Abstract
Based on the changes in the field of heart transplantation and the treatment and prognosis of patients with heart failure, these updated guidelines were composed by a committee under the supervision of both the Netherlands Society of Cardiology and the Netherlands Association for Cardiothoracic surgery (NVVC and NVT).
The indication for heart transplantation is defined as: ‘End-stage heart disease not remediable by more conservative measures’.
Contraindications are: irreversible pulmonary hypertension/elevated pulmonary vascular resistance; active systemic infection; active malignancy or history of malignancy with probability of recurrence; inability to comply with complex medical regimen; severe peripheral or cerebrovascular disease and irreversible dysfunction of another organ, including diseases that may limit prognosis after heart transplantation.
Considering the difficulties in defining end-stage heart failure, estimating prognosis in the individual patient and the continuing evolution of available therapies, the present criteria are broadly defined. The final acceptance is done by the transplant team which has extensive knowledge of the treatment of patients with advanced heart failure on the one hand and thorough experience with heart transplantation and mechanical circulatory support on the other hand. (Neth Heart J 2008;16:79-87.)
Keywords: heart transplantation, heart failure, guidelines
Although the first human heart transplantation was performed in 1967, the annual number of transplants only began to grow substantially from the 1980s, declining again after 1996 due to the shortage of donor hearts. Meanwhile, more than 73,000 heart transplants have been performed worldwide, of which more than 800 were performed in the Netherlands.1 Heart transplantation in the Netherlands began in 1984, after a long period of decision-making by the government. The use of strict and congruent protocols and yearly evaluation and reporting to the Ministry of Health were prerequisites. In 1998 the Dutch guidelines for heart transplantation were published after approval by the Netherlands Society of Cardiology (NVVC).2
In 1998 specific legislation on organ donation (WOD, Wet op de Orgaan Donatie) was instituted to regulate the fair allocation of organs and the correct handling of organ donors. It was hoped that this law, in combination with an intensive public awareness programme, would increase the number of donor organs. All initiatives so far, however, have not resulted in more donor hearts. On the contrary, the number of heart transplantations has decreased in recent years, as it has in all countries over the world (figure 1).
Figure 1A.
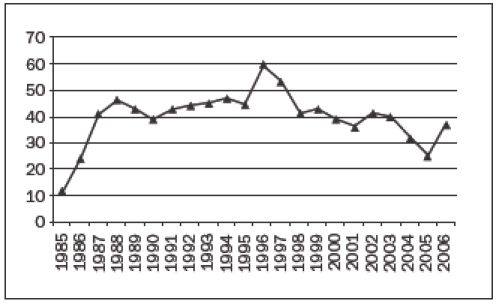
The number of heart transplantations in the Netherlands per year.
Figure 1B.
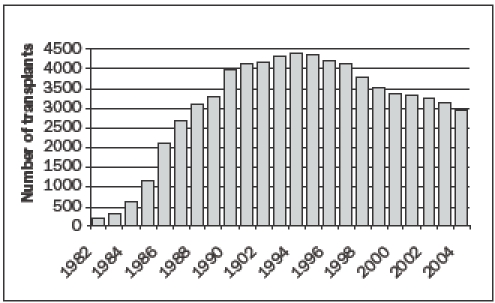
The number of heart transplantations worldwide, according to the ISHLT.
The main explanation for the low number of donor hearts in the Netherlands is the low and still decreasing mortality from traffic accidents in comparison with other European countries. The mortality from traffic accidents per million inhabitants in the Netherlands is 2 to 21⁄2 times lower than in Belgium, Spain and Austria, countries with high numbers of transplantations. 3 In these countries traffic accidents account for 70% of the organ donors, while in the Netherlands this is only ±30%. More than 65% of our donor hearts are obtained from patients who died because of stroke. In general, these patients are older than traffic victims, thus potentially have more cardiovascular problems, limiting their use as cardiac donors, where lung donation may still be possible. Worldwide the mean age of donors used for heart donation is currently 33 years, in contrast to a mean age of 41 years in the Netherlands. 1 Furthermore, in the Netherlands, the percentage of donors older than 56 years used for heart transplantations is much higher than in other Eurotransplant countries (Netherlands 12%; Belgium 8%; Austria 5%; Eurotransplant data 2002).
