Abstract
Context: Abnormal plasma nonesterified fatty acid (NEFA) metabolism may play a role in the development of type 2 diabetes.
Objectives: Our objectives were to demonstrate whether there is a defect in insulin-mediated suppression of plasma NEFA appearance (RaNEFA) and oxidation (OxNEFA) during enhanced intravascular triacylglycerol lipolysis early in the natural history of type 2 diabetes, and if so, to determine whether other mechanisms than reduced insulin-mediated suppression of intracellular lipolysis are involved.
Design: These are cross-sectional studies.
Setting: The studies were performed at an academic clinical research center.
Participants: Nine healthy subjects with both parents with type 2 diabetes (FH+) and nine healthy subjects with no first-degree relatives with type 2 diabetes (FH−) with similar anthropometric features were included in the studies.
Interventions: Pancreatic clamps and iv infusion of stable isotopic tracers ([1,1,2,3,3-2H5]-glycerol and [U-13C]-palmitate or [1,2-13C]-acetate) were performed while intravascular triacylglycerol lipolysis was simultaneously clamped by iv infusion of heparin plus Intralipid at low (fasting) and high insulin levels. Oral nicotinic acid (NA) was used to inhibit intracellular lipolysis.
Main Outcome Measures: RaNEFA and OxNEFA were determined.
Results: During heparin plus Intralipid infusion at high plasma insulin levels, and despite similar intravascular lipolytic rates, FH+ had higher RaNEFA and OxNEFA than FH− (RaNEFA: 17.4 ± 6.3 vs. 9.2 ± 4.2; OxNEFA: 4.5 ± 1.8 vs. 2.3 ± 1.5 μmol/kg lean body mass/min), independent of NA intake, gender, age, and body composition. In the presence of NA, insulin-mediated suppression of RaNEFA was still observed in FH−, but not in FH+.
Conclusions: Increased RaNEFA and OxNEFA during intravascular lipolysis at high insulin levels occur early in the natural history of type 2 diabetes.
Healthy offspring with two type 2 diabetes parents display an increase in plasma non-esterified fatty acid appearance and oxidation during stimulated intravascular triacylglycerol lipolysis and hyperinsulinemia, suggesting that impaired fatty acid storage in adipose tissues occur early in the natural history of type 2 diabetes and may contribute to ectopic fat accumulation in these individuals.
Excess exposure of nonadipose tissues to high plasma nonesterified fatty acids (NEFAs) decreases insulin-mediated glucose disposal (1), reduces glucose transport into muscle (2), stimulates endogenous glucose production (3), reduces hepatic insulin clearance (4), and impairs glucose-stimulated insulin secretion (5,6,7,8,9). The offspring of two parents with type 2 diabetes have a very high risk of developing overt type 2 diabetes (10). They also have increased postprandial triacylglycerol (TG) levels and blunted early postprandial lowering of NEFA (11). Thus, it has been suggested that in subjects with a family history of type 2 diabetes, impaired postprandial reduction of NEFA by insulin could be involved in the development of insulin resistance and type 2 diabetes through increased exposure of nonadipose tissue to NEFAs (5).
The effect of insulin on postprandial NEFA metabolism is complex. In the postprandial state, the suppression of NEFA by insulin could be altered early on in the natural history of type 2 diabetes, although this has not been previously addressed to our knowledge. In adipose tissue, insulin stimulates lipoprotein lipase (LPL)-mediated lipolysis of chylomicron and very low density lipoprotein (VLDL) TG in the circulation (i.e. stimulates intravascular TG lipolysis) (12), suppresses intracellular adipose tissue lipolysis (13), and may stimulate esterification of NEFA in adipose tissue. Thus, insulin may contribute to adipose tissue uptake of NEFAs generated from intravascular TG lipolysis (13,14), i.e. insulin may reduce NEFA spillover.
Our primary aim was to demonstrate the early occurrence in the natural history of type 2 diabetes of a defect in insulin-mediated suppression of plasma NEFA appearance rate (RaNEFA) and plasma NEFA oxidation rate (OxNEFA) during enhanced intravascular TG lipolysis. If present, we also aimed to establish whether this defect was independent of insulin-mediated regulation of both LPL and intracellular adipose tissue lipolysis, i.e. whether reduced insulin-mediated adipose tissue uptake of NEFA generated during intravascular TG lipolysis occurs early on in the natural history of type 2 diabetes.
Subjects and Methods
Subjects
Nine healthy subjects with both parents with type 2 diabetes [subjects with both parents whose diabetes started after age 30, but not requiring insulin therapy at diagnosis (FH+)] and nine healthy subjects with no first-degree relatives with type 2 diabetes (FH−) participated in the studies (Table 1). All subjects tested negative for diabetes based on a 75-g oral glucose tolerance test (15). No subject was taking any chronic medication other than stable thyroid hormone replacement therapy, had no active medical condition known to affect lipid levels or insulin sensitivity, and had no cardiovascular disease. All female subjects were premenopausal and participated during the follicular phase of their menstrual cycle. Using the questionnaire of Sallis et al. (16), habitual energy expenditure was similar in both groups (Table 1). Written informed consent was obtained before participation, in accordance with the Declaration of Helsinki and all applicable laws and regulations. Experimental protocols were duly approved by the Research Ethics Institutional Review Board at the Centre hospitalier universitaire de Sherbrooke.
Table 1.
