Abstract
Background
B-type natriuretic peptide (BNP) is secreted from cardiomyocytes and may reflect haemodynamic abnormalities predisposing to atrial fibrillation (AF). We aimed to investigate whether N-terminal pro BNP (NT-proBNP) is associated with newly detected AF in subjects obtained from the general population.
Methods
From the PREVEND programme (n=8592), we selected all subjects with an available baseline and four-year electrocardiogram and NTproBNP levels at baseline. We excluded subjects with AF at baseline and subjects with a serum creatinine >2.0 mg/dl.
Results
In total, 6494 subjects were eligible for the prospective analysis (aged 49±12 years, 49.7% men). At four years, AF was detected in 41 (0.6%) subjects. Median NT-proBNP levels at baseline in subjects with newly detected AF after four years was 62.2 (22.6 to 208.5) pg/ml as compared with 35.7 (15.9 to 68.7) pg/ml in those with sinus rhythm (p=0.001). Each 1 standard deviation increment in natural log transformed NT-proBNP was associated with a 54% (5% to 126%, p=0.028) increase in risk for AF after adjustment for other risk factors predisposing to AF. NT-proBNP levels above the sex-specific 80th percentile (97 pg/ml in women and 60 pg/ml in men) were associated with a multivariate odds ratio of 2.65 (1.22 to 5.76, p=0.01) for the occurrence of AF.
Conclusion
Plasma levels of NT-proBNP predict newly detected AF in subjects obtained from the general population independent of cardiovascular risk factors predisposing to AF. (Neth Heart J 2008;16:73-8.)
Keywords: PREVEND, BNP, atrial fibrillation, epidemiology, risk factors
A trial fibrillation (AF) is the most common sustained arrhythmia in clinical practice and is associated with an increase in total and cardiovascular mortality, as well as cardiovascular morbidity, including stroke and heart failure.1 Traditional risk factors for the development of AF are higher age, male sex, hypertension, diabetes, an enlarged left atrial volume, and a history of myocardial infarction, valvular heart disease, or congestive heart failure.2,3 Recently, the presence of systemic inflammation determined by C-reactive protein (CRP) has also been associated with the presence of AF and has been identified as predictor for future AF.4,5 Atrial biopsies in patients with AF demonstrated evidence of inflammation in patients with lone AF, which suggest a cause-effect relationship between inflammation and AF.6 Another potential mechanism related to the development of AF may be the presence of left ventricular diastolic dysfunction. Tsang et al. found that diastolic dysfunction assessed by echocardiography was associated with an increased risk for AF potentially mediated through an increase in atrial pressure and atrial stretch.7
In this regard, N-terminal pro B-type natriuretic peptide (NT-proBNP) may also be associated with future AF as NT-proBNP is secreted from cardiomyocytes, reflects haemodynamic alterations and is increased in symptomatic and asymptomatic patients with mild left ventricular dysfunction.8 In addition, NT-proBNP has been cross-sectionally associated with AF in patients who had underlying hypertension, coronary heart disease,9 or chronic heart failure,10 and even in subjects with lone AF.11 Furthermore, plasma levels of BNP have been shown to predict recurrence of AF in patients with mild heart failure12 and postoperative AF in patients undergoing cardiac surgery.13 One study, the Framingham Offspring study, demonstrated an increased risk for AF in subjects from a community-based cohort with higher BNP levels at baseline.14 These results suggest that BNP levels may identify subjects at increased risk for AF at an early stage. A second study is necessary to confirm this finding and additional adjustment for other potential markers (such as CRP) is also needed. Therefore, we aimed to investigate whether NT-proBNP levels are associated with newly detected AF in subjects obtained from the general population.
