Abstract
Single morphine injections induce a state of acute opioid dependence measured by an increase in naloxone potency to precipitate withdrawal. Repeated morphine exposure (daily/weekly intervals) results in further potentiation of naloxone potency, perhaps due to conditioning mechanisms. The current study tested the hypothesis that previously neutral stimuli could elicit a conditioned potentiation of the withdrawal response following acute bolus injections of morphine. Rats trained on an FR15 schedule for food received five morphine injections (5.6 mg/kg) at daily intervals. Four hr after morphine injection on Conditioning Days (first 4 days), naloxone (1 mg/kg)-induced suppression of responding was paired either with operant context only, or with a tone/light conditioned stimulus (CS). On Test Day low dose naloxone (0.001–0.33 mg/kg) given 4 hr post-morphine preceded the operant session. Rats exposed to naloxone repeatedly in the operant context without CS (Paired-Context Only) showed an increase in naloxone potency on Test Day relative to Unpaired Controls that received all morphine and naloxone in the home cage at a different time of day than operant testing. Rats exposed to the tone/light CS on Conditioning Days also showed a significant increase in naloxone potency relative to Unpaired Controls when the CS was represented on Test Day (Paired-CS), but not when the CS was omitted on the Test Day (Paired-CS/Test Context). Thus, conditioning processes appear to play a significant role in the early development of opioid dependence and withdrawal.
Keywords: Morphine, Dependence, Withdrawal, Abstinence, Addiction, Naloxone, Pavlovian Conditioning
1. Introduction
Leftward shifts in opioid antagonist dose-effect functions resulting from opioid agonist exposure allow quantitation of neuroadaptive changes associated with opioid dependence (Villereal and Castro, 1979; Way et al., 1969). With this method the magnitude of shift in the dose-effect function of the antagonist used to precipitate withdrawal is presumed to reflect the magnitude of the underlying state of dependence. Using this quantitative approach has revealed that even a single injection of an opioid agonist can elicit a state of “acute dependence” as measured by a variety of antagonist-precipitated withdrawal signs ranging from somatic/physiological to affective/subjective, in both humans and animal models (Adams and Holtzman, 1990; Azar et al., 2003; Azorlosa et al., 1994; Bickel et al., 1988; Cheney and Goldstein, 1971; Easterling and Holtzman, 1999; Heishman et al., 1989a, 1989b; Schulteis et al., 1997; Young, 1986).
As would be expected if acute dependence reflects the early stages in the development of a chronic opioid dependence state, repeated treatments with morphine at daily or weekly intervals can progressively increase the severity of withdrawal-like signs elicited upon antagonist administration (Adams and Holtzman, 1990; Azorlosa et al., 1994; Liu and Schulteis, 2004; Schulteis et al., 1999; Schulteis et al., 2003, 2004). Repeated experience with naloxone (i.e. repeated withdrawal) following each morphine pretreatment is necessary to produce a shift in antagonist potency under some (Liu and Schulteis, 2004; Schulteis et al., 1999; Schulteis et al., 2003, 2004) but not all experimental conditions (Azorlosa et al., 1994; Schulteis et al., 1997; Schulteis et al., 2003), implying that both naloxone-experience-dependent and naloxone-experience-independent processes contribute to the potentiation of withdrawal magnitude produced by repeated morphine exposure. It has been argued previously that naloxone-experience-independent processes reflect direct neuroadaptive responses to repeat administration of morphine itself (Schulteis et al., 2004), whereas the naloxone-experience-dependent processes may reflect underlying conditioning mechanisms (Adams and Holtzman, 1990; Schulteis et al., 1999, 2003, 2004).
Recently our group demonstrated that the contribution of repeated naloxone experience was context-dependent; repeated naloxone experience influenced subsequent withdrawal magnitude as measured by suppression of operant responding only when the naloxone experience occurred in the operant environment, and not when an equal amount of naloxone exposure occurred in the home cage environment (Schulteis et al., 2004). Thus, a conditioned association was apparently formed between naloxone-induced withdrawal and the operant context in which withdrawal was experienced.
It is well-established that withdrawal-associated stimuli in dependent humans can ultimately come to elicit withdrawal-like signs on their own through the establishment of conditioned withdrawal responses (O’Brien et al., 1976; Wikler, 1973). Evidence of conditioned withdrawal responses in models of acute opioid dependence (Adams and Holtzman, 1990; Schulteis et al., 2003, 2004) indicate that conditioning may begin to play a role very early in the development of opioid dependence. The current study sought to further establish the role of conditioning mechanisms in our acute opioid dependence model using naloxone-precipitated suppression of operant responding as a functional index of the underlying dependence state. To that end, we examined whether the naloxone-experience dependent component of the left-ward shift in the naloxone dose-effect function could become associated not only with the operant context as demonstrated previously (Schulteis et al., 2004), but also could become associated with a discrete conditioned stimulus (CS) that is paired with precipitated withdrawal from acute morphine pretreatment.
