °C in 100mM triethanolamine buffer pH 7.6, 10mM MgCl2. After 3 hours, 1μM ZnCl2, 1mM DTT and TarJ was added. After an additional 4 hours the reaction was spun in a 3000 MWCO filter to remove the enzymes. The resulting ribitol-5-phosphate product was purified using a previously established method [31]. Briefly, the reaction was applied to a 1mL Dowex 1×8−400 anion exchange resin (Cl-form). The product was purified using a stepwise gradient of HCl and found to elute with 0.02N HCl. The pH of collected product was adjusted to 7 using NaOH and the solvent was evaporated to concentrate the product. 1 unit inorganic pyrophosphatase, 2mM CTP and TarI were added to the purified ribitol-5-phosphate and the reaction was allowed to proceed for 1 hour at 30 °C in 100mM triethanolamine buffer pH 7.6, 10mM MgCl2. The resulting [2-3H]-CDP-ribitol was used in subsequent enzymatic reactions.
HPLC Assay for TarA and TarB
TarA (500nM) was incubated at RT for 2.5h with 50μM UDP-ManNAc and 100μM 1b in buffer (20mM Tris-HCl pH 7.9, 500mM NaCl). TarB (500nM) was incubated at RT for 2.5h with 50μM CDP-glycerol or 50μM CDP-ribitol and 100μM 2b in buffer (20mM Tris-HCl pH 7.5, 100mM NaCl, 10mM MgCl2). Reactions were quenched with an equal volume of DMF, applied to an analytical anion exchange HPLC column (Phenosphere 5μSAX 250 × 4.6 mm, 5 μM, Phenomenex). TarA reactions were eluted using a linear gradient of 0−5%B over 30 min (buffer A: 5mM NH4H2PO4, pH 4.5, buffer B: 750mM NH4H2PO4, pH 3.7). TarB reactions were eluted using a linear gradient from 0%B to 100%B over 25 min (buffer A: 5mM NH4H2PO4, pH 2.8, buffer B: 750mM NH4H2PO4, pH 3.7). UDP-ManNAc and UDP were monitored at 260nM. CDP-glycerol and CMP were monitored at 271nM.
HPLC-MS and MS Assays
TarA and TarB reaction conditions were the same as described above except TarA reactions utilized 50μM 2b and 150μM 1b and TarB reactions utilized 50μM 3b and 200μM CDP-glycerol. 500nM TarF was incubated with 50μM 2b, 500nM TarB and 350μM CDP-glycerol in buffer (20mM Tris-HCl, pH 7.5, 100mM NaCl and 10mM MgCl2). The products were purified using a Phenomenex Strata C18-E column by washing with 100% water and eluting product with 50:50 water:methanol. MS-TOF experiments were performed on a Waters mass spectrometer. The purified compounds (TarA, B, and F products) were run in negative ion mode using water with 0.1% ammonium formate as solvent. For ESI-MS analysis mass spectra were acquired using an Agilent 1100 series LC/MSD mass spectrometer. After the mass of the purified compound was obtained, unpurified TarA product and purified TagA product (obtained as described previously [17]) were subjected to LC-MS analysis (extracted ion of 787.3 from the LC trace) using a Zorbax 300SB-C18, 5μM, 4.6 × 250 mm (Agilent) column. The compounds were eluted at a flow rate of 0.5mL/min using a step gradient: 0−60% solution B over 8 min, 60−100% solution B over 30 sec, and 100%B for 7.5 min (solution A, water, solution B, methanol, both solutions were supplemented with 0.1% ammonium hydroxide as a solvent modifier).
TarB, TarF, TarK and TarL Enzymatic Reactions for Polyacrylamide Gel Electrophoresis Analysis
Reactions containing 1uM purified 2b and 2uM [14C]-CDP-glycerol were incubated with TarB (500nM). TarF reactions were performed using 500nM enzyme 2uM purified [14C]-labeled-3b and 4uM either CDP-ribitol or [14C]-CDP-glycerol. TarK and TarL reactions were performed by reacting 500nM enzyme with 1μM purified [14C]-labeled-4b or 1μM purified [14C]-labeled-3b and 200μM CDP-ribitol or CDP-glycerol. TarB, TarF, TarK and TarL enzymatic reactions were performed in buffer containing 20mM Tris-HCl, pH 7.5, 100mM NaCl and 10mM MgCl2. All enzymatic reactions were quenched with an equal volume of DMF after 2.5 hours. [14C]-TagB product was obtained using [14C]-CDP-glycerol following our previously established method [17].
