Abstract
Map kinases are drug targets for autoimmune disease, cancer, and apoptosis-related diseases. Drug discovery efforts have developed MAP kinase inhibitors directed toward the ATP binding site and neighboring “DFG-out” site, both of which are targets for inhibitors of other protein kinases. On the other hand, MAP kinases have unique substrate and small molecule binding sites that could serve as inhibition sites. The substrate and processing enzyme D-motif binding site is present in all MAP kinases, and has many features of a good small molecule binding site. Further, the MAP kinase p38α has a binding site near its C-terminus discovered in crystallographic studies. Finally, the MAP kinases ERK2 and p38α have a second substrate binding site, the FXFP binding site that is exposed in active ERK2 and the D-motif peptide induced conformation of MAP kinases. Crystallographic evidence of these latter two binding sites is presented.
1. Introduction
MAP kinase cascades confer switch-like responses to extracellular hormonal and stress stimuli that lead to cell fate decisions such as differentiation, proliferation, apoptosis [1, 2] and senescence [3]. The modules are comprised of a Ser/Thr kinase MAP3K that doubly phosphorylates a dual-specificity MAP2K, which in turn doubly phosphorylates, through a phosphotyrosine intermediate, a proline-directed Ser/Thr MAPK. The sigmoid response behavior is thought to be a consequence of the dissociative nature of these two double phosphorylation reactions [4]. Different MAP kinase modules respond to distinct extracellular stimuli. The extracellular signal regulated kinases 1 and 2 (ERK1/2) are activated by mitogens and growth hormones, while the c-Jun N-terminal kinases (JNKs) and p38 MAP kinases are activated by bacterial liposacchrides, interleukine-1, tumor necrosis factor-α, and cellular stresses such as osmotic shock and UV radiation [5]. ERK5 is activated by both classes of stimuli [6, 7]. Given these central regulatory roles, it is not surprising that three out of four of these best studied MAP kinases have received very significant attention as drug targets [8].
The p38 MAP kinase pathway is a therapeutic target for inflammatory diseases such as psoriasis, rheumatoid arthritis and chronic obstructive pulmonary disease [9, 10]. JNKs are drug targets for apoptosis related diseases such as Alzheimer’s disease, Parkinson’s disease, type II diabetes, hearing loss, and also for autoimmune diseases [11, 12]. ERK2 pathway components are drug targets for proliferative diseases, notably MEK1 / 2 and Raf isoforms [13, 14].
Search for anti-inflammatory agents lead to the discovery of p38α as a potential drug target [15]. A very significant effort by the drug industry has produced many more inhibitors of p38α [16]. The vast majority of these compounds have proved to be competitive with ATP, binding to the active site (reviewed for example in [10, 17]). A few inhibitors have also been found that bind to a site adjacent to the active site. This neighboring site has been termed the “DFG-out”-site, because binding at this site is always associated with conformational changes in the conserved DFG sequence (subdomain VII) [16, 18] (Fig. 1). The existence of this site relies on the conformational flexibility of the activation loop of these protein kinases, and a similar site was identified in the development of the inhibitors of MAP/ERK kinase 1 and 2 (MEK1/2) [19], c-Abl [20] and other protein kinases [21, 22].
Fig. 1.
The structure of inactive p38α with substrate and small molecule binding sites indicated. The ATP binding site, the DFG-out site, CD-docking groove, FXFP binding site, and backside site are indicated as violet ellipsoids. Helices are cyan, β-strands magenta, and loops deep salmon. Figures generated using PyMOL (Delano Scientific, San Carlos, CA).
MAP kinase modules utilize docking interactions to link module components and bind substrates (reviewed in [23-25]). Docking interactions may account for the pathway specificity among MAP kinase modules. Linear peptide docking motifs in substrates, MAP2Ks, and phosphatases bind to sites in MAPKs outside the kinase active site [26]. MAP2Ks have been shown recently to utilize other docking interactions with MAP3Ks [27].The best studied docking interactions are those between MAP kinases and “D-motifs” (originally named δ-domains, but also referred to as D-sites, D-domains, DEJL, D-boxes, kinase interaction motifs, or KIMs), linear sequences with substrates and other interacting proteins. D-motifs were identified initially in JNK substrates [28], and were later found in MAP2Ks [25] and in MAP kinase phosphatases [29]. Each of the MAP kinases p38s, JNKs and ERKs [24] and ERK5 [30] bind D-motifs. In addition, transcription factor substrates and phosphatases of the MAP kinase ERK2 and p38α have a second docking motif, named FXFP [23, 31, 32], for the sequence in the docking motif. The binding sites for these motifs may have potential for making specific inhibitors of MAP kinases. The idea of making substrate based inhibitors is established for protein kinases [33], but new for MAP kinases [33-36]. Here we discuss the “druggability” of the MAP kinase docking motif binding sites for D-motifs and FXFP, and present data on the existence of a novel small molecule binding site in p38α termed the Backside site (Fig. 1).