Methods
Study population
The population analysed in this study was obtained from the PREVEND study.15 The PREVEND (Prevention of Renal and Vascular Endstage Disease) study was designed to investigate the natural course of microalbuminuria and its relation with renal and cardiovascular disease in the general population as previously described.16 In summary, all inhabitants of the city of Groningen (the Netherlands) between the age of 28 and 75 years (n=85,421) were asked to send in a morning urine sample and to fill in a short questionnaire on demographics and cardiovascular medical history. A total of 40,856 subjects responded. All subjects with a urinary albumin concentration ≥10 mg l–1 (n=7768) and a randomly selected sample of subjects with a urinary albumin concentration <10 mg l–1 (n=3395) were invited to make two visits to an outpatient clinic. After exclusion of subjects with type 1 diabetes mellitus, females who were possibly pregnant, and females and males not able or willing to participate, a total of 6000 subjects with a urinary albumin concentration ≥10 mg l–1 and a random control sample of subjects with a urinary albumin concentration <10 mg l–1 (n=2592) completed the screening protocol and formed the baseline PREVEND cohort (n=8592). From this cohort, we excluded subjects without a baseline and four-year follow-up electrocardiogram (n=1849) and NT-proBNP (n=152) levels at baseline. In addition, we excluded subjects with AF at baseline (n=65), subjects with a serum creatinine of more than 2.0 mg/dl (n=9), and subjects with a natural log transformed NT-proBNP more than 3 standard deviations from the mean to minimise the effects of outliers in the analyses (n=23). In total, 6494 subjects were thus eligible for the current analysis. All participants gave written informed consent. The PREVEND study was approved by the local medical ethics committee and conducted in accordance with the guidelines of the Helsinki declaration.
Electrocardiography
Standard 12-lead electrocardiograms were recorded using the computer programme MEANS (Modular ECG Analysis System),17 and AF was defined according to Minnesota codes 8.3.1 and 8.3.3. Ischaemic heart disease was defined as self-reported myocardial infarction with hospitalisation or as Minnesota codes 1.1–2, 4.1–2, or 5.1–2. The presence of left ventricular hypertrophy (LVH) was identified using the Cornell voltage x QRS duration product: (R amplitude at aVL on the ECG + S amplitude at V3 on the ECG) x QRS duration in men and (RaVL+SV3+6 mm) x QRS duration in women. A threshold of 2440 mm x msec was used to identify LVH.18
Laboratory measurements
N-terminal pro-BNP was measured using an electrochemiluminiscence immunoassay ‘ECLIA’ on an Elecsys 2010 analyser (Roche Diagnostics, Mannheim, Germany). Measuring range of NT-proBNP is 5 to 35,000 pg/ml. Values below the detection limit are reported as <5 pg/ml. The urinary albumin excretion rate was measured as the mean of two 24-hour urine collections, and urinary albumin concentrations were determined by nephelometry with a threshold of 2.3 mg l–1 and intra- and inter-assay coefficients of variation of less than 2.2 and 2.6%, respectively (Dade Behring Diagnostic, Marburg, Germany). High-sensitive CRP was also determined by nephelometry with a threshold of 0.175 mg l–1 and intra- and inter-assay coefficients of less than 4.4 and 5.7%, respectively (BNII N, Dade Behring). CRP levels below the detection level were scored as 0.18 mg l–1. Plasma glucose, serum cholesterol, and serum and urinary creatinine were determined by Kodak Ektachem dry chemistry (Eastman Kodak, Rochester, NY, USA). HDL cholesterol was measured with a homogeneous method (direct HDL, no. 7D67, AEROSET System; Abbott Laboratories).
Risk factor definition
Blood pressure was measured during two visits using the automated blood pressuremeasurements after ten minutes of supine rest with a DinamapXL Model 9300 device (Johnson & Johnson Medical, Tampa, Florida, US). The last two blood pressure values of both visits were averaged. Hypertension was defined as a systolic blood pressure ≥140 mmHg or a diastolic blood pressure ≥90 mmHg or the use of antihypertensive medication. Hypercholesterolaemia was defined as a total serum cholesterol level of ≥6.5 mmol/l (251 mg/dl) or the use of lipid-lowering therapy. Diabetes was defined as a fasting plasma glucose level >7.0 mmol/l or a non-fasting plasma glucose level >11.1 mmol/l or
categorised as no smoking, or smoking (current or stopped <1 year ago).