2. Materials and Methods
2.1 Animals
Male Wistar rats (n = 120, Harlan Labs, Indianapolis, IN) weighing 300–400 g at the time of testing were used. All rats were group housed (2–3/cage) in a temperature- and humidity-controlled room with a 12 hour light/12 hour dark cycle (lights ON at 6:00 AM). Rats had ad libitum access to food until the start of operant training, and had ad libitum access to water at all times. Once operant training was begun, rats were maintained on 15 g of rat chow per day in addition to the food pellets earned in the operant boxes (total food intake was approximately 20–22 g/rat/day). All training and testing took place from 9:00 AM to 4:00 PM daily, Monday thru Friday. On days when rats were not trained in the operant boxes (Saturday and Sunday), an additional 5 g of rat chow was provided to ensure that total food intake remained relatively constant. All rats continued to gain weight at an average of 10–20 g/week throughout training and testing. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the VA San Diego Healthcare System, an AAALAC-accredited facility, and are in strict accordance with the “Guide for the Care and Use of Laboratory Animals” (revised 1996).
2.2 Drugs
Morphine sulfate was purchased from King Pharmaceuticals, Inc (Bristol, TN, USA), and naloxone HCl was purchased from Sigma (St. Louis, MO, USA). Both drugs were prepared for injection in sterile physiological saline, and all injections were made subcutaneously (SC) in a volume of 0.1–ml/100 g body weight. Doses of both drugs are expressed as the salt. Morphine was administered at a dose of 5.6 mg/kg, and naloxone was administered at doses of 0.001 – 1.0 mg/kg.
2.3 Operant Training
Eight operant chambers (Coulbourn Instruments, Columbus, OH) served as the training and testing environments. Each chamber was equipped with a food hopper located 4 cm above a grid floor, a lever located to the right of the food hopper, and an LED located above the lever. The LED illuminated for 1 sec as a food pellet (45 mg) was delivered each time a rat completed a fixed-ratio (FR) component. Rats were autoshaped to lever press for food pellets in 30 min sessions five days a week, beginning on an FR-1 schedule and progressing to an FR-15 schedule (1 sec timeout).
After 2–3 weeks on the FR-15 schedule, daily training was separated into three phases: 1) a 10-min period of lever availability (designated pre-CS session or Pre-CS), 2) a 15-min timeout period where levers were retracted, and 3) a 20-min period where levers again were made available (designated CS Session). Once responding was stable for both Pre-CS and CS sessions of the daily training regimen (less than 10% variation in mean response rate over 5 consecutive days), rats received two SC injections of saline-vehicle separated by 4 hr, with the 2nd injection timed to occur during the 15 min timeout window between sessions, exactly 5 min prior to the onset of the CS Session. This procedure was repeated on 2 consecutive days, with response rates on the 2nd day serving as the vehicle baseline to which all subsequent changes in operant responding were compared.
2.4 Dependence Induction and Conditioning Regimen
The conditioning regimen, modified from that designed by Baldwin and Koob (Baldwin and Koob, 1993), consisted of four Conditioning Days and one Test Day, each separated by 24 hr. Drug regimens and experimental procedures for all groups are summarized in Table 1.
Table 1.
Summary of Experimental Design.
| Experimental Condition (All injections in Home Cage except those followed by CS or NO CSa) | |||||
|---|---|---|---|---|---|
| Control Groups | Paired Groups | ||||
| Mor-Nal Naive | Unpaired | Paired-CS | Paired-CS/Test Context | Paired-Context Only | |
| Conditioning Sessions (Days 1–4)
T = 0 T = 4 hr T = 8 hr |
VEH VEH + CSa VEH |
VEH + CSa MOR NAL |
MOR NAL + CSa VEH |
MOR NAL + CSa VEH |
MOR VEH + NO CSa VEH |
| Test Session (Day 5)
T = 0 T = 4 hr |
MOR NAL + CSa |
MOR NAL + CSa |
MOR NAL + CSa |
MOR NAL + NO CSa |
MOR NAL + NO CSa |
Abbreviations: MOR = morphine, NAL = naloxone, VEH = vehicle, CS = conditioned stimulus present in CS Session, NO CS = Context-Only in CS Session.
On Conditioning Days and Test Days, a 10-min operant session (Pre-CS Session) preceded the CS session, with the corresponding VEH or NAL injections administered 10 min after the conclusion of the Pre-CS Session and 5 min prior to onset of the CS Session. Note that on Conditioning Days the Unpaired group received vehicle prior to the CS session, and morphine and naloxone treatment followed rather than preceded the CS session. All other groups received a vehicle injection at the time when the Unpaired group received naloxone, to maintain a constant number of injections. Naloxone dose was fixed at 1.0 mg/kg on Conditioning Days for all groups, but varied as described in Methods on the Test Day.