2. D-motif binding site
The D-motifs are probably the main specificity determinant that distinguishes among different MAP kinases [25]. In D-motif swapping experiments among MAPKAP isoforms that are differentially activated by ERK2 and p38α, the D-motif conferred pathway specificity [37]. Also, peptides derived from the D-motif of c-Jun interacting protein (JIP), which bind to the docking groove of JNK inhibit JNK activation [38] and are neuroprotective in animal models for Parkinson’s disease [39], apparently blocking apoptotic signaling through JNK. D-motifs can have different lengths. The longest have the consensus sequences X-ØH-X2-(Arg/Lys)1-2-(X)2-6-ØA-X-ØB (where ØA, ØB and ØH are hydrophobic residues (Leu, Ile, or Val), while shorter motifs can be missing the first four residues [24]. The D-motif binding site on MAP kinases is complex, and is formed of acidic patch in the C-terminal extension from the kinase core known as the ”CD domain” (for Common Docking)[26] and hydrophobic docking groove [40, 41] (also referred to as “ED”[42]). The existence of two subsites may allow separate or overlapping specificities to evolve in MAP kinase substrates [43]. Crystallographic studies of p38α [41] and JNK1 [44] revealed the hydrophobic groove to be near helices D and E. Recent data on ERK2 docking interactions [45, 46] and on the yeast MAP kinase Fus3 and Kss1 [47] has revealed interactions in the CD domain. The CD domain is an electrostatic surface depression [45] to which the ØH and basic residues bind.
2.1 The Hydrophobic Docking Groove
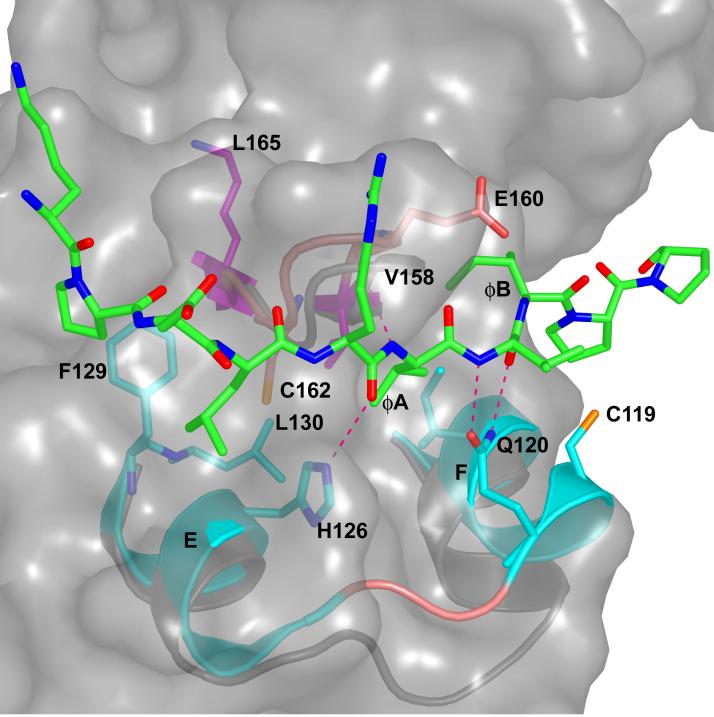
The hydrophobic docking groove appears to have the greatest potential for drug development since it has a significant hydrophobic pocket [45]. The docking groove spans across helices D and E in a crevice formed between these helices and β7-β8 reverse turn (Fig. 2). The hydrophobic pocket is occupied variably by two to three hydrophobic residues in different MAP kinases. In p38α, the ØA makes van der Waals contacts with the side chains of Ile116, Leu122 in helix D, Val158 in β7 and Cys162 in β8. The ØB residue contacts the side chains of Ala111 and Ile116 in helix D and Val158 in β7. A leucine residue in the position ØA-2 is in the groove, making contacts with Phe129 and Leu130 in helix E. The interaction also involves several hydrogen bonds. In p38α, four hydrogen bonds are made to the peptide by Gln120, His126 and the carbonyl of Glu160 (in the β7-β8 reverse turn). In contrast to the docking groove, the CD domain is a surface depression that requires the D-motif to be folded into a 2-turn helix to bind [45].
Fig. 2.
Docking groove interactions between p38α and pepMEF2A induced conformational changes. Cartoon of p38α docking groove; residues 110-129 + 157-163. Ø-2 through ØB of pepMEF2A is shown in green and all atom colors. The p38α/pepMEF2A is shown in cyan and all atom colors, and uncomplexed p38α is shown in gray. The side chains of I116, C119, Q120, H126, F129, L130, V158, and C162 and backbone of E160 p38α/pepMEF2A are displayed in ball and stick rendering. Dotted lines denote hydrogen bonds (2.6-3.2 Å). The surface of unliganded p38α in the docking groove is shown to reveal the full size of the pocket in the absence of peptide.