Characteristics of the study participants
| FH+ (n = 9)
|
FH− (n = 9)
|
P value | |||
|---|---|---|---|---|---|
| Mean ± se | Range | Mean ± se | Range | ||
| Age (yr) | 41.2 ± 11.7 | 24–56 | 34.7 ± 10.8 | 21–47 | 0.25 |
| Gender (M/F) | 4/5 | 6/3 | 0.64 | ||
| Height (cm) | 166.7 ± 10.5 | 154.0–185.0 | 170.5 ± 9.9 | 152.0–185.5 | 0.44 |
| Weight (kg) | 70.1 ± 9.3 | 55.5–84.2 | 71.0 ± 11.4 | 59.1–93.0 | 0.85 |
| BMI (kg/m2) | 25.2 ± 3.0 | 20.0–28.6 | 24.4 ± 3.3 | 20.0–30.9 | 0.59 |
| WC (cm) | 82 ± 9 | 69–95 | 84 ± 6 | 78–98 | 0.64 |
| LBM (kg) | 52.4 ± 14.1 | 36.7–74.1 | 56.3 ± 10.8 | 38.9–74.4 | 0.52 |
| Non-LBM (kg) | 17.6 ± 7.8 | 5.0–29.3 | 14.6 ± 11.4 | 4.9–36.4 | 0.53 |
| Estimated EE (kcal/d) | 2417 ± 381 | 1935–2932 | 2713 ± 522 | 1902–3468 | 0.19 |
| TG (mmol/liter) | 1.70 ± 1.59 | 0.48–5.06 | 1.09 ± 0.42 | 0.58–1.86 | 0.28 |
| TC (mmol/liter) | 4.92 ± 0.99 | 3.57–6.55 | 4.34 ± 0.51 | 3.84–5.26 | 0.14 |
| HDL-C (mmol/liter) | 1.32 ± 0.45 | 0.79–2.07 | 1.23 ± 0.27 | 0.90–1.77 | 0.60 |
| LDL-C (mmol/liter) | 2.82 ± 0.75 | 1.64–3.86 | 2.62 ± 0.54 | 1.64–3.35 | 0.53 |
EE, Energy expenditure; F, female; HDL-C, high-density lipoprotein cholesterol; LBM, lean body mass; LDL-C, low-density lipoprotein cholesterol; M, male; TC, total plasma cholesterol; WC, waist circumference.
Experimental protocols
All subjects participated in four separate study protocols (A/B and C/D), each conducted 3–4 wk apart. These protocols were modeled on our previously described protocol, which produces a sustained and similar elevation of intravascular TG lipolysis at different plasma insulin levels (17). On arrival, body weight and height were measured, and lean body mass was determined by bioelectrical impedance (Hydra ECF/ICF; Xitron Technologies, San Diego, CA). A 4-h period of intravascular TG lipolysis was achieved using an iv infusion of heparin (250 U/h Hepalean; Organon Teknika, Scarborough, Ontario, Canada) and Intralipid 20% (40 ml/h; Baxter, Mississauga, Ontario, Canada) under normoinsulinemic (Novolin R; Novo Nordisk, Bagsværd, Denmark; 0.05 mU.kg−1·min−1 continuous infusion; protocols A and C) or hyperinsulinemic (Novolin R, 0.8 mU/kg primed, 1.2 mU.kg−1·min−1 continuous infusion; protocols B and D) conditions with infusion of octreotide acetate and human GH (17). Although glucagon is suppressed by octreotide acetate, it was not replaced to avoid insulin secretion breakthrough at low insulin infusion rates (18), and because of its minimal effect on NEFA metabolism in humans (19).
During protocols A and B, a constant infusion of [U-13C]K palmitate (0.01 μmol/kg/min in 100 ml 25% human serum albumin; Cambridge Isotopes Laboratories Inc., Andover, MA) was started at time zero. In protocols C and D, [1,2-13C]sodium acetate (0.08 μmol/kg/min; Cambridge Isotopes Laboratories Inc.) was infused in place of the [U-13C]K palmitate (17). Mean variation of plasma glucose, insulin, and NEFA levels between protocols to determine fractional acetate recovery and those to determine plasma NEFA metabolism were less than 5%. The choice of [U-13C]palmitate as the NEFA tracer was based on the following: 1) palmitate, oleate, and linoleate are the most prevalent NEFAs, both in human plasma and in Intralipid, and they have similar plasma clearance rates in humans (20); 2) palmitate was previously used to trace total NEFA turnover in humans after oral fat intake (13,21); and 3) during iv infusion of heparin plus Intralipid (HI) in humans, we have shown that NEFA appearance rates determined with [U-13C]palmitate predict very well the rates determined using simultaneous palmitate and linoleate tracers (r = 0.90; P < 0.001) (22). To quantify plasma glycerol flux as a measure of whole body lipolysis, a primed continuous (1.6 μmol/kg; 0.11 μmol/kg/min) infusion of [1,1,2,3,3-2H5]glycerol (Cambridge Isotopes Laboratories Inc.) was also administered during protocols A and B (23). To prime the bicarbonate pool, a bolus infusion of sterile [1-13C]NaHCO3 1.2 μmol/kg (Cambridge Isotopes Laboratories Inc.) was administered at time zero in all four protocols (24). All tracers were pretested for sterility and nonpyrogenicity.
During the last 2 h of the protocols, nicotinic acid (NA) was given orally (100 mg at 120 and 200 min, and 150 mg at 150 and 180 min) because it has been previously shown to profoundly suppress intracellular adipose tissue lipolysis for up to 4 h (25). As also previously described (17), this allowed us to discriminate between insulin-mediated inhibition of intracellular lipolysis as opposed to insulin-mediated enhancement of adipose tissue uptake of NEFA derived from intravascular TG lipolysis.
Each protocol started with 30-min bed rest, after which blood samples were taken at 10-min intervals during baseline and steady states, i.e. during the last 30 min of the first 2 h (without NA), and again during the last 30 min of the 4-h clamp period (with NA). Blood and breath samples were collected, and urine nitrogen excretion, oxygen consumption (VO2), and CO2 excretion (VCO2) were measured as previously described (17).
Laboratory determinations
Glucose, insulin, glucagon, GH, total plasma NEFA, and TG were measured as previously described (17). Plasma glycerol and [1,1,2,3,3-2H5]glycerol enrichment were measured by gas chromatography-mass spectrometry. Individual plasma NEFA (palmitate, linoleate, oleate) and [U-13C]palmitate enrichment in plasma were measured by liquid chromatography-mass spectrometry (17). Intraassay and interassay coefficients of variation were under 6.1% for all assays. Breath 13CO2/12CO2 ratio was determined by isotope ratio mass spectrometry (Sercon Ltd., Crewe, Cheshire, UK).
