Abstract
Context: Erythrocytosis is a dose-limiting adverse effect of testosterone therapy, especially in older men.
Objective: Our objective was to compare the dose-related changes in hemoglobin and hematocrit in young and older men and determine whether age-related differences in erythropoietic response to testosterone can be explained by changes in erythropoietin and soluble transferrin receptor (sTfR) levels.
Design: We conducted a secondary analysis of data from a testosterone dose-response study in young and older men who received long-acting GnRH agonist monthly plus one of five weekly doses of testosterone enanthate (25, 50, 125, 300, or 600 mg im) for 20 wk.
Setting: The study took place at a General Clinical Research Center.
Participants: Participants included 60 older men aged 60–75 yr and 61 young men aged 19–35 yr.
Outcome Measures: Outcome measures included hematocrit and hemoglobin and serum erythropoietin and sTfR levels.
Results: Hemoglobin and hematocrit increased significantly in a linear, dose-dependent fashion in both young and older men in response to graded doses of testosterone (P < 0.0001). The increases in hemoglobin and hematocrit were significantly greater in older than young men. There was no significant difference in percent change from baseline in erythropoietin or sTfR levels across groups in either young or older men. Changes in erythropoietin or sTfR levels were not significantly correlated with changes in total or free testosterone levels.
Conclusions: Testosterone has a dose-dependent stimulatory effect on erythropoiesis in men that is more pronounced in older men. The testosterone-induced rise in hemoglobin and hematocrit and age-related differences in response to testosterone therapy may be mediated by factors other than erythropoietin and sTfR.
Polycythemia is a dose-limiting adverse effect of testosterone therapy. This study shows testosterone treatment of older men has a greater dose-dependent effect on the hemoglobin and hematocrit than treatment in younger men. However, no changes in erythropoietin or soluble transferring receptor levels were found in older or younger men, suggesting an alternate mechanism for testosterone-related polycythemia.
Increase in hematocrit is a predictable effect of testosterone therapy (1,2,3,4,5,6). Erythrocytosis is the most frequently reported adverse event in testosterone trials (2). Anecdotally, older men appear more sensitive to the erythropoietic effects of testosterone than young men (7,8,9,10), although a head-to-head comparison of the effects of testosterone therapy on hematocrit in young and older men has not been conducted. In a recent comparison of the responsiveness of young and older men to graded doses of testosterone, a greater number of older men had to be discontinued from the trial because of an increase in hematocrit above 54% (11,12); in contrast, none of the young men experienced increase in hematocrit above 54% even at a 600-mg weekly dose of testosterone enanthate. Therefore, we conducted a secondary analysis of hemoglobin and hematocrit data from the testosterone dose-response study to determine whether older men were more sensitive to the erythropoietic effects of testosterone than young men.
The Endocrine Society Guideline on Androgen Deficiency Syndromes in Men recommended hematocrit monitoring 3 months after initiation of testosterone therapy and annually thereafter (1). However, in a number of participants in our dose- response study, the hematocrit continued to rise after 12 wk. Therefore, a second objective of our analyses was to evaluate the time course of hematocrit change after testosterone therapy.
The mechanisms by which testosterone stimulates erythropoiesis are poorly understood. It has been postulated that testosterone induces erythrocytosis by stimulating erythropoietin production (13,14). The effects of testosterone on erythropoietin levels have been inconsistent in previous studies (15,16). Testosterone also acts directly on the bone marrow, increasing the number of erythropoietin-responsive cells (5,17). The soluble transferrin receptor (sTfR), involved in the intracellular transport of iron, correlates directly with the degree of erythropoietic activity in the bone marrow (18), and sTfR levels have been used as a marker of erythropoietic activity (18,19). We determined whether the increases in hematocrit during testosterone therapy are associated with dose-related increases in erythropoietin and sTfR levels. We also assessed whether differences in hemoglobin and hematocrit responses in older and younger men receiving graded doses of testosterone enanthate could be explained by age-related differences in the changes in erythropoietin and sTfR.
