Abstract
Regulation of cellular adhesion and cytoskeletal dynamics is essential for neurulation, though it remains unclear how these two processes are coordinated. Members of the Ena/VASP family of proteins are localized to sites of cellular adhesion and actin dynamics and lack of two family members, Mena and VASP, in mice results in failure of neural tube closure. The precise mechanism by which Ena/VASP proteins regulate this process, however, is not understood. In this report, we show that Xenopus Ena (Xena) is localized to apical adhesive junctions of neuroepithelial cells during neurulation and that Xena knockdown disrupts cell behaviors integral to neural tube closure. Changes in the shape of the neural plate as well as apical constriction within the neural plate are perturbed in Xena knockdown embryos. Additionally, we demonstrate that Xena is essential for cell-cell adhesion. These results demonstrate that Xena plays an integral role in coordinating the regulation of cytoskeletal dynamics and cellular adhesion during neurulation in Xenopus.
Keywords: Ena/VASP, neurulation, morphogenesis, adhesion, actin dynamics, apical wedging
Introduction
Neurulation is the morphogenetic process whereby the flat neural plate is converted into a closed neural tube (Colas and Schoenwolf, 2001). Dysregulation of this process results in neural tube defects (NTDs), a class of birth defects including anencephaly and spina bifida that occur at a rate of 1:1000 live births within the human population (Copp et al., 1990; Golden and Chernoff, 1995). Though the incidence of these defects has decreased within the population through dietary supplementation of folate (Czeizel and Dudas, 1992), our understanding of the cellular and molecular mechanisms underlying the genesis of these defects remains incomplete.
The cell behaviors underlying neurulation have been well characterized in a variety of vertebrate systems (Colas and Schoenwolf, 2001; Davidson and Keller, 1999). Neural tube formation begins with the thickening and shaping of the neural epithelium as cells elongate along their apicobasal axis. Cells of the neural plate then undergo mediolateral intercalation movements, termed neural convergent extension, which lengthens the plate along the anterioposterior axis and narrows the plate mediolaterally. Cells at specific regions called hingepoints then undergo apical constriction, which bends the neural plate and elevate the neural folds. Intrinsic and extrinsic forces then facilitate medial movement of the folds, which then meet and fuse at the midline. Lastly, the neural tube separates from the overlying epidermal tissue (Smith and Schoenwolf, 1997). The successful completion of neural tube closure requires the precise coordination of several morphogenetic behaviors including changes in cell migration, shape, and adhesion (Colas and Schoenwolf, 2001; Davidson and Keller, 1999).
A number of genes have been identified that are required for neural tube closure and a significant proportion of these genes encode proteins that regulate the actin cytoskeleton and cell adhesion. Members of the Planar Cell Polarity (PCP) pathway (Seifert and Mlodzik, 2007), including strabismus/Van Gogh (Darken et al., 2002; Kibar et al., 2001; Ybot-Gonzalez et al., 2007), Dishevelled (Wallingford and Harland, 2002; Wang et al., 2006) and Celsr1/flamingo (Curtin et al., 2003) are required for neural convergent extension movements. Other regulators of the cytoskeleton such as vinculin (Xu et al., 1998), Mena/VASP (Lanier et al., 1999; Menzies et al., 2004), Shroom (Hildebrand and Soriano, 1999), Marcks (Stumpo et al., 1995) and Abl/Arg (Koleske et al., 1998) are also necessary for proper neural tube closure. Furthermore, pharmacological disruption of actin microfilaments within the neural tube disrupts neurulation (Schoenwolf et al., 1988; Ybot-Gonzalez and Copp, 1999), indicating a critical role for the actin cytoskeleton and its regulation in neural tube closure. Several molecules involved in adhesion have also been found to be important in neural tube closure including N-cadherin (Radice et al., 1997) as well as the loss of a combination of integrins (De Arcangelis et al., 1999). These reports emphasize the important role of the actin cytoskeleton and adhesion in regulating neurulation.
Ena/VASP proteins act as regulators of actin dynamics by binding to actin filaments and promoting filament elongation while inhibiting branching (Bear et al., 2002). This function is exemplified by the localization of these proteins to filopodia and lamellipodia in migrating cells where elongation of actin filaments is needed to drive extension of the membrane critical for motility (Bear et al., 2002). Additionally, Ena and VASP are both localized to adherens junctions, important structures for cell-cell adhesion (Grevengoed et al., 2001; Vasioukhin et al., 2000), as well as focal adhesions, structures linking the cytoskeleton with integrins and the extracellular matrix (Gertler et al., 1996). These localizations suggest that Ena/VASP proteins are key links between the actin cytoskeleton and adhesive structures. Two Ena/VASP family members, Mena and VASP, are essential for neurulation in the mouse. Mena−/−VASP−/− progeny die perinatally with defects in cephalic neural tube closure and craniofacial structures (Lanier et al., 1999; Menzies et al., 2004). The underlying cellular mechanism causing these defects has not been fully examined, but data from work in cultured cells suggests that Ena/VASP proteins may play an important role in coordinating actin dynamics and the linkage of the actin cytoskeleton to cell surface adhesion receptors during neural tube closure.
In this report, we examined the function of Xenopus Ena (Xena) to gain insights into the molecular mechanisms that control neural tube closure. We found that Xena is enriched at apical cell-cell junctions within neuroepithelial cells throughout neurulation. Knockdown of Xena using an antisense morpholino disrupts neurulation and blocks several morphogenetic events essential for neural tube closure including changes in the shape of the neural plate and apical constriction and elevation of the neural folds. Additionally, neuroepithelial cells in Xena depleted embryos appear loosely adherent and reaggregation assays demonstrate that Xena is required for optimal cell-cell adhesion. Together these data demonstrate that Xena is essential for neural tube closure in Xenopus and suggest that Xena controls cell behaviors required for neurulation by regulating the actin cytoskeleton and cellular adhesion.
