Abstract
Purpose: Protein kinase A (PKA) affects cell proliferation in many cell types and is a potential target for cancer treatment. PKA activity is stimulated by cAMP and cAMP analogs. One such substance, 8-Cl-cAMP, and its metabolite 8-Cl-adenosine (8-Cl-ADO) are known inhibitors of cancer cell proliferation; however, their mechanism of action is controversial. We have investigated the antiproliferative effects of 8-Cl-cAMP and 8-CL-ADO on human thyroid cancer cells and determined PKA’s involvement.
Experimental Design: We employed proliferation and apoptosis assays and PKA activity and cell cycle analysis to understand the effect of 8-Cl-ADO and 8-Cl-cAMP on human thyroid cancer and HeLa cell lines.
Results: 8-Cl-ADO inhibited proliferation of all cells, an effect that lasted for at least 4 d. Proliferation was also inhibited by 8-Cl-cAMP, but this inhibition was reduced by 3-isobutyl-1-methylxanthine; both drugs stimulated apoptosis, and 3-isobutyl-1-methylxanthine drastically reduced 8-Cl-cAMP-induced cell death. 8-Cl-ADO induced cell accumulation in G1/S or G2/M cell cycle phases and differentially altered PKA activity and subunit levels. PKA stimulation or inhibition and adenosine receptor agonists or antagonists did not significantly affect proliferation.
Conclusions: 8-Cl-ADO and 8-Cl-cAMP inhibit proliferation, induce cell cycle phase accumulation, and stimulate apoptosis in thyroid cancer cells. The effect of 8-Cl-cAMP is likely due to its metabolite 8-Cl-ADO, and PKA does not appear to have direct involvement in the inhibition of proliferation by 8-Cl-ADO. 8-Cl-ADO may be a useful therapeutic agent to be explored in aggressive thyroid cancer.
In thyroid cancer cells, 8-Cl-cAMP and its metabolite 8-Cl-adenosine inhibit proliferation, induce cell cycle phase accumulation, and stimulate apoptosis, all without the involvement of protein kinase A, making these cAMP analogues possible therapeutic agents for treating aggressive thyroid cancer.
Thyroid cancer is the most prevalent endocrine malignancy. Although differentiated thyroid cancer has generally good prognosis, a subset of thyroid carcinomas are highly aggressive and respond poorly to available treatment (1,2,3,4,5). New therapies for metastatic, poorly differentiated, and undifferentiated thyroid cancer are needed.
Signals mediated by cAMP and protein kinase A (PKA) have been implicated in the control of cell proliferation in many cell types (6,7). The PKA holoenzyme consists of a heterotetramer of two homodimers of the regulatory subunits (R-subunits, RIα or RIβ and RIIα or RIIβ) and two catalytic subunits (Cα, Cβ, or Cγ) (8), resulting in the formation of two isozymes, type I (RI) and type II (RII). Binding of cAMP to the regulatory subunits releases the catalytic subunits, which act as serine threonine kinases and phosphorylate target molecules to control a variety of cellular functions (6,9,10).
Site-selective cAMP analogs induce growth inhibition in a variety of cancer cell types. Among the cAMP analogs initially tested, the 8-Cl derivative of cAMP (8-Cl-cAMP) was the most potent (11). Although evidence of cellular toxicity was consistently present, conflicting views arose as to the mechanism of its action. One group of studies suggested that the inhibitory effect of 8-Cl-cAMP involves its binding to R-subunits of PKA to modulate both type I and type II PKA isozymes, to reduce the RI to RII subunit ratio (11,12,13). Other studies provided evidence that 8-Cl-cAMP elicits antiproliferative effects via its extracellular conversion to a dephosphorylated metabolic breakdown product, 8-Cl-adenosine (8-Cl-ADO), by serum phosphodiesterase and 5′-nucleotidase (14,15,16,17,18,19). The intracellular level of 8-Cl-ADO, however, depends on the activity of two cellular enzymes: adenosine deaminase (Km = 17 μm), which deaminates 8-Cl-ADO to an inactive 8-Cl-inosine, and adenosine kinase (Km = 2 μm), which phosphorylates 8-Cl-ADO to an active 5′,8-chloro-AMP. Higher amounts of 5′,8-chloro-AMP are present in the cell due to the lower Km of adenosine kinase (20). Some reports suggest that 8-Cl-ADO acts through the inhibition of DNA and/or RNA polymerase (14,16) or by premature RNA chain termination (21). Finally, others show that the inhibitory effects of both 8-Cl-cAMP (22) and 8-Cl-ADO (23,24) result in apoptosis; in cells treated with 8-Cl-cAMP, apoptosis is thought to occur only in cells that accumulate in G2/M cell cycle phase (22), whereas cells treated with 8-Cl-ADO are thought to accumulate in the G0/G1 phase without induction of apoptosis (25).
In the present investigation, we studied three human thyroid cancer cell lines: poorly differentiated carcinoma (NPA), follicular carcinoma (WRO), and undifferentiated (anaplastic) cancer (ARO). As a reference cell line, we used HeLa cells. We studied the effect of 8-Cl-cAMP and 8-Cl-ADO on [3H]thymidine incorporation, cell proliferation/metabolism, the cell cycle, and apoptosis. We assessed whether the effect of 8-Cl-cAMP is due to its metabolite 8-Cl-ADO and whether the effect of both drugs is due to an action on A1, A2a and -b, and A3 adenosine surface receptors (17). We also assessed the enzymatic activity of the PKA holoenzyme and the individual PKA subunits in relationship to 8-Cl-ADO. Our data indicated that 8-Cl-cAMP through 8-Cl-ADO inhibited cell proliferation/ metabolism and induced thyroid cell accumulation in the G1/S or G2/M cell cycle phases and apoptosis. There was no apparent involvement of adenosine receptors and no direct involvement of the PKA enzyme and/or R-subunits in the above actions. The data suggest that 8-Cl-ADO (or 8-Cl-cAMP) may be a potential chemotherapeutic agent for the treatment of aggressive thyroid cancer, and its effects will not depend on PKA signaling.
