Abstract
Context: Rates of bone loss across the menopause transition and factors associated with variation in menopausal bone loss are poorly understood.
Objective: Our objective was to assess rates of bone loss at each stage of the transition and examine major factors that modify those rates.
Design, Setting, and Participants: We conducted a longitudinal cohort study of 1902 African-American, Caucasian, Chinese, or Japanese women participating in The Study of Women’s Health Across the Nation. Women were pre- or early perimenopausal at baseline.
Outcome Measure: We assessed bone mineral density (BMD) of the lumbar spine and total hip across a maximum of six annual visits.
Results: There was little change in BMD during the pre- or early perimenopause. BMD declined substantially in the late perimenopause, with an average loss of 0.018 and 0.010 g/cm2·yr from the spine and hip, respectively (P < 0.001 for both). In the postmenopause, rates of loss from the spine and hip were 0.022 and 0.013 g/cm2·yr, respectively (P < 0.001 for both). During the late peri- and postmenopause, bone loss was approximately 35–55% slower in women in the top vs. the bottom tertile of body weight. Apparent ethnic differences in rates of spine bone loss were largely explained by differences in body weight.
Conclusions: Bone loss accelerates substantially in the late perimenopause and continues at a similar pace in the first postmenopausal years. Body weight is a major determinant of the rate of menopausal BMD loss, whereas ethnicity, per se, is not. Healthcare providers should consider this information when deciding when to screen women for osteoporosis.
A study of bone mineral density changes during menopause reveals that bone loss accelerates substantially in the late perimenopause and continues at a similar pace in the first postmenopausal years. Additionally, the rate of postmenopausal bone mineral changes is related to body weight and not ethnicity.
Osteoporosis affects over 20 million Americans and leads to approximately 1.5 million fractures each year, making it one of the leading public health problems in the United States (1). The most important risk factor for bone loss in midlife women is the menopause. Women lose about 50% of their trabecular bone and 30% of their cortical bone during the course of their lifetime, about half of which is lost during the first 10 yr after the menopause (1,2). Approximately 40% of all postmenopausal women will eventually experience fractures (1,2). In 2001, the National Osteoporosis Foundation estimated that the annual cost of health care and lost productivity related to osteoporosis was $17 billion.
Despite the public health importance of postmenopausal osteoporosis, there are important gaps in our knowledge of the effect of the menopause transition on the skeletal system. Although bone loss accelerates after menses cease (3,4,5), it is not clear either when bone loss begins or what the rates of bone loss are at various stages of the menopause transition. It is important to determine when bone loss accelerates so that women and their health care providers can make informed decisions regarding the appropriate time to screen for osteoporosis and to consider therapy to prevent bone loss. There is considerable variation in rates of bone loss among women, with some women experiencing rapid bone loss during the menopause transition and others experiencing little or no bone loss (3,5,6). The basis for this variation is poorly understood. Identifying factors that are associated with rapid or slow rates of bone loss during the menopause transition could assist clinicians in making decisions that will optimize skeletal health in midlife women.
The Study of Women’s Health Across the Nation (SWAN) is a seven-center, longitudinal cohort study of the menopause transition in a community-based sample of women from multiple ethnic groups. Bone mineral density (BMD) of the lumbar spine and proximal femur has been measured annually in women at five SWAN sites. SWAN is the first large-scale, multiethnic, longitudinal cohort study to assess BMD across the entire menopause transition. Thus, SWAN provides a unique opportunity to characterize changes in BMD across the menopause transition and to assess factors that influence those changes.