2.2 Similarities and differences among MAP kinase docking grooves
The hydrophobic docking grooves are significantly different in p38α, ERK2 and JNK’s (discussed in [45]). For example, in ERK2, ERK2/Thr108 replaces p38α/Ala111, and the larger side-chain completely blocks the site occupied by ØB in p38α. In addition, ØB of ERK2-binding peptides occupy the pocket held by ØA in p38α. JNK/Ala111 binds to ØA and ØB like p38α. But the site lacks ØA-2 site, which is blocked by an arginine unique to JNK (Arg127). These differences may be contributing to the success in making specific peptide-based inhibitors of JNKs [48]. Another feature of the hydrophobic docking groove is the presence of cysteine residues. p38α has two cysteine residues that face the pocket, Cys119 and Cys162. Both ERK2 and JNK possess one cysteine, ERK2/Cys159 and JNK/Cys163. Thus, development of covalent inhibitors directed toward cysteine [49] becomes a possibility. Also, tethering, the use of mild thiol reagents to enhance affinities of compounds in fragment and small molecule libraries [50] can potentially be used to search for inhibitors targeted to the docking groove.
2.3 D-motif induced conformational changes
D-motif peptides induce conformational changes in p38α and ERK2, and possibly in JNK. The changes involve the activation loop in each case, which becomes disordered in p38α and adopts a new structure in ERK2. These conformational changes may promote greater access to the activation loop phosphorylation sites when activating MAP2Ks or phosphatases are bound in the D-motif binding site.
In p38α and ERK2, the changes are known because structures are available for the unbound and peptide-bound conformations for the identical protein (rat ERK2 and murine p38α) [41, 45]. The induced conformational changes are unique. In p38α the changes occur near helices D and E (Fig. 2). The movement appears to be required in order to simultaneously form all of the observed hydrogen bonds made to the peptides, especially the interactions of Gln120 with residue X in (ØA-X-ØB) and the interaction of His126 with the docking site peptide backbone. In ERK2, the hydrophobic docking groove is preformed. Instead, conformational changes occur near the CD domain in ERK2 [45] (not shown).
The affinities for docking peptides to MAP kinases are sub-micromolar to micromolar. Can more tightly bound inhibitors be found? The peptide induced conformational changes likely absorb intrinsic binding energy [45]. Thus an inhibitor that fits in without inducing structural rearrangements may have a better binding constant. The amount of energy absorbed by conformational changes has not been measured in MAP kinases, but is 3 kcal or more in allosteric enzymes [51]. Since the D-motif induced conformational changes are different in p38α, ERK2, and JNK, the changes probably contribute to specificity among these kinases and may be a factor to specific inhibitor binding.
3. FXFP binding site in ERK2
The MAP kinase ERK2 has a second binding site utilized by transcription factor substrates and phosphatases, the FXFP binding site [24, 52]. This site was identified by changes in deuterium exchange profiles, and is near the MAP kinase insertion and helix G [32]. Mutational studies showed that Tyr231 and Tyr261, when mutated affected FXFP binding to doubly phosphorylated ERK2 (ERK2-P2) [32, 53]. We have obtained crystallographic evidence of the location of this site in studies of D-motif peptides bound to ERK2 [45]. The refined structure of ERK2 in complex with a D-motif peptide derived from a tyrosine phosphatase revealed an extra tubular electron density near Tyr231 (Fig. 3).
Fig. 3.
ERK2/pepHePTP reveals electron density near the FXFP binding site and peptide induced conformational changes. Electron density (|2Fo-Fc|, contoured at 0.9 σ) near the helix G and the MAP kinase insertion. Although the D-motif peptide (not shown) binds in the same docking groove as MEF2A binds to p38α [45], extra electron density is observed near helix G in ERK2. Ribbon diagram of the ERK2/pepHePTP (displayed between 176-203 +225-263 is in cyan. The side chains of L198, Y231, L232, and Y261 are shown in ball and stick. Uncomplexed ERK2 is shown in gray, and F181 and L182 are shown in ball and stick. Note that this binding pocket is available in ERK2/pepHePTP but not in ERK2.
The electron density was modeled with 3 residues derived from the D-motif peptide used in the study and is included in the PDB file 2GPH. The electron density is between the MAP kinase insertion and helix G. In low activity ERK2 (PDB file 1ERK) [54], this site is occupied with activation loop residues Phe181 and Leu182. The tyrosine phosphatase peptide and MEK2 derived peptide both induced conformational changes that caused the activation loop to exit this site. This binding site is hydrophobic, and lined by Leu198, Tyr231, Leu232, Leu235, Ala258 and Tyr261. Hydrogen bonding potential is available from Asn255 and Lys257. This site is adjacent to the active site, and could potentially be accessed by inhibitors simultaneously with the active site.