Materials and Methods
Cell lines and other materials
Thyroid cancer cell lines have been extensively described elsewhere (26,27). HeLa cells were from American Type Tissue Collection (Rockville, MD); RPMI 1640 media, AIM V media, fetal bovine serum (FBS; GIBCO/Invitrogen, Carlsbad, CA), and anti-bromodeoxyuridine (anti-BrdU)-fluorescein isothiocyanate (FITC) monoclonal antibody (mAb) from Invitrogen (Carlsbad, CA); FBS from Hyclone (Logan, UT); forskolin, isoproterenol, 8-Cl-cAMP, 8-Br-cAMP, 3-propyl-6-ethyl-5-[(ethylthio)carbonyl]-2-phenyl-4-propyl-3-pyridinecarboxylate (MRS1523), N-ethyl-carbomidoadenosine (NECA), adenosine, xanthine amine congener (XAC), 3-isobutyl-1-methylxanthine (IBMX), and LY294002 from SigmaAldrich (St. Louis, MO); 8-Cl-adenosine from the NIH/NCI (Bethesda, MD); protein kinase inhibitor (PKI) and Cell Titer 96 AQ assay 3-(4,5-dimethylthiazol-2yl)-5-(3-carboxymethoxyphenol)-2-(4-sulfophenyl)-2H-tetrazolium, salt (MTS) assay] from Promega (Madison WI); annexin V-FITC, 7-amino-actinomycin D (7AAD) from BD Pharmingen (San Diego, CA); PKA assay kit and [γ−32P]triphosphate from Amersham Pharmacia (Piscataway, NJ); [3H]thymidine from MP Biomedicals (Solon, OH); and FACScalibur flow cytometer and Cell Quest software from Becton Dickinson (San Jose, CA).
Cell culture and plating
Cells were grown in RPMI 1640 media with 1% l-glutamine, 10% FBS, and 0.05% gentamicin. Population doubling times were as follows: ARO, 24 h; WRO, 36 h; HeLa, 77 h; and NPA, 79 h. Cells were plated as follows: in 24-well plates (2 × 104 cells/ml) or in 96-well plates (1 to 2.5 × 103 cells/well) for [3H]thymidine and MTS assays, respectively; in six-well plates (3 × 104 cells/ml) for apoptosis experiments; in 175-cm2 flasks (1 × 106 cells/ml) for PKA assays and gel electrophoresis; and in 75-cm2 flasks (2 × 105 cells/10 ml) for cell cycle analysis. In all experiments, cells were allowed to attach overnight before the addition of drugs.
Cell proliferation
Cell proliferation was determined by [3H]thymidine incorporation or MTS assay. Cells, incubated with media alone or with media, drugs, and 2 μCi/well [3H]thymidine, were washed with PBS (pH 7.4), and 10% TCA (23 C) added. Proteins were solubilized with 0.5 N NaOH and [3H]thymidine detected by scintillation count. For MTS assays, cells were incubated with drugs, and Cell Titer 96 AQ solution was added (37 C) before determining the absorbance at 490 nm.
Quantitation of apoptosis
Apoptosis was quantified as reported previously (10) and is the percentage of cells that excluded 7AAD and exteriorized membrane phosphatidylserine, detected by the binding of annexin V-FITC (28). Briefly, cultures treated with drugs were washed, resuspended in binding buffer, labeled with 7AAD and annexin V-FITC, and incubated, and a minimum of 20,000 events were analyzed by flow cytometry (Cell Quest software). Apoptotic cells were stained by annexin V-FITC; late apoptotic/necrotic cells were stained by both annexin V-FITC and 7AAD.
Cell cycle analysis
Cell cycle analysis was performed as previously described (10). Briefly, cells were synchronized in low-serum medium and released from synchronization with 10% FBS/RPMI. BrdU was added 1 h before each time point. After 1 h, cells were collected, washed, and resuspended in PBS followed by ethanol. Samples were centrifuged and washed with HCl/Triton X-100, resuspended in H2O, and boiled. PBS/Triton X-100 was added followed by centrifugation. Anti-BrdU-FITC mAb was added, and samples were kept in the dark. Propidium iodide was added, and a minimum of 20,000 events were analyzed by flow cytometer using CellQuest software.
Gel electrophoresis
Briefly, proteins in whole-cell lysates were separated by SDS-PAGE on 10% Tris-glycine gels (7). Proteins, transferred to nitrocellulose membranes, were probed with primary antibodies to PKA R-subunit proteins, followed by secondary antibodies against mouse or rabbit IgG. Bands were detected by ECL reagent and quantitated by densitometer scanning (Molecular Dynamics, Sunnyvale, CA). Equal sample loading was confirmed, and arbitrary values were calculated when blots were stripped and reprobed with β-actin mAb.
PKA activity determinations
PKA activity was measured as described previously (29), using [γ-32P]triphosphate, in cultures that were lysed in a PKA extraction buffer [2.5 mm Tris-HCl (pH 7.4), 0.5 mm EDTA, 0.5 mm EGTA, 10 mm β-mercaptoethanol]. Extracts were exposed to cAMP or cAMP and PKI before determination of PKA activity. All determinations were performed twice for each sample and corrected for protein content.
Statistics
Results were analyzed by ANOVA using the PROC Mixed procedure of the Statistical Analysis System (SAS Institute, Cary, NC). When more than two groups were compared, the PDIFF procedure (SAS Institute) was used to compare differences between treatment means. Differences were considered significant at P < 0.05.
Results
8-Cl-ADO inhibits cell proliferation
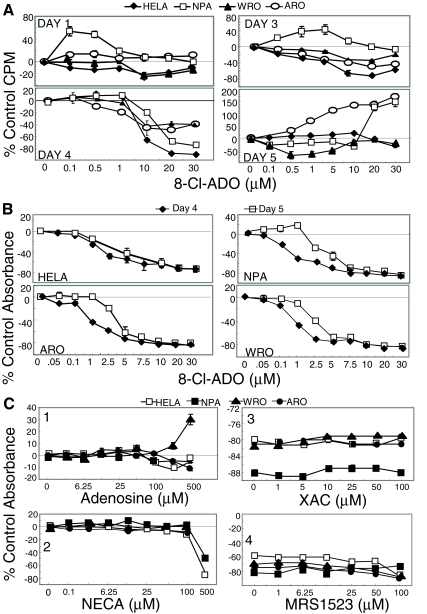
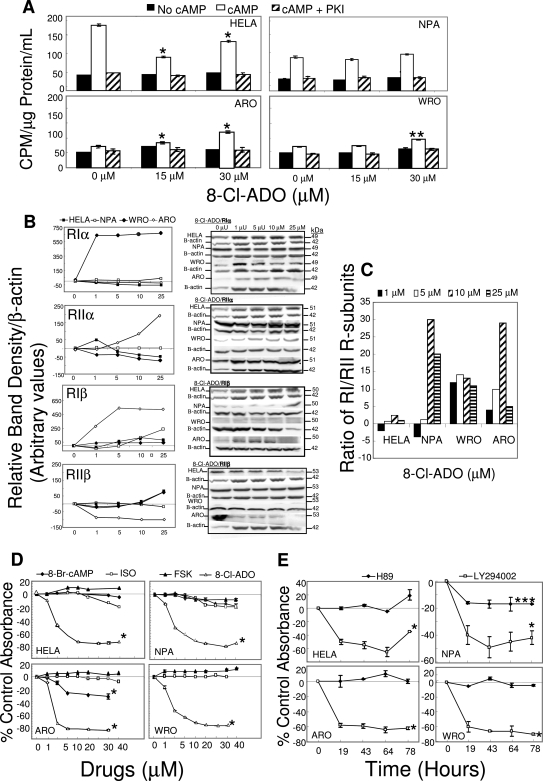
Cells were cultured with 8-Cl-ADO and [3H]thymidine (Fig. 1A) or with 8-Cl-ADO alone in cell metabolism studies (Fig. 1B). 8-Cl-ADO inhibited [3H]thymidine incorporation in all cells in a time and concentration-dependent manner. Cell numbers used are based on cell-titration experiments. A maximal inhibitory effect in the MTS assay was observed in a 4-d incubation period (data not shown). Under these conditions, optimal inhibition occurred on d 4 (to 90%, Fig. 1A; to 82%, Fig. 1B). Curves were biphasic (Fig. 1A and 0–7.5 μm 8-Cl-ADO, Fig. 1B) and inhibition was decreased on d 5. Curves (Fig. 1B) were statistically different for thyroid cells (P < 0.0001) only. High levels of inhibition occurred with both assays, with a reversal of the inhibition on d 5. Cells (Fig. 1C) were incubated (4 d) with the A1, A2, and A3 adenosine receptor agonist adenosine; the A1, A2, adenosine receptor agonist NECA; the A1, A2a, andA2b adenosine receptor antagonist XAC; or the A3 adenosine receptor antagonist MRS1523 (panels 1–4, respectively) and 20 μm 8-Cl-ADO (panels 3 and 4). The adenosine receptor agonists (except NECA at a toxic concentration, 500 μm) did not inhibit cell metabolism, and no reversal of the inhibition by 8-Cl-ADO occurred with the adenosine receptor antagonists. These data exclude the possibility that 8-Cl-ADO’s effect is by an effect on adenosine receptors.
