Abstract
Context
Obesity is associated with hypoferremia, but it is unclear if this condition is caused by insufficient iron stores or diminished iron availability related to inflammation-induced iron sequestration.
Objective
To examine the relationships between obesity, serum iron, measures of iron intake, iron stores and inflammation. We hypothesized that both inflammation-induced sequestration of iron and true iron deficiency were involved in the hypoferremia of obesity.
Design
Cross-sectional analysis of factors anticipated to affect serum iron.
Setting
Outpatient clinic visits.
Patients
Convenience sample of 234 obese and 172 non-obese adults.
Main outcome measures
Relationships between serum iron, adiposity, and serum transferrin receptor, C-reactive protein, ferritin, and iron intake analyzed by analysis of covariance and multiple linear regression.
Results
Serum iron was lower (75.8 ± 35.2 vs 86.5 ± 34.2 g/dl, P=0.002), whereas transferrin receptor (22.6 ± 7.1 vs 21.0 ± 7.2 nmol/l, P=0.026), C-reactive protein (0.75 ± 0.67 vs 0.34±0.67 mg/dl, P<0.0001) and ferritin (81.1 ± 88.8 vs 57.6 ± 88.7 mg/l, P=0.009) were higher in obese than non-obese subjects. Obese subjects had a higher prevalence of iron deficiency defined by serum iron (24.3%, confidence intervals (CI) 19.3–30.2 vs 15.7%, CI 11.0–21.9%, P=0.03) and transferrin receptor (26.9%, CI 21.6–33.0 vs 15.7%, CI 11.0–21.9%, P=0.0078) but not by ferritin (9.8%, CI 6.6–14.4 vs 9.3%, CI 5.7–14.7%, P=0.99). Transferrin receptor, ferritin and C-reactive protein contributed independently as predictors of serum iron.
Conclusions
The hypoferremia of obesity appears to be explained both by true iron deficiency and by inflammatory-mediated functional iron deficiency.
Keywords: iron deficiency, adiposity, ferritin, inflammation, C-reactive protein, transferrin receptor
Introduction
Obesity is associated with low-serum iron concentrations. The inverse relationship between iron status and adiposity was first reported in 1962, when Wenzel et al.1 unexpectedly found a significantly lower mean serum iron concentration in obese compared with non-obese adolescents. Most subsequent studies in pediatric2–4 and adult samples5–8 have shown similar results. For example, in a large study using National Health and Nutrition Examination Survey-I (NHANES-I) data, Micozzi et al.5 found that higher body mass index (BMI) was significantly associated with lower serum iron in women, and that transferrin saturation was significantly lower in the highest BMI quartile for both men and women.
The etiology of the hypoferremia of obesity is uncertain. Among the proposed causes are deficient iron intake from an iron poor diet,2 and deficient iron stores owing to greater iron requirements in obese adults because of their larger blood volume.3,9,10 The relationship between serum iron and dietary iron intake among obese adults has not been previously examined in large studies. Also, since obesity is considered a chronic inflammatory state,11 inflammatory-mediated sequestration of iron in the reticuloendothelial system, with resultant hypoferremia despite adequate or even increased iron stores could play a role in the hypoferremia of obesity. Inappropriate sequestration of iron, when severe, results in the anemia of chronic disease. Support for an inflammatory cause for the hypoferremia of obesity derives from the observation that serum ferritin concentrations, which are usually suppressed when body iron stores are low,12 tend to be high and inversely related to transferrin saturation in those with excessive adiposity.6,7,13,14 Ferritin is increased in conditions associated with inflammation because it is an acute-phase reactant;15 cytokines such as interleukin-1β and tumor necrosis factor-α (TNF-α) induce ferritin production within macrophages, hepatocytes and adipocytes.16,17 Prior studies have found that ferritin may be elevated in inflammatory conditions even in the presence of true iron deficiency.18 It remains unclear, however, if the lower serum iron and elevated ferritin seen in obesity are most reflective of a functional iron deficiency related to an inflammatory state, or if obesity is also a risk factor for true iron deficiency.
Recently, serum transferrin receptor concentration has been recognized as a useful indicator of iron status. Serum transferrin receptor is a soluble protein that is produced by proteolytic cleavage of the membrane-bound transferrin receptor. Both the expression of transferrin receptor on the cell surface and its intracellular concentration are inversely related to intracellular iron concentrations.19,20 Serum transferrin receptor increases in true iron deficiency states. Unlike ferritin, it is not an acute-phase reactant and is not elevated in chronic inflammatory states.21–23 Hence, serum transferrin receptor concentration is believed to be more reliable than ferritin to diagnose true iron deficiency in the presence of inflammation.24
The purpose of this study was to examine the relationships among serum iron and measures of inflammation, iron intake, and iron stores in obese and non-obese adults. We hypothesized that both inflammation-induced diversion of iron into the reticuloendothelial system and true iron deficiency were involved in the hypoferremia of obesity.