A development that may have negative effects on the number of heart transplantations is the shift from heart-beating donation to non-heart-beating procedures. Non-heart-beating donation can be performed in patients who are not (yet) brain-dead, but have an unfavourable prognosis and in whom therapy will be withheld. Ventilation is switched off and shortly after the ensuing circulatory arrest the patient is transferred to the operating room for the donation procedure. In this way kidneys, liver and lungs can be used for transplantation, but not the heart due to the extended period of (warm) ischaemia. Non-heartbeating donation was introduced in the Netherlands to expand the pool of donor organs beyond patients meeting the well-defined criteria for brain-death. The procedure is logistically easier for the intensive care, and shorter than a multi-organ (including the heart) procedure, however, and is therefore sometimes preferred by the relatives of the donor and also by the donor hospital. Whether this shift to non-heart-beating procedures really substitutes heart-beating donation is not quite clear and will be investigated.
Due to the above-mentioned developments, the numbers of donor hearts are not expected to increase in the near future.4
In contrast, the number of patients with heart failure is growing, due to ageing of the population and improved survival after myocardial infarction, as well as improved survival of heart failure patients.5
On the one hand, this increase in heart failure patients may result in more potential heart transplant candidates; on the other hand, the improved survival of patients with heart failure raises the question which patients will actually need a transplant.6The widespread use of β-receptor blockers, ACE inhibitors, AT-II blockers, aldosterone blockers, exercise training, cardiac resynchronisation therapy and implantable defibrillators has had a favourable impact on the prognosis of heart failure patients and warrants continuous reevaluation of existing transplant indications.7 Today, the prognosis of many stable heart failure patients is comparable with the one-year post-transplant survival of 85 to 90%, questioning the benefit of heart transplantation in these patients. Therefore fewer ambulatory patients are being transplanted. Instead, heart transplantation in patients on the waiting list, hospitalised because of acutely decompensated heart failure (ADHF), is increasing. Especially the use of ventricular assist devices (LVAD) has enabled these acutely deteriorated patients to survive until heart transplantation. Due to the low number of donor hearts, waiting time can be too long, even when the acute patient would get priority on the waiting list. The results of bridging to transplantation with LVADs in selected patients are very favourable and the patients can lead a reasonably normal life awaiting their heart transplantation.8,9
Based on the changes in the field of heart transplantation and the treatment and prognosis of patients with heart failure it was deemed necessary to update the existing guidelines of 1998. This was supported by a request of the Organ Donation Advisory Committee (BOTX, Begeleidingscommissie Orgaan Transplantatie) of the Health Care Insurance Board (CvZ, College voor Zorgverzekeringen) to provide more transparency in the acceptance or refusal for heart transplantation as well as the acceptance of donor hearts. For this reason an ad hoc committee was formed under the supervision of the NVVC and the NVT and chaired by a representative of the BOTX. The participants of this committee are mentioned in the appendix. A meeting of this committee was organised on 18 May 2006. These updated guidelines are the result of this meeting.
Criteria for acceptation on the transplant waiting list (table 1)
‘End-stage heart disease not remediable by more conservative measures’10
Table 1.
The indication and contraindications for heart transplantation.
| Indication for heart transplantation |
| - End-stage heart disease not remediable by more conservative measures |
| Contraindications |
| - Irreversible pulmonary hypertension /elevated pulmonary vascular resistance |
| - Active systemic infection |
| - Active malignancy or history of malignancy with probability of recurrence |
| - Inability to comply with complex medical regimen |
| - Severe peripheral or cerebrovascular disease |
| - Irreversible dysfunction of another organ, including diseases that may limit prognosis after heart transplantation |
In the light of the foregoing, selection of those patients who may expect to have the greatest benefit in terms of both life expectancy and quality of life from a scarce societal resource is inevitable.
Patients who should be considered for heart transplantation are those with severe symptoms of heart failure, intractable angina or rhythm disturbances, without any alternative form of treatment available and with a poor prognosis. As mentioned in the introduction, new treatment options have modified the prognostic significance of the variables traditionally used to identify heart transplant candidates, e.g. maximal oxygen consumption on exertion (VO2 max).11 Therefore, ‘end-stage’ heart disease has become a ‘moving target’; many patients referred for heart transplantation end up improving their clinical status with judicious use of newer therapies.10
The presence of a low left ventricular ejection fraction or a history of functional class III or IV symptoms of heart failure, as such, and a peak VO2 greater than 15 ml/kg/min (or >55% of predicted uptake) are insufficient indications for heart transplantation.12
The patient must be willing to and capable of undergoing intensive medical treatment, and be sufficiently emotionally stable to withstand the many uncertainties likely to occur both before and after transplantation.11
Given a one-year mortality after heart transplantation of 10 to 15%, the expected one-year mortality in a potential transplant candidate should be at least as high as that. Recent trials of patients with advanced heart failure, such as the COPERNICUS trial, demonstrated a yearly mortality of 11% in patients taking ACE inhibitors and β-blockers.13 In this trial, patients with true end-stage heart failure were not included, but it underlines the difficulty of identifying real transplant candidates.