Subjects and Methods
Study population
SWAN is a seven-site, longitudinal cohort study in community-based groups of women. At baseline, 3302 pre- or early perimenopausal women who belonged to one of five ethnic/racial groups were recruited: Caucasian (n = 1550), African-American (n = 935), Japanese (n = 281), Chinese (n = 250), and Hispanic (n = 286). Race/ethnicity was determined by self-report. Participants were enrolled at seven clinical sites in Boston, Chicago, Detroit area, Los Angeles, Hudson County (NJ), Oakland, and Pittsburgh. All seven sites enrolled Caucasians, and each site also enrolled women belonging to one prespecified minority ethnic group. Eligibility criteria for entry into the SWAN longitudinal cohort were age 42–52 yr, intact uterus and at least one ovary, no current use of estrogens or other medications known to affect ovarian function, at least one menstrual period in the 3 months before screening, and self-identification as a member of one of the five eligible ethnic groups. Recruitment techniques were designed to generate a representative sample of women at each of the seven sites. Eligibility criteria and cohort recruitment have been described in detail (7). The Chicago and Hudson County, NJ, sites did not measure BMD, leaving a potential maximum of 2413 Caucasian, African-American, Japanese, or Chinese women for BMD analyses. Of these women, 1902 had a usable baseline BMD measurement and at least one follow-up exam and are included in this report.
Determination of menopause stage
At each annual visit, menopause stage was determined based on reports about frequency and regularity of menstrual bleeding. Women were classified as premenopausal if they had experienced at least one menstrual period in the last 3 months with no change in the regularity of their menstrual bleeding during the last year. Women were classified as early perimenopausal if they had experienced at least one menstrual period in the last 3 months with some change in the regularity of their menstrual bleeding during the last year. Women were classified as late perimenopausal if they had experienced no menstrual bleeding in the last 3 months but some menstrual bleeding during the last 11 months. Women were classified as postmenopausal once they had experienced at least 12 consecutive months of amenorrhea. The postmenopausal stage was defined as beginning at the time of the woman’s final menstrual period. Once a woman had transitioned to a more advanced menopause stage, she was not reclassified into an earlier stage.
Study protocol
All subjects were seen at a SWAN center for annual measurements of BMD of the lumbar spine and proximal femur, assessment of factors possibly related to BMD using questionnaires, and measurement of height and weight. Standardized interviewer-administered or self-administered questionnaires were used to assess the following parameters: age (years), cigarette smoking, alcohol intake (drinks per day), vitamin D (International units per day), and calcium intake (milligrams per day) (8), medication use, and menopause stage. The protocol was approved by the Institutional Review Board at each center, and all women provided written informed consent.
Assessment of BMD
BMD of the posterior-anterior lumbar spine and total hip was measured by dual-energy x-ray absorptiometry (DXA) using a Hologic QDR 2000 densitometer (Hologic Inc., Waltham, MA) in Pittsburgh and Oakland or a Hologic QDR 4500A densitometer in Boston, Detroit area, and Los Angeles.
All five centers employed a standard quality control program that included daily measurement of a Hologic anthropomorphic spine phantom at each site, cross-site calibration with a single anthropomorphic spine phantom, visual review of every scan image by a local site investigator experienced in bone densitometry, and random review of 5% of scans plus all problem scans by Synarc, Inc. (Waltham, MA). Measurements of the local spine phantoms and the circulating spine phantom were analyzed by Synarc and used to adjust DXA measurements for minor temporal or geographic variations. Our short-term in vivo measurement sd values are 0.014 g/cm2 (1.4%) and 0.016 g/cm2 (2.2%) for the lumbar spine and femoral neck.
Data analysis
The primary goal of this study was to estimate and then compare rates of change of lumbar spine and total hip BMD across the four menopause stages. The rate of change in BMD during each stage was estimated using linear mixed models. Repeated measures of BMD were modeled as a function of four separate time variables, one for the cumulative amount of time spent in each of the four menopause stages. The algorithm used to determine the cumulative amount of time spent in each menopause stage is described in the Appendix (published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). Regression coefficients for these four time variables provided estimates of the annual change in BMD within each menopause stage. Models were adjusted for baseline age, menopause stage, weight, smoking, calcium intake, vitamin D intake, and alcohol intake; percent change in weight since baseline; ethnicity; and SWAN clinical site. Estimated rates of BMD change across menopause stages, weight tertiles, and ethnic groups were compared using suitable contrasts.