Calculations
The plasma palmitate appearance rate (Rapalmitate) was calculated from the C16:0 mass +16 enrichment of plasma palmitate as previously described (17). RaNEFA was calculated by multiplying Rapalmitate by the ratio of NEFA to palmitate concentration. The plasma glycerol appearance rate (Raglycerol) was determined from plasma glycerol enrichment (mass +5) corrected for infusion rate of free glycerol contained in the Intralipid infusate (17). Fractional palmitate oxidation (Foxpalmitate) was determined as described (26) with correction for the fractional acetate recovery assessed in each individual (17). The plasma palmitate oxidation rate (Oxpalmitate) was then calculated by multiplying Foxpalmitate by Rapalmitate. OxNEFA was calculated by multiplying Oxpalmitate by the ratio of NEFA to palmitate concentration (22). Net total body carbohydrate and fatty acid oxidation (CHOox and FATox, respectively) were estimated by indirect calorimetry (27). Insulin sensitivity was assessed at high insulin level by dividing the glucose infusion rate by plasma insulin level.
Statistical analysis
Data at 10-min intervals were averaged for baseline and the last 30-min clamp with and without NA at fasting and high insulin, and were expressed as mean ± sd unless otherwise specified. Within-group comparisons were performed by ANOVA for repeated measures with Scheffe’s post hoc test. ANOVA was used for between-group comparisons at baseline, with and without NA at pancreatic clamp, at fasting plasma insulin level (INSLOW), and during pancreatic clamp at hyperinsulinemia (INSHIGH). Gender distribution between FH+ and FH− was compared by Fisher’s exact test. Multivariate linear regression was used to assess the effect of FH+ or FH− on NEFA appearance during INSLOW and INSHIGH, both before and after correcting for potential confounding variables in the models. A two-tailed P value less than 0.05 was considered significant. All analyses were performed with the SAS software for Windows, version 9.1.3 (SAS Institute Inc., Cary, NC).
Results
Plasma glucose, insulin, TG, GH, and glucagon levels (Table 2)
Table 2.
Plasma levels of glucose, insulin, TG, GH, and glucagon
| Group | Low insulin level protocol
|
High insulin level protocol
|
|||||
|---|---|---|---|---|---|---|---|
| Baseline | INSLOW | INSLOW NA | Baseline | INSHIGH | INSHIGH NA | ||
| Glucose (mmol/liter) | FH− | 5.0 ± 0.6 | 5.2 ± 0.9 | 5.4 ± 0.9 | 5.1 ± 0.3 | 5.1 ± 0.6 | 5.5 ± 0.9 |
| FH+ | 5.3 ± 0.6 | 6.1 ± 0.6a | 6.0 ± 0.6 | 5.5 ± 0.6 | 5.6 ± 0.6 | 5.4 ± 0.6 | |
| Insulin (pmol/liter) | FH− | 59 ± 30 | 56 ± 15 | 62 ± 21 | 74 ± 30 | 468 ± 129b,c | 441 ± 123b,d |
| FH+ | 72 ± 39 | 57 ± 12 | 57 ± 15 | 80 ± 42 | 474 ± 87b,c | 443 ± 78b,d | |
| TG (mmol/liter) | FH− | 0.66 ± 0.24 | 1.36 ± 0.39b | 1.20 ± 0.36b | 0.68 ± 0.24 | 1.33 ± 0.39b | 1.16 ± 0.30b |
| FH+ | 1.23 ± 1.02 | 2.54 ± 2.01b | 2.39 ± 2.04b | 1.42 ± 1.02a | 2.66 ± 1.92b | 2.59 ± 1.92b | |
| GH (μg/liter) | FH− | 0.5 ± 0.6 | 0.8 ± 0.3 | 0.8 ± 0.3 | 0.7 ± 0.6 | 0.6 ± 0.3 | 0.7 ± 0.3 |
| FH+ | 1.4 ± 1.8 | 0.6 ± 0.3a | 1.0 ± 1.2 | 1.2 ± 1.8 | 0.6 ± 0.3 | 0.9 ± 0.9 | |
| Glucagon (ng/liter) | FH− | 70 ± 30 | 36 ± 15b | 35 ± 18b | 68 ± 21 | 38 ± 15b | 29 ± 15b |
| FH+ | 68 ± 18 | 31 ± 15b | 29 ± 12b | 70 ± 18 | 28 ± 12b | 27 ± 15b | |
Data are mean ± sd.
P < 0.05 vs. FH− by ANOVA.
P < 0.05 vs. baseline by ANOVA with Scheffe’s test.
P < 0.05 vs. INSLOW by ANOVA with Scheffe’s test.
P < 0.05 vs. INSLOW NA by ANOVA with Scheffe’s test.
During INSLOW, plasma glucose did not significantly change from baseline in the FH− group but increased in the FH+ group (P = 0.01). By design, plasma insulin was elevated from baseline during INSHIGH, a change not affected by NA in either group. Plasma TG tended to be higher in the FH+ group (significant only at baseline for INSHIGH and at INSHIGH NA). As expected, plasma TG significantly increased during HI infusion in both groups and was unaffected by NA. Plasma GH was largely unaffected by the protocols. Plasma glucagon was similarly reduced from baseline in both groups and was not affected by NA. Insulin sensitivity was not significantly different between the two groups (0.067 ± 0.041 vs. 0.102 ± 0.058 μmol/kg lean body mass/min per pmol/liter in FH+ and FH−, respectively; P = 0.16).
Plasma concentration of NEFA and glycerol (Table 3)
Table 3.