Materials and methods
Embryos and microinjections
Xenopus laevis embryos were obtained by fertilization of eggs from females injected with human chorionic gonadotropin (Sigma). Eggs were dejellied in 2% cysteine (Sigma), cultured in 0.33× MMR (Sive et al., 2000), and staged according to Nieuwkoop and Faber (Nieuwkoop and Faber, 1967). Capped mRNA for microinjections was synthesized using the SP6 mMessage Machine kit (Ambion) and embryos were injected in 4% ficoll in 0.33×MMR. Morpholinos (MO) were obtained from Gene Tools (Philomath, OR). Two MOs were used in this study, a splice blocking MO (sMO) and a translation blocking MO (MO2). To identify sites of putative introns for targeting by an antisense morpholino, the cDNA sequence for Xena (Xanthos et al., 2005) was compared with the genomic sequences for Xenopus tropicalis, mouse and human Ena. Primers surrounding a likely site for intron 2 were created and used to amplify and sequence this intron from Xenopus laevis genomic DNA. The sequence was then used to design a MO targeting the splice acceptor site at the boundary of intron 2 and exon 3 (Xena sMO: CACAAACCCATACCTGGTGATCCT). Xena MO2 targets a sequence in the 5'-UTR (XenaMO2: TGTGTCTCACTTTGCTCCTCCCCCG), while misMO2 has 5 basepair mismatches to that target sequence (misMO2: TGTCTCTGACTTTGGTCCTGCCACG).
Analysis of neural tube phenotypes
Phenotypes of knockdown embryos were scored using the following criteria. Normal: embryos with elevated neural folds that met at the midline along the entire length of the embryo. Mild: embryos with elevated neural folds slightly separated. Moderate: embryos with neural folds moderately separated, anterior folds are separated by about half the width of the embryo. Severe: embryos with almost no neural fold elevation, anterior folds are positioned on the lateral sides of the embryo. Endoderm: failure of blastopore to close and endoderm is exposed.
In situ hybridization
In situ hybridization was carried out as described (Harland, 1991). Digoxigenin-labeled Sox2 and Pax3 probes were synthesized using a MAXI Script kit (Ambion) and purified using MEGA Clear columns (Ambion). Probes were detected by alkaline phosphatase-conjugated anti-digoxigenin (Roche) using BM Purple substrate (Roche Diagnostics). Photographs of embryos were taken using a digital camera (Nikon Coolpix). Measurements of gene expression domains were made using NIH Image J. Statistical analysis was performed using Microsoft Excel.
Immunofluorescence
Embryos were fixed at appropriate stages in 4% formaldehyde in CSK buffer (10 mM Hepes pH 7.5, 150 mM sucrose, 100 mM KCl, 2 mM EGTA) for 1 hour at room temperature or in Dent's fix (80% methanol, 20% DMSO) overnight at 4° C. Whole embryos were washed in PBS and then embedded in 4% agarose in PBS. Embedded embryos were stored in PBS+0.1% Triton-X100 overnight at 4° C and subsequently stored in PBS. Embryos were sectioned using a vibratome into 200 μM sections. Sections were incubated in PBST, 2% BSA, 10% normal goat serum (NGS) to prevent non-specific binding of antibodies. Staining with primary and secondary (Molecular Probes) antibodies was performed in PBST, 2% BSA, 10% NGS for 2 hours at room temperature. Actin was visualized using Alexa-568 or Alexa-647 phalloidin (Molecular Probes). Images were collected using a Zeiss spinning disc confocal microscope and digital images were processed using Adobe Photoshop. Animal caps were cut at stage 9 using a Gastromaster microdissection instrument (Xenotek Engineering) and then fixed and stained as above. Neural plates were cut at appropriate stages using an eyebrow knife and fixed and stained as above.
The following antibodies and dilutions were used: anti-β-catenin monoclonal antibody (Santa Cruz Biotechnology, 1:50 dilution), anti-GFP polyclonal antibody (Santa Cruz Biotechnology, 1:250), anti-Xena polyclonal antibody (1:100) (Xanthos et al., 2005), anti-β-tubulin monoclonal antibody (E7, 1:50; Developmental Studies Hybridoma Bank, DSHB developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242.), and anti-vinculin monoclonal antibody (VN3−24, 1:25; DSHB).
RT-PCR analysis
RT-PCR analysis was performed using total RNA isolated from whole embryos and animal caps at the indicated stages. cDNA was synthesized using SuperScript III reverse transcriptase (Invitrogen). Primers used for RT-PCR: sMO forward 5'-CGCGGGGGAGGAGCAAAGTGAGACA-3' (nucleotides −28 to −4 in the 5'-UTR of the Xena cDNA) and sMO reverse 5'-TCCTGGGAGTTCAGCACCTCTAAG-3' (nucleotides 344 to 321 of the Xena coding sequence).
Western blot analysis
Animal caps were cut at st. 8 and incubated in 1× Steinberg's until sibling embryos reached st. 23. Explant lysates were and analyzed by SDS-PAGE and Western blotting as previously described (Xanthos et al., 2005). The following antibodies and dilutions were used: anti-Mena monoclonal antibody (1:50, Lebrand et al., 2004), anti-β-tubulin monoclonal antibody (E7; 1:1000, DSHB). NIH Image J was used to quantify the amount of protein present and all values were normalized to beta-tubulin.
Adhesion assay
Animal caps were cut at stage 10.5 and groups of caps were dissociated in 1× calcium-magnesium free MMR for 30 minutes until inner cells dissociated. Outer pigmented cells were removed and the media was replaced with 1X Steinberg's. Cells were alternately rotated for 5 minutes, followed by incubation for 30 minutes without rotation, six times. Cells were then incubated without rotation and observed periodically for cell clumping.
Results
Xena is enriched at apical cell-cell junctions in neuroepithelial cells
Previous work showed that Xena transcripts are highly expressed in the neural plate during neurulation (Xanthos et al., 2005) and this expression pattern, together with knockout data from mice (Lanier et al., 1999; Menzies et al., 2004), suggest that Xena may play a critical role in neurulation. As a first step in examining the potential function of Xena in neural tube formation, we characterized the subcellular distribution of Xena protein in the developing neural plate and neural tube. During early stages of neurulation (stage 14), Xena is localized to the cell cortex and is associated with pigment granules within the cytoplasm (Fig. 1A). β-catenin, a component of cadherin-catenin adhesion complexes (Aberle et al., 1996), co-localizes with Xena at cell-cell junctions (Fig. 1A, right panel). In addition, vinculin, a known Ena binding partner found within adherens junction complexes (Vasioukhin et al., 2000), also co-localizes with Xena at cell-cell contacts (Fig. 1B).
Fig. 1.