4. Backside binding pocket
In an effort to visualize protein kinase inhibitors, we soaked inhibitors for other kinases into crystals of p38α, as a model protein kinase. We anticipated that most of the kinase inhibitors tested would bind to the ATP binding site, as was the case for the p38 inhibitors SB203580 and related comounds [7]. However, two compounds, PD98059 and sulindac sulfide, bind to a site outside the active site. The MAP2K MEK1/2 inhibitor PD98059 (1-(2′-amino-3′-methoxyphenyl)-oxanaphthalen-4-one), is a flavonoid that binds to the inactive form of MEK1 non-competitively with respect to ATP [55]. The IkB kinase inhibitor sulindac sulfide (cis-5-fluoro-2-methyl-1-[p-(methylsulfinyl)benzylidene]indene-3-acetic acid, is a non-steroidal anti-inflammatory drug [56]. Previous studies have indicated that sulindac sulfide and PD98059 do not inhibit p38α activity [55,56]. However, we did find that these compounds bind p38α with IC50′s of 19 μM and 24 μM, respectively, as measured by binding-induced changes in absorbance (at 365 nm and 329 nm, respectively). Crystals of p38α prepared as described previously [57, 58] were soaked with 1 mM sulindac sulfide or PD98059, soaked for 45 min. The structures were solved using 2.6 Å data and refined to R-factors of 21% or better (Table 1). Both sulindac sulfide and PD98059 bind p38α near the hinge point between the two domains of the kinase, where the C-terminal helix unique to MAP kinases contacts the kinase core. We refer to this site as the Backside binding site.
Table 1.
X-ray Data Collection Parameters and Refinement Statistics
| p38 + Sulindac | p38 +PD98059 | |
|---|---|---|
| Diffraction data | ||
| Space group | P212121 | P212121 |
| Unit cell (Å) | a=45.59 | a=45.71 |
| b=85.54 | b=84.57 | |
| c=124.26 | c=125.34 | |
| Wavelength (Å) | 1.5418 | 1.5418 |
| Resolution (Å) | 2.5 | 2.2 |
| No. of measurements | 275364 | 264392 |
| Unique reflections | 14546 | 25889 |
| Completeness(%)(last shell) | 89.1(78.9) | 86.5(67.0) |
| Rmerge (%) (last shell) | 5.6(32.4) | 4.8(37.5) |
| Refinement | ||
| Resolution (Å) | 20-2.6 | 20-2.4 |
| No. of reflections (F>2σ) | 12448 | 15196 |
| Rcryst/Rfree (%) | 21.7/25.9 | 21.1/24.1 |
| No. of waters | 98 | 91 |
| Ramachandran Plot: | ||
| Most favoured | 77.3 | 81.1 |
| Additional allowed | 19.2 | 17.0 |
| Generously allowed | 3.5 | 1.9 |
| Disallowed | 0 | 0 |
4.1. Sulindac sulfide
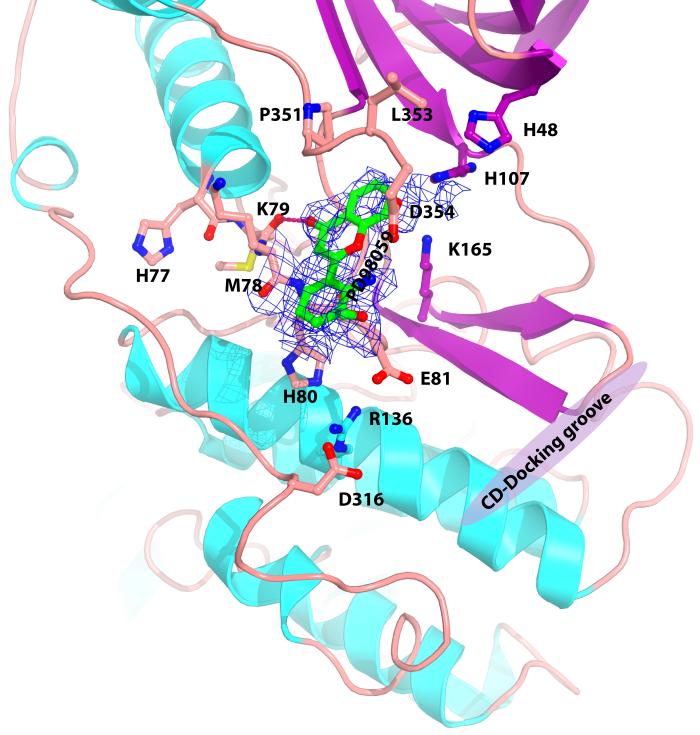
The sulfur atom of sulindac sulfide was especially visible, with a 3.5 σ electron density pinpointing the sulfur atom, and the entire molecule is visible as contoured (Fig. 4A). The indene moiety of sulindac sulfide is held between the His107 and Lys165, with ring stacking link between the indene and the His 107 side-chain. The carboxylate group in the sulindac sulfide contacts the backbone atoms Met78O, His80N, and the carboxylate of Glu81 in the linker between helix C and β-4. The benzyledene forms van der Waals contacts with the C-terminal residues Leu353 and Asp354. The indene fluorine atom in sulindac is hydrogen bonded (2.9Å) to Lys165Nε. Thus there are several specific hydrogen bonds as well as hydrophobic interactions.