Subjects and Methods
The hemoglobin and hematocrit responses to graded doses of testosterone enanthate were compared from a previous testosterone dose-response study in 61 young men, aged 18–35 yr (11,12), and 60 older men, aged 60–75 yr (11); the design and main findings of this study have been reported (11,12). This randomized, double-blinded study consisted of a 4-wk control period, a 20-wk treatment period, and a 16-wk recovery period. The study protocols were approved by the institutional review boards of Charles Drew University and Harbor-UCLA Research and Education Institute. Participants provided written, informed consent.
Exclusion criteria included a history of prostate cancer, prostate-specific antigen more than 4 ng/ml, a score of more than 7 on American Urologic Association prostatic symptom questionnaire, hematocrit greater than 48%, severe sleep apnea, diabetes mellitus, congestive heart failure, use of androgens, or participation in moderate to intense exercise training.
Participants were randomized to one of five groups to receive monthly injections of a GnRH agonist (leuprolide depot 7.5 mg; TAP, North Chicago, IL) to suppress endogenous gonadotropin production and weekly im injections of 25, 50, 125, 300, or 600 mg testosterone enanthate (Delatestryl; Savient, Iselin, NJ).
The Data and Safety Monitoring Board stopped the 600-mg testosterone enanthate dose group in December 2002 due to the number of adverse events in older men in this dose group. Subsequently, participants were randomized to the 25-, 50-, 125-, or 300-mg group.
Outcome measures
Total testosterone was measured by a RIA that has been validated against liquid chromatography tandem mass spectrometry (11). Free testosterone was measured by an equilibrium dialysis procedure that has a sensitivity of 0.22 pg/ml, and intra- and interassay coefficients of variation (CV) of 4.2 and 12.3%, respectively (20). Serum SHBG levels were measured by an immunofluorometric assay that has a sensitivity of 6.25 nmol/liter.
Quantikine ELISA (R&D Systems, Inc., Minneapolis, MN) was used to measure erythropoietin. This double-antibody sandwich method uses recombinant human erythropoietin as standard. The assay had a sensitivity of 0.6 mIU/ml with intra- and interassay CV of 2.1–3.1% and 3.7–4.2%, respectively.
sTfR concentrations were measured using a Quantikine ELISA (R&D Systems) based on the double-antibody sandwich method with a sensitivity 0.5 nmol/liter. The intra- and interassay CV are 0.3–2.5% and 0.7–3.8%, respectively.
Statistical analyses
All outcome variables were evaluated for distribution and homogeneity of variance; variables that did not meet the assumptions of homogeneity of variance or normal distribution were log-transformed. ANOVA was used to evaluate differences across dose groups stratified by age, younger vs. older. Changes within groups from baseline to treatment were evaluated with paired t tests. For determining statistical significance, α was set at 0.05. Data are presented as mean ± sem or mean percent change from baseline ± sem.
Results
Subjects
The characteristics of the participants have been described (11,12). Fifty-two young men of the 61 randomized and 52 older men of the 60 randomized completed the study. The reasons for treatment discontinuation have been described (11). Only subjects in whom hemoglobin, hematocrit, and testosterone data were available through wk 12 were included in the analysis of erythropoietin and sTfR.
Compliance
General Clinical Research Center staff administered all injections to assure compliance. All participants received all scheduled GnRH agonist injections. One participant in the 125-mg testosterone enanthate group missed one testosterone enanthate injection with an overall compliance rate of more than 99%.
Baseline characteristics
Baseline total and free testosterone, SHBG, hemoglobin, hematocrit, erythropoietin, or sTfR values did not differ among dose groups at baseline in either the young or older male group. However, older men had lower total and free testosterone and hemoglobin concentrations and higher SHBG and higher body weight and body mass index than young men, as reported (11).