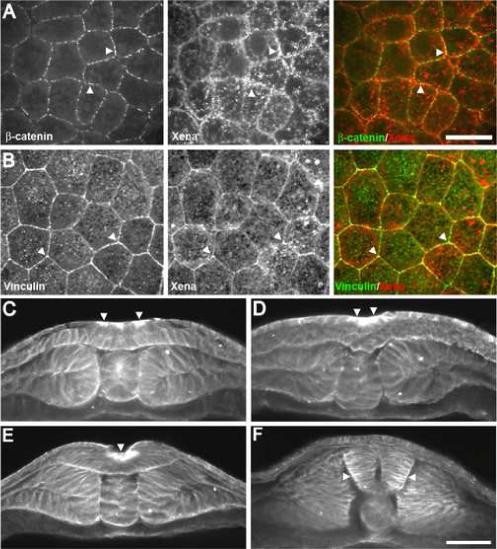
Spatial distribution of Xena protein within the neural plate during Xenopus development. (A) Xena is localized to sites of cell-cell adhesion at stage 14 in a dorsal view of the neural plate (arrowheads). (B) Xena and vinculin colocalize at sites of cell-cell adhesion in the neural plate (arrowheads). (C-F) Xena protein was detected in cross-sections using a polyclonal antibody at (C) stage 14, (D) stage 16, (E) stage 18, (F) and stage 20. Xena is enriched apically in cells of the neural plate undergoing apical wedging (arrowheads). Scale bar in A and B = 25 μm. Scale bar in C-F = 100 μm.
Three hingepoints, two dorsolateral hingepoints and one midline hingepoint, form during neural tube closure and serve as nucleating centers for neural plate folding (Schoenwolf and Smith, 1990). These areas initiate folding and undergo stereotypical changes in cell shape and actin organization. In a cross-sectional view, Xena is enriched at the apical surface of neuroepithelial cells, including at putative hingepoints (Fig. 1C-E, arrowheads). Upon fusion of the neural tube, Xena is no longer enriched at the apical surface, but instead is detected at lateral cell-cell contacts and is elevated at the basal ends of cells in the neural tube (Fig. 1F). Together these data demonstrate that Xena is localized to apical adhesive junctions and sites of dynamic actin remodeling important for driving cell shape changes during neural tube formation.
Knockdown of Xena disrupts neural tube closure
Ena/VASP proteins have been found to be critical for neural tube closure in mice in which removal of both Mena and VASP causes a high incidence of neural tube defects (Lanier et al., 1999; Menzies et al., 2004), yet the mechanism by which they control this process remains unclear. In order to elucidate the mechanism by which Ena/VASP proteins control neural tube closure, we investigated the requirement for Xena in neurulation in Xenopus. We focused on examining the function of Xena because it is highly expressed in the neural plate, whereas Xenopus VASP (Xvasp) is only weakly expressed in all epithelial cells throughout early development (K.A. Kragtorp and J.R. Miller, unpublished data) and Xevl is not expressed until after neurulation is complete (Wanner et al., 2005). Thus, Xena is the sole Ena/VASP family member expressed at high levels in the neuroepithelium and is therefore predicted to be the primary Ena/VASP protein regulating neurulation in Xenopus.
To analyze the requirement for Xena in neural tube formation, we designed an antisense morpholino (Xena sMO) that targets the splice donor site at the boundary of intron 2 and exon 3 (Fig. 2A). Binding of Xena sMO to the splice donor site of exon 2 is predicted to cause mis-splicing of exon 1 with exon 3, resulting in the removal of exon 2. This mis-splicing would remove the majority of the EVH1 domain, a critical protein-protein interaction domain, and cause a frameshift resulting in an early stop codon in the protein. RT-PCR amplification of the region surrounding the morpholino binding site indicated that injection of Xena sMO (50 ng) caused a mis-splicing event with the predicted change in size of the Xena transcript from 370 bp to 201bp (Fig. 2B). Sequencing of the smaller PCR product confirmed that injection of Xena sMO caused the expected mis-splicing of the Xena transcript resulting in the loss of exon 2, which includes the majority of the EVH1 domain, and a frameshift, which produces a premature stop codon. Based on these data, translation of the mis-spliced mRNA would produce a truncated protein comprised only of a small portion of the amino terminus and, as a result, this truncated protein is predicted to be non-functional. Examination of Xena protein expression in animal caps by western blot showed that injection of Xena sMO (50 ng) into the animal pole resulted in a 45% decrease in the levels of all isoforms of Xena protein and a 58% decrease in a neural specific isoform of Xena (Supplemental Fig. 1). Thus, Xena protein expression is significantly decreased upon injection of Xena sMO.
Fig. 2.

Xena knockdown disrupts neural tube formation. (A) Schematic showing target site of the Xena sMO. Binding of Xena sMO to the splice donor site of exon 2 is predicted to cause mis-splicing of exon 1 to exon 3 and a frameshift, resulting in an early stop codon and production of a non-functional, truncated protein. (B) RT-PCR analysis shows that injection of 50 ng of Xena sMO causes mis-splicing of Xena transcripts. The predicted mis-spliced product was confirmed by sequencing. (C) Xena depletion results in failure of neural tube closure. Xena sMO (25 ng) injected embryos at stage 15 (mid-neurulation) and stage 18 (late neurulation) displaying neural tube closure defects. Dashed lines indicate the borders of the neural folds. (D) Defects caused by Xena sMO injection can be rescued by Xena-GFP mRNA injection (500pg). Injection of Xena sMO together with 250 pg of GFP mRNA produced a majority of embryos with moderate to severe neural tube closure defects at stage 19. Injection of Xena sMO together with Xena-GFP mRNA reduced the severity of the phenotype and produced a majority of embryos with mild neural tube closure defects. Embryos were scored as described in Materials and Methods.
To determine the requirement for Xena in neural tube formation, Xena sMO (25 ng) was injected at the 4-cell stage into cells fated to give rise to the neural plate. GFP mRNA was co-injected with Xena sMO to ensure proper targeting of the injections. Consistent with a requirement for Xena in neural tube formation, injection of Xena sMO (25 ng) disrupted the elevation and mediolateral movement of the neural folds resulting in embryos with a range of neural tube defects (Fig. 2C). Injection of varying amounts of Xena sMO from 10 ng to 50 ng resulted in dose dependent defects in neural tube closure ranging from a minor effect on the narrowing of the neural plate and elevation of the neural folds (10 ng) to a complete block of neurulation (50 ng) (data not shown). All further experiments were carried out using the 25 ng dose of Xena sMO.
