Abstract
Prepulse inhibition of startle (PPI) is an operational measure of sensorimotor gating that is impaired in schizophrenia and is disrupted in rats by dopamine (DA) agonists like apomorphine (APO). Using acoustic prepulses and acoustic startle pulses, previous studies have demonstrated heritable strain differences between Sprague Dawley (SD) and Long Evans (LE) rats in the sensitivity to the PPI-disruptive effects of APO. As PPI deficits in schizophrenia are evident with both uni- and cross-modal stimuli, we tested whether strain differences in the gating-disruptive effects of APO occur with a cross-modal visual and acoustic stimulus combination. APO caused a dose-dependent disruption of both acoustic and visual PPI in SD rats. Compared to LE rats, SD rats were more sensitive to the PPI-disruptive effects of APO with both acoustic and visual PPI. These findings suggest that SD vs. LE strain differences in PPI APO sensitivity are mediated outside of the auditory system, within higher circuitry that regulates or processes multi-modal information. The present findings provide further validation for this heritable model of impaired sensorimotor gating in schizophrenia, which can be detected across multiple sensory modalities.
Introduction
Evidence suggests that vulnerability for developing schizophrenia can be inherited (Harrison and Weinberger, 2005; Sullivan, 2005) and that genes conferring this vulnerability ultimately do so via changes in brain circuitry. Great effort is being put towards identifying the genetic basis of this vulnerability through the use of endophenotypes, i.e. phenotypes that are intermediate between the genes and the more complex clinical manifestations of these diseases (Gottesman and Gould, 2003; Turetsky et al., 2007). One useful schizophrenia endophenotype may be reduced PPI of the startle reflex (Graham, 1975). Normal prepulse inhibition of startle (PPI) is a cross-species phenomenon that also occurs in humans, rats, and mice when a weak lead stimulus inhibits the response to an intense, abrupt startling stimulus. PPI is reduced in schizophrenia patients and their unaffected first-degree relatives (Braff et al., 1978; 2001; Cadenhead et al., 2000; Kumari et al., 2005) suggesting that deficient PPI may be a useful endophenotype for inherited forms of schizophrenia.
In rodents, PPI deficits can be induced by dopamine (DA) agonists such as apomorphine (APO), and recent studies have identified heritable differences in the dopaminergic regulation of PPI in both mice (Ralph and Caine, 2005) and rats (Swerdlow et al., 2004c). For example, Sprague Dawley rats from Harlan Laboratories (SD) are significantly more sensitive to the PPI-disruptive effects of dopamine (DA) agonists such as APO, compared to Long Evans rats from Harlan Laboratories (LE) (Swerdlow et al., 2001b; 2003; 2004a; 2004b; 2004c). These differences have been shown to be innate (Swerdlow et al., 2004a; 2004c) and neurochemically specific (Swerdlow et al., 2003; 2004b), cannot be explained by differences in maternal behavior (Swerdlow et al., 2004a), and appear to be linked to inherited properties of DA-linked G-protein function (Swerdlow et al., 2006). Conceivably, this heritable strain difference in the “disruptability” of PPI by DA activation may provide a useful model for understanding the basis for heritable differences in PPI in disorders such as schizophrenia and Tourette Syndrome (Castellanos et al., 1996).
To date, all evidence for this SD vs. LE strain difference comes from studies in which stimuli were acoustic prepulses and acoustic startle pulses. Arguably, models of heritable gating differences will be most relevant to neuropsychiatric disorders if they are not specific to one sensory modality, but rather involve integrated information across several stimulus modalities. Thus, PPI deficits in schizophrenia are evident with both uni- and cross-modal stimuli, and clinical symptoms of impaired gating involve multiple sensory modalities (Braff et al., 1992). In rats, pioneering work by Schwartz et al. (1976) demonstrated the ability to detect cross-modal PPI, using visual prepulses and acoustic pulses, and Campeau and Davis, (1995) and Taylor et al. (1995) later demonstrated that APO disrupts PPI elicited by visual prepulses in SD rats. In the present study, we tested whether SD vs. LE strain differences in the gating-disruptive effects of APO occur with uni-modal acoustic vs. cross-modal visual and acoustic stimulus combinations.