Statistical analysis
Continuous data are reported as mean ± standard deviation or median (interquartile range) if the data was skewed. Categorical data are presented as per group percentages. Differences between subgroups were evaluated by Student’s t-test for the normally distributed continuous variables, or with the Mann- Whitney test if data were skewed. Differences in categorical data were compared with the Χ2 test.
The following cardiovascular risk factors predisposing to AF were included in the multivariate logistic regression analyses: age, sex, presence of left ventricular hypertrophy, ischaemic heart disease, hypertension, hypercholesterolaemia, HDL cholesterol, CRP, serum creatinine level, and urinary albumin excretion.
The relationship between the presence of AF on the electrocardiogram after four years and baseline predictors was assessed by use of logistic regression modelling. For regression modelling, NT-proBNP was inserted both continuously after natural logarithmic transformation and categorically (above and below sexspecific 80th percentile values). A p value of <0.05 was considered statistically significant. All above-mentioned calculations were performed with SPSS version 12.0.1 software (SPSS, Chicago, Illinois, US).
Results
In total, 6494 subjects were eligible for the prospective analysis (age 49±12 years, 50.4% women). At four years, AF was detected in 41 (0.6%) subjects who had no AF at baseline. The clinical characteristics of subjects with sinus rhythm throughout and newly detected AF are shown in table 1. Subjects with newly detected AF were older, predominantly male, had a higher prevalence of LVH on the electrocardiogram, ischaemic heart disease, hypertension, hypercholesterolaemia, and higher levels of urinary albumin excretion and serum creatinine compared with controls. NT-proBNP levels at baseline were substantially higher in subjects who developed AF compared with subjects with sinus rhythm throughout at follow-up (table 2). Using logistic regression analysis, natural log transformed NT-proBNP levels were significantly associated with AF, even after adjustment for cardiovascular risk factors associated with AF (table 3). Similar results were found when investigating the cross-sectional relation between NT-proBNP per standard deviation and AF at start study (unadjusted odds ratio 2.92 (2.50-3.4), p<0.001). This association remained significant after adjustment for cardiovascular risk factors (2.76 (2.24-3.41), p<0.001).
Table 1.
Baseline characteristics divided subjects with sinus rhythm (SR) throughout and newly detected atrial ibrillation (AF).
| SR (n=6453) | AF (n=41) | P value | |
|---|---|---|---|
| Age (years) | 49.0±12 | 58.0±13 | <0.001 |
| Male (%) | 49.5 | 70.7 | 0.007 |
| Caucasian (%) | 96.0 | 100.0 | 0.634 |
| Body mass index (kg/m2) | 26.0±4.1 | 27.0±4.8 | 0.139 |
| Current smoking (%) | 36.3 | 31.7 | 0.544 |
| Left ventricular hypertrophy (%) | 10.2 | 22.5 | 0.011 |
| Ischaemic heart disease (%) | 16.3 | 39.0 | <0.001 |
| Systolic blood pressure (mmHg) | 128±19 | 141±28 | 0.006 |
| Diastolic blood pressure (mmHg) | 74±10 | 76±11 | 0.105 |
| Hypertension (%) | 32.7 | 63.4 | <0.001 |
| Diabetes mellitus (%) | 3.5 | 2.4 | 0.708 |
| Total cholesterol (mmol/l) | 5.6±1.1 | 6.0±1.2 | 0.029 |
| HDL cholesterol (mmol/l) | 1.3±0.4 | 1.2±0.4 | 0.057 |
| Hypercholesterolaemia (%) | 26.0 | 42.5 | 0.018 |
| Creatinine (μmol//) | 82 (74-91) | 87 (78-99) | 0.014 |
| C-reactive protein (mg/l) | 1.20 (0.54-2.80) | 1.61 (0.71-3.66) | 0.131 |
| Urinary albumin excretion (mg/l) | 9.03 (6.21-15.78) | 12.79 (6.93-24.49) | 0.015 |
Data shown as percentages for categorical variables and mean ± standard deviation for continuous variables. HDL cholesterol, creatinine, C-reactive protein, and urinary albumin excretion are expressed as median (interquartile range).