Fig. 4.
Electron density of map with coefficients (|2Fo-Fc|) contoured at 0.7 σ for p38α crystals soaked in A) sulindac sulfide, and B) PD98059. The sulfur atom of sulindac sulfide is yellow, fluorine orange, oxygen red, and nitrogen blue. Residues forming contacts, K79, E81, H107, K165, P351 and L353 are displayed in ball and stick. Residues R136 and D316 in the CD domain are also displayed. Location of D-motif docking groove shown in magenta.
Although there are no known inhibitors targeted to this site, an inhibitor developed at Boehringer Ingleheim Pharmaceuticals (called CMPD1) [59] shows deuterium exchange protection profiles that include His107 and Lys165, among other residues, suggestive of potential binding to this site.
A major question of course is whether the target of sulindac sulfide binds; IkB kinase binds the inhibitor similarly. IkB kinase does have homology with MAP kinases in this region, including the C-terminal helix that walls the binding pocket. Thus the possibility that sulindac sulfide binds Iκβ in this site cannot be ruled out.
4.2 PD98059
PD98059 binds to the same site as sulindac sulfide. The carbonyl of oxanaphthalen-4-one ring of PD98059 contacts the backbone of Met78O as with sulindac sulfide. Otherwise the interactions and the orientation are different, with PD98059 binding closer to Lys79 and Glu81 (Fig. 4b). The structure of inactive MEK1/2 is available (PDB file 1S9J) and no similar site is present [19], so PD98059 is very unlikely to bind to MEK1/2 in this region.
4.3 Proximity of the Backside site to the CD domain
It is interesting that Backside binding site is very near the CD domain. Glu81 that contacts the inhibitors is involved in an extended ionic network that includes Arg136 and Asp316, in the CD domain defined by [26] (Fig. 4A). This raises the possibility of making inhibitors that target both the Backside and docking groove. CMPD1 [59] mentioned above is an inhibitor of p38α that affects binding and phosphorylation of MAPKAPs but not transcription factor substrates. It is intriguing that MAPKAPs have the long variety of D-motifs, making more extensive interactions near the CD domain whereas transcription factors usually have the shorter variety [24], suggestive that the inhibitor does indeed bind the Backside site.
5. Conclusions and perspectives
Although MAP kinase drug discovery so far has identified inhibitors primarily to the ATP site and DFG-out site, other substrate and small molecule binding sites may prove useful in the future for the development of novel and specific inhibitors of MAP kinases. We think the allosteric effects in MAP kinases offer an especially interesting opportunity for drug development, because of the chance of binding an inhibitor that binds more tightly than the docking peptide. Also, D-motif binding site directed inhibitors could inhibit the pathway both by substrate and MAP2K binding, and also by inhibiting conformational changes. The sites discussed here are all smaller than the ATP binding site and DFG-out site, and thus it appears likely that strategies that block the ATP site may be needed to find inhibitors to these smaller sites.
Acknowledgements
We thank Tianjun Zhou for contributions to this work. This research was supported by NIH grant DK46993 and grant I1128 from the Welch Foundation. Use of the Argonne National Laboratory Structural Biology Center beamlines at the Advanced Photon Source was supported by the U. S. Department of Energy, Office of Biological and Environmental Research, under Contract No. W-31-109-ENG-38.