Classification of the severity of neural tube defects (see Materials and Methods for details) revealed that the majority of Xena sMO-injected embryos have moderate (24/34, 70.6%; Fig. 2D) or severe (7/34, 20.6%; Fig. 2D) defects in neural tube formation, whereas only a small proportion of GFP injected control siblings have mild defects (1/27, 3.7%; Fig. 2D). Importantly, while overexpression of Xena-GFP RNA alone does not affect neural tube closure or cause any other gross morphological phenotypes (data not shown), the phenotype caused by injection of Xena sMO was rescued by co-injection of Xena-GFP mRNA. The greatest proportion of co-injected embryos (19/33, 57.6%; Fig. 2D) had mild neural tube defects, a lower proportion showed moderate defects (13/33, 39.4%; Fig. 2D), and a very small proportion of embryos displayed severe defects (1/33, 3.0%; Fig. 2D). Thus, co-injection of Xena-GFP with Xena sMO resulted in significant rescue of the neural tube defects, indicating that the observed phenotype is the result of specific knockdown of Xena by the Xena sMO. The inability to completely rescue the neural tube phenotype may be due to fact that Xena-GFP protein aggregates abnormally when overexpressed in embryos (data not shown).
The specificity of the Xena knockdown phenotype was further confirmed by injection of a second morpholino (Xena MO2) that binds the 5'UTR and start site of Xena transcripts, thereby blocking translation of Xena protein. Injection of 25 ng of Xena MO2 causes a similar phenotype to that seen following injection of Xena sMO (Supplemental Fig. 2). Defects caused by injection of Xena MO2 are also rescued by coinjection of Xena-GFP mRNA and injection of a morpholino with five nucleotide mismatches (Xena misMO2) does not affect neurulation (Supplemental Fig. 2). Additionally, injection of mRNA encoding a dominant negative Mena, Mena TD-GFP (250 pg, n = 25; Vasioukhin et al., 2000), yields similar neural tube phenotypes (data not shown). Together these data demonstrate that Xena is required for proper neural tube formation during Xenopus development.
Xena is required for morphogenesis of the neural plate
The initial shaping of the neural plate requires the coordination of several morphogenetic behaviors including changes in cell shape and polarized cell migration. At the onset of neurulation, neuroepithelial cells become elongated along the apicobasal axis, causing a thickening and narrowing of the plate (Schoenwolf and Powers, 1987). This initial elongation of cells is accompanied by mediolateral intercalation of cells, which leads to further narrowing and lengthening of the neural plate (Davidson and Keller, 1999; Schoenwolf and Alvarez, 1989). These behaviors together contribute to the process of neural convergent extension, which serves to increase the overall length of the embryo and to reduce the distance between the neural folds, enabling them to fuse at the completion of neurulation (Wallingford and Harland, 2002).
In order to determine the precise role Xena plays in neurulation, we first examined the expression of two neural markers, Sox2 and Pax3. Analysis of the pan-neural marker Sox2 revealed that neural induction is not disrupted in Xena sMO injected embryos, as Sox2 is highly expressed throughout the neural plate. However, the area of Sox2 expression was consistently wider and shorter than controls at early stages of neurulation (Fig. 3A). Determination of the length-width ratio (LWR) of Sox2 expression showed that neural plates of Xena sMO-injected embryos had significantly lower LWRs compared to their GFP injected control siblings (Fig. 3B). Coinjection of Xena-GFP mRNA with Xena sMO resulted in Sox2 expression domains with higher LWRs, indicating that Xena-GFP can rescue these defects in neural plate shaping (data not shown).
Fig. 3.

Xena knockdown disrupts neural plate morphogenesis. (A) Xena sMO injected embryos have wider domains of Sox2 expression compared to control embryos. (B) Quantification of the length-width ratio (LWR) of Sox2 staining demonstrates that the LWR of Xena sMO injected embryos is significantly smaller than that of GFP injected controls (GFP n = 21, Xena sMO n = 25, student's t-test p = 6.02678*10−09). (C) Pax3 expression domains are significantly wider in Xena sMO injected embryos compared to GFP-injected controls. (D) Quantification of distance between neural folds as measured by Pax3 expression (GFP n = 17, Xena MO n = 23, student's t-test p = 4.22892*10−08).
Additionally, we examined the expression of Pax3, which marks the dorsal neural tube during later stages of neurulation. We found that the stripes of Pax3 expression in Xena sMO injected embryos appeared farther apart than those in GFP injected control siblings (Fig. 3C). Quantification of these results demonstrated that the medial movement of the neural folds is significantly affected in Xena knockdown embryos (Fig. 3D). Together, these data provide evidence for a potential role for Xena in regulating narrowing of the neural plate and elevation of the neural folds during neurulation.
Xena is required for cellular elongation and apical constriction
One of the cell behaviors underlying the shaping of the developing neural plate is the elongation of cells along their apicobasal axis. This elongation must be maintained later in neurulation and in combination with apical constriction is required to facilitate folding of the neural plate into the neural tube. To examine whether Xena might play a role in regulating cell shape, we examined the morphology of neuroepithelial cells upon injection of Xena sMO. In control embryos, neuroepithelial cells are elongated along their apical-basal axis (Fig. 4A). In contrast, cells targeted by Xena sMO appear cuboidal or even rounded (Fig. 4B) indicating that Xena depletion blocks initial apicobasal elongation of cells in the neural plate.
Fig. 4.

Xena is required for changes in cell shape and apical constriction during neurulation. (A) Stage 15 GFP injected control embryo stained for GFP and β-tubulin to outline cells. In control embryos cells are elongated and apically constricted. (B) In Xena sMO injected embryos, cells appear rounded and fail to undergo apical constriction. Scale bar = 25μM.
After the neural plate is initially shaped the neural folds elevate, causing the neural plate to bend. The bending of the plate is achieved by the apical constriction as well as basal expansion of neuroepithelial cells (Schoenwolf and Smith, 1990). Apical constriction is dependent on reorganization of the actin cytoskeleton at apical cell-cell junctions. Since Xena sMO injected embryos appear to have defects in cell shape and neural fold elevation, we examined whether Xena is required for apical constriction during neurulation. Neuroepithelial cells in GFP injected control embryos appear elongated and their apical surfaces are highly constricted (Fig. 4A, 16/16 GFP injected embryos). Cells containing Xena sMO, as revealed by the GFP tracer, showed little or no apical constriction with very rounded unelongated cells (Fig. 4B, 20/20 Xena sMO injected embryos). The absence of apical constriction in Xena sMO targeted cells coincided with a widening of the neural plate as well as defects in the elevation of the neural folds at these sites. Similar defects are observed in Xena MO2 injected embryos (7/7 affected, data not shown) whereas injection of Xena misMO2 had no effect on apical constriction (5/5, data not shown). Additionally, unilateral injection of Xena MO2 blocked elevation of the neural fold only on the injected side, indicating that Xena is required cell autonomously for elevation of neural folds (Supplemental Fig. 3). These data demonstrate that Xena is required for the normal apical constriction of neuroepithelial cells and suggest that lack of constriction may lead to defects in the shaping of the neural plate and elevation of the neural folds.