Methods
Experimental animals
Adult male SD (n = 17) and LE (n = 17) rats (225-250 g; Harlan Laboratories, Livermore, CA) were maintained on a reversed light/dark schedule with water and food available ad libitum. Rats were handled within 2 d of arrival. Testing occurred during the dark phase. All experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) and were approved by the UCSD Animal Subjects Committee (protocol #S01221).
Drugs
APO (0.01 % ascorbate/saline vehicle, 0.25 or 0.5 mg/kg) was administered subcutaneously (sc) immediately prior to testing using an application volume of 1 ml/kg.
Apparatus
Startle chambers were housed in a sound-attenuated room, and consisted of a Plexiglas cylinder 8.2 cm in diameter resting on a 12.5 × 25.5 cm Plexiglas frame within a ventilated enclosure. Noise bursts were presented via a speaker mounted 24 cm above the cylinder. Visual stimuli consisted of flashes of incandescent white light delivered via a 15 W light bulb. The light bulb was mounted to the ceiling of the chamber in a corner of the startle chamber at a distance of approximately 22 cm from the center of the rat cylinder. A piezoelectric accelerometer mounted below the Plexiglas frame detected and transduced motion from within the cylinder. Stimulus delivery was controlled by the SR-LAB microcomputer and interface assembly, which also digitized (0-4095), rectified, and recorded stabilimeter readings. One hundred 1-ms readings were collected beginning at stimulus onset. Startle amplitude was defined as the average of the 100 readings.
The light stimulus used during the drug study (40ms lights on) was quantified using a silicon detector (stock #NT53-378, Edmund Optics, Barrington, NJ) that was soldered in parallel to a 1k-Ohm resistor (RadioShack, Fort Worth, TX) and connected to an analogue oscilloscope (model #2236, Tektronix, Beaverton, OR). Changes in light intensity were displayed as voltage by the oscilloscope and recorded with a video camera (CR-HC21 NTSC, Sony, Tokyo, Japan) that was directed towards the screen of the oscilloscope. Video images were transferred to a personal computer and analyzed offline using Windows Video Maker (Microsoft, Redmond, WA). Recordings with the silicon detector were converted to approximate light intensities based on a calibration procedure during which stable light intensities were recorded in parallel on a light meter (Auto Meter IV F, Minolta Camera Co., Osaka, Japan). The 10, 20, 40 and 60 ms light flashes reached peak intensities of approximately 9, 50, 311 and 560 Lux, respectively, with the tip of the silicon detector 22 cm from the light bulb. Rise times to peak for 10, 20, 40 and 60 ms light pulses were approximately 13, 22, 42 and 61 ms, respectively, and were followed by exponential decays that reached half-maximal values approximately 40, 24, 17 and 16 ms, respectively, after the peak. The light flash did not generate any audible sound as tested when the background noise and chamber ventilation were switched off.