2.4.1 Conditioning Days
Over 4 consecutive Conditioning Days, all rats in Paired groups were injected with morphine (5.6 mg/kg SC) followed 4 hr later by precipitation of acute withdrawal by naloxone (1.0 mg/kg SC). The doses of naloxone and morphine as well as the 4 hr interval between morphine and naloxone injection were selected based upon prior work that demonstrated reliable and reproducible acute withdrawal under these conditions (Schulteis et al., 1997, 1999, 2003, 2004). Naloxone was always administered 10 min after the conclusion of the Pre-CS session, and 5 min prior to onset of the CS Session. Precipitated withdrawal in all Paired groups therefore was experienced exclusively during the CS Session. Two of the Paired groups, Paired-CS and Paired-CS/Test Context were exposed on all Conditioning Days to the discrete tone/light compound CS (7 kHz, 85 dB tone plus house light on for 5 sec, off for 2 sec, repeating). A third Paired Group was exposed only to the operant chambers without the tone/light CS (Paired-Context Only).
In addition to the three Paired groups, there were 2 control conditions. An Unpaired control group received vehicle prior to each daily operant CS session, with morphine administered 4 hr and naloxone 8 hr after the operant session in the home cage, allowing for the assessment of repeated naloxone-induced withdrawal 4 hr post-morphine that was explicitly unpaired with operant context and CS. To equalize injection history across experimental groups, all other groups received a vehicle injection at the 8 hr time-point where the Unpaired group received naloxone (see Table 1). An additional control group received vehicle in place of both morphine and naloxone on conditioning days (Mor-Nal Naive), but still received CS exposure during the CS Session on all Conditioning Days. This permitted the assessment of CS effects on Test day in the absence of any prior morphine or naloxone exposure history.
2.4.2 Test Day
On the Test Day, all experimental groups, Paired and Control, received morphine (5.6 mg/kg) followed 4 hr later by naloxone and the operant test session, with subgroups of animals in each of the 5 experimental conditions receiving one of several low doses of naloxone (0.001 – 0.33 mg/kg). This permitted construction of a dose-effect function of naloxone potency to induce suppression of responding on the Test Day, and quantitation of shifts in naloxone potency associated with varying experimental conditions. The CS was presented during the CS Session on the Test Day in all experimental conditions except Paired-Context Only and Paired-CS/Test Context (see Table 1), since the effect produced by naloxone in response to operant context alone was to be assessed in these groups.
2.5 Data Analysis
Data on all experimental treatment days were expressed as % of response rate in the corresponding interval (Pre-CS or CS Session) on the Vehicle Baseline Day. Changes in responding during the Pre-CS Session across Conditioning and Test Days were assessed with mixed design ANOVAs in which experimental condition served as a between-subjects factor, and treatment days as a within-subjects factor. Follow-up comparisons consisted of interaction contrasts of pairs or trios (control or paired) of experimental conditions. Because all sub-groups within a given experimental condition differed only in the dose of naloxone administered 5 min prior to the CS Session on the Test Day, all animals within each of the 5 experimental groups had identical treatment histories up to and including the Pre-CS Session on the Test Day. Therefore, all sub-groups within a given experimental condition were pooled for the analysis of responding during the Pre-CS Session.
To minimize animal subject requirements while ensuring that naloxone was tested in the linear portion of its dose-effect function, different dose ranges of naloxone were tested under different experimental conditions as described above. This precluded analysis of naloxone dose-effects in the CS Session using standard ANOVA techniques. Thus, to compare naloxone potency across different experimental conditions during the CS Session, quantitative probit dose-response analysis was performed according to the method of Litchfield and Wilcoxon using computer software (Tallarida and Murray, 1987). Using this procedure, ED50 values (and 95% confidence limits) for naloxone were calculated under each experimental condition, and potency ratios (with 95% confidence limits) served as the measure of statistical reliability of any observed shifts in naloxone potency under different experimental conditions. This same approach has been used previously in our laboratory in quantitative analyses of naloxone-precipitated withdrawal from acute (Azar et al., 2003; Schulteis et al., 2003) and chronic (Schulteis et al., 1994) morphine dependence.
3. Results
As shown in Figure 1, responding in the Pre-CS Session varied significantly across treatment days as a function of experimental condition. An overall 2-factor mixed design ANOVA revealed a significant main effect of experimental condition (between-subjects: F [4,95] = 23.51, p < 0.0001), a significant main effect of treatment day (within-subjects: F [4,380] = 51.04, p < 0.0001), and a significant condition x day interaction (F [16,380] = 11.54, p < 0.0001). An interaction contrast comparing the Control groups (Mor-Nal Naive, Unpaired) still indicated a significant experimental condition x treatment day interaction (F [4,172] = 5.04, p < 0.001). The Unpaired group showed slightly lower response rates on Conditioning Days 2–4 than the Mor-Nal Naive group, and this is likely due to the lack of morphine history in the latter group. Consistent with a prior report (Liu and Schulteis, 2004), it is possible that a modest degree of spontaneous withdrawal from morphine on Conditioning Days 2–4 may be demonstrable during the Pre-CS session in groups exposed repeatedly to morphine, which includes the Unpaired control group.