Xena regulates apical actin organization during neurulation
Actin is highly concentrated at the apical ends of neuroepithelial cells during apical constriction and neural plate bending, where it is thought to play a critical role in generating the physical forces required for bending of the neural plate and elevation of the neural folds. Since Ena/VASP proteins are known actin regulatory proteins, we hypothesized that Xena knockdown may affect apical actin organization. In control embryos, actin is concentrated apically in the developing neural tube throughout neurulation, persisting at these sites until fusion of the neural folds is complete (Fig. 5A, B, 13/13 GFP injected embryos). In contrast, actin does not accumulate apically in cells containing Xena sMO (Fig. 5C, bracket, 20/20 Xena sMO injected embryos), though actin does accumulate apically in neighboring cells lacking Xena sMO (Fig. 5C, arrowhead). The correlation between the lack of apical actin and defects in apical constriction suggest that Xena may regulate apical constriction and bending of the neural plate by facilitating the accumulation or remodeling of actin at apical cell-cell junctions.
Fig. 5.

Xena is required for apical accumulation of actin during apical constriction. (A) Distribution of actin during neural tube closure in control embryos. Actin accumulates apically (arrowheads) in cells undergoing apical constriction and persists apically until the neural tube is completely fused. (B) Stage 18 GFP injected control embryo. Actin is concentrated apically in cells undergoing apical constriction (arrowhead). (C) Stage 18 Xena sMO/GFP injected embryo. No apical accumulation of actin is observed in Xena sMO/GFP positive cells. Xena knockdown cells remain cuboidal and fail to undergo apical constriction (bracket). Notice the higher density of apical actin in GFP negative cells (arrowheads). Scale bar in A = 100 μM. Scale bar in B and C = 20μM.
Xena is required for proper cell-cell adhesion
Cell-cell adhesion is important for a number of processes during neurulation including regulation of cell shape, directed migration, and apical constriction (Oda and Tsukita, 2001; Wallingford, 2005). Xena co-localizes with components of adherens junctions in the neural plate (Fig. 1A,B) suggesting that it may regulate cell behaviors during neurulation through the modulation of adhesive interactions. To explore this possibility, we utilized a reaggregation assay to determine whether Xena is required for cell-cell adhesion. Animal caps from control and Xena sMO injected embryos were dissociated in Ca2+/Mg2+-free media and were then transferred to complete media and allowed to reform cell-cell adhesions. Cells from control animal caps formed large, tight clumps of cells indicative of the reformation of strong cell-cell contacts (Fig. 6A). However, cells from Xena sMO-injected animal caps failed to re-adhere to one another and only formed small, loosely adherent clumps of cells (Fig. 6B). Consistent with a role for Xena in cell adhesion, we observed a reduction in the level of α-catenin in neural plates in cells containing Xena sMO (Fig. 6D, 4/4 Xena sMO injected embryos) compared to control GFP injected embryos (Fig. 6C). Additionally, α-catenin levels were elevated in cells where Xena sMO was co-expressed with Xena-GFP mRNA, indicating that Xena-GFP can rescue the morpholino induced phenotype (Fig. 6E, 3/3 Xena sMO + Xena-GFP mRNA injected embryos). These results indicate that Xena is required for cell-cell adhesion and suggest that impairment of adhesion in Xena knockdown embryos may have an important contribution to the observed neural tube defects.
Fig. 6.

Xena is required for cell-cell adhesion. (A) Reaggregation assays show that dissociated cells from control explants form numerous tightly adherent aggregates whereas (B) cells from Xena sMO injected embryos fail to reaggregate and mostly stay as single cells. (C) Neural plate of stage 15 GFP-injected control embryo. α-catenin is localized apically at the cortex of cells in the neuroepithelial cells of neural plate (arrowheads). (D) Neural plate of stage 15 Xena sMO/GFP injected embryo. α-catenin is diminished at cell-cell junctions and is distributed cytoplasmically in cells containing Xena sMO/GFP (arrowheads). (E) Neural plate of stage 15 Xena sMO/Xena-GFP injected embryo. α-catenin is localized cortically in cells containing Xena sMO/Xena-GFP (arrowheads). Notice that α-catenin is enriched at sites of cell-cell adhesion when compared to levels in surrounding uninjected cells. Scale bar in C, D and E = 50 μM.
Discussion
In this report we analyzed the role of Xena in regulating morphogenetic processes during neurulation. We found that Xena is enriched at apical junctions of cells in the neural plate during neural tube formation and that knockdown of Xena disrupts neural tube closure. Examination of neural markers revealed that remodeling of the neural plate shape appears to be disrupted. These defects coincide with disruption of cell shape changes within the neural plate including inhibition of apical constriction, which coincides with a reduction of apical actin accumulation. Finally, we observed that Xena depleted cells are unable to reaggregate, indicating a requirement for Xena in cell-cell adhesion. Together, these results demonstrate that Xena is essential for neural tube formation in Xenopus and provide evidence for a model in which Xena regulates the morphogenetic processes that drive neurulation through modulation of the actin cytoskeleton and cellular adhesion.
Our work provides additional evidence supporting an important role for Ena/VASP proteins in neural tube formation. Previous studies reported that neural tube closure is disrupted in Mena−/−;profilin+/− mice (Lanier et al., 1999) and when Mena and VASP are both removed (Menzies et al., 2004). These data indicated that Ena/VASP family members play redundant roles in neural tube formation in mice. In contrast, we found that knockdown of Xena on its own disrupted neural tube formation in Xenopus. This lack of redundancy in Xenopus is likely due to the lack expression of other Ena/VASP family members in the neural plate. Xevl is not expressed until after neurulation is complete (Wanner et al., 2005) while Xvasp is expressed at very low levels in all epithelial cells during neurulation (K. A. Kragtorp and J. R. Miller, unpublished data) and thus may not be present at levels high enough to compensate for the loss of Xena. Thus, while the requirement for Ena/VASP function in neural tube formation in conserved, changes in the expression levels and/or spatial domains of Ena/VASP transcripts may account for the lack of redundancy seen in Xenopus. It will be interesting to see whether Ena is the primary functional family member during neurulation in other vertebrates.