Startle testing procedure
Pilot experiments demonstrated insufficient PPI in response to light stimuli when startle chambers were constantly illuminated. To increase salience of the visual stimuli, all subsequent experiments were carried out with the house lights switched-off. A parametric test session was carried out in untreated animals to 1) optimize the stimulus characteristic of the visual prepulse with respect to PPI and 2) to assign rats to matched APO dose groups in the subsequent drug experiment. For the parametric experiment, rats were placed in the dark chambers for a 5 min acclimation period with a 70 dB(A) background noise. The rats were then exposed to a series of trial types which were presented in pseudorandom order: (1) 40 ms - 120 dB(A) noise burst (P-ALONE); (2) P-ALONE preceded 100 ms (onset-to-onset) by a 20 ms noise burst 10 dB above background (PP10dB+P-ALONE); (3-6) P-ALONE preceded 100ms (onset-to-onset) by a light flash of either 10 ms (LI10ms+P-ALONE), 20 ms (LI20ms+P-ALONE), 40 ms (LI40ms +P-ALONE) or 60 ms (LI60ms +P-ALONE) duration; (7) a 20 ms noise burst 10 dB above background (PP10dB-ALONE); (8) a 60 ms light flash (LI60ms -ALONE). Interspersed between any of these trial types was a trial in which no stimulus was presented, but motor activity was measured (NOSTIM trials). Not taking NOSTIM trials into account, the session began with 3 consecutive P-ALONE trials and ended with 3 consecutive P-ALONE trials; between these trials was one block consisting of 8 trails of each of the other 8 active stimulus types. Intertrial intervals were variable and averaged 15 s. NOSTIM trials were not included in the calculation of inter-trial intervals. Total session duration was 22.5 min.
Based on the parametric test session, a duration of 40ms was selected for the light flash in the drug experiment. Rats were assigned to APO dose groups (0, 0.25, 0.5 mg/kg) based on average %PPI derived from responses to acoustic (PP10dB+P-ALONE) and visual (40ms; LI40ms+P-ALONE) prepulse trails of the test session.
Drug testing began 1 d after the parametric test session for a total of 3 test days in a within subject, pseudo-random balanced dose order design, with 3 d between tests. After the injection of vehicle or APO, rats were immediately placed in the dark startle chambers for a 5 min acclimation period with a 70 dB(A) background noise. The rats were then exposed to a series of trial types presented in pseudorandom order: (1) P-ALONE; (2) PP10dB+P-ALONE; (3) LI40ms+P-ALONE; (4) PP10dB-ALONE); and (5) a 40ms light flash (LI40ms-ALONE). Interspersed between any of these trial types was a NOSTIM trial. Not taking NOSTIM trials into account the session began with 3 consecutive P-ALONE trials and ended with 3 consecutive P-ALONE trials; between these trials were two blocks, each consisting of 6 P-ALONE, 6 LI40ms+P-ALONE, 6 PP10dB+P-ALONE, 3 LI40ms-ALONE and 3 PP10dB-ALONE trails. Intertrial intervals were variable and averaged 15 s. NOSTIM trials were not included in the calculation of inter-trial intervals. Total session duration was 18.5 min.
Data analysis
PPI was defined as 100-[(startle amplitude on prepulse trials / startle amplitude on P-ALONE trials) × 100], and was analyzed by mixed design ANOVAs. The values for the acoustic startle P-ALONE were used in separate calculations of both unimodal (acoustic prepulse + P-ALONE stimuli) and cross-modal (light prepulse + acoustic P-ALONE stimuli) PPI. Other ANOVAs were used to assess P-ALONE magnitude, as well as activity recorded in the aftermath of prepulses alone, or NOSTIM trials. Post-hoc comparisons were conducted using Fisher’s PLSD. Alpha was 0.05.
Results
Light prepulse duration and cross-modal PPI
Separate ANOVAs of PPI were carried out for the two prepulse modalities. No strain effects on PPI were detected in response to acoustic prepulses (F = 2.30, df 1,32, ns). The ANOVA of PPI for visual prepulses revealed a significant main effect of strain (SD > LE, F=12.39, df 1,32, p<0.002) and light duration (F=42.05, df 3, 96, p<0.0001), but no strain × light duration interaction (F<1, df 3,96, ns; Figure 1). Post-hoc analyses revealed that %PPI exhibited an “inverted-U” function: highest values for the 40 ms light flash, lowest for the 10ms light flash, and intermediate values for the 60 ms and 20 ms light flash (40 ms > 60 ms > 20 ms > 10 ms, p<0.005 for all comparisons). Acoustic startle magnitude did not differ between strains (F<1, df 1,32, ns; Figure 1, inset).
Figure 1.