Hormones
As reported (11), both total and free testosterone increased with testosterone enanthate dose and were significantly higher in older men than young men in the 125-, 300-, and 600-mg dose groups (P < 0.05).
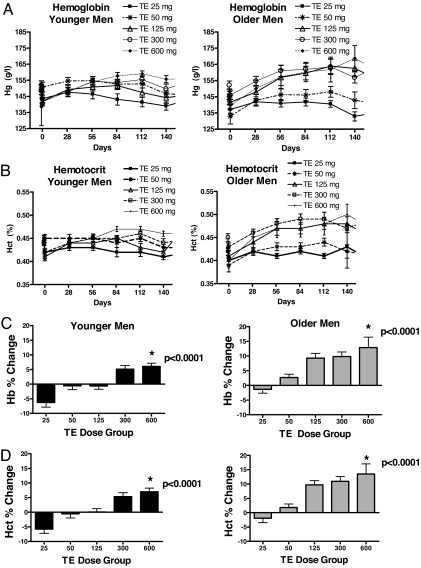
Hemoglobin and hematocrit (Fig. 1)
Figure 1.
A, Hemoglobin in young (left panel) and older (right panel) men during the 20-wk treatment period. Data are mean ± sem. Hg, Hemoglobin. B, Hematocrit in young (left panel) and older (right panel) men during the 20-wk treatment period. Data are mean ± sem. Hct, Hematocrit. C, Mean ± sem percent changes in hemoglobin (Hg) in young (left panel) and older (right panel) men during testosterone treatment. D, Mean ± sem percent changes in hematocrit in young (left panel) and older (right panel) men during testosterone treatment. TE, Testosterone enanthate.
Hemoglobin and hematocrit changed in a linear, dose-dependent fashion in both young and older men (P < 0.0001). Total and free testosterone concentrations during treatment were correlated significantly with changes in hemoglobin (total testosterone r = 0.57, P < 0.0001; free testosterone r = 0.52, P < 0.0001) and hematocrit (total testosterone r = 0.63, P < 0.0001; free testosterone r = 0.59, P < 0.0001).
Because older men had higher total and free testosterone levels than younger men, we assessed whether incremental increases in hemoglobin and hematocrit were greater in older men after adjusting for total and free testosterone levels. This analysis revealed that even after adjusting for total and free testosterone levels, the percent change in hemoglobin and hematocrit was significantly different in older and young men (P ≤ 0.0001 for each).
Changes in hematocrit also were correlated with treatment estradiol concentrations (Pearson correlation coefficient ρ = 0.48, P = 0.0003, for young men, and ρ= 0.68, P = 0.0001, for older men). However, estradiol concentrations were highly correlated with total testosterone concentrations (ρ for young men = 0.65, P < 0.0001; ρ for older men = 0.91, P < 0.0001). After adjusting for testosterone levels, the changes in hematocrit and hemoglobin were not significantly correlated with estradiol concentrations.
Time course of hemoglobin and hematocrit change (Fig. 1)
Hemoglobin and hematocrit started to increase within 1 month after treatment initiation and continued to increase in many subjects after wk 12. A majority of treatment discontinuations due to erythrocytosis occurred after wk 12. On average, the hematocrit in older men peaked later and at a higher level than younger men. Thirty-eight percent of older men in the 25-mg group, 58% in the 50-mg group, 75% in the 125-mg group, 85% in the 300-mg group, and 83% in the 600-mg group, respectively, achieved peak hematocrit levels after wk 12. Similarly, 42% of young men in the 125-mg group, 70% in the 300-mg group, and 85% in the 600-mg group achieved peak hematocrit levels after wk 12.