Plasma levels of NEFA, palmitate, oleate, linoleate, and glycerol
| Group | Low insulin level protocol
|
High insulin level protocol
|
|||||
|---|---|---|---|---|---|---|---|
| Baseline | INSLOW | INSLOW NA | Baseline | INSHIGH | INSHIGH NA | ||
| NEFA (μmol/liter) | FH− | 477 ± 171 | 1041 ± 210a | 807 ± 159a,b | 416 ± 192 | 470 ± 147b | 440 ± 102c |
| FH+ | 483 ± 117 | 1324 ± 297a,d | 1027 ± 267a,b,d | 480 ± 144 | 683 ± 195b,d | 710 ± 213c,d | |
| Palmitate (μmol/liter) | FH− | 102 ± 33 | 164 ± 33a | 125 ± 24b | 92 ± 36 | 66 ± 21b | 62 ± 15 |
| FH+ | 109 ± 30 | 186 ± 51a | 134 ± 36b | 102 ± 30 | 81 ± 27b | 84 ± 24c,d | |
| Oleate (μmol/liter) | FH− | 207 ± 66 | 283 ± 69a | 197 ± 51b | 181 ± 69 | 105 ± 36b | 91 ± 24a,c |
| FH+ | 206 ± 63 | 323 ± 93a | 215 ± 66b | 180 ± 54 | 131 ± 48b | 131 ± 48c,d | |
| Linoleate (μmol/liter) | FH− | 92 ± 93 | 443 ± 93a | 407 ± 90a | 84 ± 33 | 203 ± 75a,b | 209 ± 54a,c |
| FH+ | 91 ± 36 | 510 ± 210a | 430 ± 162a | 89 ± 33 | 264 ± 108a,b | 282 ± 102a,c | |
| Glycerol (μmol/liter) | FH− | 67 ± 15 | 170 ± 15a | 173 ± 18a | 62 ± 9 | 149 ± 9a | 155 ± 18a |
| FH+ | 61 ± 12 | 186 ± 30a | 166 ± 27a | 69 ± 15 | 170 ± 36a | 170 ± 30a | |
Data are mean ± sd.
P < 0.05 vs. baseline by ANOVA with Scheffe’s test.
P < 0.05 vs. INSLOW by ANOVA with Scheffe’s test.
P < 0.05 vs. INSLOW NA by ANOVA with Scheffe’s test.
P < 0.05 vs. FH− by ANOVA.
Total NEFA, and plasma palmitate, oleate, and glycerol levels were similar at baseline in both groups. At INSLOW, HI infusion significantly increased total NEFA, palmitate, oleate, and linoleate levels from baseline in both groups. During both INSLOW and INSHIGH, total NEFAs were significantly higher in the FH+ group. As expected after the HI infusion during INSLOW, the increase in total NEFA was significantly reduced by NA to a similar extent in both groups. Increases in plasma palmitate and oleate, but not linoleate, were also significantly blunted by NA at INSLOW in both groups. At INSHIGH, total NEFA, palmitate, and oleate did not significantly change from baseline, whereas plasma linoleate increased significantly in both groups. Neither total NEFA, nor palmitate, oleate, or linoleate were significantly affected by NA at INSHIGH in either group. As expected during HI infusion, in both groups, total NEFA, palmitate, oleate, and linoleate were significantly lower at INSHIGH vs. INSLOW. During INSHIGH plus NA, plasma total NEFA, palmitate, and oleate were significantly higher in the FH+ group. In both groups, plasma glycerol was significantly increased from baseline to the same extent during INSLOW or INSHIGH, with or without NA.
Indirect calorimetry and net substrate oxidation rates (Table 4)
Table 4.
Indirect calorimetry and net substrate oxidation rates
| Group | Low insulin level protocol
|
High insulin level protocol
|
|||||
|---|---|---|---|---|---|---|---|
| Baseline | INSLOW | INSLOW NA | Baseline | INSHIGH | INSHIGH NA | ||
| VO2a | FH− | 3.72 ± 0.57 | 3.74 ± 0.54 | 3.79 ± 0.63 | 3.74 ± 0.54 | 2.88 ± 0.57 | 3.85 ± 0.63 |
| FH+ | 4.20 ± 1.11 | 4.23 ± 0.99 | 4.37 ± 1.20 | 4.01 ± 0.90 | 4.27 ± 1.08 | 4.43 ± 1.23b | |
| VCO2a | FH− | 3.07 ± 0.45 | 3.05 ± 0.48 | 3.13 ± 0.51 | 3.15 ± 0.39 | 3.46 ± 0.48b,c | 3.53 ± 0.45b,d |
| FH+ | 3.49 ± 0.84 | 3.39 ± 0.72 | 3.47 ± 0.87 | 3.56 ± 0.87 | 3.88 ± 1.11 | 4.12 ± 1.41b,d | |
| RQ | FH− | 0.83 ± 0.06 | 0.82 ± 0.06 | 0.83 ± 0.06 | 0.85 ± 0.03 | 0.89 ± 0.06c | 0.92 ± 0.03b,d |
| FH+ | 0.84 ± 0.03 | 0.81 ± 0.03 | 0.80 ± 0.03 | 0.89 ± 0.06 | 0.91 ± 0.06c | 0.92 ± 0.06d | |
| CHOoxe | FH− | 11.4 ± 4.2 | 10.4 ± 4.5 | 11.5 ± 4.2 | 13.0 ± 2.4 | 18.2 ± 5.4b,c | 20.5 ± 3.6b,d |
| FH+ | 13.5 ± 3.3 | 10.5 ± 3.3 | 9.9 ± 2.7 | 18.4 ± 7.2f | 22.0 ± 10.2c | 25.1 ± 14.7d | |
| FAToxe | FH− | 3.8 ± 1.2 | 4.0 ± 1.2 | 3.9 ± 1.2 | 3.4 ± 1.2 | 2.5 ± 1.5 | 1.9 ± 1.5d |
| FH+ | 4.1 ± 1.8 | 4.8 ± 1.8 | 5.2 ± 2.1 | 2.6 ± 1.2 | 2.3 ± 1.5c | 1.8 ± 1.8d | |
Data are mean ± sd.
Expressed in ml/kg lean body mass/min.
P < 0.05 vs. baseline by ANOVA with Scheffe’s test.
P < 0.05 vs. INSLOW by ANOVA with Scheffe’s test.
P < 0.05 vs. INSLOW NA by ANOVA with Scheffe’s test.
Expressed in μmol/kg lean body mass/min.
P < 0.05 vs. FH− by ANOVA.
VO2 did not change from baseline during any of the experimental protocols in the FH− group but significantly increased from baseline at INSHIGH plus NA in FH+ subjects. VCO2 and respiratory quotient (RQ) increased at INSHIGH in both groups, especially with NA. At INSLOW, net CHOox did not change with HI infusion, with or without NA, but, in both groups, was significantly higher than baseline at INSHIGH. VO2, VCO2, RQ, and net CHOox and FATox were not significantly different between groups.