Abbreviations
- CD-domain
Common docking domain
- D-Motif
Docking motif or docking site in MAP kinase substrates and MAP kinase processing enzymes
- DFG-out
unusual conformation in well conserved DFG in subdomain VII
- ED
hydrophobic docking groove (that is flanked by D and E residues)
- ERK
Extracellular response kinase
- JNK
c-Jun N-terminal kinase
- MAPK
Mitogen activated protein kinase
- MAP2K
Mitogen activated protein kinase kinase
- MAPKAP kinase
Mitogen activated protein kinase activated protein kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, Wright A, Vanderbilt C, Cobb MH. MAP kinases. Chem. Rev. 2001;101:2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- 2.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 3.Deng Q, Liao R, Wu BL, Sun P. High intensity ras signaling induces premature senescence by activating p38 pathway in primary human fibroblasts. J. Biol. Chem. 2004;279:1050–1059. doi: 10.1074/jbc.M308644200. [DOI] [PubMed] [Google Scholar]
- 4.Ferrell JE, Jr., Bhatt RR. Mechanistic studies of the dual phosphorylation of mitogen-activated protein kinase. J. Biol. Chem. 1997;272:19008–19016. doi: 10.1074/jbc.272.30.19008. [DOI] [PubMed] [Google Scholar]
- 5.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Tournier C. Regulation of cellular functions by the ERK5 signalling pathway. Cell Signal. 2006;18:753–760. doi: 10.1016/j.cellsig.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Canagarajah BJ, Boehm JC, Kassisa S, Cobb MH, Young PR, Abdel-Meguid S, Adams JL, Goldsmith EJ. Structural basis of inhibitor selectivity in MAP kinases. Structure. 1998;6:1117–1128. doi: 10.1016/s0969-2126(98)00113-0. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Shen B, Lin A. Novel strategies for inhibition of the p38 MAPK pathway. Trends Pharmacol. Sci. 2007;28:286–295. doi: 10.1016/j.tips.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat. Rev. Drug. Discov. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 10.Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta. 2005;1754:253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Manning AM, Davis RJ. Targeting JNK for therapeutic benefit: from junk to gold? Nat. Rev. Drug Discov. 2003;2:554–565. doi: 10.1038/nrd1132. [DOI] [PubMed] [Google Scholar]
- 12.Resnick L, Fennell M. Targeting JNK3 for the treatment of neurodegenerative disorders. Drug Discov. Today. 2004;9:932–939. doi: 10.1016/S1359-6446(04)03251-9. [DOI] [PubMed] [Google Scholar]
- 13.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 14.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat. Rev. Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 15.Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 16.Wrobleski ST, Doweyko AM. Structural comparison of p38 inhibitor-protein complexes: a review of recent p38 inhibitors having unique binding interactions. Curr. Top. Med. Chem. 2005;5:1005–1016. doi: 10.2174/1568026054985894. [DOI] [PubMed] [Google Scholar]
- 17.Adams JL, Badger AM, Kumar S, Lee JC. p38 MAP kinase: molecular target for the inhibition of pro-inflammatory cytokines. Prog. Med. Chem. 2001;38:1–60. doi: 10.1016/s0079-6468(08)70091-2. [DOI] [PubMed] [Google Scholar]
- 18.Regan J, Capolino A, Cirillo PF, Gilmore T, Graham AG, Hickey E, Kroe RR, Madwed J, Moriak M, Nelson R, Pargellis CA, Swinamer A, Torcellini C, Tsang M, Moss N. Structure-activity relationships of the p38alpha MAP kinase inhibitor 1-(5-tert-butyl-2-p-tolyl-2H-pyrazol-3-yl)-3-[4-(2-morpholin-4-yl-ethoxy)n aph-thalen-1-yl]urea (BIRB 796) J. Med. Chem. 2003;46:4676–4686. doi: 10.1021/jm030121k. [DOI] [PubMed] [Google Scholar]
- 19.Ohren JF, Chen H, Pavlovsky A, Whitehead C, Zhang E, Kuffa P, Yan C, McConnell P, Spessard C, Banotai C, Mueller WT, Delaney A, Omer C, Sebolt-Leopold J, Dudley DT, Leung IK, Flamme C, Warmus J, Kaufman M, Barrett S, Tecle H, Hasemann CA. Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nat. Struct. Mol. Biol. 2004;11:1192–1197. doi: 10.1038/nsmb859. [DOI] [PubMed] [Google Scholar]
- 20.Nagar B, Bornmann WG, Pellicena P, Schindler T, Veach DR, Miller WT, Clarkson B, Kuriyan J. Crystal structures of the kinase domain of c-Abl in complex with the small molecule inhibitors PD173955 and imatinib (STI-571) Cancer Res. 2002;62:4236–4243. [PubMed] [Google Scholar]
- 21.Sebolt-Leopold JS, English JM. Mechanisms of drug inhibition of signalling molecules. Nature. 2006;441:457–462. doi: 10.1038/nature04874. [DOI] [PubMed] [Google Scholar]