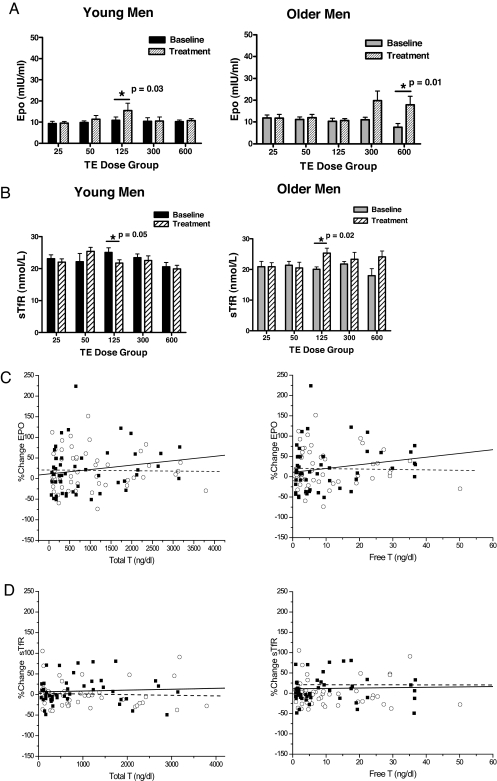
Erythropoietin levels
Changes in erythropoietin levels did not differ among the five dose groups in either young or older men (ANOVA, young men P = 0.71, older men P = 0.64, Fig. 2A). Serum erythropoietin levels were not significantly different between young and older men. The percent change in erythropoietin levels was also not significantly different between young and older men. There was no significant difference in percent change from baseline in erythropoietin levels across dose groups in either young or older men.
Figure 2.
A, Serum erythropoietin (Epo) levels in young (left panel) and older (right panel) men during the 20-wk treatment period. Data are mean ± sem. Overall ANOVA did not reveal an age or dose effect. B, Serum sTfR levels in young (left panel) and older (right panel) men during the 20-wk treatment period. Data are mean ± sem. Overall ANOVA did not reveal an age or dose effect. C, Changes in serum erythropoietin levels in young (○ with dashed line) and older men (▪ with solid line) are plotted against serum total testosterone (left panel) and free testosterone (right panel) levels during testosterone treatment. There was no significant correlation between total or free testosterone and change in erythropoietin levels in either young or older men. There was no age effect on the relationship between percent change in erythropoietin levels and testosterone levels. D, Changes in serum sTfR levels in young (○ with dashed line) and older men (▪ with solid line) are plotted against serum total testosterone (left panel) and free testosterone (right panel) levels during testosterone treatment. There was no significant correlation between total or free testosterone during treatment and change in StFR levels in either young or older men. There was no age effect on the relationship between percent change in sFtR levels and testosterone levels. T, Testosterone; TE, testosterone enanthate.
Total and free testosterone levels did not significantly correlate with erythropoietin levels at baseline in either young or older men. Also, total and free testosterone levels did not correlate significantly with changes in erythropoietin levels in young or older men (Fig. 2C).
sTfR levels
sTfR levels were not significantly different between young and older men at baseline. The changes in sTfR levels did not differ among the five dose groups either in young or in older men (ANOVA, young men P = 0.08, older men P = 0.054, Fig. 2B). The percent change in sTfR levels during treatment was not significantly different between the young and older men. There was no significant difference in percent change from baseline in serum sTfR levels across testosterone dose groups in either younger or older men (Fig. 2B).
Testosterone dose or serum total and free testosterone levels were not significantly correlated with changes in sTfR levels (Fig. 2D).
Discussion
Despite the strong association of testosterone dose and erythrocytosis, we found no dose-dependent effect of testosterone enanthate on erythropoietin or sTfR levels. Testosterone-associated increase in hemoglobin was not accompanied by a significant increase in erythropoietin or sTfR levels. Thus, the greater increase in hematocrit and hemoglobin observed in older men during testosterone therapy is not explained by the changes in erythropoietin and sTfR levels.