Figure 1.
8-Cl-ADO inhibits [3H]thymidine uptake and cell metabolism in thyroid cancer cells and in HeLa cells, with no effect on adenosine receptors. A, 8-Cl-ADO inhibits [3H]thymidine uptake in a time- and concentration-dependent manner. Cells in 24-well culture plates were incubated for 1–5 d with increasing 8-Cl-ADO concentrations (0–30 μm), in the presence of 2 μCi [3H]thymidine per well. Counts per minute (CPM) were determined by scintillation count, and values were calculated as percent of control counts per minute. One representative experiment is shown. B, Cells in 96-well culture plates were incubated for 4 and 5 d with 0–30 μm 8-Cl-ADO. C, Cells in 96-well culture plates were incubated for 4 d with adenosine (0–500 μm, panel 1), NECA (0–500 μm, panel 2), XAC (0–100 μm) plus 20 μm 8-Cl-ADO or 8-Cl-ADO alone (panel 3), and MRS1523 (0–100 μm) plus 20 μm 8-Cl-ADO, or 8-Cl-ADO alone (panel 4). In B and C, Cell Titer 96 AQ solution was added 3.5 h before plates were read using an ELISA reader at 460 nm. Results in B and C are percentage of control absorbance. In C (panels 3 and 4), zero represents 20 μm 8-Cl-ADO alone. Points are mean ± sem of four wells (A), mean ± sem of four experiments (B), and mean ± sem of three experiments (C); P < 0.0001 (B).
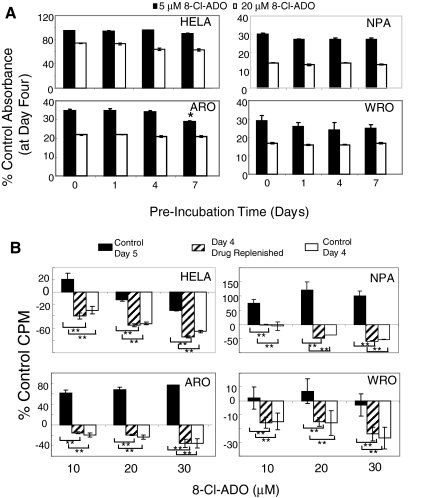
8-Cl-ADO undergoes intracellular metabolism
We preincubated 8-Cl-ADO in complete media (to 7 d; 37 C) without cells, followed by incubation with cells (4 d in the same solution; Fig. 2A). No significant effect on the inhibition of cell metabolism by preincubated 8-Cl-ADO was observed in all cell types, except ARO (−14%, 5 μm, 7 d; P = 0.032). Thus, the reduced inhibition on d 5 (Fig. 1, A and B) is not due to extracellular drug metabolism. Cells were incubated with 8-Cl-ADO for 4 and 5 d (control d 4 and 5), or the drug was replenished on d 4 (d 4 drug replenished) and [3H]thymidine incorporation determined on d 5 (Fig. 2B). In all cells, decreased inhibition occurred on control d 5 (P < 0.0001) compared with control d 4. In cultures where drug was replenished on d 4 and assayed on d 5, the inhibition of control d 4 was maintained. These data suggest an intracellular mechanism of drug metabolism.
Figure 2.
Loss of inhibition with 8-Cl-ADO with prolonged incubation time: intracellular drug metabolism. A, 8-Cl-ADO (5 and 20 μm) was preincubated (minus cells) for 0–7 d at 37 C before its addition to cells for an additional 4 d; B, cells in 24-well culture plates were incubated with 8-Cl-ADO (20 μm) and [3H]thymidine (2 μCi per well) for 4 and 5 d. In some cultures, 8-Cl-ADO was replenished on d 4 and cultures assayed on d 5. Levels of intranuclear counts per minute (CPM) were determined by scintillation count, and values expressed as percent of control counts per minute. In A, Cell Titer 96 AQ solution was added 3.5 h before plates were read using an ELISA plate reader. Results are expressed as percentage of control absorbance. Points are mean ± sd of two experiments (A) and mean ± sem of three experiments (B); *, P = 0.032 (A); **, P < 0.0001 (B).