Subjects and methods
Subjects
A convenience sample of adults was recruited from the Washington, DC metropolitan area through advertisements inviting ‘healthy volunteers’ or ‘overweight’ adults to participate in a calcium supplementation study.25 Potential subjects were excluded for reporting significant medical illnesses such as diabetes, bone, kidney, liver or gallbladder disease, a history of cancer, gastrointestinal bleeding or inflammatory diseases, for using medications that affect body weight or for having intentional weight change of more than 3% of body weight in the preceding 3 months. The research protocol was approved by the National Institute of Child Health and Human Development Institutional Review Board, and signed consent was obtained from all subjects. Financial compensation was provided for subjects’ time and inconvenience.
Subjects reported after a 10-h overnight fast to undergo a detailed history and physical examination. Subjects were weighed in hospital gowns using a digital scale (Life Measurement Instruments, Concord, CA, USA) that was calibrated with a known weight before each subject’s measurement. Height was measured using a stadiometer also calibrated before each measurement (Holtain Ltd, Crymych, UK). Whole-body dual energy X-ray absorptiometry (DEXA) for estimation of fat mass (Delphi A, LX 20 Beckman, Bedford, MA, USA, software version 11.2) was performed in 332 subjects. Socioeconomic status (SES) was determined by Hollingshead score,26 grouped as follows: categories I and II were ‘higher,’ category III was ‘mid’ and categories IV and V constituted ‘lower’ SES.
Laboratory assays
Serum iron and transferrin were measured by the NIH clinical chemistry laboratory with a Beckman 20 LX analyzer (Beckman Coulter Inc., Fullerton, CA, USA). For serum iron, an iron ferrozine complex method was used with sensitivity 5 µg/dl; serum transferrin was measured using a turbidi-metric method with sensitivity 70 mg/dl. Serum iron <50 µg/dl was considered to constitute iron deficiency based on the manufacturer’s guidelines. Transferrin saturation was calculated by finding the molar ratio of serum iron and twice the serum transferrin (because each transferrin molecule can bind two atoms of iron) using the formula transferrin saturation = (serum iron (µg/dl)/transferrin (mg/dl))*71.2. Serum ferritin was measured by immunometric assay (IMMULITE 2000, EURO/DPC Ltd, Los Angeles, CA, USA); sensitivity was 0.4 µg/l. Ferritin <9 µg/l was considered consistent with iron deficiency based on NIH laboratory normative data. Hemoglobin and mean corpuscular volume (MCV) were measured using flow cytometry (CELL-DYN® 4000, Abbott Laboratories, Abbott Park, IL, USA). Soluble (serum) transferrin receptor was measured by a Nichols Advantage chemiluminescence immunoassay (Nichols Institute Diagnostics, San Juan Capistrano, CA, USA), with calculated sensitivity of 0.1 nmol/l and intraassay coefficient of variation of 4.6–6.5% and interassay coefficient of variation of 6.1–10.5%. The manufacturer’s expected reference range for this assay was 6.4–25.7 nmol/l, and iron deficiency was defined as a value >25.7 nmol/l per the manufacturer’s recommendation. Normal reference ranges cited in the literature report that serum transferrin receptor is dependent on the ethnic origin of the patient with African Americans having higher values than non-African Americans. 27 C-reactive protein was measured by a high-sensitivity assay (IMMAGE Immunochemistry Systems, Beckman Coulter Inc., Fullerton, CA, USA). Reference interval for this assay was <0.8 mg/dl and sensitivity 0.1 mg/dl.
Recorded iron intake
Dietary iron intake was measured by a 7-day food record. Subjects were given written instructions to record all foods and beverages consumed over 7 consecutive days. Food records were reviewed in person with subjects by a registered dietitian to maximize accuracy and completeness, and analyzed for dietary iron intake using the Nutrition Data System for Research (NDS-R) software versions 4.04_32 and 4.05_33, developed by the Nutrition Coordinating Center (University of Minnesota, Minneapolis, MN, USA).28 As is recommended,29 invalid food records (n=2) were excluded if subjects reported a biologically implausible amount of calories per day (less than 600 cal or more than 3500 cal per day for women and less than 800 cal or more than 4200 cal per day for men). Iron from supplements is not measured by the food record method, but food records are considered the gold standard for dietary intake assessment. To estimate intake of iron from supplements, we used responses to questions regarding dietary supplement use during the past year on the Diet History Questionnaire (DHQ), a validated, 36-page booklet listing 124 separate food items developed by the National Cancer Institute for use in epidemiological research.29 The estimated amount of iron intake from multivitamin supplements was determined by assigning an average value to the amount of iron in multivitamins. DHQs were analyzed using Diet*Calc Analysis Program (Version 1.3.2., National Cancer Institute, Applied Research Program, June 2003). We added dietary iron intake from food records to supplemental iron intake from DHQs to estimate total daily iron intake.