Table 2.
Levels of NT-proBNP among subjects with sinus rhythm (SR) throughout and newly detected atrial fibrillation (AF). Results expressed as median (interquartile range) or as percentage above sex-specific 80th percentile of NT-proBNP (97 pg/ml in women and 60 pg/ml in men).
| SR (n=6453) | AF (n=41) | P value | |
|---|---|---|---|
| NT-proBNP (pg/ml) | 35.7 (15.9-68.7) | 62.2 (22.6-208.5) | 0.001 |
| % above sex-specific 80th percentile NT-proBNP | 19.8 % (1276/6453) | 53.7 % (22/41) | <0.001 |
Table 3.
Odds ratios for atrial fibrillation after four years in subjects without atrial fibrillation at baseline.
| Odds ratio (95% CI) | P value | |
|---|---|---|
| NT-proBNP (pg/ml) per SD | ||
| Model 1 | 1.94 (1.41-2.68) | <0.001 |
| Model 2 | 1.69 (1.20-2.38) | 0.003 |
| Model 3 | 1.54 (1.05-2.26) | 0.028 |
| % Above sex-specific 80th percentile NT-proBNP | ||
| Model 1 | 4.70 (2.54-8.71) | <0.001 |
| Model 2 | 2.93 (1.47-5.82) | 0.002 |
| Model 3 | 2.65 (1.22-5.76) | 0.014 |
A significant difference was found in NT-proBNP levels between women and men (median [interquartile range] NT-proBNP was 49.9 [27.9-84.1] pg/ml for women vs. 22.6 [9.6-49.2] pg/ml for men, p<0.001). Using a sex-specific 80th percentile cut-off value for NT-proBNP gave similar results as using NT-proBNP as continuous variable (table 3).
Furthermore, a significant interaction was present between the presence of LVH on the electrocardiogram and NT-proBNP levels on the odds for AF (figure 1, p=0.007). Even though the association between NT-proBNP levels and AF was much greater in subjects with LVH (odds ratio 12.63 (5.63-28.31), p<0.001), NT-proBNP levels above the sex-specific 80th percentile cut-off value remained significantly associated with AF in subjects without LVH (odds ratio 2.70 (1.31-5.57), p=0.007). In addition, a similar interaction was present between hypertension and NTproBNP levels on the odds for AF (p=0.019). As expected, hypertension was strongly associated with the presence of LVH (odds ratio 2.20 (1.87-2.59), p<0.001). The interaction between LVH and NTproBNP remained significant after adjusting for the presence of hypertension (p=0.011). Interestingly, subjects with hypertension and LVH at baseline who developed AF had significantly higher NT-proBNP levels than subjects with hypertension and LVH who did not have AF at follow-up (208.5 pg/ml vs. 52.5 pg/ml, p<0.001).
Figure 1.
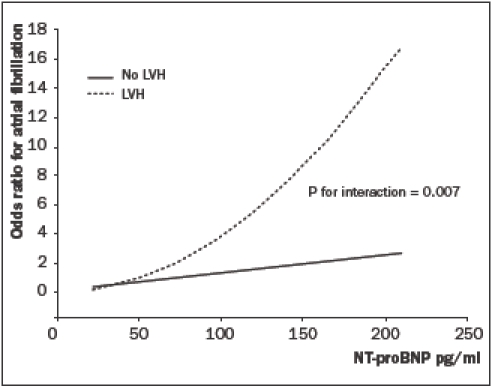
Interaction between N-terminal B-type natriuretic peptide (NT-proBNP) and left ventricular hypertrophy (LVH) on the odds for atrial fibrillation.
Discussion
In this population-based study, NT-proBNP levels predict newly detected AF after four years of followup and this association was independent of other established risk factors for AF, such as higher age, male gender, hypertension, and presence of left ventricular hypertrophy. In addition, a similar association was found between NT-proBNP levels and AF on the electrocardiogram at baseline.