% PPI and startle magnitude (inset) in response to either an acoustic prepulse (10 dB above background) or a visual prepulse of either 10, 20, 40 or 60 ms duration in SD rats (open bars) and LE rats (solid bars). Startle magnitude was similar in SD and LE rats (ns). Both rat strains had similar % PPI in response to acoustic prepulses (ns). For both strains % PPI to visual prepulses was strongly depended on prepulse duration (p < 0.0001). PPI was lowest at a prepulse duration of 10 ms and reached peak values at 40 ms. SD rats exhibited more PPI to visual prepulses than did LE rats (p < 0.002).
APO effects on uni- and cross-modal PPI
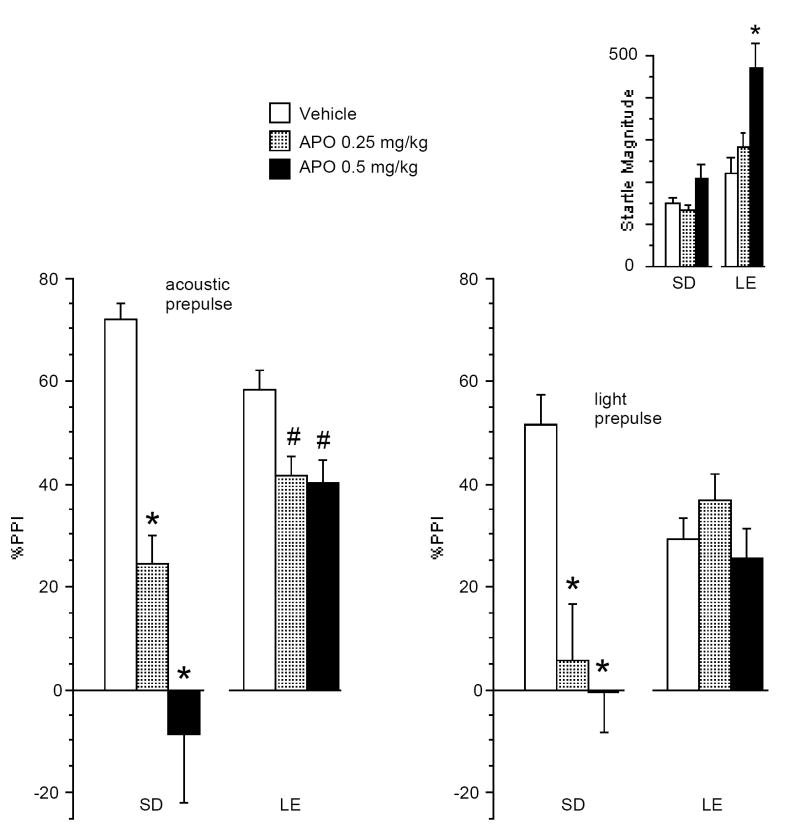
ANOVA of %PPI revealed significant main effects of strain (F=6.14, df 1,32, p<0.02) and APO dose (F=22.81, df 2,64, p<0.0001), and a significant strain × dose interaction (F=12.38, df 2,64, p<0.0001). There was a significant effect of prepulse modality (acoustic > light; F=5.36, df 1,32, p<0.03), and a significant interaction of APO dose × modality (F=3.88, df 2,64, p<0.03), but no significant interaction of dose × modality × strain (F=2.80, df 1,32, ns). Separate ANOVAs for each prepulse modality revealed significant interactions of strain × dose (acoustic: F = 9.52, df 2,64, p<0.0002; visual: F = 9.26, df 2,64, p<0.0003), in each case reflecting greater sensitivity to the PPI-disruptive effects of APO in SD rats (Figure 2). Post-hoc comparisons revealed significant PPI-disruptive effects of APO in SD rats with both acoustic (F=19.73, df 2,32, p<0.0001) and visual prepulses (F=10.62, df 2,32, p<0.0004). For LE rats, APO significantly reduced PPI with acoustic prepulses (F=5.04, df 2,32, p<0.015), but not with visual prepulses (F=1.87, df 2,32, ns).