- 22.Mol CD, Fabbro D, Hosfield DJ. Structural insights into the conformational selectivity of STI-571 and related kinase inhibitors. Curr. Opin. Drug. Discov. Devel. 2004;7:639–648. [PubMed] [Google Scholar]
- 23.Jacobs D, Glossip D, Xing H, Muslin AJ, Kornfeld K. Multiple docking sites on substrate proteins form a modular system that mediates recognition by ERK MAP kinase. Genes Dev. 1999;13:163–175. [PMC free article] [PubMed] [Google Scholar]
- 24.Sharrocks AD, Yang SH, Galanis A. Docking domains and substrate-specificity determination for MAP kinases. Trends Biochem. Sci. 2000;25:448–453. doi: 10.1016/s0968-0004(00)01627-3. [DOI] [PubMed] [Google Scholar]
- 25.Bardwell L. Mechanisms of MAPK signalling specificity. Biochem. Soc. Trans. 2006;34:837–841. doi: 10.1042/BST0340837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanoue T, Adachi M, Moriguchi T, Nishida E. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat. Cell. Biol. 2000;2:110–116. doi: 10.1038/35000065. [DOI] [PubMed] [Google Scholar]
- 27.Takekawa M, Tatebayashi K, Saito H. Conserved docking site is essential for activation of mammalian MAP kinase kinases by specific MAP kinase kinase kinases. Mol. Cell. 2005;18:295–306. doi: 10.1016/j.molcel.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Kallunki T, Deng T, Hibi M, Karin M. c-Jun can recruit JNK to phosphorylate dimerization partners via specific docking interactions. Cell. 1996;87:929–939. doi: 10.1016/s0092-8674(00)81999-6. [DOI] [PubMed] [Google Scholar]
- 29.Zhou B, Wu L, Shen K, Zhang J, Lawrence DS, Zhang ZY. Multiple regions of MAP kinase phosphatase 3 are involved in its recognition and activation by ERK2. J. Biol. Chem. 2001;276:6506–6515. doi: 10.1074/jbc.M009753200. [DOI] [PubMed] [Google Scholar]
- 30.Barsyte-Lovejoy D, Galanis A, Clancy A, Sharrocks AD. ERK5 is targeted to myocyte enhancer factor 2A (MEF2A) through a MAPK docking motif. Biochem. J. 2004;381:693–699. doi: 10.1042/BJ20031940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galanis A, Yang SH, Sharrocks AD. Selective targeting of MAPKs to the ETS domain transcription factor SAP-1. J. Biol. Chem. 2001;276:965–973. doi: 10.1074/jbc.M007697200. [DOI] [PubMed] [Google Scholar]
- 32.Lee T, Hoofnagle AN, Kabuyama Y, Stroud J, Min X, Goldsmith EJ, Chen L, Resing KA, Ahn NG. Docking motif interactions in MAP kinases revealed by hydrogen exchange mass spectrometry. Mol. Cell. 2004;14:43–55. doi: 10.1016/s1097-2765(04)00161-3. [DOI] [PubMed] [Google Scholar]
- 33.Bogoyevitch MA, Barr RK, Ketterman AJ. Peptide inhibitors of protein kinases-discovery, characterisation and use. Biochim. Biophys. Acta. 2005;1754:79–99. doi: 10.1016/j.bbapap.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 34.Mayor F, Jr., Jurado-Pueyo M, Campos PM, Murga C. Interfering with MAP kinase docking interactions: implications and perspective for the p38 route. Cell Cycle. 2007;6:528–533. doi: 10.4161/cc.6.5.3920. [DOI] [PubMed] [Google Scholar]
- 35.Hancock CN, Macias AT, Mackerell AD, Jr., Shapiro P. Mitogen activated protein (MAP) kinases: development of ATP and non-ATP dependent inhibitors. Med. Chem. 2006;2:213–222. doi: 10.2174/157340606776056151. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro P. Discovering new MAP kinase inhibitors. Chem. Biol. 2006;13:807–809. doi: 10.1016/j.chembiol.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Smith JA, Poteet-Smith CE, Malarkey K, Sturgill TW. Identification of an extracellular signal-regulated kinase (ERK) docking site in ribosomal S6 kinase, a sequence critical for activation by ERK in vivo. J. Biol. Chem. 1999;274:2893–2898. doi: 10.1074/jbc.274.5.2893. [DOI] [PubMed] [Google Scholar]
- 38.Ho DT, Bardwell AJ, Grewal S, Iverson C, Bardwell L. Interacting JNK-docking sites in MKK7 promote binding and activation of JNK mitogen-activated protein kinases. J. Biol. Chem. 2006;281:13169–13179. doi: 10.1074/jbc.M601010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arthur PG, Matich GP, Pang WW, Yu DY, Bogoyevitch MA. Necrotic death of neurons following an excitotoxic insult is prevented by a peptide inhibitor of c-jun N-terminal kinase. J. Neurochem. 2007;102:65–76. doi: 10.1111/j.1471-4159.2007.04618.x. [DOI] [PubMed] [Google Scholar]
- 40.Gum RJ, Young PR. Identification of two distinct regions of p38 MAPK required for substrate binding and phosphorylation. Biochem. Biophys. Res. Commun. 1999;266:284–289. doi: 10.1006/bbrc.1999.1787. [DOI] [PubMed] [Google Scholar]