Statistical analyses
The primary analysis was an analysis of covariance, with serum iron as the dependent variable. Independent variables included serum transferrin receptor, ferritin, and C-reactive protein concentrations, total daily iron intake, and obesity as the categorical variable of interest (obese vs non-obese); age, sex, race and SES were treated as covariates. These data were analyzed using SPSS for Windows version 14.0 (Chicago, IL, USA). Unless otherwise indicated, data are reported as mean ±s.d. adjusted for covariates. We also examined these same variables and covariates in multiple linear regression models with BMI and fat mass as the continuous variables of interest and in logistic regression models to predict iron deficiency. Tests were performed for collinearity. Correlations between measures were evaluated using Spearman correlation coefficients. Categorical data were compared by contingency table analyses and are reported as percentages with 95th percentile CI. P<0.05 was considered significant.
Results
A total of 172 healthy non-obese (BMI <30 kg/m2), and 234 obese (BMI ≥30 kg/m2) adults were studied (Table 1). There was a higher proportion of Caucasians in the non-obese group and a higher proportion of African Americans in the obese group (P=0.009). SES was also different among study participants (P=0.001), with a greater proportion of obese subjects having low SES.
Table 1.
Subject characteristics
| Characteristics | Obese (n = 234) | Non-obese (n = 172) | P-value |
|---|---|---|---|
| Age (years) | 38.6 ± 9.7 | 37.2± 11.2 | 0.20 |
| Range | (18.7−64.0) | (19.3−70.6) | |
| Race (%) | |||
| Caucasian | 52.1 | 64.5 | 0.009 |
| African American | 38.9 | 24.4 | |
| Other | 9.0 | 11.1 | |
| Socioeconomic status (%) | |||
| High | 34.1 | 41.6 | 0.001 |
| Mid | 43.3 | 50.0 | |
| Low | 22.6 | 8.4 | |
| BMI (kg/m2) | 38.4±6.3 | 25.6±3.1 | <0.0001 |
| Range | (30.1−60.5) | (17.8−29.9) | |
| Body fat, kg (n=332) | (43.7±9.2) | (22.9±6.4) | <0.0001 |
| Range | (21.2−68.7) | (8.1−38.7) |
Abbreviation: BMI, body mass index.
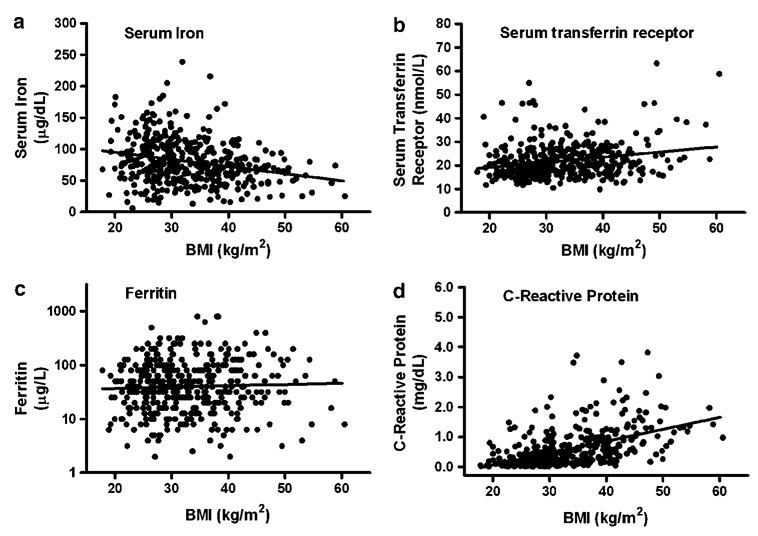
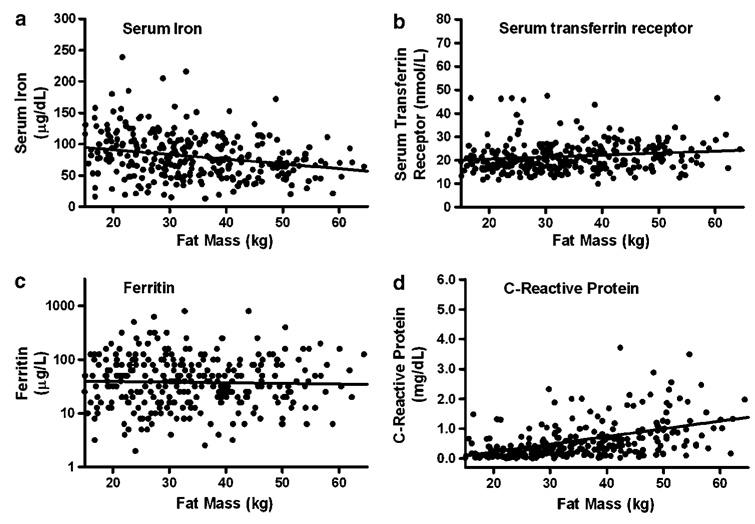
Serum iron was negatively correlated with BMI and fat mass in univariate regression (Figure 1a and Figure 2a) and in multiple regression analyses that included sex, race, age and SES (P=0.0003 and P=0.0004, respectively). Transferrin saturation and MCV were also negatively correlated with BMI and fat mass (all P<0.05) adjusting for the same factors. Serum transferrin receptor (Figure 1b and Figure 2b; P<0.05) and C-reactive protein (Figure 2b and d; P<0.0001) were positively correlated with BMI and