The importance of Ena/VASP proteins in various actin-dependent processes indicates that Xena may regulate multiple morphogenetic processes during neural tube formation. Ena/VASP proteins localize to dynamic regions of the cell associated with increased actin remodeling, such as lamellipodia and filopodia (Gertler et al., 1996; Rottner et al., 1999) and newly formed cell-cell adhesions (Scott et al., 2006). Ena/VASP proteins bind F-actin and are thought to regulate the three dimensional organization of the actin network within cellular protrusions (Bear et al., 2002). Additionally, Ena/VASP proteins are localized to focal adhesions and cell-cell adhesion complexes where they are thought to modulate the connection between the actin cytoskeleton and cell surface adhesion receptors (Kragtorp and Miller, 2006; Reinhard et al., 1992; Scott et al., 2006; Vasioukhin et al., 2000). Thus, the defects observed in Xena depleted embryos, including failure of convergent extension and defects in cell shape and cell adhesion, suggest that Xena may regulate directed cell migration, actin-mediated cell shape changes, and cell adhesion during neural tube formation. The interconnectedness of these processes, for example cell adhesion is critical for regulating both cell migration and cell shape, make it difficult to discern the primary function for Xena during neurulation. Yet, our data allow us to propose several potential models to explain how Xena may control cell behaviors required for neural tube formation.
Neural convergent extension is dependent on polarized cell motility that drives the narrowing and elongation of the neural plate. In Xenopus, neuroepithelial cells display monopolar protrusive activity and migrate towards the dorsal midline to drive convergent extension of the neural plate (Elul and Keller, 2000). Neural convergent extension requires PCP signaling (Ciruna et al., 2006; Curtin et al., 2003; Kibar et al., 2001; Wallingford and Harland, 2002; Wang et al., 2006) and is necessary for bringing the neural folds into close enough proximity to allow the neural folds to meet and fuse at the midline (Wallingford and Harland, 2002). While we can not say conclusively that loss of Xena disrupts neural convergent extension movements, Xena depletion perturbs shaping of the neural plate indicating that Xena is a component of the regulatory machinery coordinating cell movements in the neural plate. The well-established roles for Ena/VASP proteins in cell migration suggest that Xena may regulate shaping of the neural plate by modulating actin dynamics at the leading edge of migrating neuroepithelial cells. Our data also raise the interesting possibility that PCP signaling may control protrusive activity by regulating Ena/VASP activity. Further studies will be required to test this idea and define the precise role for Xena in controlling shaping of the neural plate and whether it regulates neural convergent extension movements.
Apical constriction is a common morphogenetic process involved in bending epithelial sheets. During neurulation, apical constriction drives bending of the neural plate at specific hingepoints, which together with medial movement of the neural folds promotes the meeting and fusion of the neural folds at the midline. Apical constriction coincides with apical accumulation of actin and activation of myosin II (Bertet et al., 2004; Nikolaidou and Barrett, 2004; Shimizu et al., 2005), suggesting a mechanism involving myosin-mediated contraction of the apical actin network. In Xena depleted embryos, apical constriction fails to occur and as a result elevation of the neural folds is significantly compromised. Actin fails to accumulate apically in Xena depleted embryos suggesting that Xena may be required for the establishment and/or contraction of the apical actin network. Interestingly, altering Ena/VASP activity in cultured cells can affect cell contractility (Galler et al., 2006; Hoffman et al., 2006). Furthermore, recent studies in Drosophila show that Abelson (Abl) kinase regulates the localization and activity of Ena to control apical constriction of the ventral furrow (Fox and Peifer, 2007). Mice lacking Abl and Arg kinases develop neural tube defects and actin organization in neuroepithelial cells is altered (Koleske et al., 1998). Together, these studies indicate that precise regulation of Ena/VASP activity is required for proper cell contractility and that Abl-Ena/VASP signaling may represent a conserved mechanism for regulating actin organization during apical constriction. Whether Ena/VASP and Abl work together with other modulators of apical constriction such as Shroom (Dietz et al., 2006; Haigo et al., 2003; Hildebrand, 2005; Hildebrand and Soriano, 1999; Lee et al., 2007) is an open question. Future analyses of Ena/VASP function will shed additional insights into the mechanisms coordinating actin assembly and apical constriction.
Our work provides support for a key role for Xena in modulating cell-cell adhesion. Xena is enriched at cell-cell contacts in the neural plate where it co-localizes with β-catenin and vinculin. In addition, Xena depleted cells fail to reaggregate providing direct evidence that Xena is required for optimal cell-cell adhesion. Studies in mammalian cells indicate that Ena/VASP proteins promote F-actin accumulation and assembly at cell-cell contacts and loss of Ena/VASP function perturbs cell adhesion (Scott et al., 2006; Vasioukhin et al., 2000). Interestingly, Mena was found to primarily accumulate at newly formed adhesions (Scott et al., 2006) suggesting that Ena/VASP proteins may preferentially function to modulate forming or dynamic adhesions. Morphogenetic movements are dependent on the making and breaking of adhesions suggesting that Xena could participate in this process through a function in modulating cell adhesion in addition to a role in cell migration. Cell shape changes, including apical constriction, are also dependent on cell adhesion. Cell surface adhesion complexes are required to transmit intracellular forces produced by contraction of the apical cytoskeleton to the membrane and surrounding cells. Thus, it is possible that Xena may regulate apical constriction through modulation of actin organization and/or cell-cell adhesion. Further work will help define the roles for Xena and provide mechanistic insights into the conserved mechanisms that control neural tube formation
Neurulation is driven by the coordination of several morphogenetic processes including directed cell migration and changes in cell shape and adhesion. This study demonstrates that Xena is a key regulator of neural convergent extension movements, apical constriction, and cellular adhesion during neurulation. Xena may control these morphogenetic processes by providing a key regulatory link between the actin cytoskeleton and cell surface adhesion receptors, highlighting the importance of coordinating the regulation of actin and adhesion during morphogenesis. By analyzing the molecular pathways that control morphogenesis, our work can shed light on the conserved mechanisms that coordinate cell behaviors to drive neural tube formation in vertebrates.