Fig. 1.
Responding during the Pre-CS Session, prior to naloxone injection and CS onset, declines in a time-dependent fashion in all paired groups receiving naloxone-withdrawal experience in the operant context (*p < 0.05 Paired groups vs Unpaired), but not in control groups. Data represent mean (± SEM) percent of baseline response rate. Because rats within a given experimental group (paired or control) did not differ in their treatment until the naloxone injection after the Pre-CS Session and prior to the CS Session on the Test Day, all rats within an experimental group are represented in this graph (n = 15–24/group).
A second interaction contrast comparing all Paired groups (Paired-Context Only, Paired-CS/Test Context, Paired-CS) revealed a significant main effect of treatment day (F [4,208] = 50.03, p < 0.0001), but no significant main effect of experimental condition (F [2,52] = 2.48, N.S.), or condition x day interaction (F [8,208] = 1.97, N.S.). This pattern is explained by a time-dependent decline in responding during the Pre-CS Session as a function of treatment day in all 3 Paired treatment conditions. Finally, each Paired group was compared in a separate interaction contrast to the Unpaired control group, which had an identical history of morphine and naloxone exposure to the Paired groups, but not in temporal contiguity with the CS Session. All three comparisons revealed a significant experimental day x treatment condition interaction (all F’s > 16.09, p’s < 0.0001), indicating that the time-dependent decline in responding during the Pre-CS Session beginning on Conditioning Day 3 was specific to groups in which naloxone-induced acute withdrawal was explicitly paired with the operant context (Paired-Context Only) or both operant context and the tone/light CS (Paired- CS/Test Context, Paired-CS). This decline in responding during the Pre-CS Session occurred prior to any naloxone or CS exposure on a given Conditioning Day, and reflects suppression in response to the operant context alone. Thus, as reported previously (Schulteis et al., 2004), context-specific suppression of operant responding is rapidly acquired under conditions where said context is predictive of naloxone-induced withdrawal from acute morphine treatment, as it was when naloxone was administered 10 min after the Pre-CS Session on each Conditioning Day in all Paired groups, but not in the Unpaired control group.
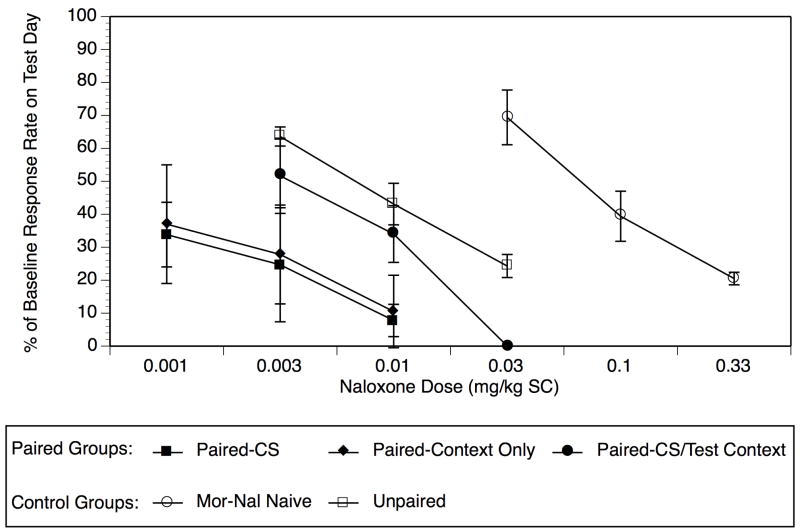
Figure 2 illustrates the naloxone-dose effect functions derived during the CS Session on the Test Day as a function of experimental condition, and Table 2 presents the ED50 value under each experimental condition as well as potency ratio comparisons across conditions. Naloxone dose-dependently suppressed responding in the group receiving morphine and naloxone for the very first time on the Test Day (Mor-Nal Naive), with an ED50 of 0.07 mg/kg. Relative to this single morphine treatment control condition, repeated treatment with morphine and naloxone (4 hr later) explicitly unpaired with the operant context and CS (Unpaired control) resulted in a significant 10.44-fold increases in naloxone potency. This shift in naloxone potency in the Unpaired group is comparable to what has been reported previously for groups receiving repeated morphine outside of any operant context (Schulteis et al., 2004), and is presumably due to the direct neuroadaptive response to repeated morphine.
Fig. 2.
Shifts in naloxone potency during the CS Session on the Test Day varied as a function of experimental group. Relative to Unpaired controls, rats exposed to naloxone repeatedly in the operant context without a tone/light CS showed a significant shift to the left when re-exposed to the operant environment on the Test Day (Paired-Context Only). Rats presented repeatedly with the CS on Conditioning Days showed a significant shift to the left in the naloxone dose-response curve relative to Unpaired Controls when the CS was represented on Test Day (Paired-Train CS/Test CS), but not when the CS was omitted on the Test Day (Paired-Train CS/Test Context). Data represent mean (± SEM) percent of baseline response rate. Sample size varied from 7–11/data point for each of 3 naloxone doses within each experimental group. Statistical analysis of the data consisted of potency ratio analysis (Tallarida and Murray, 1987), and is summarized in Table 2.