fat mass in univariate studies (Figure 1b, d and Figure 2b, d) and in multiple linear regression analyses that included sex, race, age and SES (all P<0.01). Ferritin had a non-significant univariate relationship with BMI and fat mass (Figure 1c and Figure 2c), but was positively associated with both BMI and fat mass in multiple linear regression analyses (both P<0.05). In univariate regression, transferrin receptor was negatively correlated with both ferritin (r2=0.04, P<0.0001) and hemoglobin (r2=0.15, P<0.0001).
Figure 1.
Univariate associations between BMI and (a) serum iron (r2 = 0.06, P<0.0001), (b) serum transferrin receptor (r2=0.05, P<0.0001), (c) ferritin (r2=0.002, P=0.11) and (d) C-reactive protein (r2=0.20, P<0.0001).
Figure 2.
Univariate associations between fat mass and (a) serum iron (r2=0.075, P<0.0001), (b) serum transferrin receptor (r2=0.03, P=0.002), (c) ferritin (r2=0.001, P=0.59) and (d) C-reactive protein (r2=0.22, P<0.0001).
Obese subjects had lower-adjusted mean serum iron (75.8±35.2 vs 86.5±34.2 µg/dl, P=0.002), transferrin saturation (20.3±9.9 vs 23.0±9.9%, P=0.005), and MCV (85.9±5.4 vs 88.0±5.4 fl, P<0.0001) than non-obese subjects (Table 2). Mean serum transferrin receptor was higher in obese than non-obese subjects (22.6±7.1 vs 21.0±7.2 nmol/l, P=0.026). Mean ferritin (81.1±88.8 vs 57.6±88.7 µg/l, P=0.009) and C-reactive protein (0.75±0.67 vs 0.34±0.67 mg/dl, P<0.0001) were also higher in obese than non-obese subjects.
Table 2.
Laboratory and iron intake data
| Obese (n=234) | Non-obese (n=172) | P-value | |
|---|---|---|---|
| Serum iron (µg/dl) | 75.8±35.2 | 86.5±34.2 | 0.002 |
| (Range) | (13−239) | (6−205) | |
| Transferrin saturation (%) | 20.3±9.9 | 23.0±9.9 | 0.005 |
| (Range) | (3−78) | (2−54) | |
| Mean corpuscular volume (fl) | 85.9±5.4 | 88.0±5.4 | <0.0001 |
| (Range) | (57.7−95.8) | (70.3−100) | |
| Hemoglobin (g/dl) | 13.5±1.1 | 13.5±1.1 | 0.88 |
| (Range) | (8.7−17.0) | (7.6−16.7) | |
| Ferritin (µg/l) | 81.1±88.8 | 57.6±88.7 | 0.009 |
| (Range) | (2.2−838) | (2.2−507) | |
| Serum transferrin receptor (nmol/l) | 22.6±7.1 | 21.0±7.2 | 0.026 |
| (Range) | (9.9−63.3) | (11.6−54.9) | |
| C-reactive protein (mg/dl) | 0.75±0.67 | 0.34±0.67 | <0.0001 |
| (Range) | (0.28−3.82) | (0.01−7.31) | |
| Iron intake – food record (mg/day) | 16.0±5.9 | 16.1±5.9 | 0.91 |
| (Range) | (7.0−45.7) | (2.5−45.5) | |
| Supplemental iron intake (mg/day) | 3.7±7.8 | 5.4±7.8 | 0.05 |
| (Range) | (0−39.4) | (0−39.4) | |
| Total iron intake (mg/day) | 19.4±10.0 | 21.5±10.0 | 0.06 |
| (Range) | (7.3−62.3) | (2.5−63.7) |
Results are adjusted for covariates as described in subjects and methods.