- 41.Chang CI, Xu BE, Akella R, Cobb MH, Goldsmith EJ. Crystal structures of MAP kinase p38 complexed to the docking sites on its nuclear substrate MEF2A and activator MKK3b. Mol. Cell. 2002;9:1241–1249. doi: 10.1016/s1097-2765(02)00525-7. [DOI] [PubMed] [Google Scholar]
- 42.Tanoue T, Nishida E. Molecular recognitions in the MAP kinase cascades. Cell Signal. 2003;15:455–462. doi: 10.1016/s0898-6568(02)00112-2. [DOI] [PubMed] [Google Scholar]
- 43.Barsyte-Lovejoy D, Galanis A, Sharrocks AD. Specificity determinants in MAPK signaling to transcription factors. J. Biol. Chem. 2002;277:9896–9903. doi: 10.1074/jbc.M108145200. [DOI] [PubMed] [Google Scholar]
- 44.Heo YS, Kim SK, Seo CI, Kim YK, Sung BJ, Lee HS, Lee JI, Park SY, Kim JH, Hwang KY, Hyun YL, Jeon YH, Ro S, Cho JM, Lee TG, Yang CH. Structural basis for the selective inhibition of JNK1 by the scaffolding protein JIP1 and SP600125. Embo J. 2004;23:2185–2195. doi: 10.1038/sj.emboj.7600212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou T, Sun L, Humphreys J, Goldsmith EJ. Docking interactions induce exposure of activation loop in the MAP kinase ERK2. Structure. 2006;14:1011–1019. doi: 10.1016/j.str.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Liu S, Sun JP, Zhou B, Zhang ZY. Structural basis of docking interactions between ERK2 and MAP kinase phosphatase 3. Proc. Natl. Acad. Sci. U S A. 2006;103:5326–5331. doi: 10.1073/pnas.0510506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Remenyi A, Good MC, Bhattacharyya RP, Lim WA. The role of docking interactions in mediating signaling input, output, and discrimination in the yeast MAPK network. Mol. Cell. 2005;20:951–962. doi: 10.1016/j.molcel.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 48.Bogoyevitch MA. Therapeutic promise of JNK ATP-noncompetitive inhibitors. Trends Mol. Med. 2005;11:232–239. doi: 10.1016/j.molmed.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 49.Powers JC, Asgian JL, Ekici OD, James KE. Irreversible inhibitors of serine, cysteine, and threonine proteases. Chem. Rev. 2002;102:4639–4750. doi: 10.1021/cr010182v. [DOI] [PubMed] [Google Scholar]
- 50.Erlanson DA, Wells JA, Braisted AC. Tethering: fragment-based drug discovery. Annu. Rev. Biophys. Biomo. Struct. 2004;33:199–223. doi: 10.1146/annurev.biophys.33.110502.140409. [DOI] [PubMed] [Google Scholar]
- 51.Goldsmith EJ. Allosteric enzymes as models for chemomechanical energy transducing assemblies. Faseb J. 1996;10:702–708. doi: 10.1096/fasebj.10.7.8635687. [DOI] [PubMed] [Google Scholar]
- 52.Zhang J, Zhou B, Zheng CF, Zhang ZY. A bipartite mechanism for ERK2 recognition by its cognate regulators and substrates. J. Biol. Chem. 2003;278:29901–29912. doi: 10.1074/jbc.M303909200. [DOI] [PubMed] [Google Scholar]
- 53.Polychronopoulos S, Verykokakis M, Yazicioglu MN, Sakarellos-Daitsiotis M, Cobb MH, Mavrothalassitis G. The transcriptional ETS2 repressor factor associates with active and inactive Erks through distinct FXF motifs. J. Biol. Chem. 2006;281:25601–25611. doi: 10.1074/jbc.M605185200. [DOI] [PubMed] [Google Scholar]
- 54.Zhang F, Strand A, Robbins D, Cobb MH, Goldsmith EJ. Atomic structure of the MAP kinase ERK2 at 2.3 A resolution. Nature. 1994;367:704–711. doi: 10.1038/367704a0. [DOI] [PubMed] [Google Scholar]
- 55.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. U S A. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamamoto Y, Yin MJ, Lin KM, Gaynor RB. Sulindac inhibits activation of the NF-kappaB pathway. J. Biol. Chem. 1999;274:27307–27314. doi: 10.1074/jbc.274.38.27307. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z, Harkins PC, Ulevitch RJ, Han J, Cobb MH, Goldsmith EJ. The structure of mitogen-activated protein kinase p38 at 2.1-A resolution. Proc. Natl. Acad. Sci. U S A. 1997;94:2327–2332. doi: 10.1073/pnas.94.6.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee SJ, Zhou T, Goldsmith EJ. Crystallization of MAP kinases. Methods. 2006;40:224–233. doi: 10.1016/j.ymeth.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Davidson W, Frego L, Peet GW, Kroe RR, Labadia ME, Lukas SM, Snow RJ, Jakes S, Grygon CA, Pargellis C, Werneburg BG. Discovery and characterization of a substrate selective p38alpha inhibitor. Biochemistry. 2004;43:11658–11671. doi: 10.1021/bi0495073. [DOI] [PubMed] [Google Scholar]