Acknowledgments
The authors acknowledge Drs. Randall Moon (University of Washington), John Wallingford (University of Texas), and Frank Gertler (MIT) for generously providing reagents used in this work. We also thank members of the Miller lab and Drs. Lorene Lanier, Duncan Clarke, and Laura Gammill (University of Minnesota) for numerous discussions and insightful comments. This work was supported by NIH Pre-Doctoral Training Grant HD07480 and March of Dimes research grant 6-FY04−66.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplemental Figure Legends
Supplemental Figure 1. Injection of Xena sMO results in a reduction in Xena protein in stage 23 animal caps. Multiple isoforms of the Xena protein are detected in Xenopus and the major isoforms migrate at approximately 80−, 88−, 100−, and 140 kDa (Xanthos et al., 2005). Injection of Xena sMO reduces the amount o protein present in each of these bands, but does not change the size of the isoforms. Total Xena protein is reduced to 55% of control GFP-injected levels and the higher molecular weight Xena (+) protein is reduced to 42% of control levels. Protein quantification was normalized to β-tubulin expression.
Supplemental Figure 2. Injection of Xena MO2 disrupts neural tube closure. (A) Injection of Xena MO2 results in wider neural folds compared to control siblings in stage 19 embryos. (B) Defects in neural tube closure can be rescued by coinjection of Xena-GFP mRNA and are not seen when a five basepair mismatch MO2 is injected. Uninjected n=29, Xena MO2 n=27, Xena MO2 + Xena-GFP n=28, mismatch MO2 n=23. Embryos were scored as described in Materials and Methods.
Supplemental Figure 3. Unilateral injection of Xena MO2 results in failure of neural fold elevation. Staining for Xena (A, red in C) reveals a reduction in Xena protein levels in cells containing MO2 (outlined by dotted line in A). Cells containing Xena MO2 are marked by the GFP tracer (B, green in C), which was coinjected with Xena MO2. Knockdown of Xena protein results in development of a shorter and broader neural fold (bracket in B and C) compared to that seen on the uninjected side of the embryo (arrowhead).
References
- Aberle H, et al. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–23. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Bear JE, et al. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 2002;109:509–21. doi: 10.1016/s0092-8674(02)00731-6. [DOI] [PubMed] [Google Scholar]
- Bertet C, et al. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–71. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- Ciruna B, et al. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature. 2006;439:220–4. doi: 10.1038/nature04375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas JF, Schoenwolf GC. Towards a cellular and molecular understanding of neurulation. Dev Dyn. 2001;221:117–45. doi: 10.1002/dvdy.1144. [DOI] [PubMed] [Google Scholar]
- Copp AJ, et al. The embryonic development of mammalian neural tube defects. Prog Neurobiol. 1990;35:363–403. doi: 10.1016/0301-0082(90)90037-h. [DOI] [PubMed] [Google Scholar]
- Curtin JA, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–33. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–5. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- Darken RS, et al. The planar polarity gene strabismus regulates convergent extension movements in Xenopus. Embo J. 2002;21:976–85. doi: 10.1093/emboj/21.5.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LA, Keller RE. Neural tube closure in Xenopus laevis involves medial migration, directed protrusive activity, cell intercalation and convergent extension. Development. 1999;126:4547–56. doi: 10.1242/dev.126.20.4547. [DOI] [PubMed] [Google Scholar]
- De Arcangelis A, et al. Synergistic activities of alpha3 and alpha6 integrins are required during apical ectodermal ridge formation and organogenesis in the mouse. Development. 1999;126:3957–68. doi: 10.1242/dev.126.17.3957. [DOI] [PubMed] [Google Scholar]
- Dietz ML, et al. Differential actin-dependent localization modulates the evolutionarily conserved activity of Shroom family proteins. J Biol Chem. 2006;281:20542–54. doi: 10.1074/jbc.M512463200. [DOI] [PubMed] [Google Scholar]
- Elul T, Keller R. Monopolar protrusive activity: a new morphogenic cell behavior in the neural plate dependent on vertical interactions with the mesoderm in Xenopus. Dev Biol. 2000;224:3–19. doi: 10.1006/dbio.2000.9746. [DOI] [PubMed] [Google Scholar]
- Fox DT, Peifer M. Abelson kinase (Abl) and RhoGEF2 regulate actin organization during cell constriction in Drosophila. Development. 2007;134:567–78. doi: 10.1242/dev.02748. [DOI] [PubMed] [Google Scholar]
- Galler AB, et al. VASP-dependent regulation of actin cytoskeleton rigidity, cell adhesion, and detachment. Histochem Cell Biol. 2006;125:457–74. doi: 10.1007/s00418-005-0091-z. [DOI] [PubMed] [Google Scholar]
- Gertler FB, et al. Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell. 1996;87:227–39. doi: 10.1016/s0092-8674(00)81341-0. [DOI] [PubMed] [Google Scholar]
- Golden JA, Chernoff GF. Multiple sites of anterior neural tube closure in humans: evidence from anterior neural tube defects (anencephaly). Pediatrics. 1995;95:506–10. [PubMed] [Google Scholar]
- Grevengoed EE, et al. Abelson kinase regulates epithelial morphogenesis in Drosophila. J Cell Biol. 2001;155:1185–98. doi: 10.1083/jcb.200105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigo SL, et al. Shroom induces apical constriction and is required for hingepoint formation during neural tube closure. Curr Biol. 2003;13:2125–37. doi: 10.1016/j.cub.2003.11.054. [DOI] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–95. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD. Shroom regulates epithelial cell shape via the apical positioning of an actomyosin network. J Cell Sci. 2005;118:5191–203. doi: 10.1242/jcs.02626. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD, Soriano P. Shroom, a PDZ domain-containing actin-binding protein, is required for neural tube morphogenesis in mice. Cell. 1999;99:485–97. doi: 10.1016/s0092-8674(00)81537-8. [DOI] [PubMed] [Google Scholar]
- Hoffman LM, et al. Genetic ablation of zyxin causes Mena/VASP mislocalization, increased motility, and deficits in actin remodeling. J Cell Biol. 2006;172:771–82. doi: 10.1083/jcb.200512115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibar Z, et al. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet. 2001;28:251–5. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- Koleske AJ, et al. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron. 1998;21:1259–72. doi: 10.1016/s0896-6273(00)80646-7. [DOI] [PubMed] [Google Scholar]
- Kragtorp KA, Miller JR. Regulation of somitogenesis by Ena/VASP proteins and FAK during Xenopus development. Development. 2006;133:685–95. doi: 10.1242/dev.02230. [DOI] [PubMed] [Google Scholar]
- Lanier LM, et al. Mena is required for neurulation and commissure formation. Neuron. 1999;22:313–25. doi: 10.1016/s0896-6273(00)81092-2. [DOI] [PubMed] [Google Scholar]