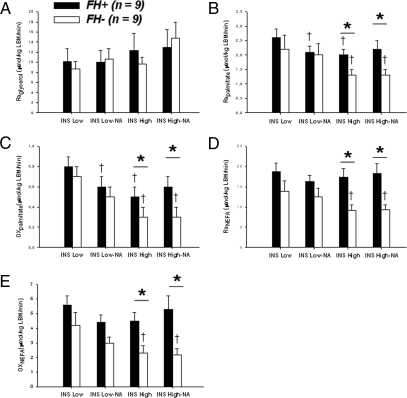
Glycerol and NEFA metabolic rates (Fig. 1)
Figure 1.
Raglycerol (A), Rapalmitate (B), and oxidation rate (Oxpalmitate) (C). Plasma NEFA appearance rate (RaNEFA) (D) and oxidation rate (OxNEFA) (E). †, P < 0.05 vs. INSLOW by ANOVA with Scheffe’s test. *, P < 0.05 vs. FH− by ANOVA. FH− (open bars). FH+ (closed bars). Data are mean ± se. LBM, Lean body mass.
Plasma glycerol enrichment (mass +5 tracer to tracee ratio) was similar throughout the experimental protocols in both groups (data not shown). Raglycerol corrected for exogenous free glycerol contained in Intralipid was also similar in both groups and was not significantly affected by either insulin or NA (Fig. 1A). Expressing Raglycerol per unit of nonlean body mass did not affect these results (data not shown).
In the FH− group, plasma palmitate enrichment (mass +16 tracer to tracee ratio) was 0.012 ± 0.006 on average and was similar throughout the experimental protocols (P = 0.19). In the FH+ group, plasma palmitate enrichment was lower during INSLOW without NA compared with the other experimental conditions (0.006 ± 0.003, 0.008 ± 0.003, 0.009 ± 0.003, and 0.008 ± 0.003 in INSLOW, INSLOW NA, INSHIGH, and INSHIGH NA respectively; P = 0.002). During INSHIGH, plasma palmitate enrichment was lower in FH+ (with or without NA; P = 0.07 and P = 0.05, respectively). At INSLOW, Rapalmitate was similar in both groups and tended to be lower after NA. At INSHIGH, Rapalmitate was significantly higher in FH+ with or without NA, and was lower than at INSLOW (Fig. 1B). Foxpalmitate was not significantly changed by insulin or NA and was similar in both groups (data not shown). During INSLOW, Oxpalmitate was similar in both groups but tended to be lower with NA. During INSHIGH, Oxpalmitate was significantly lower than during INSLOW, whereas NA blunted this difference in both groups. Oxpalmitate was significantly higher in FH+ subjects, but only during INSHIGH (Fig. 1C). In FH− subjects, RaNEFA and OxNEFA tended to be reduced by NA at INSLOW, and were significantly reduced by insulin with and without NA. Neither NA nor insulin significantly changed RaNEFA or OxNEFA in FH+ subjects. RaNEFA and OxNEFA were significantly higher in FH+ subjects during INSHIGH, but not during INSLOW (Fig. 1, D and E).
Effect of family history of type 2 diabetes on NEFA metabolism with and without adjustment for other potential confounding variables
During INSLOW, FH+ subjects had significantly higher NEFA (P = 0.03), but not higher RaNEFA or OxNEFA, an effect that was no longer significant after accounting for age or gender. During INSHIGH, the FH+ group had significantly higher NEFA (P = 0.02), higher RaNEFA (P = 0.005), and higher OxNEFA (P = 0.01) compared with the FH− group. These associations remained significant after correction for age, gender, body mass index (BMI), nonlean body mass, lean body mass, or waist circumference. Partial mismatch in gender between FH+ and FH− was unlikely to be responsible for the differences we observed at high insulin levels because plasma NEFA levels, RaNEFA and OxNEFA were not significantly different between men and women (data not shown). However, we found a significant inverse correlation between plasma NEFA level (r = −0.52; P = 0.03), RaNEFA (r = −0.51; P = 0.03), and OxNEFA (r = −0.47; P = 0.05), and insulin sensitivity determined during INSHIGH.
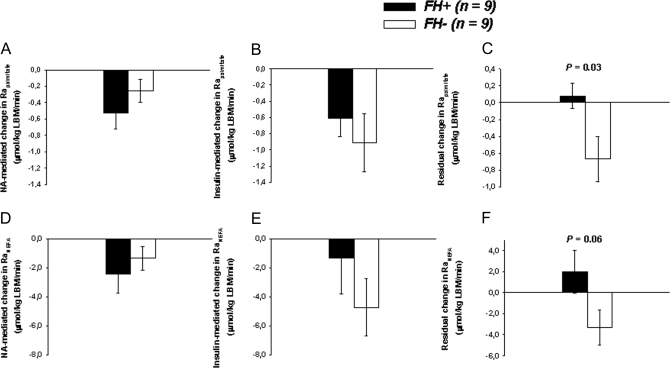
Comparison of the effects of insulin with and without NA on RaNEFA (Fig. 2)
Figure 2.
NA-mediated change (INSLOW NA minus INSLOW) (A), insulin-mediated change (INSHIGH minus INSLOW) (B), and insulin-mediated change during NA intake (INSHIGH NA minus INSLOW NA) (C) in Rapalmitate. NA-mediated change (D), insulin-mediated change (E), and insulin-mediated change during NA intake (residual) (F) in plasma NEFA appearance rate (RaNEFA). FH− (open bars). FH+ (closed bars). P values are from comparison of the two groups of participants by ANOVA. Data are mean ± se. LBM, Lean body mass.
Reduction of Rapalmitate and RaNEFA (Fig. 2, A and D, respectively) by NA at INSLOW was not significantly different between the FH+ and FH− groups. In the absence of NA, insulin-mediated reductions of Rapalmitate or RaNEFA (INSLOW minus INSHIGH) were also not significantly different between the two groups (Fig. 2, B and E, respectively). In contrast in FH−, insulin reduced Rapalmitate and RaNEFA in the presence of NA (INSLOW NA minus INSHIGH NA), but these effects were absent in FH+ (P = 0.03 and P = 0.06 for difference in change of Rapalmitate and RaNEFA, respectively, between FH+ vs. FH− subjects) (Fig. 2, C and F).