Considering the difficulties in defining end-stage heart disease, estimating prognosis in the individual patient and the continuing evolution of available therapies, the present criteria are broadly defined. The decision to accept a patient for transplantation is made after careful evaluation by the transplant team with broad experience in this increasingly complex field and will try to draw up the most optimal management plan for the individual patient. This includes optimal pharmacological and non-pharmacological management, such as implantable cardioverter defibrillator, cardiac resynchronisation therapy, revascularisation and alternative surgical options. Patients should only be considered for transplantation when they are on optimal therapy.6
The general experience is that the majority of patients referred for transplantation are never listed and that those who are listed are rarely listed immediately after referral.1,14Deferring transplantation in eligible patients not believed to need immediate listing appears safe and may potentially increase their overall survival, as the post-transplant course is associated with a limited life expectancy.1,14
Estimation of prognosis in patients with heart failure
Estimation of the prognosis in individual patients is extremely difficult because of the large variability in the clinical course of heart failure. Stable periods alternate with acute deteriorations, which may or may not stabilise again.
No single test or measurement has enough predictive power to stratify patients.15
In patients with stable heart failure, measurement of peak oxygen consumption with exercise (VO2 max) can be used to select those with the worst prognosis. In general a peak VO2 ≤14 ml/kg/min, or less than 50% of predicted for age and gender during anaerobic exercise (respiratory quotient, RQ ≥1.05) is thought to delineate a group of patients who potentially benefit from heart transplantation.16 In patients on β-blockers a survival advantage of heart transplantation at one and three years has only been demonstrated in those with a peak VO2 <12 ml/kg/min.17
In addition to peak VO2 the ventilatory response to exercise (VE/VCO2, EqCO2) can be used as a prognostic marker, because this can be measured throughout the entire exercise duration and is independent of patient motivation. The VE/VCO2 slope during exercise is steeper in patients with more severe heart failure and can be regarded as a continuous risk factor for mortality. A VE/VCO2 slope >35 identifies an increased risk for early mortality and this risk is even higher when this slope is >40 to 45.18-20
The combination of several other noninvasive measures can contribute to the estimation of prognosis in patients with heart failure. Of the many risk factors available, seven have been used and validated in patients undergoing transplant evaluation: the Heart Failure Survival Score (HFSS) (table 2).21 Although this risk score was made in the days before widespread use of β-blockers, this scoring system also provides effective risk stratification in patients on β-blockers.22
Table 2.
The Heart Failure Survival score.
| Clinical characteristic | Value (α) | Coefficient (β) | Product |
|---|---|---|---|
| Ischaemic cardiomyopathy | Yes = 1 | +0.6931 | α×β |
| No = 0 | |||
| Resting heart rate | beats/min | +1.9440 | α×β |
| LV-EF | % | –0.0464 | α×β |
| Mean BP | mmHg | –0.0255 | α×β |
| IVCD | >120 msec = 1 | ||
| <120 msec = 0 | +0.6083 | α×β | |
| Peak VO2 | ml/kg/min | –0.0546 | α×β |
| Serum sodium | mmol/l | –0.0470 | α×β |
LV-EF=left ventricular ejection fraction, BP=blood pressure, IVCD=intraventricular conduction delay. The Heart Failure Survival Score (HFSS) is calculated by taking the absolute value of the sums of the products of each component’s variable value and its model coefficient. Low-risk strata: ≥8.10, medium-risk strata: 7.20 to 8.09, high-risk strata: <7.20.
Nowadays, the levels of BNP or NT-pro-BNP and their reactions to therapy can also be taken into consideration as predictors of a poor prognosis, although large studies are lacking.23,24
The timing of evaluation is an important aspect of the risk assessment in heart failure patients. It will be clear that the risk score is considerably worse in severely congested patients and can be improved by increasing the medication. Therefore, evaluation should only be done in optimally treated patients. Worsening of the patient’s condition over time, for instance a gradual decrease in peak VO2 in consecutive exercise tests, or repeated admissions in hospital for the treatment of decompensation, may also delineate transplant candidates.25
The estimation of prognosis in hospitalised patients with acute heart failure is even more difficult than in stable, ambulatory patients. Some patients deteriorate so rapidly that only an urgent heart transplantation or mechanical support can save them. Others, however, stabilise and may show gradual improvement in the course of months or years. This is especially the case in patients with a first manifestation of a cardiomyopathy.26
The HFSS is not validated for patients hospitalised with acute heart failure.
The Acute Decompensated Heart Failure National Registry (ADHERE) identified three variables at hospital admission which correlated with increased mortality: serum urea >15 mmol/l, systolic blood pressure <115 mmHg. and serum creatinine >240 μmol/l (figure 2).27
Figure 2.