- Lebrand C, et al. Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron. 2004;42:37–49. doi: 10.1016/s0896-6273(04)00108-4. [DOI] [PubMed] [Google Scholar]
- Lee C, et al. Shroom family proteins regulate {gamma}-tubulin distribution and microtubule architecture during epithelial cell shape change. Development. 2007;134:1431–41. doi: 10.1242/dev.02828. [DOI] [PubMed] [Google Scholar]
- Menzies AS, et al. Mena and vasodilator-stimulated phosphoprotein are required for multiple actin-dependent processes that shape the vertebrate nervous system. J Neurosci. 2004;24:8029–38. doi: 10.1523/JNEUROSCI.1057-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin). North-Holland; Amsterdam: 1967. [Google Scholar]
- Nikolaidou KK, Barrett K. A Rho GTPase signaling pathway is used reiteratively in epithelial folding and potentially selects the outcome of Rho activation. Curr Biol. 2004;14:1822–6. doi: 10.1016/j.cub.2004.09.080. [DOI] [PubMed] [Google Scholar]
- Oda H, Tsukita S. Real-time imaging of cell-cell adherens junctions reveals that Drosophila mesoderm invagination begins with two phases of apical constriction of cells. J Cell Sci. 2001;114:493–501. doi: 10.1242/jcs.114.3.493. [DOI] [PubMed] [Google Scholar]
- Radice GL, et al. Developmental defects in mouse embryos lacking N-cadherin. Dev Biol. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- Reinhard M, et al. The 46/50 kDa phosphoprotein VASP purified from human platelets is a novel protein associated with actin filaments and focal contacts. Embo J. 1992;11:2063–70. doi: 10.1002/j.1460-2075.1992.tb05264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottner K, et al. VASP dynamics during lamellipodia protrusion. Nat Cell Biol. 1999;1:321–2. doi: 10.1038/13040. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Alvarez IS. Roles of neuroepithelial cell rearrangement and division in shaping of the avian neural plate. Development. 1989;106:427–39. doi: 10.1242/dev.106.3.427. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, et al. A reexamination of the role of microfilaments in neurulation in the chick embryo. Anat Rec. 1988;220:87–102. doi: 10.1002/ar.1092200111. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Smith JL. Epithelial cell wedging: A fundamental cell behaviour contributing to hinge point formation during epithelial morphogenesis. In: Keller RE, Fristom D, editors. Control of morphogenesis by specific cell behaviors. Vol. 1. W.B. Saunders Co.; London: 1990. pp. 325–334. [Google Scholar]
- Schoenwolf GC, Powers ML. Shaping of the chick neuroepithelium during primary and secondary neurulation: role of cell elongation. Anat Rec. 1987;218:182–95. doi: 10.1002/ar.1092180214. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Smith JL. Mechanisms of neurulation: traditional viewpoint and recent advances. Development. 1990;109:243–70. doi: 10.1242/dev.109.2.243. [DOI] [PubMed] [Google Scholar]
- Scott JA, et al. Ena/VASP proteins can regulate distinct modes of actin organization at cadherin-adhesive contacts. Mol Biol Cell. 2006;17:1085–95. doi: 10.1091/mbc.E05-07-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8:126–38. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, et al. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol. 2005;168:941–53. doi: 10.1083/jcb.200411179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive HL, et al. Early Development of Xenopus lavis: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2000. [Google Scholar]
- Smith JL, Schoenwolf GC. Neurulation: coming to closure. Trends Neurosci. 1997;20:510–7. doi: 10.1016/s0166-2236(97)01121-1. [DOI] [PubMed] [Google Scholar]
- Stumpo DJ, et al. MARCKS deficiency in mice leads to abnormal brain development and perinatal death. Proc Natl Acad Sci U S A. 1995;92:944–8. doi: 10.1073/pnas.92.4.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin V, et al. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–19. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- Wallingford JB. Neural tube closure and neural tube defects: studies in animal models reveal known knowns and known unknowns. Am J Med Genet C Semin Med Genet. 2005;135:59–68. doi: 10.1002/ajmg.c.30054. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development. 2002;129:5815–25. doi: 10.1242/dev.00123. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–78. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner SJ, et al. Molecular cloning and expression of Ena/Vasp-like (Evl) during Xenopus development. Gene Expr Patterns. 2005;5:423–8. doi: 10.1016/j.modgep.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Xanthos JB, et al. Cloning and developmental expression of Xenopus Enabled (Xena). Dev Dyn. 2005;233:631–7. doi: 10.1002/dvdy.20358. [DOI] [PubMed] [Google Scholar]
- Xu W, et al. Vinculin knockout results in heart and brain defects during embryonic development. Development. 1998;125:327–37. doi: 10.1242/dev.125.2.327. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Copp AJ. Bending of the neural plate during mouse spinal neurulation is independent of actin microfilaments. Dev Dyn. 1999;215:273–83. doi: 10.1002/(SICI)1097-0177(199907)215:3<273::AID-AJA9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, et al. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development. 2007;134:789–99. doi: 10.1242/dev.000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure Legends
Supplemental Figure 1. Injection of Xena sMO results in a reduction in Xena protein in stage 23 animal caps. Multiple isoforms of the Xena protein are detected in Xenopus and the major isoforms migrate at approximately 80−, 88−, 100−, and 140 kDa (Xanthos et al., 2005). Injection of Xena sMO reduces the amount o protein present in each of these bands, but does not change the size of the isoforms. Total Xena protein is reduced to 55% of control GFP-injected levels and the higher molecular weight Xena (+) protein is reduced to 42% of control levels. Protein quantification was normalized to β-tubulin expression.
Supplemental Figure 2. Injection of Xena MO2 disrupts neural tube closure. (A) Injection of Xena MO2 results in wider neural folds compared to control siblings in stage 19 embryos. (B) Defects in neural tube closure can be rescued by coinjection of Xena-GFP mRNA and are not seen when a five basepair mismatch MO2 is injected. Uninjected n=29, Xena MO2 n=27, Xena MO2 + Xena-GFP n=28, mismatch MO2 n=23. Embryos were scored as described in Materials and Methods.
Supplemental Figure 3. Unilateral injection of Xena MO2 results in failure of neural fold elevation. Staining for Xena (A, red in C) reveals a reduction in Xena protein levels in cells containing MO2 (outlined by dotted line in A). Cells containing Xena MO2 are marked by the GFP tracer (B, green in C), which was coinjected with Xena MO2. Knockdown of Xena protein results in development of a shorter and broader neural fold (bracket in B and C) compared to that seen on the uninjected side of the embryo (arrowhead).