Discussion
We found that impaired suppression of systemic RaNEFA by insulin during enhanced intravascular TG lipolysis occurs before the development of overt hyperglycemia and hyperinsulinemia in the natural history of type 2 diabetes, and is independent of obesity. Our experimental protocol induced similar increases in intravascular TG lipolysis in both groups, but FH+ subjects displayed significantly higher NEFA levels during high insulin infusion, an effect due to an increase in RaNEFA. Increased RaNEFA was associated with higher OxNEFA independently of age, gender, or body composition. During INSLOW, although NEFA levels were also higher in FH+, the difference in RaNEFA and OxNEFA between FH+ and FH− was not significant. Thus, our results suggest that postprandial elevation of NEFA observed in FH+ subjects (11) may occur, at least in part, from failure of insulin to fully suppress systemic appearance of NEFA, a defect that appears to be independent of intravascular TG lipolysis. RaNEFA is elevated in the postprandial state in obese patients with type 2 diabetes compared with younger and leaner healthy individuals. Our results demonstrate that impaired suppression of systemic RaNEFA by insulin during enhanced intravascular TG lipolysis occurs before the development of overt hyperglycemia and hyperinsulinemia in the natural history of type 2 diabetes, and is independent of obesity. The results of the present study apply to individuals with BMIs ranging from 20.0–30.9 kg/m2.
Insulin-mediated reduction of RaNEFA during HI infusion can be totally accounted for by inhibition of intracellular adipose tissue lipolysis in FH+ but not in FH− subjects. Even after NEFA suppression by NA, a further insulin-mediated decline in RaNEFA still occurred in the FH− group. NA resulted in profound suppression of plasma NEFA levels (25) and intracellular lipolysis (17). Furthermore, NA-induced suppression of RaNEFA did not differ significantly between FH+ or FH− subjects. NA reduced RaNEFA through a mechanism independent of and competing with that of insulin (28). We have also shown previously that this HI iv infusion protocol resulted in steady-state plasma NEFA levels and kinetics between 90 and 240 min during infusion (22). Thus, we believe that suppression of intracellular lipolysis together with steady stimulation of intravascular lipolysis was achieved in the present protocol. Our results suggest that in the presence of experimentally increased intravascular TG lipolysis, some component of insulin-mediated reduction of RaNEFA in FH− subjects results from a mechanism other than suppression of adipose tissue lipolysis. Moreover, our data suggest that the observed increase in RaNEFA during hyperinsulinemia and HI infusion in FH+ subjects resulted, at least in part, from failure of insulin to act on this NEFA-suppressing mechanism. Direct adipose tissue uptake of NEFA was recently demonstrated in vivo in humans (21,29). As previously suggested (5), insulin-facilitated uptake of NEFA from intravascular TG lipolysis in the local microcirculation of adipose tissue (14) could possibly be impaired early on in the natural history of type 2 diabetes. Therefore, we propose that NEFA uptake by adipocytes is probably defective in FH+.
The increased oxidative disposal of NEFA in the FH+ group observed in the present study is also consistent with higher exposure for organs known to oxidize NEFA (e.g. liver, heart, and skeletal muscle) in subjects at high risk for developing type 2 diabetes. Increased postprandial deposition of dietary fatty acids in liver and skeletal muscle was previously reported in subjects with type 2 diabetes (30). In healthy subjects, dietary fatty acids start to appear in plasma TG within hours of oral intake (21,31,32). Indeed, a fraction of plasma NEFA derived from intravascular TG lipolysis is available in the systemic circulation during both the fasting and postprandial state (21,33,34). One potential mechanism by which this increased NEFA availability may occur is reduced insulin-mediated suppression of NEFA spillover from intravascular TG lipolysis during the postprandial state.
Despite higher OxNEFA in FH+, net FATox in our protocol was not different between the two groups. Thus, a larger fraction of the fatty acids oxidized in FH+ subjects during the postprandial state may come from the circulation, with consequent sparing of lean tissue intracellular stores of TG. Over time, this NEFA overload from plasma could potentially contribute to intramyocellular lipid accumulation, typical of insulin-resistant individuals and seen early in the offspring of type 2 diabetics (35). Such a defect in NEFA disposal in adipose tissues could contribute to the gradual development or maintenance of insulin resistance in muscle, and to the worsening of β-cell failure and glucose tolerance over time (5). It might also contribute to enhanced susceptibility to the lipid-induced β-cell failure observed in the offspring of type 2 diabetics (7). Others have shown that the insulin-resistant offspring of type 2 diabetics display impaired skeletal muscle oxidative metabolism during fasting (36), an effect associated with impaired muscle mitochondrial substrate oxidation (37). Thus, both impaired muscle oxidative metabolism together with altered RaNEFA regulation by insulin during the postprandial state possibly occur in the offspring of type 2 diabetics and predispose them to ectopic fat accumulation.
Partial mismatch of gender between FH+ and FH− subjects is a potential limitation of our study. Our heparin-Intralipid-insulin infusion model with hyperinsulinemia may also have caused a further increase in LPL activity in the adipose tissue microcirculation, an effect that could have been different in the FH+ vs. FH− subjects (38). However, we found no difference in Raglycerol after correction for exogenous glycerol infusion (a marker of total TG lipolysis) in FH+ or FH− subjects during the experimental protocols. We cannot totally exclude that higher plasma TG levels in FH+ subjects may have caused more intravascular TG lipolysis in the microcirculation of other tissues that can use glycerol, i.e. skeletal muscle, heart, or liver, an effect that would increase NEFA appearance in the face of similar glycerol appearance rates. However, this seems unlikely due to the net spillover of NEFA from adipose tissue, but not from muscle, in the postprandial state in healthy humans (21), suggesting that the most important source of NEFA spillover is adipose tissue. Nevertheless, this point suggests that lean tissue in FH+ subjects metabolizes more circulating NEFA despite possible overexposure to fatty acids produced from intravascular TG lipolysis in the local microcirculation.