Because body weight and ethnicity were powerful predictors of baseline BMD in these women (9), we performed additional analyses to isolate the effects of these variables on rates of change of BMD in each menopause stage. First, interaction terms between ethnicity and each of the four time variables were tested to determine whether rates of change within each stage varied across ethnic groups. As has been done previously (9), these analyses were also performed on a subset of the population in which there was considerable overlap of body weight among all four ethnic groups (50–78 kg). This subset contained 1133 women (587 Caucasians, 198 African-Americans, 181 Japanese, and 167 Chinese). Because there were still weight differences between ethnic groups in the restricted sample, models were still adjusted for the variables listed above, including weight. Second, interaction terms between baseline weight tertiles and each of the four time variables were tested to determine the effect of weight on rates of change in each menopause stage. This analysis was then stratified by ethnicity to assess the effect of weight on rates of change within each ethnic group separately. For these latter analyses, weight tertiles were generated using cutoff points specific to each ethnic group.
Data were censored at the time a participant started hormone or other antiresorptive therapy (including bisphosphonates, calcitonin, raloxifene, or tamoxifen), had GnRH agonist therapy, had two consecutive visits in which she reported recent glucocorticoid use, or underwent a hysterectomy or bilateral oophorectomy.
Results
Clinical characteristics
The clinical characteristics of the entire cohort (n = 1902) and the four ethnic groups are shown in Table 1. On average, African-American women were 9 kg heavier than Caucasians and 26–27 kg heavier than Chinese and Japanese women. Median calcium intake was highest in Caucasian women.
Table 1.
Clinical characteristics of the study cohort
| Characteristic | All subjects | African-American | Caucasian | Chinese | Japanese |
|---|---|---|---|---|---|
| n | 1902 | 494 | 944 | 221 | 243 |
| Baseline age (yr) | 46.3 ± 2.7 | 46.2 ± 2.7 | 46.2 ± 2.7 | 46.4 ± 2.6 | 46.7 ± 2.7 |
| Baseline menopause stage | |||||
| Premenopausal, n (%) | 1075 (57) | 253 (51) | 526 (56) | 143 (65) | 153 (63) |
| Early perimenopausal, n (%) | 827 (43) | 241 (49) | 418 (44) | 78 (35) | 90 (37) |
| Baseline BMD (g/cm2) | |||||
| Lumbar spine | 1.10 ± 0.14 | 1.14 ± 0.15 | 1.06 ± 0.13 | 1.04 ± 0.13 | 1.02 ± 0.12 |
| Total hip | 0.96 ± 0.15 | 1.05 ± 0.15 | 0.95 ± 0.13 | 0.85 ± 0.10 | 0.89 ± 0.11 |
| Follow-up data | |||||
| Average duration of follow up (yr) | 3.9 ± 1.5 | 3.8 ± 1.5 | 3.8 ± 1.6 | 4.3 ± 1.2 | 4.2 ± 1.3 |
| Average no. of follow-up visits | 3.7 ± 1.5 | 3.5 ± 1.5 | 3.6 ± 1.5 | 4.1 ± 1.3 | 3.9 ± 1.4 |
| Premenopause (n)a | 1070 | 250 | 525 | 142 | 153 |
| Observed time (yr), median (IQR) | 0.9 (2.1) | 1.0 (1.8) | 1.3 (2.5) | 0.5 (2.0) | 0.4 (2.0) |
| Early perimenopause (n)a | 1661 | 420 | 816 | 206 | 219 |
| Observed time (yr), median (IQR) | 2.5 (2.9) | 2.5 (3.1) | 2.4 (2.9) | 2.9 (3.0) | 2.8 (3.0) |
| Late perimenopause (n)a | 388 | 114 | 189 | 37 | 48 |
| Observed time (yr), median (IQR) | 0.5 (1.1) | 0.6 (1.2) | 0.5 (0.97) | 0.4 (0.72) | 0.6 (1.1) |
| Postmenopause (n)a | 453 | 119 | 194 | 69 | 71 |
| Observed time (yr), median (IQR) | 1.9 (2.0) | 2.1 (2.3) | 1.8 (1.9) | 1.9 (1.6) | 1.8 (1.7) |