Figure 2.
Effects of APO on % PPI and startle magnitude (inset) in response to acoustic vs. visual prepulses in SD and LE rats. ANOVA revealed a significant main effect for strain, APO dose, and prepulse modality (p<0.02, p<0.0001, and p<0.03 respectively) and significant interactions for APO dose × prepulse modality and strain × APO dose (p<0.03 and p<0.0001, respectively), with greater APO sensitivity on PPI for SD than LE rats. PPI APO sensitivity was greater for SD vs. LE rats with prepulses from both acoustic (p<0.0002) and visual modalites (p<0.0003). Fisher’s PLSD post-hoc tests revealed that APO reduced acoustic PPI in SD rats (*p<0.0005 for 0.25 and 0.5 mg/kg doses vs. vehicle) and in LE rats (# p<0.02 for 0.25 and 0.5 mg/kg vs. vehicle). Visual PPI was reduced by APO in SD rats (*p<0.0005 for 0.25 and 0.5 mg/kg doses vs. vehicle), but not in LE rats (ns). Startle response magnitude was increased by APO at the highest dose in LE rats (*p<0.0005 for 0.5 mg/kg dose vs. vehicle), but not in SD rats (ns).
ANOVA of startle magnitude on P-ALONE trials revealed significant main effects of strain (LE > SD; F=7.99, df 1,32, p<0.009) and APO dose (F=14.19, df 2,64, p<0.0001), and a significant strain × dose interaction (F=4.86, df 2,64, p<0.015). This interaction reflected significant startle-enhancing effects of APO in LE rats (F=13.68, df 2,32, p<0.0001), but not in SD rats (F=2.18, df2,32, ns).
To assess the impact of APO effects on startle on PPI differences, a separate analysis was carried out for subsets of rats constructed by eliminating the extreme responders until the effects of apomorphine on startle magnitude were numerically balanced across strains (mean (SEM) of startle magnitude in these subsets of rats: SD: vehicle = 122 (26), APO 0.25 mg/kg = 113 (23), APO 0.5 mg/kg 122 (20), n =5; LE: vehicle = 136 (46), APO 0.25 mg/kg = 126 (20), APO 0.5 mg/kg 160 (26); n=5). ANOVA of startle magnitude on P-ALONE trials in these rats confirmed a lack of effect of strain (F<1, df 1,8, ns), APO dose (F<1, df 2,16, ns), and strain × dose (F<1, df 2,16, ns). ANOVA of %PPI in these subgroups confirmed the key results: a significant main effect of APO dose (F=10.42, df 2,16, p<0.002) and a significant strain × dose interaction (F=5.57, df 2,16, p<0.02). No significant effects of strain, prepulse modality, APO dose × modality, and dose × modality × strain were detected. Separate ANOVAs for each strain revealed significant effects of APO dose in SD rats (F = 14.86, df 2,8, p< 0.005), but not in LE rats (F<1, df 2,8, ns) confirming greater APO PPI sensitivity in SD rats than LE rats with both uni- and cross-modal stimuli, independent of APO effects on startle. Simple regression analyses provided confirmatory information, revealing no significant correlations of APO effects on startle magnitude vs. PPI, for any APO dose or prepulse modality within either SD or LE strains.
Inspection of motor activity (cage displacement) on prepulse alone trials in vehicle-treated rats revealed small signals that differed across stimulus modalities. ANOVA of prepulse-induced motor activity revealed no significant effects of strain or APO dose, and no significant interactions of dose × strain, or dose × strain × modality (all comparisons ns). Much of this motor “signal” reflected ongoing, rather than stimulus-triggered, movement: subtraction of NOSTIM activity from activity measured in the aftermath of light prepulses yielded values indistinguishable from zero units for both SD and LE rats. Separate analysis of NOSTIM levels revealed significant effects of APO that were more robust in SD than LE rats. However, neither correlational analyses nor ANCOVAs revealed any consistent relationship between differential APO effects on PPI and motor activity across strains.