Polyacrylamide Gel Electrophoresis Assay for TarB, TarF, TarK and TarL
The acrylamide gels used are similar to those described previously [32]. Bio-Rad minigels (7.0 cm × 8.3 cm (H × W); 1.0mm thickness) were constructed of 20% acrylamide/0.25M TBE by mixing 4mL of a prepared acrylamide stock solution (Protogel, 30% (w/v) acrylamide: 0.8% (w/v) bisacrylamide), 0.25 mL water, 1.69 mL 10× TBE solution, 5 uL TEMED, and 20 uL 10% ammonium persulfate. 2 uL of quenched enzymatic reactions were added to 2 uL of a 2× loading buffer consisting of 50% glycerol and 0.1% bromophenol blue. The gels were electrophoresed in 0.25M TBE buffer running at a constant 100V for 107 minutes using the Bio-Rad Mini-PROTEAN system. The gels were dried between two sheets of cellophane and the dried gels were exposed to a tritium storage phosphor screen (GE Healthcare) for approximately 48 hours. The screen was imaged using a Typhoon 9400 imager and analyzed using the ImageQuant TL computer software.
Paper Chromatography Assay to Determine the Incorporation of RboP Repeats
6μL reactions containing 0.5μM purified 4b and 100μM [2-3H]-CDP-ribitol and 500nM TarL enzyme were incubated at room temperature for 2.5 hours in 20mM Tris base pH 7.5, 10mM MgCl2, and 100mM NaCl. The reaction was performed in triplicate. The reactions were quenched with 6μL DMF and 3μL spotted onto 3MM Whatman paper in triplicate. The reactions were spotted 3cm from the bottom of the paper and developed using 5:3 isobutyric acid:1M NH4OH. The solvent was allowed to run to the top of the 20cm paper. The paper was dried and the origin cut out and placed in eppendorf tubes. 750μL of water was added and the tubes were placed at 100 °C for 4 hours. Heating samples allowed for a consistent amount of radioactive quenching (10% for 14C and 20% for 3H). 5mL of Ultima Gold scintillation cocktail was added and the samples were analyzed using a scintillation counter.
WTA Polyacrylamide Gel Electrophoresis for TarL and Natural WTAs
A 150μL reaction containing 1μM purified 1b and 4μM UDP-ManNAc was incubated at room temperature for 30 minutes with 500nM TarA in 20mM Tris-HCl pH 7.9 and 500mM NaCl. 5μM CDP-glycerol, 400μM CDP-ribitol, and 10mM MgCl2 was added. 600mL of water was then added to dilute the higher concentration of salt that was found to be slightly inhibitory to later stage enzymes and 500nM each of TarB, TarF and TarL was added and the reaction was allowed to proceed for and additional 2 hours at room temperature. The reactions were quenched with 600uL MeOH and evaporated using a speed vacuum. The reaction was resuspended in 15uL of 50:50 water:DMF and 120mM NaOH (so as to mimic the final step in WTA isolation from cells where the linkage between ManNAc and GroP is cleaved). The reaction mixture was shaken at room temperature for 15 minutes and the reaction was loaded onto a 16 cm (1.0mm thickness) polyacrylamide gel. For the separating gel a 30% total acrylamide and 6% crosslinking solution was made by adding 340mg Bisacrylamide to 30mL Protogel solution. 19.9mL of this acrylamide solution was added to 10mL 3M Tris(HCl), pH 8.5, 300μL 10% ammonium persulfate and 30μL TEMED. The stacking gel was prepared by mixing 1mL of Protogel solution, 3 mL of 3M Tris(HCl), pH 8.5, 6mL water, 100μL 10% ammonium persulfate and 10μL TEMED. The running buffer consisted of 0.1M Tris base and 0.1M Tricine, pH 8.1. A lane containing bromophenol blue was used as marker of gel progress. The gel was electrophoresed until the bromophenol blue was 1 inch from the bottom. The gel was stained using alcian blue and silver staining as described previously [33]. Wild-type WTA was a gift from Timothy Meredith.
Acknowledgements
This work was supported by the National Institutes of Health grant. S. B. is funded by an NSF Fellowship. We thank Dr. Timothy Meredith (Harvard Medical School, Boston MA) for providing extracted WTAs from S. aureus NCTC8325. We thank Xiao Fang for assistance in cloning TarI and TarJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplementary Table 1 – Primer Pairs for Cloning
(BamHI sites shown in bold and XhoI sites shown by underline.)