| Physical characteristics | |||||
| Weight (kg) | 72.6 ± 19.4 | 83.8 ± 19.1 | 74.3 ± 18.3 | 57.9 ± 10.5 | 56.5 ± 8.8 |
| Height (cm) | 162.3 ± 6.5 | 163.5 ± 6.1 | 164.1 ± 6.2 | 157.9 ± 5.5 | 157.1 ± 4.8 |
| BMI (kg/m2) | 27.4 ± 6.8 | 31.3 ± 7.1 | 27.6 ± 6.6 | 23.2 ± 3.8 | 22.9 ± 3.5 |
| Baseline smoking status | |||||
| Current smokers, n (%) | 275 (15) | 120 (25) | 125 (13) | 1 (0) | 29 (12) |
| Past smokers, n (%) | 474 (25) | 103 (21) | 308 (33) | 9 (4) | 54 (22) |
| Never smokers, n (%) | 1139 (60) | 262 (54) | 508 (54) | 211 (96) | 158 (66) |
| Alcohol consumption | |||||
| None, n (%) | 975 (51) | 278 (56) | 385 (41) | 175 (80) | 137 (57) |
| Average <1 drink/d, n (%) | 775 (41) | 192 (39) | 461 (49) | 39 (18) | 83 (34) |
| Average ≥1 drink/d, n (%) | 146 (8) | 24 (5) | 95 (10) | 6 (3) | 21 (9) |
| Baseline Ca intake (mg/d), median (IQR) | 792 (726) | 649 (578) | 930 (796) | 730 (645) | 755 (722) |
| Baseline Vit, D intake (IU/d), median (IQR) | 181 (349) | 152 (311) | 200 (350) | 145 (220) | 195 (373) |
Data are presented as mean ± sd unless specified otherwise. IQR, Interquartile range.
Number of women who had observed time in specified menopause stage. Because a woman could have had observed time in more than one menopause stage, the sum of the numbers is greater than the total number of study subjects.
The maximal number of follow-up visits was six (baseline plus five follow-up exams) in five yr. On average, women had 4.7 DXA exams (baseline plus 3.7 follow-ups) and were followed for 3.9 yr (Table 1). Two hundred women (11%) remained premenopausal, 1702 (89%) transitioned to early perimenopause or beyond, 616 women (32%) transitioned to late perimenopause or beyond, and 453 women (24%) became postmenopausal during the follow-up period.
Rates of change in BMD in various menopause stages
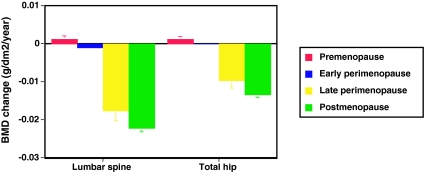
Figure 1 shows the annual rate of change in lumbar spine and total hip BMD, adjusted for covariates, in each menopause stage for the entire cohort. There was little change in lumbar spine or total hip BMD during the pre- or early perimenopause. Bone loss accelerated markedly in the late perimenopause, with an average loss of 0.018 g/cm2·yr (1.6%) and 0.010 g/cm2·yr (1.0%) from the spine and hip, respectively (P < 0.001 for both). In postmenopausal women, rates of spine and hip bone loss were 0.022 g/cm2·yr (2.0%) and 0.013 g/cm2·yr (1.4%), respectively (P < 0.001 for both). Ethnicity, baseline weight, and change in weight since baseline were significantly associated with lumbar spine BMD. Ethnicity, baseline weight, change in weight since baseline, smoking, baseline menopause stage, and clinical site were significantly associated with hip BMD.
Figure 1.
Annual rate of change in BMD of the lumbar spine and total hip in premenopausal (red bars), early perimenopausal (blue bars), late perimenopausal (yellow bars), and postmenopausal (green bars) women (n = 1902). Rates of change were estimated from multivariable linear mixed models and adjusted for multiple covariates. Error bars represent 95% confidence limits. Comparisons were made across status categories: early peri- vs. premenopausal, P < 0.001 (spine) and P = 0.002 (hip); late peri- vs. early perimenopausal, P < 0.001 (spine) and P < 0.001 (hip); and post- vs. late perimenopausal, P = 0.002 (spine) and P < 0.001 (hip).