Discussion
We previously reported greater sensitivity of SD than LE rats to the PPI-disruptive effects of systemically administered DAergic agonists, including APO, d-amphetamine and quinpirole (Swerdlow et al., 2001b; 2003; 2004a; 2004b; 2004c). These studies demonstrated the effects of dopamine agonists on PPI under conditions in which both prepulse and pulse were acoustic stimuli. These effects on acoustic PPI were replicated in the present study. The APO-disruption of visual PPI in SD rats reported here also confirm findings from an earlier report by Campeau and Davis (1995). Importantly, in the present study, we demonstrated for the first time that heritable strain differences in the gating-disruptive effects of APO extend to cross-modal PPI, i.e. PPI elicited by a visual prepulse and an acoustic pulse.
Conceivably, relatively reduced PPI in LE rats after vehicle treatment might contribute to the blunted impact of APO on acoustic and visual PPI, via a “floor effect”. However, a floor effect cannot explain the present findings, because in both modalities, PPI after APO treatment was substantially greater in LE than SD rats. Differential motor responses to prepulses might also conceivably contribute to SD vs. LE difference in PPI APO sensitivity, but the present data also do not support such an interpretation: analyses of motor activity after prepulses revealed that both strain × APO dose (p=0.28) and strain × APO dose × modality (p=0.76) interactions failed to reach statistical significance despite substantial power afforded by the current sample sizes (n=17 per strain). Finally, strain-specific APO effects on startle magnitude cannot account for the observed strain differences in APO sensitivity for acoustic and visual PPI, because these PPI differences were evident even among “matched” subsets of SD and LE rats that exhibited comparable APO effects on startle.
The present findings have several implications. First, they provide another level of homology between the clinical data of uni- and cross-modal gating deficits in several different brain disorders, and an animal model of heritable differences in uni- and cross-modal PPI “disruptability”. Thus, PPI deficits in schizophrenia patients are detected with both acoustic prepulses and pulses, and with acoustic prepulses and tactile (air puff) pulses (Braff et al., 1992). Similarly, PPI deficits in Tourette Syndrome are evident with either acoustic or tactile stimuli, the latter including both facial shocks (Castellanos et al., 1996) and air puffs (Swerdlow et al., 2001a). Thus, at least in these two heritable brain disorders, gating deficits are not modality-specific.
Second, the present findings provide a basis for strong inference regarding the anatomical substrates responsible for these heritable gating differences. Thus, the simplest explanation for the strain-specific APO effects on both acoustic and cross-modal PPI is that these effects are mediated by circuitry that processes integrated information, at a level beyond the convergence of separate auditory and visual sensory streams. Such circuitry also regulates PPI, and exhibits strain differences in the effects of DA receptor stimulation on cellular events. We have hypothesized that the nucleus accumbens (NAC) may be one critical substrate contributing to these heritable differences in PPI APO sensitivity, based in part on evidence that LE and SD rats differ significantly in the effects of APO on NAC measures of activity within DA-linked signal transduction pathways, including DA-stimulated GTPγS binding (Swerdlow et al., 2006), phosphorylation of cyclic AMP binding protein (CREB) in the NAC (Saint Marie et al., 2007) and NAC FOS activation (Saint Marie et al., 2006), and that these strains also differ significantly in their characteristic gene activation patterns within NAC signal pathways (Shilling et al., 2007). If the present study had revealed that SD vs. LE differences in PPI APO sensitivity were limited to unimodal acoustic stimuli, it would be very difficult to argue that cellular mechanisms in the NAC mediate the heritable differences in gating phenotypes in this animal model, and by extension, in schizophrenia or other complex brain disorders.