References
- 1.Neuhaus FC, Baddiley J. A continuum of anionic charge: structures and functions of D-alanyl-teichoic acids in gram-positive bacteria. Microbiol Mol Biol Rev. 2003;67:686–723. doi: 10.1128/MMBR.67.4.686-723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weidenmaier C, Kokai-Kun JF, Kristian SA, Chanturiya T, Kalbacher H, Gross M, Nicholson G, Neumeister B, Mond JJ, Peschel A. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat Med. 2004;10:243–245. doi: 10.1038/nm991. [DOI] [PubMed] [Google Scholar]
- 3.Weidenmaier C, Peschel A, Xiong YQ, Kristian SA, Dietz K, Yeaman MR, Bayer AS. Lack of wall teichoic acids in Staphylococcus aureus leads to reduced interactions with endothelial cells and to attenuated virulence in a rabbit model of endocarditis. J Infect Dis. 2005;191:1771–1777. doi: 10.1086/429692. [DOI] [PubMed] [Google Scholar]
- 4.Bera A, Biswas R, Herbert S, Kulauzovic E, Weidenmaier C, Peschel A, Gotz F. Influence of wall teichoic acid on lysozyme resistance in Staphylococcus aureus. J Bacteriol. 2007;189:280–283. doi: 10.1128/JB.01221-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vinogradov E, Sadovskaya I, Li J, Jabbouri S. Structural elucidation of the extracellular and cell-wall teichoic acids of Staphylococcus aureus MN8m, a biofilm forming strain. Carbohydr Res. 2006;341:738–743. doi: 10.1016/j.carres.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Walter J, Loach DM, Alqumber M, Rockel C, Hermann C, Pfitzenmaier M, Tannock GW. D-alanyl ester depletion of teichoic acids in Lactobacillus reuteri 100−23 results in impaired colonization of the mouse gastrointestinal tract. Environ Microbiol. 2007;9:1750–1760. doi: 10.1111/j.1462-2920.2007.01292.x. [DOI] [PubMed] [Google Scholar]
- 7.Gross M, Cramton SE, Gotz F, Peschel A. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect Immun. 2001;69:3423–3426. doi: 10.1128/IAI.69.5.3423-3426.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weidenmaier C, Kristian SA, Peschel A. Bacterial resistance to antimicrobial host defenses--an emerging target for novel antiinfective strategies? Curr Drug Targets. 2003;4:643–649. doi: 10.2174/1389450033490731. [DOI] [PubMed] [Google Scholar]
- 9.Heckels JE, Archibald AR, Baddiley J. Studies on the linkage between teichoic acid and peptidoglycan in a bacteriophage-resistant mutant of Staphylococcus aureus H. Biochem J. 1975;149:637–647. doi: 10.1042/bj1490637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokoyama K, Miyashita T, Araki Y, Ito E. Structure and functions of linkage unit intermediates in the biosynthesis of ribitol teichoic acids in Staphylococcus aureus H and Bacillus subtilis W23. Eur J Biochem. 1986;161:479–489. doi: 10.1111/j.1432-1033.1986.tb10469.x. [DOI] [PubMed] [Google Scholar]
- 11.Harrington CR, Baddiley J. Biosynthesis of wall teichoic acids in Staphylococcus aureus H, Micrococcus varians and Bacillus subtilis W23. Involvement of lipid intermediates containing the disaccharide N-acetylmannosaminyl N-acetylglucosamine. Eur J Biochem. 1985;153:639–645. doi: 10.1111/j.1432-1033.1985.tb09348.x. [DOI] [PubMed] [Google Scholar]
- 12.Fiedler F, Glaser L. The synthesis of polyribitol phosphate. I. Purification of polyribitol phosphate polymerase and lipoteichoic acid carrier. J Biol Chem. 1974;249:2684–2689. [PubMed] [Google Scholar]
- 13.Lazarevic V, Abellan FX, Moller SB, Karamata D, Mauel C. Comparison of ribitol and glycerol teichoic acid genes in Bacillus subtilis W23 and 168: identical function, similar divergent organization, but different regulation. Microbiology. 2002;148:815–824. doi: 10.1099/00221287-148-3-815. [DOI] [PubMed] [Google Scholar]
- 14.Qian Z, Yin Y, Zhang Y, Lu L, Li Y, Jiang Y. Genomic characterization of ribitol teichoic acid synthesis in Staphylococcus aureus: genes, genomic organization and gene duplication. BMC Genomics. 2006;7:74. doi: 10.1186/1471-2164-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward JB. Teichoic and teichuronic acids: biosynthesis, assembly, and location. Microbiol Rev. 1981;45:211–243. doi: 10.1128/mr.45.2.211-243.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhavsar AP, Truant R, Brown ED. The TagB protein in Bacillus subtilis 168 is an intracellular peripheral membrane protein that can incorporate glycerol phosphate onto a membrane-bound acceptor in vitro. J Biol Chem. 2005;280:36691–36700. doi: 10.1074/jbc.M507154200. [DOI] [PubMed] [Google Scholar]