Three hundred and thirty-eight subjects completed 7-day food records, and 395 subjects completed the DHQ. Adjusted mean daily dietary iron intake was not different between obese and non-obese subjects (16.0±5.9 vs 16.1±5.9 mg/day, P=0.91). When supplemental iron intake was added to dietary intake, obese subjects reported ingesting slightly, but not significantly, less iron per day (19.4±10.0 mg/day vs 21.5±10.0 mg/day, P=0.06). This difference was primarily caused by a small disparity between obese and non-obese subjects in their reported supplemental iron intake (3.7 ± 7.8 vs 5.4 ± 7.8 mg/day, P=0.05). Multiple linear regression analysis to predict serum iron including age, sex, race, SES and total daily iron intake found that adiposity, whether estimated by BMI (P=0.0005) or body fat mass (P=0.0003), was an independent predictor of serum iron, whereas total daily iron intake was not a significant independent predictor (P=0.51).
To examine the relative contributions of markers of true iron deficiency and measures of inflammation, we used another multiple regression model to predict serum iron with ferritin, serum transferrin receptor, C-reactive protein, BMI, iron intake and demographic variables as regressors. Ferritin, serum transferrin receptor, C-reactive protein, sex and race were each independent predictors of serum iron (r2=0.31), and BMI no longer independently predicted iron once these variables were included in the model. Ferritin contributed only 1% of the total variance predicted by this model, whereas transferrin receptor alone accounted for 76%. Total dietary iron intake was not a significant predictor of serum iron in this model.
Low-serum iron levels were more prevalent in obese than non-obese adults (24.3%, CI 19.3–30.2% vs 15.7%, CI 11.0–21.9%, P=0.03). Among obese subjects, 26.9% (CI 21.6–33.0%) had iron deficiency based on serum transferrin receptor concentrations vs 15.7% (CI 11.0–21.9%) of non-obese subjects (P=0.0078). When the criterion for iron deficiency was defined by the laboratory reference range for low ferritin, there was no statistically significant difference in the prevalence of iron deficiency between obese (9.8%, CI 6.6–14.4%) and non-obese subjects (9.3%, CI 5.7–14.7%, P=0.99). Using logistic regression to predict iron deficiency as determined by serum transferrin receptor concentrations, a model including race, sex, age, SES and obesity status (obese vs non-obese) suggested obese adults had a higher odds ratio (OR) relative to non-obese adults of having iron deficiency (OR 1.8, 95% CI 1.1–3.0, P=0.04). Using ferritin as the marker of iron deficiency, in a similar logistic regression model, obese subjects did not have different odds of iron deficiency (OR 0.9, 95% CI 0.56–2.4, P=0.71).
Discussion
Compared with non-obese control subjects, obese adults, in addition to having lower serum iron, transferrin saturation and MCV, had significantly higher serum transferrin receptor concentrations, suggesting their adiposity was associated with an increased risk for true iron deficiency.8 By contrast, ferritin and C-reactive protein concentrations were higher in obese subjects and were positively correlated with BMI, findings consistent with the observation that obesity is an inflammatory state that increases acute-phase reactants. Multiple regression analysis showed that while the best model to predict serum iron included ferritin, C-reactive protein and transferrin receptor, most of the predicted variance was accounted for by transferrin receptor alone, although inflammatory indices were also independent predictors. Dietary iron was not an independent predictor of serum iron. Further, BMI was no longer an independent predictor of serum iron in this model suggesting that both true iron deficiency and inflammation are explanations for the low serum iron observed in obese adults.