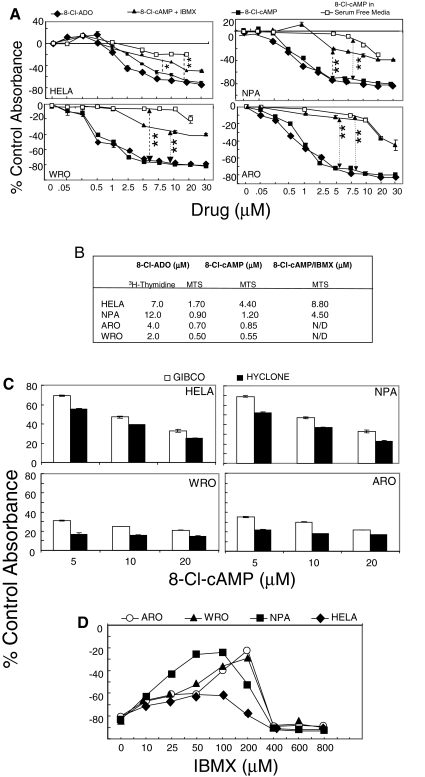
8-Cl-cAMP is metabolized to 8-Cl-ADO
Cells were incubated (4 d) with 8-Cl-ADO, 8-Cl-cAMP, and 8-Cl-cAMP plus the phosphodiesterase inhibitor IBMX (50 μm) in complete media containing Hyclone serum and 8-Cl-cAMP in AIM V serum-free media (Fig. 3A). Although for 8-Cl-ADO, IC50 values were greater in [3H]thymidine than in MTS assays, in MTS assays, 8-Cl-cAMP inhibited cell metabolism at IC50 values similar to that of 8-Cl-ADO (see IC50 values, Fig. 3B). However, the inhibition by 8-Cl-cAMP was greatly reduced by IBMX (to 62%, P < 0.0001–0.01) and further reduced in serum-free media (to 75%, P < 0.0001; Fig. 3A). No inhibition occurred with IBMX alone, and IBMX did not alter the inhibition by 8-Cl-ADO (data not shown). The level of inhibition by 8-Cl-cAMP also depended on the source of FBS (e.g. Hyclone vs. GIBCO), with media containing Hyclone serum producing the greatest inhibition by 8-Cl-cAMP (Fig. 3C). Accordingly, IBMX showed a decrease in the inhibition (20–72%, HeLa to ARO cells, respectively) by 8-Cl-cAMP (25 μm; Fig. 3D). A sharp decline was observed in the recovery from inhibition, likely due to toxicity at high IBMX concentrations (400–800 μm). Therefore, the data suggest that 1) 8-Cl-cAMP is metabolized to 8-Cl-ADO and 2) the inhibitory effect of 8-Cl-cAMP is due to its by-product 8-Cl-ADO.
Figure 3.
Metabolism of 8-Cl-cAMP to 8-Cl-ADO: effect of IBMX and serum-free media on extracellular drug metabolism. A, Cells in 96-well culture plates were incubated with increasing concentrations (0–30 μm) of 8-Cl-ADO, 8-Cl-cAMP, or 8-Cl-cAMP plus IBMX (50 μm) in media containing 10% FBS (Hyclone). Cells were also incubated with 8-Cl-cAMP in serum-free medium. All cultures were incubated for 4 d. B, List of IC50 values from [3H]thymidine and MTS assays. C, Cells in 96-well culture plates were incubated (4 d) with increasing concentrations of 8-Cl-cAMP in media containing serum from GIBCO/Invitrogen or from Hyclone. D, Cells in 96-well culture plates were incubated (4 d) with increasing concentrations of IBMX (0–800 μm) and/or 8-Cl-cAMP (25 μm). In A, C, and D, Cell Titer 96 AQ solution was added for 3.5 h before plates were read using an ELISA plate reader at 460 nm. Results are expressed as percentage of control absorbance and are mean ± sem of six experiments (A) and mean ± sd of two experiments (C and D). *, P = 0.01; **, P < 0.0001 (A).
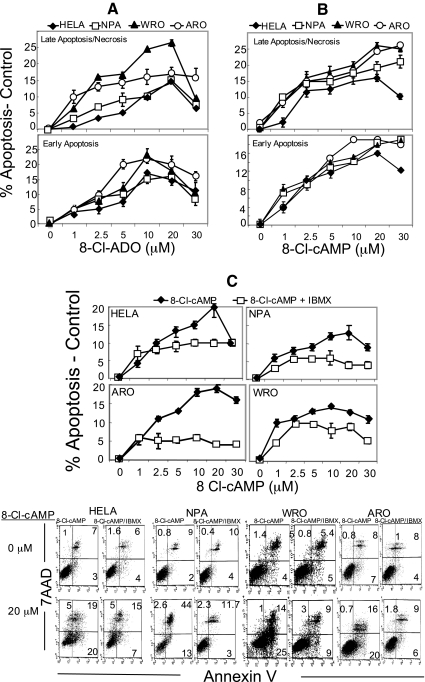
8-Cl-ADO and 8-Cl-cAMP induce apoptosis
Cells were incubated (4 d) with 8-Cl-ADO, 8-Cl-cAMP, or 8-Cl-cAMP plus 50 μm IBMX (Fig. 4, A–C, respectively) in media containing Hyclone serum. Both 8-Cl-ADO and 8-Cl-cAMP induced apoptosis in all cell types in a concentration-dependent manner. Apoptosis induced by 8-Cl-cAMP was reduced (to 65%; P = 0.004 to <0.001) by IBMX. In cells treated with 8-Cl-ADO or 8-Cl-cAMP (Fig. 4, A and B, respectively), apoptosis led to significantly high levels of necrosis (Fig. 4, A and B, upper panels). Detectable total cell death (early apoptosis plus necrosis) was high (to 57%) in all cell types. Thus, values given for apoptosis exclude apoptotic cell death in cells that had disintegrated during earlier incubation times. Total cell death, however, was similar to levels of inhibition seen in cell metabolism and [3H]thymidine studies (Fig. 1, A and B) on d 4.
Figure 4.
8-Cl-cAMP and 8-Cl-ADO stimulate apoptosis in HeLa cells and in thyroid cancer cells. IBMX inhibits 8-Cl-cAMP-induced apoptosis. Cells were incubated with increasing concentrations of 8-Cl-ADO (A), 8-Cl-cAMP (B), or 8-Cl-cAMP plus (50 μm) IBMX (C). Apoptosis was assessed by annexin V-FITC/7AAD assay staining and measured by FACScalibur flow cytometry. Points are mean ± sem of three experiments (A), four experiments (B), and three experiments (C), and results are expressed as percentage of apoptosis (minus apoptosis in untreated cells). Dot plots in C are of one representative experiment. P = 0.004 to <0.0001 (C).