Rat strain differences in drug sensitivity might reflect genetic or epigenetic influences on a number of different biological systems, and many such differences undoubtedly arise from mechanisms with little relevance to the genesis of neuropsychiatric disorders. The specific strain differences described in these studies have been pursued experimentally from the “top-down”, i.e. from the levels of possible fostering effects and maternal-pup interactions, to different parametric behavioral manipulations, through possible pharmacodynamic mediators, neurochemical and neuroanatomical substrates, and more recently to regionally specific signal transduction mechanisms and gene expression. Both the neurobiological mechanisms under investigation (genetic control of NAC DA-linked signal transduction) and the phenotype (low vs. high DA-mediated PPI “disruptability”) are ones that could be reasonably viewed as being of potential relevance to human brain disorders. Conceivably, within the molecular pathways controlling these mechanisms and phenotypes will be targets for therapeutic interventions in heritable disorders of impaired PPI, such as schizophrenia and Tourette Syndrome.
This study was not designed to provide a full parametric mapping of stimulus-response characteristics for cross-modal PPI in SD and LE rats. Nonetheless, we note that, compared to albino SD rats, pigmented LE rats exhibited less PPI in response to visual prepulses for all prepulse durations. This observation is consistent with reports of greater sensitivity to photic stimulation within the superior colliculus in albino vs. pigmented rats (Thomas et al., 2005).
In summary, heritable differences in the sensitivity to the gating-disruptive effects of DA stimulation in SD and LE rats occur when gating is produced with either uni- or cross-modal stimulus pairs. The simplest explanation for the present findings is that these SD vs. LE differences are mediated by circuitry outside the auditory system, within forebrain circuitry that integrates information streams across multiple sensory modalities. The present findings provide further validation for this heritable model of impaired sensorimotor gating in schizophrenia, which is also detected across multiple sensory modalities.
Acknowledgments
Research supported by MH01436 and MH68366. The authors gratefully acknowledge the assistance of Maria Bongiovanni in manuscript preparation, Dr. Martin Vogel and Richard F. Sharp for technical expertise and David Ko for critical discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Archives of General Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Swerdlow NR, Shafer KM, Diaz M, Braff DL. Modulation of the startle response and startle laterality in relatives of schizophrenic patients and in subjects with schizotypal personality disorder: evidence of inhibitory deficits. The American Journal of Psychiatry. 2000;157:1660–1668. doi: 10.1176/appi.ajp.157.10.1660. [DOI] [PubMed] [Google Scholar]
- Campeau S, Davis M. Prepulse inhibition of the acoustic startle reflex using visual and auditory prepulses: disruption by apomorphine. Psychopharmacology. 1995;117:267–274. doi: 10.1007/BF02246101. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Fine EJ, Kaysen D, Marsh WL, Rapoport JL, Hallett M. Sensorimotor gating in boys with Tourette’s syndrome and ADHD: preliminary results. Biological Psychiatry. 1996;39:33–41. doi: 10.1016/0006-3223(95)00101-8. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. The American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Graham FK. Presidential Address, 1974. The more or less startling effects of weak prestimulation. Psychophysiology. 1975;12:238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Molecular Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- Kumari V, Das M, Zachariah E, Ettinger U, Sharma T. Reduced prepulse inhibition in unaffected siblings of schizophrenia patients. Psychophysiology. 2005;42:588–594. doi: 10.1111/j.1469-8986.2005.00346.x. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Caine SB. Dopamine D1 and D2 agonist effects on prepulse inhibition and locomotion: comparison of Sprague-Dawley rats to Swiss-Webster, 129X1/SvJ, C57BL/6J, and DBA/2J mice. The Journal of Pharmacology and Experimental Therapeutics. 2005;312:733–741. doi: 10.1124/jpet.104.074468. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Neary AC, Shoemaker J, Swerdlow NR. Apomorphine effects on CREB phosphorylation in the nucleus accumbens of rat strains that differ in PPI sensitivity. Biological Psychiatry. 2007;61:37s. [Google Scholar]