The relation between NT-proBNP and AF is potentially confounded by factors such as sex, age and structural cardiac abnormalities predisposing to the risk of AF. The present data confirm the observation of prior studies that NT-proBNP levels are higher in women compared with men,19,20 but the relation between NT-proBNP and AF remained highly signifycant when using sex-specific cut-off values. Age was next to NT-proBNP levels the strongest predictor of AF in this population-based population. The association between NT-proBNP and AF was still highly significant after adjustment for age, but the odds ratio decreased substantially indicating that age is indeed a strong confounding factor in the relation between NTproBNP and AF. These results confirm data from prior reports demonstrating a strong relation between NTproBNP levels and ageing.19
Interestingly, a significant interaction was found between NT-proBNP levels and LVH on the risk for AF. These data suggest that subjects with haemodynamic abnormalities and structural abnormalities have the highest risk for developing AF. Subjects without structural abnormalities, but with haemodynamic alterations as assessed with NT-proBNP are also at increased risk for AF; however, this association is less pronounced.
Adjustment for other potential confounding factors such as inflammation measured by CRP, renal function, and vascular abnormalities as assessed by urinary albumin excretion did not change the results substantially. These data confirm the findings from the Framingham Offspring Study14 after additional adjustment for novel markers (e.g. CRP).
A likely explanation for the observed independent association between NT-proBNP and AF might be the presence of increased cardiac filling pressures through subclinical cardiac abnormalities such as atrial enlargement, cardiac fibrosis, hypertrophy, and systolic and diastolic dysfunction.21 All these factors are related to elevated levels of NT-proBNP and could potentially cause or mediate AF. Recently, Tsang et al. found a strong association between diastolic filling abnormalities and the development of AF in elderly men and women.7 Diastolic relaxation abnormalities may lead to increased atrial pressures during diastole, which in turn may result in a larger left atrial volume, which is strongly related to the development of AF. Supporting this concept, the stretching of the atrial wall may increase the level of atrial natriuretic factor, which is also predictive for AF.22 Even asymptomatic subjects from the general population with mild left ventricular systolic dysfunction have increased BNP levels.20 In addition, the beneficial effects of ACE inhibition on incident AF possibly through favourable modification of the remodelling processes in patients with heart failure support this relation between structural abnormalities and AF.23
Elevated levels of NT-proBNP might also be related to AF itself.24 A previous study demonstrated an acute decrease in BNP levels after sinus rhythm restoration without any differences in echocardiographic parameters.25 The same phenomenon was found in patients with normal left ventricular function and absence of clinical heart failure.9 These results suggest that AF itself may cause an increase in BNP levels and needs to be taken into account when interpreting BNP values in cardiac patients with AF. In PREVEND, blood was drawn on the same day as the electrocardiogram was recorded. In our prospective analyses, we excluded subjects with AF at baseline and it is therefore unlikely that the observed increase of NTproBNP in subjects with incident AF was caused by the AF itself.
Struthers et al. suggested that NT-proBNP might be used in diabetics as prescreening test for left ventricular dysfunction and that subjects with diabetes and an elevated NT-proBNP may need to be referred for an echocardiogram to evaluate cardiac function.26 In addition, Nielsen et al. showed that selection for echocardiography based on BNP is more cost-effective than referring all subjects for an echocardiogram.27 Next to screening for left ventricular dysfunction, BNP measurements may also identify subjects at high risk for developing AF probably through haemodynamic alterations and structural cardiac abnormalities such as left atrial enlargement. Early therapy targeted at remodelling such as ACE inhibition might prevent heart failure in diabetic subjects, but also incident AF as shown in Val-HeFT and SOLVD.23,28 Future studies are needed to investigate whether BNP measurements in high-risk subjects such as diabetics and hypertensive patients to detect haemodynamic alterations at an early stage might be useful to prevent remodelling and adverse complications including AF.