Table 2.
ED50 Values and Potency Ratios for Naloxone-Induced Suppression of Operant Response Rates on Test Day as a Function of Experimental Condition.
| Potency Ratio (95% CL) vs...
|
|||||
|---|---|---|---|---|---|
| Experimental Condition | ED50 Value (mg/kg) (95% CL)a | Mor-Nal Naive | Unpaired | Paired-Context Only | Paired- CS/Test Context |
| Mor-Nal Naive | 0.07 (0.030 -- 0.178) | ------- | ------- | ------- | ------- |
| Unpaired | 0.007 (0.002 -- 0.026) | 10.44* (2.12 -- 51.50) | ------- | ------- | ------- |
| Paired-Context Only | 0.0005 (0.00007 -- 0.004) | 141.73* (15.77 -- 1274) | 13.57* (1.22 -- 150.80) | ------- | 8.41* (1.00 -- 70.47) |
| Paired- CS/Test Context | 0.004 (0.002 -- 0.009) | 16.85* (5.49 -- 51.89) | 1.61, N.S. (0.36 -- 7.21) | ------- | ------- |
| Paired- CS | 0.0005 (0.0001 -- 0.002) | 156.55* (24.47 -- 1002) | 14.99* (1.83 -- 122.80) | 1.10, N.S. (0.08 --14.69) | 9.29* (1.58 -- 54.69) |
p < 0.05 as determined by relative potency analysis (Tallarida and Murray 1986), N.S. = not significant.
95% confidence limits (CL) for calculated ED50 and potency ratio provided in parentheses.
In most paired groups there were further significant shifts to the left in naloxone potency on the Test Day relative to the Unpaired condition. For example, relative to the Unpaired control condition, rats receiving naloxone in association with the operant-context only on Conditioning Days (Paired-Context Only) demonstrated a significant 13.57-fold increase in naloxone potency when placed back into the operant context on the Test Day. In rats receiving naloxone on Conditioning Days in association with both operant context and the tone/light CS, a similar 14.99-fold increase in naloxone potency was noted relative to Unpaired Controls when the CS was re-presented on the Test Day (Paired- CS). However, no significant shift in naloxone potency relative to the Unpaired condition was noted when the CS was omitted on Test Day in rats trained with both CS and context on Conditioning Days (Paired- CS/Test Context).
4. Discussion
The current study confirms prior reports of potentiation of naloxone-precipitated withdrawal severity produced by repeated acute bolus injections of a moderate dose of morphine (Adams and Holtzman, 1990; Schulteis et al., 1997, 1999; Schulteis et al., 2003, 2004; White-Gbadebo and Holtzman, 1994). Prior reports also have indicated that under certain experimental conditions repeated naloxone withdrawal experience can further potentiate the observed withdrawal response beyond what is produced by repeated morphine alone, putatively through conditioning mechanisms (Adams and Holtzman, 1990; Schulteis et al., 1999, 2003, 2004). For example, we previously demonstrated that potentiation of withdrawal severity across repeated morphine pretreatments at 24 hr intervals occurred when naloxone-induced withdrawal was always experienced within the operant context, but not when the repeated withdrawal was instead experienced in the home cage (Schulteis et al., 2004). The current study confirms and extends these findings of context-dependent effects of naloxone experience. Thus, relative to an Unpaired group that received ALL of its naloxone-withdrawal experience 8 hr after each daily operant training session in the home cage, a group that received repeated naloxone in the operant context (Paired-Context Only) showed a nearly 14-fold shift in naloxone potency (see Figure 2 and Table 2). In addition, during the Pre-CS Session, the 10 min operant session that always preceded naloxone injection, a significant suppression of response rate emerged in all three Paired groups on Conditioning Days 3–4 and the Test Day (see Figure 1). This time-dependent suppression was substantially greater in groups that were repeatedly experiencing naloxone-induced withdrawal in the operant context beginning 10 min after the Pre-CS Session relative to the Unpaired group that received the same amount of morphine and naloxone exposure, but not in temporal contiguity with daily operant sessions. These data are therefore consistent with our earlier suggestion (Schulteis et al., 2004) that the experience of all unique elements provided by the morphine acute dependence induction and naloxone withdrawal regimen resulted in the formation of a new episodic context within an otherwise familiar operant environment. This novel contextual representation reliably predicts the onset of a state of acute opioid withdrawal, with a corresponding shift to withdrawal-related behaviors (e.g. suppression of responding) upon subsequent exposure to this withdrawal-predictive context.