- Saint Marie RL, Neary AC, Shoemaker JM, Swerdlow NR. The effects of apomorphine and D-amphetamine on striatal c-Fos expression in Sprague-Dawley and Long Evans rats and their F1 progeny. Brain Research. 2006;1119:203–214. doi: 10.1016/j.brainres.2006.08.045. [DOI] [PubMed] [Google Scholar]
- Schwartz GM, Hoffman HS, Stitt CL, Marsh RR. Modification of the rat’s acoustic startle response by antecedent visual stimulation. Journal of Experimental Psychology. 1976;2:28–37. doi: 10.1037//0097-7403.2.1.28. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Saint Marie RL, Shoemaker J, Breier M, Mora AB, Ko D, Neary AC, Swerdlow NR. Strain differences in gating-disruptive effects of apomorphine: Relationship to accumbens gene expression. Biological Psychiatry. 2007;61:38s. doi: 10.1016/j.biopsych.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF. The genetics of schizophrenia. PLoS Medicine. 2005;2:e212. doi: 10.1371/journal.pmed.0020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Karban B, Ploum Y, Sharp R, Geyer MA, Eastvold A. Tactile prepuff inhibition of startle in children with Tourette’s syndrome: in search of an “fMRI-friendly” startle paradigm. Biological Psychiatry. 2001a;50:578–585. doi: 10.1016/s0006-3223(01)01164-7. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Krupin AS, Bongiovanni MJ, Shoemaker JM, Goins JC, Hammer RP., Jr Heritable differences in the dopaminergic regulation of behavior in rats: relationship to D2-like receptor G-protein function. Neuropsychopharmacology. 2006;31:721–729. doi: 10.1038/sj.npp.1300877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Platten A, Kim YK, Gaudet I, Shoemaker J, Pitcher L, Auerbach P. Sensitivity to the dopaminergic regulation of prepulse inhibition in rats: evidence for genetic, but not environmental determinants. Pharmacology, Biochemistry, and Behavior. 2001b;70:219–226. doi: 10.1016/s0091-3057(01)00598-6. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Auerbach PP, Pitcher L, Goins J, Platten A. Heritable differences in the dopaminergic regulation of sensorimotor gating. II. Temporal, pharmacologic and generational analyses of apomorphine effects on prepulse inhibition. Psychopharmacology. 2004a;174:452–462. doi: 10.1007/s00213-003-1480-4. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Crain S, Goins J, Onozuka K, Auerbach PP. Sensitivity to drug effects on prepulse inhibition in inbred and outbred rat strains. Pharmacology, Biochemistry, and Behavior. 2004b;77:291–302. doi: 10.1016/j.pbb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Platten A, Pitcher L, Goins J, Auerbach PP. Heritable differences in the dopaminergic regulation of sensorimotor gating. I. Apomorphine effects on startle gating in albino and hooded outbred rat strains and their F1 and N2 progeny. Psychopharmacology. 2004c;174:441–451. doi: 10.1007/s00213-003-1481-3. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Stephany N, Wasserman LC, Talledo J, Shoemaker J, Auerbach PP. Amphetamine effects on prepulse inhibition across-species: replication and parametric extension. Neuropsychopharmacology. 2003;28:640–650. doi: 10.1038/sj.npp.1300086. [DOI] [PubMed] [Google Scholar]
- Taylor MK, Ison JR, Schwarzkopf SB. Effects of single and repeated exposure to apomorphine on the acoustic startle reflex and its inhibition by a visual prepulse. Psychopharmacology. 1995;120:117–27. doi: 10.1007/BF02246183. [DOI] [PubMed] [Google Scholar]
- Thomas BB, Aramant RB, Sadda SR, Seiler MJ. Light response differences in the superior colliculus of albino and pigmented rats. Neuroscience Letters. 2005;385:143–147. doi: 10.1016/j.neulet.2005.05.034. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophrenia Bulletin. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]