8-Cl-ADO alters cell proliferation independently of the PKA signaling pathway
Basal levels of PKA activity (Fig. 5A) were similar in all cell lines (∼40 cpm/μg protein/ml). After exposure to cAMP, PKA activity was significantly increased in WRO and ARO cells (20 and 40%, respectively; P = 0.0005 to <0.0001) only at the high 8-Cl-ADO concentration (30 μm) and in ARO cells (10%; P < 0.0001) at the lower 8-Cl-ADO concentration (15 μm). However, PKA activity was decreased by 85 and 40% in HeLa cells at 15 and 30 μm, respectively (P < 0.0001). No effect was seen at 15 μm in NPA or WRO cells. Thus, there was no consistent effect of 8-Cl-ADO on HeLa or thyroid cells. After exposure to cAMP and 8-Cl-ADO, PKA activity was reduced to basal levels (minus cAMP) by the PKA-specific inhibitor PKI. Although PKA R-subunit levels were altered by 8-Cl-ADO, they were differentially affected (Fig. 5B) with no reduction in the ratio of RI- to RII-type subunits (Fig. 5C). The data indicate a differential effect of 8-Cl-ADO on PKA activity in HeLa vs. thyroid cells with a small stimulatory effect in thyroid cells and a much greater inhibition in HeLa cells. Because the PKB/Akt cell signaling pathway also promotes cell proliferation in cancer cells (30), we determined whether the PKA and the PKB/Akt signaling pathways were involved in the inhibition of cell proliferation/metabolism by 8-Cl-ADO. Cultures were incubated (4 d) with PKA pathway stimulants [8-Br-cAMP, forskolin (FSK), and isoproterenol (ISO), Fig. 5D] and the PKA and PKB pathway inhibitors H89 (50 nm) and LY294002 (30 μm), respectively (Fig. 5E). PKA stimulants had little or no effect on cell proliferation/metabolism (Fig. 5D). Although in ARO cells a small inhibition occurred with 8-Br-cAMP (to 35%; P < 0.0001), it was 2.6-fold less than that exerted by 8-Cl-ADO in the same cell line. LY294002 inhibited cell metabolism by 50–70% (P < 0.0001), whereas H89 inhibited metabolism only in NPA cells (to 17%; P = 0.01). This suggests a small stimulatory effect of 8-Cl-ADO on PKA activity in thyroid cells but, in general, no direct involvement of the PKA cell signaling pathway in cell proliferation/metabolism. Instead, the data confirm that the PKB/Akt cell signaling pathway is involved in cell proliferation/metabolism (30).
Figure 5.
Differential effect of 8-Cl-ADO on PKA activity in thyroid cancer cells and in HeLa cells: no significant effect of PKA pathway stimulants or the PKA activity inhibitor H89 on cell metabolism/proliferation. A, Cells in 175-cm2 flasks were incubated with 8-Cl-ADO (15 and 30 μm) for 4 d, and cell extracts were exposed to cAMP or to cAMP plus the PKA-specific inhibitor PKI. PKA activity levels were then determined. Results are expressed as cpm [γ32P]dATP per microgram protein per milliliter and are mean ± sem of three experiments. B, Cells in 175-cm2 flasks were incubated with 8-Cl-ADO (0–25 μm) for 4 d and lysed, and gel electrophoresis and immunoblot assay were performed using antibodies to PKA R-subunits. C, Ratio of PKA R-subunits RIα plus RIβ/ RIIα plus RIIβ from B above. D, Cells in 96-well culture plates were incubated for 4 d with increasing concentrations (0–40 μm) of the PKA pathway stimulants 8-Br-cAMP, isoproterenol (ISO), and forskolin (FSK), or with 8-Cl-ADO. E, cells were incubated with the PKA pathway inhibitor, H89 (50 nm) or with the PKB (Akt)-pathway inhibitor LY294002 (30 μm) for 0–78 h. Cell titer 96 AQ solution was added to cultures in D and E for 3.5 h, before plates were read using an ELISA plate reader at 460 nm. Points in B are mean ± sem of three experiments, expressed as percentage of control band density/Β-actin (arbitrary values). Bands are representative of one experiment. Results are expressed as percentage of control absorbance ± sem of three experiments (D) or mean ± sd of two experiments (E). *, P < 0.0001 (A, D, and E); **, P = 0.0005 (A); ***, P = 0.01 (E) compared with baseline.
Accumulation of cells in G1/S or G2/M phases of the cell cycle
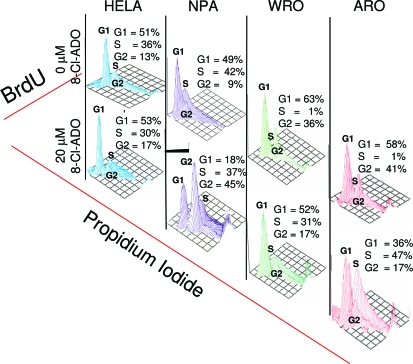
Cell cycle analysis of synchronized cells treated with 8-Cl-ADO (20 μm, 72 h), indicated that NPA cells accumulate in the G2/M phase of the cell cycle, whereas WRO and ARO cells accumulate in G1/S. No phase accumulation was observed with HeLa cells (Fig. 6).
Figure 6.
8-Cl-ADO induces the accumulation of cells in G1/S and G2/M phases of the cell cycle. Cells, synchronized (72 h) in low-serum (0.1%) media were incubated for 3 d with or without 8-Cl-ADO (20 μm) in complete medium. Cells were pulse labeled with BrdU, followed by the addition of anti-BrdU-FITC mAb and propidium iodide. Cell cycle analysis was determined by FACScalibur flow cytometry, using Cell Quest software. On the y-axis is the percentage of newly formed DNA (BrdU) content as indicated by anti-BrdU-FITC mAb in each cell cycle phase on the x-axis is propidium iodide. Three-dimensional plots are from one representative experiment.
Discussion
Our study addressed the questions of 1) whether or not 8-Cl-ADO has growth-inhibitory effects on human thyroid cancer and HeLa cells and 2) whether the inhibitory effects of the parent compound, 8-Cl-cAMP, are due to its metabolic by-product, 8-Cl-ADO. We also examined possible mechanisms of action of 8-Cl-ADO on cell growth. We first showed that 8-Cl-ADO inhibited cell proliferation/metabolism and [3H]thymidine incorporation in all thyroid and HeLa cell lines (Fig. 1). IC50 values obtained by MTS assay were comparable to those reported for other cancer cell lines (19,31,32,33). However, IC50 values determined by [3H]thymidine assay were about 10-fold higher than those obtained by MTS assay (Fig. 3B), possibly due to the difference in sensitivity of the two assays (34).
The inhibition by 8-Cl-ADO also decreased with time (on d 5 of incubation) (Fig. 1, A and B). By preincubating 8-Cl-ADO (37 C, up to 7 d without cells), followed by incubation with cells and the same preincubated drug, or by replenishing the drug on d 4, we determined whether the decrease in inhibition was due to intracellular (Fig. 2B) and/or extracellular (Fig. 2A) drug metabolism, respectively. We found no difference in the inhibitory effect with the preincubated drug, and the decrease in inhibition on d 5 could be eliminated by replenishing the drug on d 4. This suggests that 8-Cl-ADO is not metabolized by the media at 37 C but may be degraded within the cell. As shown by others (17,19,35), the possibility exists that by d 5, intracellular adenosine deaminase may convert the active 8-Cl-ADO to an inactive product, 8-Cl-inosine, thereby reducing inhibition.