In this large cohort study, no information was obtained about several known predictors of AF, e.g., hyperthyroidism, history of infection, valve disorders and echocardiographic features such as markers of diastolic dysfunction. In addition, a relatively small number of subjects developed AF during four years and a survivor bias most likely occurred. Routine measurement of BNP levels to solely predict new onset AF in subjects from the general population should not be recommended considering the low incidence of AF and low sensitivity. The type of AF (paroxysmal or persistent) could not be determined either, because one standard 12-lead electrocardiogram was taken which took two minutes. Next to NT-proBNP, atrial natriuretic peptide and N-terminal atrial natriuretic peptide might also be important biomarkers for AF.29 However, strong points of this study are the large size of the population, the computerised ECG analysis thereby avoiding intra- and inter-observer bias, and the measurement of novel biomarkers such as CRP.
In summary, NT-proBNP levels predicted newly detected AF in subjects obtained from the general population. This relation was independent of cardiovascular risk factors predisposing to AF. In addition, a significant interaction was found between the presence of LVH and NT-proBNP levels on the risk for AF. Subjects with LVH and an increased NT-proBNP level had a significantly higher risk of AF.
Acknowledgements
We thank Roche Diagnostics (Mannheim, Germany) for supplying equipment and reagents for proBNP assays. We thank Jacko J. Duker for performing the assays. This study is financially supported by grant E.013 of the Dutch Kidney Foundation, and by grant NHS 99.103 of the Netherlands Heart Foundation. F.W. Asselbergs is a research fellow of the Netherlands Heart Foundation (2003T010) and the Interuniversity Cardiology Institute of the Netherlands.
References
- 1.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 1998;82:2N-9N. [DOI] [PubMed] [Google Scholar]
- 2.Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation 1994;89:724-30. [DOI] [PubMed] [Google Scholar]
- 3.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation 1997;96:2455-61. [DOI] [PubMed] [Google Scholar]
- 4.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, et al. Inflammation as a risk factor for atrial fibrillation. Circulation 2003;108:3006-10. [DOI] [PubMed] [Google Scholar]
- 5.Asselbergs FW, van den Berg MP, Diercks GF, van Gilst WH, van Veldhuisen DJ. C-reactive protein and microalbuminuria are associated with atrial fibrillation. Int J Cardiol 2005;98:73-7. [DOI] [PubMed] [Google Scholar]
- 6.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation 1997;96:1180-4. [DOI] [PubMed] [Google Scholar]
- 7.Tsang TS, Gersh BJ, Appleton CP, Tajik AJ, Barnes ME, Bailey KR, et al. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol 2002;40:1636-44. [DOI] [PubMed] [Google Scholar]
- 8.McDonagh TA, Robb SD, Murdoch DR, Morton JJ, Ford I, Morrison CE, et al. Biochemical detection of left-ventricular systolic dysfunction. Lancet 1998;351:9-13. [DOI] [PubMed] [Google Scholar]
- 9.Wozakowska-Kaplon B. Effect of sinus rhythm restoration on plasma brain natriuretic peptide in patients with atrial fibrillation. Am J Cardiol 2004;93:1555-8. [DOI] [PubMed] [Google Scholar]
- 10.Rienstra M, Van Gelder IC, van den Berg MP, Boomsma F, van Veldhuisen DJ. Natriuretic peptides in patients with atrial fibrillation and advanced chronic heart failure: determinants and prognostic value of (NT-)ANP and (NT-pro)BNP. Europace 2006; 8: 482-7. [DOI] [PubMed] [Google Scholar]
- 11.Ellinor PT, Low AF, Patton KK, Shea MA, Macrae CA. Discordant atrial natriuretic peptide and brain natriuretic peptide levels in lone atrial fibrillation. J Am Coll Cardiol 2005;45:82-6. [DOI] [PubMed] [Google Scholar]