Rates of change in BMD in various menopause stages within ethnic groups
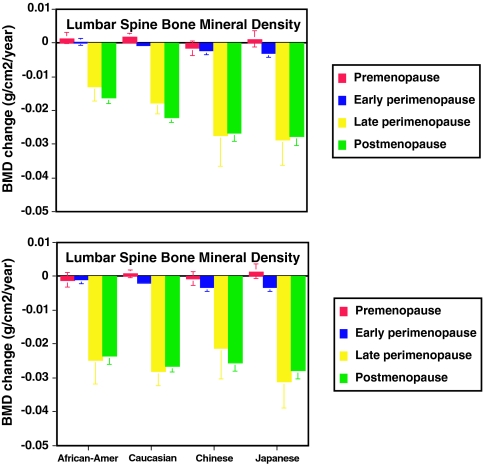
Figure 2 shows the annual rate of change in lumbar spine BMD, adjusted for covariates, during each menopause stage for each ethnic group. As in the whole cohort, BMD loss accelerated markedly in the late perimenopause in each ethnic group. In general, lumbar spine BMD loss was most rapid in Japanese and Chinese women, intermediate in Caucasian women, and slowest in African-American women during the late perimenopause and postmenopause (Fig. 2A; P < 0.001 for ethnic differences in late peri- and postmenopausal rates). When the analysis was constrained to a subset of the cohort in which there was considerable overlap of body weight among all four ethnic groups, ethnic differences in rates of late peri- and postmenopausal spine BMD loss were eliminated (Fig. 2B; P = 0.37 and P = 0.11 for the late peri- and postmenopause, respectively).
Figure 2.
Top panel, Annual rate of change in BMD of the lumbar spine in premenopausal (red bars), early perimenopausal (blue bars), late perimenopausal (yellow bars), and postmenopausal (green bars) African-American (n = 494), Caucasian (n = 944), Chinese (n = 221), and Japanese (n = 243) women from the full cohort. Rates of change were estimated from multivariable linear mixed models and adjusted for multiple covariates. Error bars represent 95% confidence limits. Comparisons were made between ethnic groups (P values for lumbar spine in pre-, early peri-, late peri-, and postmenopausal women, respectively): African-American vs. Caucasian, 0.65, 0.11, 0.10, and <0.001; African-American vs. Chinese, 0.031, <0.001, 0.006, and <0.001; African-American vs. Japanese, 0.87, <0.001, <0.001, and <0.001; Caucasian vs. Chinese, 0.004, 0.014, 0.06, and 0.003; Caucasian vs. Japanese, 0.58, <0.001, 0.009, and <0.001; Chinese vs. Japanese, 0.09, 0.43, 0.81, and 0.51. Bottom panel, Annual rate of change in BMD of the lumbar spine in premenopausal (red bars), early perimenopausal (blue bars), late perimenopausal (yellow bars), and postmenopausal (green bars) African-American (n = 198), Caucasian (n = 587), Chinese (n = 167), and Japanese (n = 181) women weighing between 50 and 78 kg. Rates of change were estimated from multivariable linear mixed models and adjusted for multiple covariates. Error bars represent 95% confidence limits. Comparisons were made between ethnic groups, with P values for lumbar spine in pre-, early peri-, late peri-, and postmenopausal women, respectively, as follows: African-American vs. Caucasian, 0.13, 0.24, 0.43, and 0.07; African-American vs. Chinese, 0.80, 0.007, 0.56, and 0.31; African-American vs. Japanese, 0.10, 0.010, 0.24, and 0.02; Caucasian vs. Chinese, 0.22, 0.041, 0.18, and 0.52; Caucasian vs. Japanese, 0.59, 0.06, 0.52, and 0.34; Chinese vs. Japanese, 0.16, 0.87, 0.11, and 0.17.