We addressed the ongoing controversy as to whether or not 8-Cl-cAMP acts as a pro-drug and is metabolized by cellular or serum phosphodiesterase and 5′-nucleotidase (18,19,31,35) to the highly potent 8-Cl-ADO. We first found that 8-Cl-cAMP inhibits cell proliferation in all cell lines and that the inhibition is comparable to that of 8-Cl-ADO when Hyclone instead of GIBCO/Invitrogen serum (Fig. 3, A–C) was used. The inhibition by 8-Cl-cAMP was greatly reduced by IBMX (Fig. 3, A and D) and further reduced in serum-free media in thyroid cells. There was, however, less reduction in inhibition in HeLa cells with IBMX and serum-free media. These studies and those of others (15,16,17,18,33,35,36) suggest that 8-Cl-cAMP is metabolized to 8-Cl-ADO by serum phosphodiesterase (and possibly 5′-nucleotidase) and that the source of the serum plays a large part in this conversion. These data may explain the large difference in IC50 values for 8-Cl-ADO and 8-Cl-cAMP that has also been reported by others (17,31).
One objective of our study was to examine the mechanisms of inhibition of cell proliferation by 8-Cl-ADO and 8-Cl-cAMP. We measured apoptosis (Fig. 4) in 8-Cl-ADO- and 8-Cl-cAMP-treated cells using the annexin V/7AAD assay (37). Treatment with 8-Cl-ADO (Fig. 4A) and 8-Cl-cAMP (Fig. 4B) for 4 d resulted in apoptosis in all cell lines. The rank order of apoptosis (data not shown) was similar to the order of the IC50 values (Fig. 3B) for both drugs as determined by the MTS assay. Both drugs caused rapid late apoptosis/necrosis (Fig. 4, A and B, upper panels). Early apoptosis was decreased at the higher 8-Cl-ADO and 8-Cl-cAMP concentration (30 μm). This may be accounted for by a population of cells that underwent cell death earlier in the incubation. These cells would disintegrate by the fourth day and would not be assessed by the assay. Because IBMX decreased 8-Cl-cAMP-induced antiproliferation (Fig. 3, A and D), we determined whether IBMX also reduces 8-Cl-cAMP-induced apoptosis. 8-Cl-cAMP-induced apoptosis (Fig. 4C) was reduced with IBMX, as was late apoptosis/necrosis (data not shown). These data show that both drugs induce apoptosis and confirm that 8-Cl-cAMP, acting as a pro-drug, is converted to 8-Cl-ADO to induce an inhibition of proliferation and apoptosis.
Next, we examined the involvement of the PKA cell signaling pathway in the inhibition by 8-Cl-ADO. Because 2-Cl-adenosine activates adenosine receptors that stimulate adenyl cyclase and PKA activity to affect cell proliferation in some cell lines (17,38), the possibility exists that the inhibition by 8-Cl-ADO is due to its action as an adenosine receptor agonist, similar to 2-Cl-adenosine. However, in our studies, incubations with adenosine receptor agonists and antagonists with and without 8-Cl-ADO (Fig. 1C) showed no effect on proliferation/metabolism. The effect of 8-Cl-ADO on PKA activity (Fig. 5A) was also examined. PKA activity was altered in a differential manner by 8-Cl-ADO, with decreased PKA activity in HeLa cells and a small increase in activity in thyroid cells, mostly at the high 8-Cl-ADO concentration (30 μm). 8-Cl-ADO did not affect basal PKA activity or PKA activity determined in the presence of the PKA inhibitor PKI. 8-Cl-ADO did differentially alter levels of PKA R-subunits (Fig. 5B). These changes, however, were contrary to those reported previously (12,13,39), with no reduction in the ratio of RI to RII subunits (Fig. 5C).
Known stimulants of PKA activity (Fig. 5D) at 8-Cl-ADO inhibitory concentrations showed little or no inhibition of cell metabolism, compared with that with 8-Cl-ADO. Likewise, the PKA pathway inhibitor H89 had no or only a small inhibitory effect (to 17%, only in NPA cells), whereas inhibition was greatly induced (to 70%) by the PKB/Akt pathway inhibitor, LY294002, as seen by others with 8-Cl-cAMP (40). These data, and the fact that 8-Cl-ADO has no effect on adenosine receptors (Fig. 1C) suggested that although in thyroid cells, there is a small stimulation of PKA activity at high 8-Cl-ADO concentrations and a differential effect on R-subunit levels, PKA most likely does not mediate changes in growth directly but may act indirectly by modulating the activity of factors that can affect tumor cell growth, as surmised by other investigators (31). The PKA cell signaling pathway and the activity of the PKA holoenzyme are in all probability not directly involved in the inhibition by 8-Cl-ADO. These studies point to an effect of the drugs on the PKB/Akt cell signaling pathway (40); more studies, however, are required to determine the mechanism of action of 8-Cl-ADO on signaling by PKB/Akt.
As in other studies (22,25), our results show that 8-Cl-ADO-induced inhibition of thyroid cell metabolism is related to cell cycle arrest. Initially (Fig. 1A), we showed that the incorporation of [3H]thymidine on d 5 is increased by 8-Cl-ADO, whereas in MTS assays, the inhibition of proliferation (at the higher 8-Cl-ADO concentrations) on d 5 remains the same as on d 4 (Fig. 1B). These data suggest that cells treated with high 8-Cl-ADO concentrations retain the ability to incorporate [3H]thymidine (Fig. 1A) but are unable to divide (Fig. 1B). Indeed, cell cycle analysis of 8-Cl-ADO-treated cells shows that by 3 d of incubation, NPA cells accumulate in G2/M phase of the cell cycle, whereas WRO and ARO cells accumulate in G1/S phase (Fig. 6), indicating cell cycle arrest by the drug. Our results with NPA cells show cell cycle arrest in G2/M and confirm the findings of others (22). However, our results in all cell types are contrary to those of others who show cell accumulation in G0/G1, with no induction of apoptosis (25). These results may be due to differences in cell type, methods used, intracellular drug metabolism, and cell cycle transit time. Also, although we have shown cell accumulation in G1/S and G2/M cell cycle phases, as well as apoptosis with 8-Cl-ADO, the existing data do not determine whether the cells that accumulate in G1/S or in G2/M are the same cell population that undergoes apoptosis.
In summary, our data indicate that proliferation of HeLa and three widely used thyroid cancer cell lines can be inhibited by 8-Cl-ADO and 8-Cl-cAMP. We showed that 1) inhibition of proliferation by 8-Cl-cAMP is via the 8-Cl-cAMP metabolic breakdown product 8-Cl-ADO, which may also undergo intracellular degradation over time; 2) cells accumulate in G1/S or G2/M of the cell cycle and undergo apoptosis, suggesting that 8-Cl-ADO may cause cell cycle arrest, although HeLa cells also undergo apoptosis without an apparent phase accumulation; and 3) PKA activity and the PKA R-subunits have no direct effects on 8-Cl-cAMP or 8-Cl-ADO actions. Because we found subtle differences in the effect of 8-Cl-ADO on the four cell types tested, generalizations cannot be made for the effect of 8-Cl-ADO on all cancer cell types; thus, a thorough determination of inhibition of proliferation and mechanism of action should be established for each cancer cell type. Finally, these data may indicate that for aggressive thyroid cancer, 8-Cl-ADO may be a useful, if not powerful, drug.