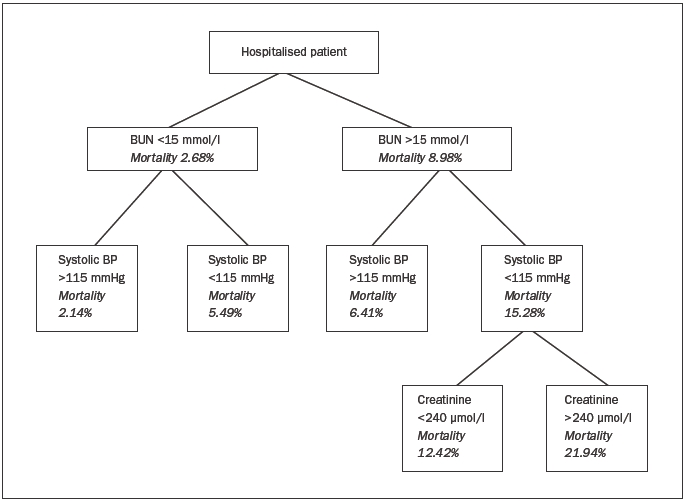
Predictors of in-hospital mortality according to the Acute Decompensated Heart Failure National Registry (ADHERE). BUN=blood urea nitrogen.
The Heart Failure Mortality Predicting Score predicts 30-day and one-year mortality using several admission data, including age, systolic blood pressure, respiratory rate, serum urea, hyponatraemia and some comorbid conditions.28 An electronic version of this risk score is available at: http://www.ccort.ca/CHFriskmodel.asp.
Both risk scores, however, apply more to the general heart failure population which is older, than to patients thought of as realistic transplant candidates. Regular consultation of a heart transplant centre to discuss therapeutic options in these difficult patients is therefore advisable.25
Given the dynamic nature of the clinical course of heart failure, patients on the waiting list for heart transplantation, as well as patients deemed too good for transplantation at first evaluation, should be regularly re-evaluated (see under Decision-making).
The implications of comorbidities (table 1)
Irreversible pulmonary hypertension / elevated pulmonary vascular resistance
Irreversible elevated PVR is generally poorly tolerated by the right ventricle of the donor heart. This may result in acute right-sided failure, sometimes resulting in the perioperative death of the recipient.29,30 An absolute cut-off value, however, does not exist. Elevated pulmonary vascular resistance has to be seen as an incremental risk factor from low to high values.
Therefore, in patients evaluated for transplantation, a right heart catheterisation is mandatory. As mentioned before, this should only be done in an optimally treated patient.
A vasodilator challenge should be administered when the pulmonary artery systolic pressure is ≥50 mmHg and either the transpulmonary gradient (TPG = PA mean-PCWP) is ≥15 mmHg or the PVR is >3 Wood units (>240 dynes.sec.cm-5). The drugs usually used for this acute challenge are prostacyclin, nitroglycerin and nitroprusside. Other drugs, such as nitric oxide, dobutamine and milrinone, can also be used.
A severely increased risk of right heart failure and mortality after heart transplantation is thought to be present:31
When the PVR is >5 Wood units (>400 dynes.sec.cm-5), or the PVRI is >6 Wood units.m2 in children), or the TPG exceeds 16 to 20 mmHg.
If the systolic pulmonary artery pressure exceeds 60 mmHg in conjunction with any one of the preceding three variables.
If the PVR can be reduced to <2.5 with a vasodilator only at the cost of a fall in arterial systolic blood pressure <85 mmHg.
Active systemic infection
An active systemic infection at the time of heart transplantation, when recipients are treated with high doses of immunosuppressive drugs, is still seen as an important contraindication, at least in the short term. Persistent infections, such as HIV, pose a problem, as the chronic use of immunosuppressive drugs in this already immunodeficient population is generally thought to give rise to serious complications. There are scarce, but growing data of organ transplantation in these patients, although data about long-term outcome are lacking. Given the increasing shortage of donor hearts, one has to wonder if these patients really are the optimal candidates for this form of therapy.
Active malignancy or history of malignancy with probability of recurrence
Active neoplasm from origins other than the skin is an absolute contraindication to heart transplantation due to the limited survival rates.31 Patients with a history of malignancy can be considered for heart transplantation when the risk of tumour recurrence is low, preferably after a reasonable time of complete remission, depending on the tumour type, response to therapy and negative metastatic work-up.