The stimulatory effects of testosterone on erythropoiesis are widely recognized (1,2,5,17). Androgens have been used to treat anemia of chronic renal failure (21). Erythrocytosis is the most frequent dose-limiting adverse event associated with testosterone therapy (1,2). Both testosterone dose and mode of delivery affect the magnitude of hematocrit elevation (1,2,3,12). Testosterone ester injections have been reported to be associated with a higher risk of erythrocytosis than oral or transdermal testosterone (3,7,8,22); however, it is unclear whether the higher frequency of erythrocytosis observed with injectable testosterone esters is due to the higher dose of testosterone delivered by the injections or the higher peaks of testosterone levels.
The role of erythropoietin in mediating testosterone-induced increase in hematocrit has remained unclear. Men and women have significant differences in testosterone as well as hematocrit levels; however, their erythropoietin levels are not significantly different (23). sTfR levels are also comparable in men and women (19). Dickerman et al. (15) observed that a majority of subjects with androgen-induced erythrocytosis had low rather than high erythropoietin levels. Earlier studies (24,25) of androgen therapy in aplastic anemia showed that androgens are effective despite markedly elevated levels of erythropoietin before therapy. Studies in patients with kidney disease also have found no erythropoietin response to androgen therapy (26). These data suggest that the androgen effects on erythropoiesis may be mediated through mechanisms other than erythropoietin and sTfR levels.
It is possible that testosterone stimulates erythropoiesis through a direct effect on the bone marrow hematopoietic stem cells (15,19); these direct erythropoietic effects involve IGF-I induction (19) through androgen receptor-mediated mechanisms (27). Androgens have been shown to stimulate erythroid colony-forming units in the bone marrow (13,28) and promote their differentiation into erythropoietin-responsive cells. Testosterone enhances intestinal iron absorption, iron incorporation in red blood cells, and hemoglobin synthesis (17). Androgen-treated men with end-stage renal disease have been reported to have longer erythrocyte survival and higher levels of 2,3- diphosphoglycerate than controls (5,29,30). The molecular mechanisms by which testosterone regulates erythropoiesis and that account for the age-related differences in androgen responsiveness need further investigation.
Our data confirm clinical experience that the frequency of erythrocytosis is greater in older men receiving testosterone therapy than in young men (11). The greater increments in hematocrit in older men may in part be due to the higher testosterone levels achieved in older men, but the age effect persisted even after adjusting for the higher total and free testosterone levels in older men. The mechanisms of this age-related difference in the erythropoietic response to testosterone are unknown. It is possible that aging is associated with increased production of an erythropoietic inhibitor and that testosterone inhibits the production of this inhibitor.
These data have implications for testosterone therapy of androgen deficiency syndromes in men. The Endocrine Society Guideline for Testosterone Therapy recommended hematocrit monitoring at baseline, 3 months, and annually thereafter (1). Our data show that hematocrits continue to increase in some men even after 3 months; additional hematocrit monitoring at 6 months is justified to detect further increases that may warrant treatment adjustment between 3 and 6 months.
Current screening strategies in the development of selective androgen receptor modulators (SARMs) favor the selection of SARMs that are anabolic on the skeletal muscle and that spare the prostate (31); however, erythrocytosis, the most frequent adverse event in androgen trials, has received inadequate attention (2). Although increase in hemoglobin might be beneficial in older men with anemia, the long-term health consequences of erythrocytosis are unknown. In epidemiological studies, high hematocrits are associated with increased risk of cerebrovascular disease and mortality (32). Clinical trials of erythropoietin in patients with end-stage renal disease suggest that higher hematocrit targets may be associated with greater frequency of cardiovascular events and mortality than lower targets (33,34). A similar systematic investigation of optimal hematocrit targets in androgen-deficient men receiving testosterone therapy is desirable. Also, further refinements of the strategies for screening of SARMs are needed to facilitate selection of molecules that have minimal effect on erythropoiesis.