Acknowledgments
We thank Dr. Deloris E. Koziol for her assistance in the statistical analysis of the data. We also thank Dr. Ioannis Bossis (now at the University of Maryland School of Veterinary Medicine, College Park, MD) for his involvement in the initial stages of this project and his advice on handling the cell lines and some of the first experiments, which were essential for the subsequent successful completion of this project.
Footnotes
This work was supported by NICHD, NIH, intramural project Z01-HD-000642-04 to C.A.S. and in part by a 2005–2007 Bench-to-Bedside award to C.A.S. and Y. Cho-Chung (NCI, NIH) supported by the NIH Clinical Center, NICHD, NCI, and the NIH Office of Rare Diseases. Support was also provided by the NIH Intramural Research Program, NCI, Center for Cancer Research, and NIH Grant R01 CA085915.
First Published Online December 11, 2007
Abbreviations: 7AAD, 7-Amino-actinomycin D; BrdU, bromodeoxyuridine; 8-Cl-ADO, 8-Cl-adenosine; FBS, fetal bovine serum; FITC, fluorescein isothiocyanate; IBMX, 3-isobutyl-1-methylxanthine; mAb, monoclonal antibody; MRS1523, 3-propyl-6-ethyl-5-[(ethylthio)carbonyl]-2-phenyl-4-propyl-3-pyridinecarboxylate; MTS, 3-(4,5-dimethylthiazol-2yl)-5-(3-carboxymethoxyphenol)-2-(4-sulfophenyl)-2H-tetrazolium, salt; NECA, N-ethyl-carbomidoadenosine; PKA, protein kinase A; PKI, protein kinase inhibitor; R-subunits; regulatory subunits; XAC, xanthine amine congener.
References
- Sakamoto A 2004 Definition of poorly differentiated carcinoma of the thyroid: the Japanese experience. Endocr Pathol 15:307–311 [DOI] [PubMed] [Google Scholar]
- Nikiforov YE 2004 Genetic alterations involved in the transition from well-differentiated to poorly differentiated and anaplastic thyroid carcinomas. Endocr Pathol 15:319–327 [DOI] [PubMed] [Google Scholar]
- Delellis RA, Lloyd RV, Hertz PU, Eng C 2004 Tumors of the thyroid and parathyroid. In: Delellis RA, Lloyd RV, Hertz PU, Eng C, eds. WHO classification of tumors: pathology and genetics. Lyon, France: IARC Press; 51–80 [Google Scholar]
- Liska J, Altanerova V, Galbavy S, Stvrtina S, Brtko J 2005 Thyroid tumors: histological classification and genetic factors involved in the development of thyroid cancer. Endocr Res 39:73–83 [PubMed] [Google Scholar]
- Ain KB 1998 Anaplastic thyroid carcinoma: behavior, biology and therapeutic approaches. Thyroid 8:715–726 [DOI] [PubMed] [Google Scholar]
- Robinson-White A, Stratakis CA 2002 Protein kinase A signaling: “cross talk” with other pathways in endocrine cells. Ann NY Acad Sci 968:256–270 [DOI] [PubMed] [Google Scholar]
- Robinson-White A, Hundley TR, Shiferaw M, Bertherat J, Sandrini F, Stratakis CA 2003 Protein kinase-A activity in PRKAR1A-mutant cells, and regulation of mitogen-activated protein kinases ERK1/2. Hum Mol Genet 12:1475–1484 [DOI] [PubMed] [Google Scholar]
- Tasken K, Shalhegg BS, Tasken KA, Solberg R, Knutsen HK, Levy FO, Sandberg M, Larsen T, Johansen AK, Vang T, Scharder HP, Reinton NT, Torgersen KM, Hansson V, Jahnsen T 1997 Structure, function and regulation of human cAMP-dependent protein kinase. Adv Second Messenger Prosphoprotein Res 31:191–204 [DOI] [PubMed] [Google Scholar]
- Scott JD 1991 Cyclic nucleotide-dependent protein kinase. Pharmacol Ther 50:123–145 [DOI] [PubMed] [Google Scholar]
- Robinson-White AJ, Leitner WW, Aleem E, Kaldis P, Bossis I, Stratakis CA 2006 PRKAR1A inactivation leads to increased proliferation and decreased apoptosis in human B lymphocytes. Cancer Res 66:10603–10612 [DOI] [PubMed] [Google Scholar]
- Katsaros D, Tortora G, Tagliaferri P, Clair T, Ally S, Neckers L, Robins RK, Cho-Chung YS 1987 Site-selective cyclic AMP analogs provide a new approach in the control of cancer cell growth. FEBS Lett 223:97–103 [DOI] [PubMed] [Google Scholar]
- Ally S, Tortora G, Clair T, Grieco D, Merlo G, Katsaros D, Ogreid D, Doskeland SO, Jahnsen T, Cho-Chung YS 1988 Selective modulation of protein kinase isozymes by the site-selective analog 8-chloroadensoine 3′,5′-cyclic monophosphate provides a biological means for control of human colon cancer cell growth. Proc Natl Acad Sci USA 85:6319–6322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlff C, Clair T, Cho-Chung YS 1993 8-Cl-cAMP induces truncation and down-regulation of the RI α-subunit and up-regulation of the RII β-subunit of cAMP-dependent protein kinase leading to type II holoenzyme-dependent growth inhibition and differentiation of HL-60 leukemia cells. J Biol Chem 268:5774–5782 [PubMed] [Google Scholar]
- Bennett Jr LL, Chang C-H, Allan PW, Adamson DJ, Rose LM, Brockman RW, Secrist III JA, Shortnacy A, Montgomery JA 1985 Metabolism and metabolic effects of halopurine nucleosides in tumor cells in culture. Nucleotides 4:107–116 [Google Scholar]
- Gandhi V, Ayers M, Halgren RG, Krett NL, Newman RA, Rosen ST 2001 8-Chloro-cAMP and 8-chloro-adenosine act by the same mechanism in multiple myeloma cells. Cancer Res 61:5474–5479 [PubMed] [Google Scholar]
- Langeveld CH, Jongenelen CAM, Theeuwes JWM, Baak JPA, Heimans JJ, Stoff JC, Peters GJ 1997 The antiproliferative effect of 8-chloro-adenosine, an active metabolite of 8-chloro-cyclic adenosine monophosphate and disturbances in nucleic acid synthesis and cell cycle kinetics. Biochem Pharm 53:141–148 [DOI] [PubMed] [Google Scholar]
- Langeveld CH, Jongenelen CAM, Heimans JJ, Stoff JC 1992 Growth inhibition of human glioma cells induced by 8-chloroadenosine, an active metabolite of 8-chloro adenosine 3′:5′-monophosphate. Cancer Res 52:3994–3999 [PubMed] [Google Scholar]