In healthy adults, iron balance is regulated mostly through absorption from the small intestine and by tissue storage of iron. A unifying explanation for our findings may stem from the recently elucidated roles of two iron-regulating proteins that are made by adipocytes: hepcidin and lipocalin-2. Hepcidin is a small peptide hormone secreted by the liver and by adipocytes.30 Hepcidin is an acute-phase reactant,31 and its expression is increased in chronic inflammatory states32,33 including obesity.30 Hepcidin can inhibit enterocyte iron absorption34 and has further been shown to inhibit the release of non-heme iron from macrophages.35 Because each of these actions diminishes the amount of bioavailable body iron, it has been suggested that when hepcidin is induced by inflammation, hepcidin is the key iron regulator that causes the hypoferremia and anemia of chronic disease.36 Although liver hepcidin expression is positively associated with transferrin saturation (i.e., those with greater iron concentrations have appropriate feedback regulation to limit iron absorption and bioavailability by increasing hepatic hepcidin), adipocyte hepcidin expression, has a positive correlation with BMI, with a trend toward a negative association with transferrin saturation.30 Therefore, lower bioavailable iron among obese adults might potentially be related to the greater adipose hepcidin. Although hepcidin expression is more than 100-fold higher in hepatocytes than in adipocytes, secreted hepcidin from both tissues may have relevance for humans because in obesity, adipose tissue mass may be 20-fold greater than liver mass. Lipocalin-2 is a siderophore binding protein which is upregulated in inflammatory states and functions to limit the availability of iron to invading pathogens.37Recent evidence suggests that white adipose tissue is the dominant site of expression of lipocalin-2.38 Circulating lipocalin-2 concentrations are increased in db/db (leptin receptor deficient) mice, and lipocalin-2 mRNA expression is upregulated in db/db adipose tissue and liver.39 Adipocyte lipocalin-2 expression is induced by cytokines such as interleukin-1 and TNF-α. Furthermore, in humans circulating lipocalin-2 concentration is positively correlated with adiposity.39 Whether or not lipocalin-2 is responsible for iron sequestration within adipocytes in obesity remains to be studied. In sum, it is possible that the proinflammatory cytokines induced by the obese state increase hepcidin and lipocalin-2 expression and upregulate ferritin synthesis in reticuloendothelial cells18 resulting in diminished absorption of iron in the setting of increased storage of iron, whether within the reticuloendothelial system or within adipocytes. Clinically, one would expect this to result in a combination of nutritional iron deficiency and functional iron deficiency, consistent with the results of this study. Further studies are needed that examine both hepcidin and lipocalin-2 concentrations in obese individuals to elucidate their relationships with serum iron.
Insufficient iron bioavailability for metabolic requirements may also be a factor in the hypoferremia of obesity.2 Since two-thirds of body iron is found in erythrocytes, and blood volume has been shown to be directly proportional to body mass,9 an increased need for iron in obese individuals is possible.3,10 Basal iron losses (and therefore iron requirements) are clinically estimated using formulae that take body weight into account.40 Implicit in such calculations is the assumption that iron requirements are increased in states of weight increase such as obesity. Our data do not confirm that obese subjects have a lower dietary iron intake than non-obese subjects or that iron intake is a predictor of serum iron concentrations. However, insufficient iron absorption could play a clinically important role in the iron deficiency of obesity given that inflammation-induced hepcidin may reduce iron absorption in obese individuals. It thus remains possible that obese individuals do not meet their dietary iron requirements.
Using serum transferrin receptor to predict the presence of iron deficiency, we found higher odds of iron deficiency in obese vs non-obese subjects. However, using ferritin, which tends to be elevated in obesity-related inflammatory states, we did not show a difference between obese and non-obese subjects in the prevalence of iron deficiency. Elevated transferrin receptor levels correlate well with a lack of stainable iron in bone marrow in normal subjects as well as in patients with rheumatoid arthritis, and transferrin receptor reportedly has a higher sensitivity than ferritin to diagnose iron deficiency in patients with ferritin elevated from acute-phase reactions.24,41,42 Similar to other inflammatory conditions, obesity appears to be a state in which transferrin receptor is a useful adjunct to ferritin in the diagnosis of iron deficiency.
Limitations of this study include the lack of a gold standard for evaluation of iron status. Although transferrin receptor concentrations may be increased by stimulated erythropoiesis, as seen in hemolytic anemia, hereditary spherocytosis and thalassemia, problems with erythropoiesis are unlikely to be present in study subjects selected to be obese but otherwise healthy. Further, transferrin receptor was significantly negatively correlated with ferritin and hemoglobin, findings consistent with iron deficiency rather than increased erythropoiesis. Future studies obtaining bone marrow aspirates for stainable iron are needed to confirm iron deficiency in obese subjects with high transferrin receptor concentrations. Another limitation is that this study was cross-sectional in design, and, therefore no conclusions regarding cause and effect relationships can be made. Strengths of this study include the large sample size, the racial and ethnic diversity of participants, and the use of DEXA to measure fat mass.
In conclusion, as assessed by soluble transferrin receptor and transferrin saturation, obesity is associated with iron deficiency. The etiology appears to be multifactorial, and may include inadequate bioavailable iron relative to body weight, as well as diminished intestinal absorption and decreased iron bioavailability induced by inflammatory adipokines found in those with excessive adiposity. The diagnosis of iron deficiency in obese individuals may be missed if clinicians rely primarily on the falsely normal ferritin concentrations, which are likely increased by chronic inflammation rather than by iron overload. The precise mechanisms of the obesity-related, inflammation-induced effect on serum iron remain to be elucidated.