Inability to comply with complex medical regimen
Compliance, the capacity to adhere to a complex lifelong regime of drug therapy, lifestyle changes and regular follow-up, is a crucial element in attaining longterm success after transplantation.31 This includes the adequate use of all medication, because suboptimal use of immunosuppressive medications plays a roll in most acute rejections occurring more than six months after transplantation and it is also related to subsequent cardiac allograft vasculopathy (chronic rejection).32
Also substance abuse (alcohol, drugs) and tobacco use have to be taken into consideration as it is thought that especially substance abuse is an important predictor of noncompliance.33 Tobacco use continues to be the foremost avoidable cause of death in the Western world with an enormous impact on cardiovascular diseases and malignancies. Small studies have demonstrated increased incidence of coronary allograft vasculopathy and malignancy, along with decreased survival in those patients who return to smoking after transplantation.34 Active tobacco smoking during the previous six months is a risk factor for poor outcomes after transplantation and therefore considered a relative contraindication.31
To evaluate the patient’s ability to comply with instructions including drug therapy, a psychosocial assessment should be performed before listing for transplantation.
Severe peripheral or cerebrovascular disease
Systemic vascular disease may contribute to both poor prognosis for survival as well as poor quality of life on a noncardiac basis and should therefore be considered as a major comorbidity that can preclude eligibility for heart transplantation.10 The severity of symptoms and the potential options for revascularisation may affect this decision. It has been suggested that the progression of vascular disease may be accelerated after heart transplantation, especially in patients transplanted for ischaemic heart disease.35
Irreversible dysfunction of another organ
Comorbidities can have an important impact on the decision about acceptance for transplantation and should be searched for in every patient. All comorbidities which adversely influence prognosis after transplantation should be weighed individually.
In this respect, renal function is a very important risk factor for mortality post transplantation.1,36 Irreversible renal dysfunction with a GFR <40 ml/min, as estimated by the creatinine clearance or sMDRD equation, can be considered as a relative contraindication for heart transplantation.31 In general, renal function will further deteriorate after heart transplantation, partly as a result of the nephrotoxic immunosuppressive drugs. The incidence of chronic renal failure (GFR <29 ml/min), five years after heart transplantation, is estimated to be 7 to 21% and severely compromises prognosis.37 Many patients after heart transplantation end up on dialysis or even secondary kidney transplantation.
Although combined transplantation of a heart and a kidney from the same donor is now technically feasible, it should only be considered in the most appropriate individuals to maximise the supply of limited organs.31
Other comorbidities which should be emphasised are diabetes mellitus and obesity. In the early years of heart transplantation, diabetes mellitus was considered an absolute contraindication by all centres. With growing experience it was recognised that selected patients with uncomplicated diabetes demonstrated the same prognosis after heart transplantation as patients without diabetes. This was recently confirmed in a large study on 20,000 heart transplant recipients of which 3600 had diabetes before transplantation. Patients with diabetes-related complications, including renal failure (serum creatinine >220 μmol/l), peripheral vascular disease, cerebrovascular accident and severe obesity had a significantly worse survival than nondiabetics, however. Therefore, diabetes with complications should be considered as a relative contraindication.38
Regarding obesity, there are many data on its adverse influence on prognosis.1 One study demonstrated a five-year mortality post-transplantation almost twice as high in obese patients (BMI >30 kg/m2) in comparison with normal-weight patients (53 vs. 27%, respectively).39 Given the poor outcome of obesity after transplantation, weight loss should be mandatory to achieve a BMI <30 kg/m2 before listing for transplantation.31
All other diseases that may limit prognosis after heart transplantation should be discussed on an individual basis.
Donor selection and management
This subject is extensively covered in the new protocol of the Netherlands Transplant Society (NTS, Nederlandse Transplantatie Stichting),40 which has been published recently.
Here, it suffices to say that in principle every braindead patient is regarded as a potential multi-organ donor and that heart-beating donation is preferred over non-heart-beating procedures. For heart donation, the upper age limit is ±65 years. The only absolute specific cardiac contraindication for heart donation is the presence of important heart disease, such as angina pectoris, myocardial infarction, prior coronary bypass surgery, moderate to severe valvular disease, cardiomyopathy and important arrhythmias. General contraindications for all donations are, for example, untreated sepsis, malignancies and active infections.
In the work-up of a potential heart donor, the medical history, an electrocardiogram and a transthoracic echocardiogram (TTE) are essential, besides haemodynamic data and markers for cardiac damage, including troponin. If the left ventricular function cannot be reliably evaluated by TTE, because of insufficient acoustic window in a ventilated patient, transoesophageal echocardiography (TEE) is mandatory. In haemodynamically unstable patients, a Swan-Ganz catheter should be used to optimise the filling status of the patient. Given the generally older age of donors in the Netherlands, coronary angiography can be helpful to rule out significant coronary artery disease in elderly donors or other patients with risk factors for coronary artery disease.