- Robbins SK, Houlbrook S, Priddle JD, Harris AL 2001 8-Cl-adenosine is an active metabolite of 8-Cl-cAMP responsible for its in vitro antiproliferative effects on CHO mutants hypersensitive to cytostatic drugs. Cancer Chemother Pharmacol 48:451–458 [DOI] [PubMed] [Google Scholar]
- Halgren RG, Traynor AE, Pillay S, Zell JL, Heller KF, Krett NL, Rosen ST 1998 8Cl-cAMP cytotoxicity in both steroid sensitive and insensitive multiple myeloma cell lines is mediated by 8Cl-adenosine. Blood 92:2893–2898 [PubMed] [Google Scholar]
- Phillips E, Newsholme EA 1979 Maximum activities, properties and distribution of 5′-nucleotidase, adenosine kinase and adenosine deaminase in rat and human brain. J Neurochem 33:553–558 [DOI] [PubMed] [Google Scholar]
- Stellrecht CM, Rodriguez Jr CO, Ayres M, Gandhi V 2003 RNA-directed actions of 8-chloro-adenosine in multiple myeloma cells. Cancer Res 63:7968–7974 [PubMed] [Google Scholar]
- Kim SN, Ahn YH, Kim SG, Park SD, Cho-Chung YS, Hong SH 2001 8-Cl-cAMP induces cell cycle specific apoptosis in human cancer cells. Int J Cancer 93:33–41 [DOI] [PubMed] [Google Scholar]
- Zhang S, Zheng D, Liu S 1998 [8 Chloro-adenosine induced apoptosis in various human tumor cell lines]. Zhonghua Zhong Liu Za Zhi (Chinese) 20:88–89 [PubMed] [Google Scholar]
- Yin Y, Allen PD, Jia I, Kelsey SM, Newland AC 2001 8-Cl-adenosine mediated cytotoxicity and sensitization of T-lymphoblast leukemia cells to TNFα-induced apoptosis is via inactivation of NF-kB. Leukemia Res 25:423–431 [DOI] [PubMed] [Google Scholar]
- Dransfield DT, Griner RD, Ray S, Keskintepe M, Bollag WB 2001 8 Cl-adenosine induces growth arrest without differentiation of primary mouse epidermal keratinocytes. J Invest Dermatol 117:1588–1593 [DOI] [PubMed] [Google Scholar]
- Asakawa H, Kobayashi T, Komoike Y, Yanagawa T, Takahashi M, Wakasugi E, Maruyama H, Tamaki Y, Matsuzawa Y, Monden M 1996 Establishment of anaplastic thyroid carcinoma cell lines for analysis of chemosensitivity and carcinogenesis. J Clin Endocrinol Metab 81:3547–3552 [DOI] [PubMed] [Google Scholar]
- Yates CM, Patel A, Oakley K, Helms A, Tuttle RM, Francis GL 2006 Erythropoietin in thyroid cancer. J Endocrinol Invest 29:320–329 [DOI] [PubMed] [Google Scholar]
- Homburg CH, deHass M, von dem Borne AE, Verhoeven AJ, Reutelingsperger CP, Roo SD 1995 Human neutrophils lose their surface FcγRIII and acquire annexin V binding sites during apoptosis in vitro. Blood 85:532–540 [PubMed] [Google Scholar]
- Robinson-White A, Meoli E, Stergiopoulos S, Horvath A, Boikos S, Bossis I, Stratakis CA 2006 PRKARIA mutation and protein kinase A interactions with other signaling pathways in the adrenal cortex. J Clin Endocrinol Metab 9:2380–2388 [DOI] [PubMed] [Google Scholar]
- Vasko V, Saji M, Hardy E, Kruhlak M, Larin A, Savchenko V, Miyakawa M, Isozaki O, Murakami H, Tsushima T, Burman KD, De Micco C, Ringel MD 2004 Akt activation and localization correlate with tumor invasion and oncogene expression in thyroid cancer. J Med Genet 41:161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb D, Steinberg RA 2002 Anti-proliferative effects of 8-Chloro-cAMP and other cAMP analogs are unrelated to their effects on protein kinase A regulatory subunit expression. J Cell Physiol 192:216–224 [DOI] [PubMed] [Google Scholar]
- Taylor CW, Yeoman LC 1992 Inhibition of colon tumor cell growth by 8-chloro-cAMP is dependent upon its conversion to 8-chloro-adenosine. Anticancer Drugs 3:485–491 [DOI] [PubMed] [Google Scholar]
- Juranic Z, Radulovic S, Joksimovic J, Juranic I 1998 The mechanism of 8-Cl-cAMP action. J Exp Clin Cancer Res 17:269–275 [PubMed] [Google Scholar]
- Gieni RS, Li Y, HayGlass KT 1995 Comparison of [3H]thymidine incorporation with MTT- and MTS-based bioassays for human and murine IL-2 and IL-4 analysis. Tetrazolium assays provide markedly enhanced sensitivity. J Immunol Methods 187:85–93 [DOI] [PubMed] [Google Scholar]
- Lange-Carter CA, Vuillequez JJ, Malkinson AM 1993 8-Chloroadenosine mediates 8-chloro-cyclic AMP-induced down-regulation of cyclic AMP-dependent protein kinase in normal and neoplastic mouse lung epithelial cells by a cyclic AMP-independent mechanism. Cancer Res 53:393–400 [PubMed] [Google Scholar]
- Han Z, Chatterjee D, Wyche JH 1993 Proliferation of nontransformed cells is inhibited by adenosine metabolite of but not by parental 8-Cl-cyclic AMP. J Pharmacol Exp Ther 265:790–794 [PubMed] [Google Scholar]
- Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C 1995 A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labeled annexin V. J Immunol Methods 184:39–51 [DOI] [PubMed] [Google Scholar]
- Williams M, Abreu M, Jarvis MF, Noronha-Blob L 1987 Characterization of adenosine receptors in the PC12 pheochromocytoma cell line using radioligand binding: evidence for A-2 selectivity. J Neurochem 48:498–502 [DOI] [PubMed] [Google Scholar]
- Ramage AD, Langdon SP, Ritchie AA, Burn DJ, Miller WR 1995 Growth inhibition by 8-chlorocyclic AMP of human HT29 colorectal and ZR-75–1 breast carcinoma xenographs is associated with selective modulation of protein kinase A isozymes. Eur J Cancer 31A:969–973 [DOI] [PubMed] [Google Scholar]
- Motti ML, Califano D, Troncone G, De Marco C, Migliaccio I, Palmieri E, Pezzullo L, Palombini L, Fusco A, Viglietto G 2005 Complex regulation of the cyclin-dependent kinase inhibitor p27kip1 in thyroid cancer cells by the PI3K/AKT pathway: regulation of p27kip1 expression and localization. Am J Pathol 166:737–749 [DOI] [PMC free article] [PubMed] [Google Scholar]