Acknowledgements
This work is supported by ZO1 HD-00641 (to JAY) from the National Institute of Child Health and Human Development and by Y2-OD-2067 (to JAY) from the Office of Dietary Supplements, National Institutes of Health, DHHS.
References
- 1.Wenzel BJ, Stults HB, Mayer J. Hypoferraemia in obese adolescents. Lancet. 1962;2:327–328. doi: 10.1016/s0140-6736(62)90110-1. [DOI] [PubMed] [Google Scholar]
- 2.Seltzer CC, Mayer J. Serum iron and iron-binding capacity in adolescents. Ii. Comparison of obese and nonobese subjects. Am J Clin Nutr. 1963;13:354–361. doi: 10.1093/ajcn/13.6.354. [DOI] [PubMed] [Google Scholar]
- 3.Pinhas-Hamiel O, Newfield RS, Koren I, Agmon A, Lilos P, Phillip M. Greater prevalence of iron deficiency in overweight and obese children and adolescents. Int J Obes Relat Metab Disord. 2003;27:416–418. doi: 10.1038/sj.ijo.0802224. [DOI] [PubMed] [Google Scholar]
- 4.Nead KG, Halterman JS, Kaczorowski JM, Auinger P, Weitzman M. Overweight children and adolescents: a risk group for iron deficiency. Pediatrics. 2004;114:104–108. doi: 10.1542/peds.114.1.104. [DOI] [PubMed] [Google Scholar]
- 5.Micozzi MS, Albanes D, Stevens RG. Relation of body size and composition to clinical biochemical and hematologic indices in US men and women. Am J Clin Nutr. 1989;50:1276–1281. doi: 10.1093/ajcn/50.6.1276. [DOI] [PubMed] [Google Scholar]
- 6.Whitfield JB, Treloar S, Zhu G, Powell LW, Martin NG. Relative importance of female-specific and non-female-specific effects on variation in iron stores between women. Br J Haematol. 2003;120:860–866. doi: 10.1046/j.1365-2141.2003.04224.x. [DOI] [PubMed] [Google Scholar]
- 7.Rossi E, Bulsara MK, Olynyk JK, Cullen DJ, Summerville L, Powell LW. Effect of hemochromatosis genotype and lifestyle factors on iron and red cell indices in a community population. Clin Chem. 2001;47:202–208. [PubMed] [Google Scholar]
- 8.Lecube A, Carrera A, Losada E, Hernandez C, Simo R, Mesa J. Iron deficiency in obese postmenopausal women. Obesity (Silver Spring) 2006;14:1724–1730. doi: 10.1038/oby.2006.198. [DOI] [PubMed] [Google Scholar]
- 9.Newman BH. Vasovagal reaction rates and body weight: findings in high- and low-risk populations. Transfusion. 2003;43:1084–1088. doi: 10.1046/j.1537-2995.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- 10.Failla ML, Kennedy ML, Chen ML. Iron metabolism in genetically obese (ob/ob) mice. J Nutr. 1988;118:46–51. doi: 10.1093/jn/118.1.46. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 12.Bezwoda WR, Bothwell TH, Torrance JD, MacPhail AP, Charlton RW, Kay G, et al. The relationship between marrow iron stores, plasma ferritin concentrations and iron absorption. Scand J Haematol. 1979;22:113–120. doi: 10.1111/j.1600-0609.1979.tb00411.x. [DOI] [PubMed] [Google Scholar]
- 13.Ford ES, Cogswell ME. Diabetes and serum ferritin concentration among U.S. adults. Diabetes Care. 1999;22:1978–1983. doi: 10.2337/diacare.22.12.1978. [DOI] [PubMed] [Google Scholar]
- 14.Gillum RF. Association of serum ferritin and indices of body fat distribution and obesity in Mexican American men – the Third National Health and Nutrition Examination Survey. Int J Obes Relat Metab Disord. 2001;25:639–645. doi: 10.1038/sj.ijo.0801561. [DOI] [PubMed] [Google Scholar]
- 15.Baynes R, Bezwoda W, Bothwell T, Khan Q, Mansoor N. The non-immune inflammatory response: serial changes in plasma iron, iron-binding capacity, lactoferrin, ferritin and C-reactive protein. Scand J Clin Lab Invest. 1986;46:695–704. doi: 10.3109/00365518609083733. [DOI] [PubMed] [Google Scholar]
- 16.Rogers JT. Ferritin translation by interleukin-1 and interleukin-6: the role of sequences upstream of the start codons of the heavy and light subunit genes. Blood. 1996;87:2525–2537. [PubMed] [Google Scholar]
- 17.Torti SV, Kwak EL, Miller SC, Miller LL, Ringold GM, Myambo KB, et al. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988;263:12638–12644. [PubMed] [Google Scholar]
- 18.Fitzsimons EJ, Brock JH. The anaemia of chronic disease. Bmj. 2001;322:811–812. doi: 10.1136/bmj.322.7290.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baynes RD. Assessment of iron status. Clin Biochem. 1996;29:209–215. doi: 10.1016/0009-9120(96)00010-k. [DOI] [PubMed] [Google Scholar]