This notion is entirely consistent with recent theories of contextual conditioning, which emphasize that a “context” consists not merely of fixed geometric features of the environment but also includes multi-modal sensory (visual, tactile, olfactory etc.) cues and temporal or episodic context (Anagnostaras et al., 2001; Moser and Paulsen, 2001). Therefore, multiple possible representations of a given spatial context may be encoded in an episodically unique fashion depending on the other elements that may be present or absent at a given point in time (“episode”). Within the framework of our acute opioid dependence model, the newly formed predictive context likely includes features that are unique to the days on which withdrawal was experienced such as drug administration cues, and perhaps even the interoceptive cues provided by the drugs themselves (Adams and Holtzman, 1990; Sokolowska et al., 2002; Schulteis et al., 2004).
The current study extends beyond our prior work in clearly demonstrating that superimposition of a more salient external stimulus (tone/light CS) can overshadow the salience of episodic context. In a group exposed to the CS within the operant context on Conditioning Days, and also tested with the CS on the Test Day (Paired CS), there was a 15-fold shift in naloxone potency relative to the Unpaired group. This shift in naloxone potency relative to the Unpaired control condition was not observed when the CS was presented on Conditioning Days but omitted on the Test Day (Paired- CS/Test Context; see Figure 2 and Table 2). In fact, context exerted a greater effect on naloxone potency when it was the only reliable cue (Paired-Context Only) than when it was combined with the CS on conditioning days (Paired-CS/Test Context; see Figure 2 and Table 2). Thus, a discrete CS that was uniquely presented ONLY in direct contiguity with naloxone-precipitated withdrawal was able to overshadow and hence weaken the influence of the operant context during the CS Session. Using a similar tone/light CS to the one employed herein, we have recently demonstrated that conditioned withdrawal responses to this unique stimulus are measurable without any morphine or naloxone administered on the test day (Amitai et al., 2004). This suggests that the cues provided by drug administration and the interoceptive stimulus properties of morphine and/or naloxone need not be present at all to evoke a state of conditioned withdrawal from acute morphine, if novel predictive stimuli are available.
Taken together with earlier results, the current study suggests that conditioned associations with precipitated acute opioid withdrawal as measured with suppression of responding (Schulteis et al., 1999; Schulteis et al., 2003, 2004; Sokolowska et al., 2002) or place aversion (Azar et al., 2003; Parker et al., 2002; Parker and Joshi, 1998) paradigms appear to be rapidly formed and quite robust. For example, naloxone potency to suppress operant response rates (Schulteis et al. 2003, 2004, current study) after as few as four acute bolus doses of morphine (5.6 mg/kg) is comparable to its potency after chronic exposure to high levels of morphine (Schulteis et al. 1994), but only if conditions are present that permit context-specific or cue-specific conditioning. In the absence of conditioning, the severity of withdrawal following 4 doses of morphine is substantially less than that observed under conditions of chronic exposure. In addition, as few as two or even one pairing of a unique environment with naloxone-precipitated acute withdrawal results in robust conditioned place aversions (Azar et al. 2003; Parker and Joshi 1998; Parker et al 2002). Finally, context-specific conditioned withdrawal-like responses are formed even when successive morphine treatments (and conditioning opportunities) were separated by intervals of six weeks (Schulteis et al. 1999). These data therefore suggest that the neural substrates mediating the response-disruptive and aversive stimulus effects of naloxone in opioid-dependent rats show particularly rapid and long-lasting neuroadaptive response to limited acute treatment with morphine, and that conditioning processes make a significant contribution to the development of this response.
This may have important implications for the role of conditioned drug-like and drug-opposite (e.g. withdrawal) responses in the development and maintenance of patterns of compulsive drug use (Childress et al., 1999; Di Chiara et al., 1999; Everitt et al., 2001; O’Brien et al., 1976; Wikler, 1973). In particular, the data derived from acute opioid dependence models suggest that conditioning mechanisms are influencing the magnitude of drug withdrawal responses at the very onset of opioid dependence, when use of opioids may still be occasional rather than habitual, and perhaps also most amenable to intervention.
It has been argued that further development of effective treatment and intervention strategies for habitual drug use and relapse after abstinence requires a full understanding of both unconditioned and conditioned drug-like and drug-opposite (i.e. withdrawal) responses (Di Chiara et al., 1999; Everitt et al., 2001; Koob and Le Moal, 2001). The model of conditioned withdrawal from acute opioid dependence developed herein aids these development efforts by providing a more complete appreciation of the contributions of all potential conditioned stimuli that can become associated with opioid withdrawal after just a few bolus doses of morphine. For example, it is now clear that even a relatively familiar drug-taking environment can rapidly acquire new episodic contextual relevance as a stimulus predictive of acute opioid withdrawal, when it is the most salient predictive cue available (e.g. Paired-Context Only in current study; see also Amitai et al., 2004; Schulteis et al., 2004). The interoceptive drug cue provided by morphine itself may contribute to this novel episodic contextual representation to facilitate a more lasting and robust conditioned withdrawal response (current study, Amitai et al., 2004; Schulteis et al., 2003, 2004; Sokolowska at al., 2002), but is not absolutely necessary to evoke a conditioned withdrawal response under all conditions (Amitai et al., 2004). Finally, the presence of discrete novel stimuli (e.g. drug paraphernalia) can overshadow the salience of the familiar environmental features, with the novel stimuli then becoming the predominant predictive stimuli (Paired-CS vs. Paired-CS/Test Context).