Footnotes
This research was supported primarily by the National Institute of Aging Grant 1RO1AG14369-01. Additional support was provided by National Institute of Diabetes, Digestive and Kidney Diseases Grants 1RO1 DK59627, 2RO1 DK49296-08, and 1R01DK070534 (to S.B.) and Building Interdisciplinary Research Careers in Women’s Health Grant Award K12HD0434 (to A.D.C.).
Disclosure Statement: A.D.C., B.K., K.M.L., T.C., and A.B.S. have nothing to declare. S.B. has received a research grant from Solvay Pharmaceuticals and drug supplies for National Institutes of Health-funded studies.
First Published Online December 26, 2007
Abbreviations: CV, Coefficients of variation; SARM, selective androgen receptor modulator; sTfR, soluble transferrin receptor.
References
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM 2006 Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 91:1995–2010 [DOI] [PubMed] [Google Scholar]
- Calof O, Singh AB, Lee ML, Urban RJ, Kenny AM, Tenover JL, Bhasin S 2005 Adverse events associated with testosterone supplementation of older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Med Sci 60:1451–1457 [DOI] [PubMed] [Google Scholar]
- Jockenhovel F, Vogel E, Reinhardt W, Reinwein D 1997 Effects of various modes of androgen substitution therapy on erythropoiesis. Eur J Med Res 2:293–298 [PubMed] [Google Scholar]
- Basaria S, Dobs AS 1999 Risks versus benefits of testosterone therapy in elderly men. Drugs Aging 15:131–142 [DOI] [PubMed] [Google Scholar]
- Molinari PF 1982 Erythropoietic mechanisms of androgens: a critical review and clinical implications. Haematologica 67:442–460 [PubMed] [Google Scholar]
- Viallard JF, Marit G, Mercie P, Leng B, Reiffers J, Pellegrin JL 2000 Polycythaemia as a complication of transdermal testosterone therapy. Br J Haematol 110:237–238 [DOI] [PubMed] [Google Scholar]
- Sih R, Morley JE, Kaiser FE, Perry 3rd HM, Patrick P, Ross C 1997 Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab 82:1661–1667 [DOI] [PubMed] [Google Scholar]
- Dobs AS, Meikle AW, Arver S, Sanders SW, Caramelli KE, Mazer NA 1999 Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab 84:3469–3478 [DOI] [PubMed] [Google Scholar]
- Hajjar RR, Kaiser FE, Morley JE 1997 Outcomes of long-term testosterone replacement in older hypogonadal males: a retrospective analysis. J Clin Endocrinol Metab 82:3793–3796 [DOI] [PubMed] [Google Scholar]
- Wang C, Swedloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G, Matsumoto AM, Weber T, Berman N 2000 Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. Testosterone Gel Study Group. J Clin Endocrinol Metab 85:2839–2853 [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, Yarasheski KE, Sinha-Hikim I, Dzekov C, Dzekov J, Magliano L, Storer TW 2005 Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab 90:678–688 [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, Chen X, Yarasheski KE, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha-Hikim I, Shen R, Storer TW 2001 Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab 281:E1172–E1181 [DOI] [PubMed] [Google Scholar]
- Malgor LA, Valsecia M, Verges E, De Markowsky EE 1998 Blockade of the in vitro effects of testosterone and erythropoietin on Cfu-E and Bfu-E proliferation by pretreatment of the donor rats with cyproterone and flutamide. Acta Physiol Pharmacol Ther Latinoam 48:99–105 [PubMed] [Google Scholar]
- Rishpon-Meyerstein N, Kilbridge T, Simone J, Fried W 1968 The effect of testosterone on erythropoietin levels in anemic patients. Blood 31:453–460 [PubMed] [Google Scholar]
- Dickerman RD, Pertusi R, Miller J, Zachariah NY 1999 Androgen-induced erythrocytosis: is it erythropoietin? Am J Hematol 61:154–155 [DOI] [PubMed] [Google Scholar]