- 20.Baynes RD, Cook JD. Current issues in iron deficiency. Curr Opin Hematol. 1996;3:145–149. doi: 10.1097/00062752-199603020-00007. [DOI] [PubMed] [Google Scholar]
- 21.Thomas C, Kirschbaum A, Boehm D, Thomas L. The diagnostic plot: a concept for identifying different states of iron deficiency and monitoring the response to epoetin therapy. Med Oncol. 2006;23:23–36. doi: 10.1385/MO:23:1:23. [DOI] [PubMed] [Google Scholar]
- 22.Baillie FJ, Morrison AE, Fergus I. Soluble transferrin receptor: a discriminating assay for iron deficiency. Clin Lab Haematol. 2003;25:353–357. doi: 10.1046/j.0141-9854.2003.00548.x. [DOI] [PubMed] [Google Scholar]
- 23.Beguin Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin Chim Acta. 2003;329:9–22. doi: 10.1016/s0009-8981(03)00005-6. [DOI] [PubMed] [Google Scholar]
- 24.Punnonen K, Irjala K, Rajamaki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052–1057. [PubMed] [Google Scholar]
- 25.Anon. Supplemental calcium in overweight people, NCT00030238. [Date accessed: January, 6, 2006];Bethesda, MD 20892: NIH Clinical Research Studies. 2002 http://www.clinicaltrialsgov/ct/show/NCT00030238.
- 26.Hollingshead AB. Hollingshead two factor index of social position (1957) In: Miller DC, editor. Handbook of Research Design and Social Measurement. 5th edn. Newbury Park, CA: Sage Publications; 1991. pp. 351–359. [Google Scholar]
- 27.Allen J, Backstrom KR, Cooper JA, Cooper MC, Detwiler TC, Essex DW, et al. Measurement of soluble transferrin receptor in serum of healthy adults. Clin Chem. 1998;44:35–39. [PubMed] [Google Scholar]
- 28.Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88:1268–1271. [PubMed] [Google Scholar]
- 29.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America’s Table Study. Am J Epidemiol. 2001;154:1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 30.Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, et al. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology. 2006;131:788–796. doi: 10.1053/j.gastro.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 32.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 33.Weinstein DA, Roy CN, Fleming MD, Loda MF, Wolfsdorf JI, Andrews NC. Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood. 2002;100:3776–3781. doi: 10.1182/blood-2002-04-1260. [DOI] [PubMed] [Google Scholar]
- 34.Laftah AH, Ramesh B, Simpson RJ, Solanky N, Bahram S, Schumann K, et al. Effect of hepcidin on intestinal iron absorption in mice. Blood. 2004;103:3940–3944. doi: 10.1182/blood-2003-03-0953. [DOI] [PubMed] [Google Scholar]
- 35.Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci USA. 2005;102:1324–1328. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barasch J, Mori K. Cell biology: iron thievery. Nature. 2004;432:811–813. doi: 10.1038/432811a. [DOI] [PubMed] [Google Scholar]
- 38.Rosen E. North Atlantic Association for the Study of Obesity 2006. Boston, MA: NAASO, the Obesity Society; 2006. Oct 22, ROS and inflammation. http://www.naaso.org/annualmeeting06/final_program.pdf. [Google Scholar]
- 39.Wang Y, Lam KS, Kraegen EW, Sweeney G, Zhang J, Tso AW, et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem. 2006 doi: 10.1373/clinchem.2006.075614. E-pub ahead of print. Published online at: http://www.clinchem.org/cgi/doi/10.1373/clinchem.2006.075614. [DOI] [PubMed] [Google Scholar]
- 40.Anon. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc: a report of the Panel on Micronutrients Food and Nutrition Board, Institute of Medicine. Washington DC: National Academy Press; 2001. Iron; pp. 290–393. [Google Scholar]
- 41.Means RT, Jr, Allen J, Sears DA, Schuster SJ. Serum soluble transferrin receptor and the prediction of marrow aspirate iron results in a heterogeneous group of patients. Clin Lab Haematol. 1999;21:161–167. doi: 10.1046/j.1365-2257.1999.00224.x. [DOI] [PubMed] [Google Scholar]
- 42.Mast AE, Blinder MA, Gronowski AM, Chumley C, Scott MG. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin Chem. 1998;44:45–51. [PubMed] [Google Scholar]