Patient-oriented allocation of donor organs is done by Eurotransplant (ET), according to blood group, body size, medical urgency and waiting time. The final acceptance of a donor heart is the responsibility of the transplantation team, which will weigh all the donor data in combination with the actual situation of the potential recipient. Although donor age especially has increased in our country, it still plays an important role in this decision because the results of heart transplantations with older donor hearts (≥50 years) are worse than with younger donor hearts. This relates to early postoperative mortality, but also correlates with the early presence of transplant-related coronary artery disease.41-43 Donor age therefore has to be seen as an important continuous risk factor for mortality postheart transplantation, especially combined with long ischaemic times of the donor heart.1 The higher risk of using hearts from older donors will always be weighed against not transplanting at all due to lack of a younger donor.
Decision-making
As stated before, the indications and contraindications for heart transplantation as well as the guidelines for the acceptance of donor hearts are broadly defined. The final acceptance is done by the transplant team which has extensive knowledge of the treatment of patients with advanced heart failure on the one hand and thorough experience with heart transplantation and mechanical circulatory support on the other hand. Heart transplantation is a very laborious treatment modality for only a few patients. It requires a dedicated team of specialists, consisting of at least a cardiologist trained in infectiology and immunology, a cardiothoracic surgeon, an anaesthesiologist, and specialised nurses.
One has to realise that, in contrast to other medical therapies, heart transplantation is a form of therapy with very limited ‘resources’ and therefore requires extensive judgement to make the most optimal use of this modality.
That is why it is also important that outpatients on the waiting list for heart transplantation should be regularly re-evaluated (every six months) preferably with cardiopulmonary exercise testing. If they have improved significantly, they may be candidates for delisting.31
If a patient or his/her referring physician does not agree with the decision of the transplant team, a second opinion in one of the other centres is possible.
The heart transplantation centres will organise a meeting, twice a year, in the presence of outside observers, e.g. referring cardiologists, to discuss referred patients and the reasons for listing or not-listing. Furthermore, potential donor offers can be discussed. In this way the whole process of decision-making will hopefully be more transparent for those concerned.
Referral
Referral of a patient to a transplant centre should be preceded by sending extensive written information including a summary of the complete medical history and current data (table 3).
Table 3.
Requested information for referral of a potential heart transplant candidate.
| - Summary of the complete medical history (cardiac as well as non-cardiac) |
| - Actual medication and history of intolerance to medication |
| - Surgery report in case of prior cardiac surgery |
| - Heart catheterisation data (left- and right-sided pressures, cardiac output, PVR, SVR and coronary angiography) |
| - Evaluation of the present status of the patient: |
| a) Functional class and predominant symptoms/problems |
| b) Physical examination including peripheral/carotid vessels, and oral cavity (dental status) |
| c) ECG |
| d) Chest X-ray |
| e) Blood type and Rhesus factor, electrolytes, renal and liver function, glucose, ESR or CRP, Hb, white blood count and |
| differentiation, platelets |
| Serology for HBV and HCV and HIV |
| Urine analysis for protein, glucose and sediment |
| Stool tests for blood loss |
| f) Echocardiogram (dimensions, systolic and diastolic ventricular function, estimation of right-sided pressures, valvular |
| abnormalities) |
| g) Exercise test, preferably with determination of peak VO2 |
| h) Pulmonary function testing |
Appendix
The committee consisted of the following persons:
Chairman: Professor P. Sergeant
N. de Jonge, A.H.M.M. Balk, C. Klöpping, A. Oosterom, A. Oomen, E.T. Bal, J-W. Lammers, A. Golüke, D. Nicastia, M. Koole, H.F. Verwey, P.W. Boonstra, P. Doevendans, J.H. Kirkels, J Brügeman, M.E. Erasmus, R.J.M. Klautz, M.L.M. Versteegh, C. Lucas, C.H. Peels, H.A. van Swieten, E. de Buijzer, K. Calsikan, A.P.W.M. Maat, J.R. Lahpor.