- Alexanian R 1969 Erythropoietin and erythropoiesis in anemic man following androgens. Blood 33:564–572 [PubMed] [Google Scholar]
- Naets JP, Wittek M 1968 The mechanism of action of androgens on erythropoiesis. Ann NY Acad Sci 149:366–376 [DOI] [PubMed] [Google Scholar]
- Beguin Y 2003 Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin Chim Acta 329:9–22 [DOI] [PubMed] [Google Scholar]
- T’Sjoen GG, Beguin Y, Feyen E, Rubens R, Kaufman JM, Gooren L 2005 Influence of exogenous oestrogen or (anti-) androgen administration on soluble transferrin receptor in human plasma. J Endocrinol 186:61–67 [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I, Arver S, Beall G, Shen R, Guerrero M, Sattler F, Shikuma C, Nelson JC, Landgren BM, Mazer NA, Bhasin S 1998 The use of a sensitive equilibrium dialysis method for the measurement of free testosterone levels in healthy, cycling women and in human immunodeficiency virus-infected women. J Clin Endocrinol Metab 83:1312–1318 [DOI] [PubMed] [Google Scholar]
- Handelsman DJ, Liu PY 1998 Androgen therapy in chronic renal failure. Baillieres Clin Endocrinol Metab 12:485–500 [DOI] [PubMed] [Google Scholar]
- Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL 2005 Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab 90:1502–1510 [DOI] [PubMed] [Google Scholar]
- Miller ME, Chandra M, Garcia JF 1985 Clinical applications of measurement of serum immunoreactive levels of erythropoietin. Ann NY Acad Sci 459:375–381 [DOI] [PubMed] [Google Scholar]
- Sanchez-Medal L, Gomez-Leal A, Duarte L, Guadalupe Rico M 1969 Anabolic androgenic steroids in the treatment of acquired aplastic anemia. Blood 34:283–300 [PubMed] [Google Scholar]
- Shahidi NT, Diamond LK 1961 Testosterone-induced remission in aplastic anemia of both acquired and congenital types. Further observations in 24 cases. N Engl J Med 264:953–967 [DOI] [PubMed] [Google Scholar]
- Raich PC, Korst DR 1978 Plasma erythropoietin levels in patients undergoing long-term hemodialysis. Arch Pathol Lab Med 102:73–75 [PubMed] [Google Scholar]
- Claustres M, Sultan C 1988 Androgen and erythropoiesis: evidence for an androgen receptor in erythroblasts from human bone marrow cultures. Horm Res 29:17–22 [DOI] [PubMed] [Google Scholar]
- Moriyama Y, Fisher JW 1975 Increase in erythroid colony formation in rabbits following the administration of testosterone. Proc Soc Exp Biol 149:178–180 [DOI] [PubMed] [Google Scholar]
- Goodman J, Bessman AN 1975 Effect of nortestosterone decanoate on red cell 2,3 diphosphoglycerate and hematocrit in hemodialysis patients. Clin Pharmacol Ther 17:167–170 [DOI] [PubMed] [Google Scholar]
- Solomon LR, Hendler ED 1987 Androgen therapy in haemodialysis patients. II. Effects on red cell metabolism. Br J Haematol 65:223–230 [DOI] [PubMed] [Google Scholar]
- Bhasin S, Calof OM, Storer TW, Lee ML, Mazer NA, Jasuja R, Montori VM, Gao W, Dalton JT 2006 Drug insight: testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat Clin Pract 2:146–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carallo C, Pujia A, Irace C, De Franceschi MS, Motti C, Gnasso A 1998 Whole blood viscosity and haematocrit are associated with internal carotid atherosclerosis in men. Coron Artery Dis 9:113–117 [PubMed] [Google Scholar]
- Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D 2006 Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355:2085–2098 [DOI] [PubMed] [Google Scholar]
- Drueke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A 2006 Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355:2071–2084 [DOI] [PubMed] [Google Scholar]