Rates of change in BMD by menopause stages within weight tertiles
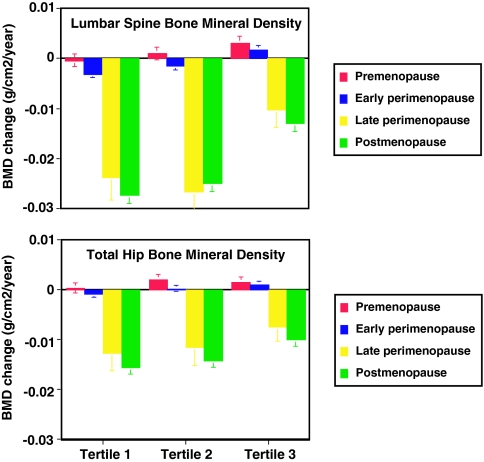
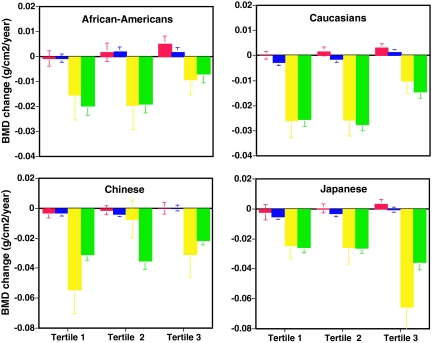
Figure 3 shows the annual rates of change in lumbar spine and total hip BMD, adjusted for covariates, during each menopausal stage by tertile of baseline body weight. In each weight tertile, BMD loss accelerated substantially in the late perimenopause. There were significant differences in rates of late peri- and postmenopausal spine BMD loss across weight tertiles (P < 0.001 for both). Rates of postmenopausal hip BMD loss also differed significantly across weight tertiles (P < 0.001). BMD loss was most rapid in women in the lowest tertile of body weight and slowest in women in the highest tertile of body weight. In late peri- and postmenopausal women, rates of spine and hip bone loss were approximately 35–55% slower in women in the top tertile vs. the bottom tertile of body weight. Similar patterns of bone loss in relation to weight tertiles were observed for the lumbar spine (Fig. 4) and total hip (data not shown) in African-American, Caucasian, and Chinese women but not in Japanese women.
Figure 3.
Annual rate of change in BMD of the lumbar spine (top panel) and total hip (bottom panel) in premenopausal (red bars), early perimenopausal (blue bars), late perimenopausal (yellow bars), and postmenopausal (green bars) women divided by tertiles of baseline body weight (tertile 1, < 60.7 kg; tertile 2, 60.7–77.3 kg; tertile 3, >77.3 kg) in the entire cohort. Rates of change were estimated from multivariable linear mixed models and adjusted for multiple covariates. Error bars represent 95% confidence limits. Comparisons were made between tertile groups, with P values for lumbar spine in pre-, early peri-, late peri-, and postmenopausal women, respectively, as follows: tertile 1 vs. 3, <0.001, <0.001, <0.001, and <0.001; tertile 2 vs. 3, 0.033, <0.001, <0.001, and <0.001; tertile 1 vs. 2, 0.13, 0.002, 0.39, and 0.044; and with P values for total hip in pre-, early peri-, late peri-, and postmenopausal women, respectively: tertile 1 vs. 3, 0.15, <0.001, 0.027, and <0.001; tertile 2 vs. 3, 0.51, 0.08, 0.09, and <0.001; tertile 1 vs. 2, 0.032, 0.017, 0.68, and 0.17.
Figure 4.