References
- 1.Taylor DO, Edwards LB, Boucek MM, Trulock EP, Waltz DA, Keck BM, et al. Registry of the International Society for Heart and Lung Transplantation: Twenty-third official adult heart transplantation report-2006. JHLTX 2006;25:869-79. [DOI] [PubMed] [Google Scholar]
- 2.Balk AHHM, Maat APWM, Weimar W, de Jonge N, et al. Heart transplantation: guidelines for the referring cardiologist. Cardiologie 1998;5:702-15. [Google Scholar]
- 3.Coppen R, Marquet RL, Friele RD. Het donorpotentieel. Een vergelijking van het donorpotentieel in Nederland en 9 andere West Europese landen. Nivel 2003. [Google Scholar]
- 4.Kirkels JH, de Jonge N, Klöpping C, Lahpor JR, van Herwerden LA, Balk AHMM, et al. Heart transplantation in the Netherlands: quo vadis? Neth Heart J 2006;14:425-30. [PMC free article] [PubMed] [Google Scholar]
- 5.Redfield MM. Heart failure-an epidemic of uncertain proportions. N Engl J Med 2002;347:1442-4. [DOI] [PubMed] [Google Scholar]
- 6.Gardner RS, McDanagh TA, MacDonald M, Dargie HJ, Murday AJ, Petrie MC. Who needs a heart transplant? Eur Heart J 2006;27:770-2. [DOI] [PubMed] [Google Scholar]
- 7.Butler J, Khadim G, Paul KM, Davis SF, Kronenberg MW, Chomsky DB, et al. Selection of patients for heart transplantation en the current era of heart failure therapy. J Am Coll Cardiol 2004;43:787-93. [DOI] [PubMed] [Google Scholar]
- 8.Lahpor JR, de Jonge N, van Swieten HA, Wesenhagen H, Klöpping C, Geertman JH, et al. Left ventricular assist device as bridge to transplantation in patients with end-stage heart failure. Eight year experience with the implantable HeartMate LVAS. Neth Heart J 2002;10:267-71. [PMC free article] [PubMed] [Google Scholar]
- 9.de Jonge N, Kirkels H, Lahpor JR, Klöpping C, Hulzebos EJ, Brutel de la Rivière A, et al. Exercise performance in patients with end-stage heart failure after implantation of a left ventricular assist device and after heart transplantation: an outlook for permanent assisting? J Am Coll Cardiol 2001;37:1794-9. [DOI] [PubMed] [Google Scholar]
- 10.Hunt SA, Kouretas PC, Balsam LB, Robbins RC. Heart transplantation. In Zipes DP, Libby P, Bonow RO, Braunwald E. Braunwald’s Heart Disease. 7th edition. Elsevier Saunders 2005. [Google Scholar]
- 11.The task force for the diagnosis and treatment of chronic heart failure of the European Society of Cardiologie. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005). Eur Heart J 2005;26:1115-40. [DOI] [PubMed] [Google Scholar]
- 12.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult-summary article. Circulation 2005;112:1825-52. [Google Scholar]
- 13.Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651-8. [DOI] [PubMed] [Google Scholar]
- 14.Lewis EF, Tsang SW, Fang JC, et al. Frequency and impact of delayed decisions regarding heart transplantation on long-term outcomes in patients with advanced heart failure. J Am Coll Cardiol 2004; 43:794-802. [DOI] [PubMed] [Google Scholar]
- 15.Hunt SA, Frazier OH. Mechanical circulatory support and cardiac transplantation. Circulation 1998;97:2079-90. [DOI] [PubMed] [Google Scholar]
- 16.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Wilson JR. Value of peak oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991;83:778-86. [DOI] [PubMed] [Google Scholar]
- 17.Peterson LR, Schechtman KB, Ewald GA, et al. Timing of cardiac transplantation in patients with heart failure receiving betaadrenergic blockers. J Heart Lung Transplant 2003;22:1141-8. [DOI] [PubMed] [Google Scholar]
- 18.Chua TP, Ponikowski P, Harrington D, et al. Clinical correlates and prognostic significance of the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol 1997;29:1585-90. [DOI] [PubMed] [Google Scholar]
- 19.Robbins M, Francis G, Pashkow FJ, et al. Ventilatory and heart rate responses to exercise. Better predictors of heart failure mortality than peak oxygen consumption. Circulation 1999;100: 2411-7. [DOI] [PubMed] [Google Scholar]
- 20.Gitt AK,Wasserman K, Kilkowskis C, et al. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation 2002;106:3079-84. [DOI] [PubMed] [Google Scholar]
- 21.Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation 1997;95:2660-7. [DOI] [PubMed] [Google Scholar]
- 22.Koelling TM, Joseph S, Aaronson KD. Heart failure survival score continues to predict clinical outcomes in patients with heart failure receiving beta-blockers. J Heart Lung Transplant 2004;23:1414-22. [DOI] [PubMed] [Google Scholar]