- 12.Mabuchi N, Tsutamoto T, Maeda K, Kinoshita M. Plasma cardiac natriuretic peptides as biochemical markers of recurrence of atrial fibrillation in patients with mild congestive heart failure. Jpn Circ J 2000;64:765-71. [DOI] [PubMed] [Google Scholar]
- 13.Wazni OM, Martin DO, Marrouche NF, Latif AA, Ziada K, Shaaraoui M, et al. Plasma B-type natriuretic peptide levels predict postoperative atrial fibrillation in patients undergoing cardiac surgery. Circulation 2004;110:124-7. [DOI] [PubMed] [Google Scholar]
- 14.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 2004;350:655-63. [DOI] [PubMed] [Google Scholar]
- 15.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002;106:1777-82. [DOI] [PubMed] [Google Scholar]
- 16.Smilde TD, Asselbergs FW, Hillege HL, Voors AA, Kors JA, Gansevoort RT, et al. Mild renal dysfunction is associated with electrocardiographic left ventricular hypertrophy. Am J Hypertens 2005;18:342-7. [DOI] [PubMed] [Google Scholar]
- 17.van Bemmel JH, Kors JA, van Herpen G. Methodology of the modular ECG analysis system MEANS. Methods Inf Med 1990;29:346-53. [PubMed] [Google Scholar]
- 18.Devereux RB, Bella J, Boman K, Gerdts E, Nieminen MS, Rokkedal J, et al. Echocardiographic left ventricular geometry in hypertensive patients with electrocardiographic left ventricular hypertrophy: The LIFE Study. Blood Press 2001;10:74-82. [DOI] [PubMed] [Google Scholar]
- 19.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC Jr. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002;40:976-82. [DOI] [PubMed] [Google Scholar]
- 20.Costello-Boerrigter LC, Boerrigter G, Redfield MM, Rodeheffer RJ, Urban LH, Mahoney DW, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol 2006;47:345-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SH, Jung JH, Choi SH, Lee N, Park WJ, Oh DJ, et al. Determinants of brain natriuretic peptide levels in patients with lone atrial fibrillation. Circ J 2006;70:100-4. [DOI] [PubMed] [Google Scholar]
- 22.Yamada T, Fukunami M, Shimonagata T, Kumagai K, Ogita H, Asano Y, et al. Prediction of paroxysmal atrial fibrillation in patients with congestive heart failure: a prospective study. J Am Coll Cardiol 2000;35:405-13. [DOI] [PubMed] [Google Scholar]
- 23.Maggioni AP, Latini R, Carson PE, Singh SN, Barlera S, Glazer R, et al. Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the Valsartan Heart Failure Trial (Val-HeFT). Am Heart J 2005;149:548-57. [DOI] [PubMed] [Google Scholar]
- 24.Shelton RJ, Clark AL, Goode K, Rigby AS, Cleland JG. The diagnostic utility of N-terminal pro-B-type natriuretic peptide for the detection of major structural heart disease in patients with atrial fibrillation. Eur Heart J 2006;27:2353-61. [DOI] [PubMed] [Google Scholar]
- 25.Inoue S, Murakami Y, Sano K, Katoh H, Shimada T. Atrium as a source of brain natriuretic polypeptide in patients with atrial fibrillation. J Card Fail 2000;6:92-6. [DOI] [PubMed] [Google Scholar]
- 26.Struthers AD, Morris AD. Screening for and treating left-ventricular abnormalities in diabetes mellitus: a new way of reducing cardiac deaths. Lancet 2002;359:1430-2. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen OW, McDonagh TA, Robb SD, Dargie HJ. Retrospective analysis of the cost-effectiveness of using plasma brain natriuretic peptide in screening for left ventricular systolic dysfunction in the general population. J Am Coll Cardiol 2003;41:113-20. [DOI] [PubMed] [Google Scholar]
- 28.Vermes E, Tardif JC, Bourassa MG, Racine N, Levesque S, White M, et al. Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction: insight from the Studies Of Left Ventricular Dysfunction (SOLVD) trials. Circulation 2003;107:2926-31. [DOI] [PubMed] [Google Scholar]
- 29.Tuinenburg AE, van Veldhuisen DJ, Boomsma F, van den Berg MP, de Kam PJ, Crijns HJ. Comparison of plasma neurohormones in congestive heart failure patients with atrial fibrillation versus patients with sinus rhythm. Am J Cardiol 1998;81:1207-10. [DOI] [PubMed] [Google Scholar]