The metabolic abnormalities observed in FH+ participants in our study were subtle and observed only at high insulin levels during iv infusion of HI. Of note, the FH+ subjects in our study tended to be more insulin resistant. The significant inverse correlations between plasma NEFA levels, RaNEFA, OxNEFA, and insulin sensitivity suggest that plasma NEFA intolerance occurs concomitantly with early decrease in insulin-mediated glucose disappearance. Whether NEFA intolerance occurs at this early stage under more physiological conditions (i.e. during the postprandial state) will require more studies.
In conclusion, under experimental hyperinsulinemia, we observed that nondiabetic offspring of type 2 diabetics have significantly increased RaNEFA and OxNEFA during stimulated intravascular TG lipolysis. Our results suggest that defective suppression of systemic NEFA spillover from intravascular TG lipolysis by insulin occurs early on in the natural history of type 2 diabetes and may contribute to ectopic fat storage in these individuals.
Acknowledgments
We thank Mr. Tommy Gagnon for performing gas chromatography-mass spectrometry measurements in this study.
Footnotes
This work was supported by a grant from the Canadian Institutes of Health Research (MOP 53094). Institutions involved in the present studies were supported by grants from the National Institutes of Health at the Biomedical Mass Spectrometry Resource (NIH NCRR-00954) and the Clinical Nutrition Research Unit (NIH P30-DK56341) of the Washington University School of Medicine, the Fonds de recherche en santé du Québec, and an institutional grant of the Centre de Recherche Clinique Étienne-Le Bel.
Disclosure Summary: A.C.C. was a new investigator of the Canadian Institutes of Health Research and is currently the recipient of a Fonds de recherche en santé du Québec Junior 2 Scholarship Award. P.B. is the recipient of the Schering-Université de Sherbrooke Department of Medicine Fellowship Award and a Fellowship from the Canadian Diabetes Association. S.C.C. holds the Canada Research Chair in Brain Metabolism and Aging, and his component of this project was supported by Natural Sciences and Engineering Research Council of Canada and the Canadian Foundation for Innovation. R.D. holds the Canada Research Chair in Genetics, Mutagenesis and Cancer. J-P.B. currently holds a Fonds de recherche en santé du Québec Junior 2 Scholarship Award.
First Published Online January 8, 2008
Abbreviations: BMI, Body mass index; CHOox, carbohydrate oxidation; FATox, fatty acid oxidation; FH+, family history of both parents with type 2 diabetes; FH−, no first-degree family history of type 2 diabetes; Foxpalmitate, fractional palmitate oxidation; HI, heparin plus Intralipid; INSHIGH, pancreatic clamp at hyperinsulinemia; INSLOW, pancreatic clamp at fasting plasma insulin level; LPL, lipoprotein lipase; NA, nicotinic acid; NEFA, nonesterified fatty acid; OxNEFA, plasma nonesterified fatty acid oxidation rate; Oxpalmitate, plasma palmitate oxidation rate; Raglycerol, plasma glycerol appearance rate; RaNEFA, plasma nonesterified fatty acid appearance rate; Rapalmitate, plasma palmitate appearance rate; RQ, respiratory quotient; TG, triacylglycerol; VCO2, CO2 excretion; VLDL, very low density lipoprotein; VO2, oxygen consumption.
References
- Boden G, Lebed B, Schatz M, Homko C, Lemieux S 2001 Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes 50:1612–1617 [DOI] [PubMed] [Google Scholar]
- Roden M, Krssak M, Stingl H, Gruber S, Hofer A, Furnsinn C, Moser E, Waldhausl W 1999 Rapid impairment of skeletal muscle glucose transport/phosphorylation by free fatty acids in humans. Diabetes 48:358–364 [DOI] [PubMed] [Google Scholar]
- Lam TK, Carpentier A, Lewis GF, van de Werve G, Fantus IG, Giacca A 2003 Mechanisms of the free fatty acid-induced increase in hepatic glucose production. Am J Physiol Endocrinol Metab 284:E863–E873 [DOI] [PubMed] [Google Scholar]
- Carpentier A, Mittelman SD, Lamarche B, Bergman RN, Giacca A, Lewis GF 1999 Acute enhancement of insulin secretion by FFA in humans is lost with prolonged FFA elevation. Am J Physiol 276(6 Pt 1):E1055–E1066 [DOI] [PubMed] [Google Scholar]
- Lewis GF, Carpentier A, Adeli K, Giacca A 2002 Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev 23:201–229 [DOI] [PubMed] [Google Scholar]
- Carpentier A, Zinman B, Leung N, Giacca A, Hanley AJ, Harris SB, Hegele RA, Lewis GF 2003 Free fatty acid-mediated impairment of glucose-stimulated insulin secretion in nondiabetic Oji-Cree individuals from the Sandy Lake community of Ontario, Canada: a population at very high risk for developing type 2 diabetes. Diabetes 52:1485–1495 [DOI] [PubMed] [Google Scholar]
- Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W, Bajaj M, Mandarino L, DeFronzo R, Cusi K 2003 A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes 52:2461–2474 [DOI] [PubMed] [Google Scholar]
- Storgaard H, Jensen CB, Vaag AA, Volund A, Madsbad S 2003 Insulin secretion after short- and long-term low-grade free fatty acid infusion in men with increased risk of developing type 2 diabetes. Metabolism 52:885–894 [DOI] [PubMed] [Google Scholar]
- Jensen CB, Storgaard H, Holst JJ, Dela F, Madsbad S, Vaag AA 2003 Insulin secretion and cellular glucose metabolism after prolonged low-grade Intralipid infusion in young men. J Clin Endocrinol Metab 88:2775–2783 [DOI] [PubMed] [Google Scholar]
- Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR 1992 Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet 340:925–929 [DOI] [PubMed] [Google Scholar]
- Axelsen M, Smith U, Eriksson JW, Taskinen MR, Jansson, PA 1999 Postprandial hypertriglyceridemia and insulin resistance in normoglycemic first-degree relatives of patients with type 2 diabetes. Ann Intern Med 131:27–31 [DOI] [PubMed] [Google Scholar]