- 23.Rothenburger M, Wichter T, Schmid C. Aminoterminal pro type Bnatriuretic peptide as a predictive and prognostic marker in patients with chronic heart failure. J Heart Lung Transplant 2004;23:1189-97. [DOI] [PubMed] [Google Scholar]
- 24.Koglin J, Pehlivanli S, Schwaiblmair M, Vogeser M, Cremer P, Von Scheidt W. Role of brain natriuretic peptide in risk stratification of patients with congestive heart failure. J Am Coll Cardiol 2001;38:1934-41. [DOI] [PubMed] [Google Scholar]
- 25.de Jonge N, Vantrimpont PJMJ. Treatment of end-stage heart failure. Neth Heart J 2004;12:548-54. [PMC free article] [PubMed] [Google Scholar]
- 26.Steimle AE, Stevenson LW, Fonarow GC, et al. Prediction of improvement in recent onset cardiomyopathy after referral for heart transplantation. J Am Coll Cardiol 1994;23:553-9. [DOI] [PubMed] [Google Scholar]
- 27.Fonarow GC, Adam KF, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acute decompensated heart failure: classification and regression tree analysis. JAMA 2005;293:572-80. [DOI] [PubMed] [Google Scholar]
- 28.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA 2003;290:2581-7. [DOI] [PubMed] [Google Scholar]
- 29.Kirklin JK, Naftel DC, Kirklin JW, Blackstone EH, White-Williams C, Bourge RC. Pulmonary vascular resistance and the risk of heart transplantation. J Heart Lung Transplant 1988;7:331-6. [PubMed] [Google Scholar]
- 30.Erickson KW, Costanzo-Nordin MR, O’Sullivan EJ, et al. Influence of preoperative transpulmonary gradient on late mortality after orthotopic heart transplantation. J Heart Lung Transplant 1990;9:526-37. [PubMed] [Google Scholar]
- 31.Mehra MR, Kobashigawa J, Starling R, et al. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates-2006. J Heart Lung Transplant 2006;25:1024-42. [DOI] [PubMed] [Google Scholar]
- 32.Dew MA, Kormos RL, Roth LH, Murali S, DiMartini A, Griffith BP. Early post-transplant medical compliance and mental health predict physical morbidity and mortality one to three years after heart transplantation. J Heart Lung Transplant 1999;18:549-62. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro PA, Williams DL, Foray AT, Gelman IS, Wukich N, Sciacca R. Psychosocial evaluation and prediction of compliance problems and morbidity after heart transplantation. Transplantation 1995;60:1462-6. [DOI] [PubMed] [Google Scholar]
- 34.Radovancevic B, Poindexter S, Birovljev S, et al. Risk factors for development of accelerated coronary artery disease in cardiac transplant recipients. Eur J Cardiothorac Surg 1990;4:309-12. [DOI] [PubMed] [Google Scholar]
- 35.Vantrimpont PJ, van Dalen BM, van Riemsdijk-van Overbeeke IC, Maat AP, Balk AH. Abdominal aortic aneurysms after heart transplantation. J Heart Lung Transplant 2004;23:171-7. [DOI] [PubMed] [Google Scholar]
- 36.Wetering van de J, Weimar CH, Balk AH, Roodnat JL, Holweg CT, Baan CC, et al. The impact of transforming growth factor beta-1 gene polymorphism on end-stage renal failure after heart transplantation. Transplantation 2006;82:1744-8. [DOI] [PubMed] [Google Scholar]
- 37.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003;349:931-40. [DOI] [PubMed] [Google Scholar]
- 38.Russo MJ, Chen JM, Kimberly NH, et al. Survival after heart transplantation is not diminished among recipients with uncomplicated diabetes mellitus. An analysis of the united network of organ sharing database. Circulation 2006;114:2280-7. [DOI] [PubMed] [Google Scholar]
- 39.Lietz K, John R, Burke EA, et al. Pretransplant cachexia and morbid obesity are predictors of increased mortality after heart transplantation. Transplantation 2001;72:277-83. [DOI] [PubMed] [Google Scholar]
- 40.Modelprotocol postmortale orgaan-en weefseldonatie 2006/2007. Onder redactie van J Ringers. Nederlandse Transplantatie Stichting. [Google Scholar]
- 41.Lietz K, Ranjit J, Mancini DM, Edwards NM. Outcomes in cardiac transplant recipients using allograft from older donors versus mortality on the transplant waiting list. Implications for donor selection criteria. J Am Coll Cardiol 2004;43:1553-61. [DOI] [PubMed] [Google Scholar]
- 42.Smits JM, Vanhaecke J, Haverich A, de Vries E, et al. Three-year survival rates for all consecutive heart-only and lung-only transplants performed in Eurotransplant, 1997-1999. Clin Transpl 2003:89-100. [PubMed] [Google Scholar]
- 43.Duport N, Pessione F, Chalem Y, Tuppin P. Marginal heart graft: extended donor criteria and their impact on graft survival. Organs Tissue 2005;2:93-100. [Google Scholar]