We recently reported that the brain sites most sensitive to the response-suppressing effects of the quaternary naloxone analog methylnaloxonium are elements of the “extended amygdala” such as the nucleus accumbens and the bed nucleus of the stria terminalis (Liu et al. 2002). This same system is critical to the positive hedonic effects of opioids and other drugs of abuse (Di Chiara et al., 1999; Koob and Le Moal, 2001). Projections of the basolateral amygdala to nucleus accumbens, bed nucleus of the stria terminalis, and central amygdala are critical to the formation of context- and cue-conditioned associations with the rewarding properties of heroin and cocaine (Alderson et al., 2000; Kalivas and McFarland, 2003; Kantak et al., 2002; See et al., 2003). Our recent report that excitotoxic lesions of the basolateral amygdala disrupt cue-conditioned withdrawal in rats chronically dependent on morphine is therefore consistent with this notion that circuitry contributing to conditioned reward and conditioned withdrawal may overlap (Schulteis et al., 2000). The utility of the conditioned withdrawal model for acute dependence as developed herein lies most immediately in its application to further differentiation of the neural substrates mediating unconditioned withdrawal, cue-conditioned withdrawal, and context-conditioned withdrawal.
Acknowledgments
This study was supported by a VA Merit Award (Dept. of Veterans Affairs Biomedical Research) and PHS grant DA10475 (National Institute on Drug Abuse) to GS. Portions of these data were reported previously at the 2004 meeting of the Society for Neuroscience (Amitai et al., 2004).
References
- Adams JU, Holtzman SG. Pharmacologic characterization of the sensitization to the rate-decreasing effects of naltrexone induced by acute opioid pretreatment in rats. J Pharmacol Exp Ther. 1990;253(2):483–9. [PubMed] [Google Scholar]
- Alderson HL, Robbins TW, Everitt BJ. The effects of excitotoxic lesions of the basolateral amygdala on the acquisition of heroin-seeking behaviour in rats. Psychopharmacology (Berl) 2000;153(1):111–9. doi: 10.1007/s002130000527. [DOI] [PubMed] [Google Scholar]
- Amitai N, Liu J, Schulteis G. 2004 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; Cue-conditioned morphine withdrawal in acutely dependent rats. CD-ROM, 2002; program no. 594.4. [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11(1):8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Azar MR, Jones BC, Schulteis G. Conditioned place aversion is a highly sensitive index of acute opioid dependence and withdrawal. Psychopharmacology (Berl) 2003;170(1):42–50. doi: 10.1007/s00213-003-1514-y. [DOI] [PubMed] [Google Scholar]
- Azorlosa JL, Stitzer ML, Greenwald MK. Opioid physical dependence development: effect of single versus repeated morphine pretreatments and of subjects’ opioid exposure history. Psychopharmacology (Berl) 1994;114:71–80. doi: 10.1007/BF02245446. [DOI] [PubMed] [Google Scholar]
- Baldwin HA, Koob GF. Rapid induction of conditioned opiate withdrawal in the rat. Neuropsychopharmacology. 1993;8(1):15–21. doi: 10.1038/npp.1993.3. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Stitzer ML, Liebson IA, Bigelow GE. Acute physical dependence in man: effects of naloxone after brief morphine exposure. J Pharmacol Exp Ther. 1988;244(1):126–32. [PubMed] [Google Scholar]
- Cheney DL, Goldstein A. Tolerance to opioid narcotics: time course and reversibility of physical dependence in mice. Nature. 1971;232(5311):477–8. doi: 10.1038/232477a0. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156(1):11–8. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Cadoni C, Carboni E. Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Ann N Y Acad Sci. 1999;877:461–85. doi: 10.1111/j.1749-6632.1999.tb09283.x. [DOI] [PubMed] [Google Scholar]
- Easterling KW, Holtzman SG. Discriminative stimulus effects of naltrexone after a single dose of morphine in the rat. J Pharmacol Exp Ther. 1999;288(3):1269–77. [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36(2–3):129–38. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Stitzer ML, Bigelow GE, Liebson IA. Acute opioid physical dependence in humans: effect of varying the morphine-naloxone interval. I J Pharmacol Exp Ther. 1989a;250(2):485–91. [PubMed] [Google Scholar]