- Fielding BA, Frayn KN 1998 Lipoprotein lipase and the disposition of dietary fatty acids. Br J Nutr 80:495–502 [DOI] [PubMed] [Google Scholar]
- Miles JM, Wooldridge D, Grellner WJ, Windsor S, Isley WL, Klein S, Harris WS 2003 Nocturnal and postprandial free fatty acid kinetics in normal and type 2 diabetic subjects: effects of insulin sensitization therapy. Diabetes 52:675–681 [DOI] [PubMed] [Google Scholar]
- Evans K, Clark ML, Frayn KN 1999 Effects of an oral and intravenous fat load on adipose tissue and forearm lipid metabolism. Am J Physiol 39(2 Pt 1):E241–E248 [DOI] [PubMed] [Google Scholar]
- American Diabetes Association 2004 Diagnosis and classification of diabetes mellitus. Diabetes Care 27(Suppl 1):S5–S10 [DOI] [PubMed] [Google Scholar]
- Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, Paffenbarger Jr RS 1985 Physical activity assessment methodology in the Five-City Project. Am J Epidemiol 121:91–106 [DOI] [PubMed] [Google Scholar]
- Carpentier A, Frisch F, Cyr D, Généreux P, Patterson BW, Giguère R, Baillargeon JP 2005 On the suppression of plasma nonesterified fatty acids by insulin during enhanced intravascular lipolysis in humans. Am J Physiol Endocrinol Metab 289:E849–E856 [DOI] [PubMed] [Google Scholar]
- Jensen MD, Caruso M, Heiling V, Miles JM 1989 Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes 38:1595–1601 [DOI] [PubMed] [Google Scholar]
- Gravholt CH, Moller N, Jensen MD, Christiansen JS, Schmitz O 2001 Physiological levels of glucagon do not influence lipolysis in abdominal adipose tissue as assessed by microdialysis. J Clin Endocrinol Metab 86:2085–2089 [DOI] [PubMed] [Google Scholar]
- Mittendorfer B, Liem O, Patterson BW, Miles JM, Klein S 2001 What does the measurement of whole-body fatty acid rate of appearance in plasma by using a fatty acid tracer really mean? Diabetes 52:1641–1648 [DOI] [PubMed] [Google Scholar]
- Bickerton AS, Roberts R, Fielding BA, Hodson L, Blaak EE, Wagenmakers AJ, Gilbert M, Karpe F, Frayn KN 2007 Preferential uptake of dietary fatty acids in adipose tissue and muscle in the postprandial period. Diabetes 56:168–176 [DOI] [PubMed] [Google Scholar]
- Carpentier AC, Frisch F, Brassard P, Lavoie F, Bourbonnais A, Cyr D, Giguère R, Baillargeon JP 2007 Mechanism of insulin-stimulated clearance of plasma nonesterified fatty acids in humans. Am J Physiol Endocrinol Metab 292:E693–E701 [DOI] [PubMed] [Google Scholar]
- Klein S, Young VR, Blackburn LA, Bistrian BR, Wolfe RR 1986 Palmitate and glycerol kinetics during brief starvation in normal weight young adult and elderly subjects. J Clin Invest 78:928–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe RR 1992 Measurement of substrate oxidation. In: Radioactive and stable isotope tracers in biomedicine: principles and practice of kinetic analysis. New-York: Wiley-Liss; 235–282 [Google Scholar]
- Boden G, Chen X, Iqbal N 1998 Acute lowering of plasma fatty acids lowers basal insulin secretion in diabetic and nondiabetic subjects. Diabetes 47:1609–1612 [DOI] [PubMed] [Google Scholar]
- Blaak EE, Aggel-Leijssen DP, Wagenmakers AJ, Saris WH, van Baak MA 2000 Impaired oxidation of plasma-derived fatty acids in type 2 diabetic subjects during moderate-intensity exercise. Diabetes 49:2102–2107 [DOI] [PubMed] [Google Scholar]
- Frayn KN 1983 Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55:628–634 [DOI] [PubMed] [Google Scholar]
- Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, Pfeffer K, Offermanns S 2003 PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med 9:352–355 [DOI] [PubMed] [Google Scholar]
- Shadid S, Koutsari C, Jensen MD 2007 Direct free fatty acid uptake into human adipocytes in vivo–relation to body fat distribution. Diabetes 56:1369–1375 [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Carey PE, Snaar JE, Deelchand DK, Cook DB, Neely RD, English PT, Firbank MJ, Morris PG, Taylor R 2005 Real-time assessment of postprandial fat storage in liver and skeletal muscle in health and type 2 diabetes. Am J Physiol Endocrinol Metab 288:E789–E797 [DOI] [PubMed] [Google Scholar]
- Barrows BR, Timlin MT, Parks EJ 2005 Spillover of dietary fatty acids and use of serum nonesterified fatty acids for the synthesis of VLDL-triacylglycerol under two different feeding regimens. Diabetes 54:2668–2673 [DOI] [PubMed] [Google Scholar]
- Heath RB, Karpe F, Milne RW, Burdge GC, Wootton SA, Frayn KN 2007 Dietary fatty acids make a rapid and substantial contribution to VLDL-triacylglycerol in the fed state. Am J Physiol Endocrinol Metab 292:E732–E739 [DOI] [PubMed] [Google Scholar]
- Miles JM, Park YS, Walewicz D, Russel-Lopez C, Windsor S, Isley WL, Coppack SW, Harris WS 2004 Systemic and forearm triglyceride metabolism: fate of lipoprotein lipase-generated glycerol and free fatty acids. Diabetes 53:521–527 [DOI] [PubMed] [Google Scholar]
- Roust LR, Jensen MD 1993 Postprandial free fatty acid kinetics are abnormal in upper body obesity. Diabetes 42:1567–1573 [DOI] [PubMed] [Google Scholar]
- Perseghin G, Scifo P, DeCobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, DelMaschio A, Luzi L 1999 Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans–a H-1-C-13 nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes 48:1600–1606 [DOI] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI 2004 Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350:664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Befroy DE, Falk PK, Dufour S, Mason GF, de Graaf RA, Rothman DL, Schulman GI 2007 Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes 56:1376–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panarotto D, Remillard P, Bouffard L, Maheux P 2002 Insulin resistance affects the regulation of lipoprotein lipase in the postprandial period and in an adipose tissue-specific manner. Eur J Clin Invest 32:84–92 [DOI] [PubMed] [Google Scholar]