- 17.Ginsberg C, Zhang YH, Yuan Y, Walker S. In vitro reconstitution of two essential steps in wall teichoic acid biosynthesis. ACS Chem Biol. 2006;1:25–28. doi: 10.1021/cb0500041. [DOI] [PubMed] [Google Scholar]
- 18.Schertzer JW, Bhavsar AP, Brown ED. Two conserved histidine residues are critical to the function of the TagF-like family of enzymes. J Biol Chem. 2005;280:36683–36690. doi: 10.1074/jbc.M507153200. [DOI] [PubMed] [Google Scholar]
- 19.Schertzer JW, Brown ED. Purified, recombinant TagF protein from Bacillus subtilis 168 catalyzes the polymerization of glycerol phosphate onto a membrane acceptor in vitro. J Biol Chem. 2003;278:18002–18007. doi: 10.1074/jbc.M300706200. [DOI] [PubMed] [Google Scholar]
- 20.D'Elia MA, Pereira MP, Chung YS, Zhao W, Chau A, Kenney TJ, Sulavik MC, Black TA, Brown ED. Lesions in teichoic acid biosynthesis in Staphylococcus aureus lead to a lethal gain of function in the otherwise dispensable pathway. J Bacteriol. 2006;188:4183–4189. doi: 10.1128/JB.00197-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badurina DS, Zolli-Juran M, Brown ED. CTP:glycerol 3-phosphate cytidylyltransferase (TarD) from Staphylococcus aureus catalyzes the cytidylyl transfer via an ordered Bi-Bi reaction mechanism with micromolar K(m) values. Biochim Biophys Acta. 2003;1646:196–206. doi: 10.1016/s1570-9639(03)00019-0. [DOI] [PubMed] [Google Scholar]
- 22.Pereira MP, Brown ED. Bifunctional catalysis by CDP-ribitol synthase: convergent recruitment of reductase and cytidylyltransferase activities in Haemophilus influenzae and Staphylococcus aureus. Biochemistry. 2004;43:11802–11812. doi: 10.1021/bi048866v. [DOI] [PubMed] [Google Scholar]
- 23.Walsh C, Wright G. Introduction: antibiotic resistance. Chem Rev. 2005;105:391–394. doi: 10.1021/cr030100y. [DOI] [PubMed] [Google Scholar]
- 24.Marra A. Targeting virulence for antibacterial chemotherapy: identifying and characterising virulence factors for lead discovery. Drugs R D. 2006;7:1–16. doi: 10.2165/00126839-200607010-00001. [DOI] [PubMed] [Google Scholar]
- 25.Hung DT, Shakhnovich EA, Pierson E, Mekalanos JJ. Small-molecule inhibitor of Vibrio cholerae virulence and intestinal colonization. Science. 2005;310:670–674. doi: 10.1126/science.1116739. [DOI] [PubMed] [Google Scholar]
- 26.Gotz F. Staphylococci in colonization and disease: prospective targets for drugs and vaccines. Curr Opin Microbiol. 2004;7:477–487. doi: 10.1016/j.mib.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Bae T, Banger AK, Wallace A, Glass EM, Aslund F, Schneewind O, Missiakas DM. Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc Natl Acad Sci U S A. 2004;101:12312–12317. doi: 10.1073/pnas.0404728101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bigham EC, Gragg CE, Hall WR, Kelsey JE, Mallory WR, Richardson DC, Benedict C, Ray PH. Inhibition of arabinose 5-phosphate isomerase. An approach to the inhibition of bacterial lipopolysaccharide biosynthesis. J Med Chem. 1984;27:717–726. doi: 10.1021/jm00372a003. [DOI] [PubMed] [Google Scholar]
- 29.Zolli M, Kobric DJ, Brown ED. Reduction precedes cytidylyl transfer without substrate channeling in distinct active sites of the bifunctional CDP-ribitol synthase from Haemophilus influenzae. Biochemistry. 2001;40:5041–5048. doi: 10.1021/bi002745n. [DOI] [PubMed] [Google Scholar]
- 30.Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC. Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Invest. 1996;98:378–385. doi: 10.1172/JCI118803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartlett GR. Methods for the isolation of glycolytic intermediated by column chromatography with ion exchange resins. J Biol Chem. 1959;234:459–465. [PubMed] [Google Scholar]
- 32.Pollack JH, Neuhaus FC. Changes in wall teichoic acid during the rod-sphere transition of Bacillus subtilis 168. J Bacteriol. 1994;176:7252–7259. doi: 10.1128/jb.176.23.7252-7259.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolters PJ, Hildebrandt KM, Dickie JP, Anderson JS. Polymer length of teichuronic acid released from cell walls of Micrococcus luteus. J Bacteriol. 1990;172:5154–5159. doi: 10.1128/jb.172.9.5154-5159.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 – Primer Pairs for Cloning
(BamHI sites shown in bold and XhoI sites shown by underline.)