- Heishman SJ, Stitzer ML, Bigelow GE, Liebson IA. Acute opioid physical dependence in postaddict humans: naloxone dose effects after brief morphine exposure. J Pharmacol Exp Ther. 1989b;248(1):127–34. [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168(1–2):44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002;22(3):1126–36. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Liu J, McElfresh A, Reis S, Fuqua L, Schulteis G. 2002 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; Neuroadaptive processes in limbic and basal forebrain reward circuitry contribute to acute opioid dependence. CD-ROM, 2002; program no. 310.10. [Google Scholar]
- Liu J, Schulteis G. Brain reward deficits accompany naloxone-precipitated withdrawal from acute opioid dependence. Pharmacol Biochem Behav. 2004;79(1):101–8. doi: 10.1016/j.pbb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Moser EI, Paulsen O. New excitement in cognitive space: between place cells and spatial memory. Curr Opin Neurobiol. 2001;11(6):745–51. doi: 10.1016/s0959-4388(01)00279-3. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Testa T, O’Brien TJ, Greenstein R. Conditioning in human opiate addicts. Pavlov J Biol Sci. 1976;11(4):195–202. doi: 10.1007/BF03000314. [DOI] [PubMed] [Google Scholar]
- Parker LA, Cyr JA, Santi AN, Burton PD. The aversive properties of acute morphine dependence persist 48 h after a single exposure to morphine: evaluation by taste and place conditioning. Pharmacol Biochem Behav. 2002;72(1–2):87–92. doi: 10.1016/s0091-3057(01)00724-9. [DOI] [PubMed] [Google Scholar]
- Parker LA, Joshi A. Naloxone-precipitated morphine withdrawal induced place aversions: effect of naloxone at 24 hours postmorphine. Pharmacol Biochem Behav. 1998;61(3):331–3. doi: 10.1016/s0091-3057(98)00104-x. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Ahmed SH, Morse AC, Koob GF, Everitt BJ. Conditioning and opiate withdrawal. Nature. 2000;405(6790):1013–4. doi: 10.1038/35016630. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Heyser CJ, Koob GF. Opiate withdrawal signs precipitated by naloxone following a single exposure to morphine: potentiation with a second morphine exposure. Psychopharmacology (Berl) 1997;129(1):56–65. doi: 10.1007/s002130050162. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Heyser CJ, Koob GF. Differential expression of response-disruptive and somatic indices of opiate withdrawal during the initiation and development of opiate dependence. Behav Pharmacol. 1999;10(3):235–42. doi: 10.1097/00008877-199905000-00001. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Gold LH, Stinus L, Koob GF. Relative sensitivity to naloxone of multiple indices of opiate withdrawal: a quantitative dose-response analysis. J Pharmacol Exp Ther. 1994;271(3):1391–8. [PubMed] [Google Scholar]
- Schulteis G, Morse AC, Liu J. Repeated experience with naloxone facilitates acute morphine withdrawal: potential role for conditioning processes in acute opioid dependence. Pharmacol Biochem Behav. 2003;76(3–4):493–503. doi: 10.1016/j.pbb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Morse AC, Liu J. Conditioning processes contribute to severity of naloxone-precipitated withdrawal from acute opioid dependence. Psychopharmacology (Berl) 2004 doi: 10.1007/s00213-004-1843-5. [DOI] [PubMed] [Google Scholar]
- See RE, Fuchs RA, Ledford CC, McLaughlin J. Drug addiction, relapse, and the amygdala. Ann N Y Acad Sci. 2003;985:294–307. doi: 10.1111/j.1749-6632.2003.tb07089.x. [DOI] [PubMed] [Google Scholar]
- Sokolowska M, Siegel S, Kim JA. Intraadministration associations: conditional hyperalgesia elicited by morphine onset cues. J Exp Psychol Anim Behav Process. 2002;28(3):309–20. [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. Manual of Pharmacologic Calculations with Computer Programs. 2. New York: Springer-Verlag; 1987. [Google Scholar]
- Villereal J, Castro A. A reformulation of the dual-action model of opioid dependence: Opioid-specific neuronal kindling. In: Beers RF, Bassett EG, editors. Mechanisms of Pain and Analgesic Compounds. New York: Raven Press; 1979. pp. 407–28. [Google Scholar]
- Way EL, Loh HH, Shen FH. Simultaneous quantitative assessment of morphine tolerance and physical dependence. J Pharmacol Exp Ther. 1969;167(1):1–8. [PubMed] [Google Scholar]
- White-Gbadebo D, Holtzman SG. Acute sensitization to opioid antagonists. Pharmacol Biochem Behav. 1994;47(3):559–66. doi: 10.1016/0091-3057(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Wikler A. Dynamics of drug dependence. Implications of a conditioning theory for research and treatment. Arch Gen Psychiatry. 1973;28(5):611–6. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- Young AM. Effects of acute morphine pretreatment on the rate-decreasing and antagonist activity of naloxone. Psychopharmacology (Berl) 1986;88(2):201–8. doi: 10.1007/BF00652241. [DOI] [PubMed] [Google Scholar]