Annual rate of change in BMD of the lumbar spine in premenopausal (red bars), early perimenopausal (blue bars), late perimenopausal (yellow bars), and postmenopausal (green bars) women divided by tertiles of body weight in African-American (tertile 1, <73.4 kg; tertile 2, 73.4–91.5 kg; tertile 3, >91.5 kg), Caucasian (tertile 1, <64.0 kg; tertile 2, 64.0–78.1 kg; tertile 3, >78.1 kg), Chinese (tertile 1, <52.9 kg; tertile 2, 52.9–59.8 kg; tertile 3, >59.8 kg), and Japanese (tertile 1, <52.4 kg; tertile 2, 52.4–58.6 kg; tertile 3, >58.6 kg) women. Rates of change were estimated from multivariable linear mixed models and adjusted for multiple covariates. Error bars represent 95% confidence limits. Note that a relatively small number of Chinese and Japanese women in each weight tertile transitioned to late perimenopause or beyond (Chinese: tertile 1, n = 24; tertile 2, n = 24; tertile 3, n = 30; Japanese: tertile 1, n = 31; tertile 2, n = 31; tertile 3, n = 25) or became postmenopausal (Chinese: tertile 1, n = 22; tertile 2, n = 23; tertile 3, n = 24; Japanese: tertile 1, n = 24; tertile 2, n = 25; tertile 3, n = 22) during the follow-up period. Comparisons were made between tertile groups, with P values for African-Americans in pre-, early peri-, late peri-, and postmenopausal women, respectively, as follows: tertile 1 vs. 3, 0.008, 0.039, 0.30, and <0.001; tertile 2 vs. 3, 0.18, 0.88, 0.08, and <0.001; tertile 1 vs. 2, 0.31, 0.028, 0.57, and 0.78; with P values for Caucasians in pre-, early peri-, late peri-, and postmenopausal women, respectively: tertile 1 vs. 3, 0.012, <0.001, <0.001, and <0.001; tertile 2 vs. 3, 0.25, <0.001, <0.001, and <0.001; tertile 1 vs. 2, 0.21, 0.11, 0.95, and 0.29; with P values for Chinese in pre-, early peri-, late peri-, and postmenopausal women, respectively: tertile 1 vs. 3, 0.21, 0.015, 0.038, and <0.001; tertile 2 vs. 3, 0.56, 0.002, 0.020, and <0.001; tertile 1 vs. 2, 0.44, 0.57, <0.001, and 0.24; and with P values for Japanese in pre-, early peri-, late peri-, and postmenopausal women, respectively: tertile 1 vs. 3, 0.08, <0.001, 0.001, and 0.002; tertile 2 vs. 3, 0.18, 0.028, 0.002, and 0.003; tertile 1 vs. 2, 0.42, 0.14, 0.89, and 0.79.
Discussion
In this study, we have shown that BMD changes little during the pre- or early perimenopause but then begins to decline substantially during the late perimenopause. BMD continues to decline rapidly during the early postmenopausal years. The annual rates of loss during these intervals were approximately 1.8–2.3% in the spine and 1.0–1.4% in the hip. If bone loss were to continue at these rates for 5 yr, the average woman’s BMD would decline 7–10% in the spine and 5–7% in the hip, amounts that are associated with approximately 50–100% higher fracture rates (10). Rates of BMD loss in the late peri- and postmenopause stages were considerably faster in women in the lowest than in the highest tertile of body weight. Although there appeared to be ethnic differences in rates of BMD loss, these differences were largely eliminated when ethnic differences in body weight were controlled.
Weight and ethnicity were powerful apparent modifiers of the rate of BMD loss. Because weight and ethnicity were strongly related, we used two strategies to isolate their impact. First, we performed stratified analyses in which the effect of weight was examined in each ethnic group. As in the full cohort, rates of late perimenopausal and postmenopausal bone loss were significantly slower in both African-American and Caucasian women in the highest than in the lowest tertile of body weight. This finding demonstrates that the effect of weight on rates of bone loss was independent from ethnicity. The failure to find an effect of weight in the Chinese or Japanese women is likely because these women tended to be of a very homogeneous, light weight thus making it unlikely to see slowing of loss in the upper tertile. Moreover, the number of Japanese and Chinese women in each weight tertile of the late peri- and postmenopause is so small that the estimates of rates of bone loss may be unstable. It is possible, however, that the relationship between weight and rates of bone loss varies by ethnicity. Additional data are needed to clarify this issue. Second, the effect of ethnicity was examined in a sub-cohort of women selected to maximize the overlap of body weight across ethnic groups. Whereas rates of spine BMD loss tended to be slower in African-Americans and higher in Asians in the whole cohort, these differences were largely eliminated when analyses were restricted to women with more homogenous body weights. Thus, ethnicity did not appear to have an independent effect on rates of bone loss.
The effect of the menopause transition on changes in BMD has been assessed in both cross-sectional and longitudinal studies, but findings have been inconsistent. Some cross-sectional studies report that bone mass of the spine or proximal femur is lower in perimenopausal than in premenopausal women (11,12,13,14,15), whereas others have not detected any differences in BMD before the menopause (4,16). Some longitudinal studies of pre- or early perimenopausal women failed to detect significant decreases in spine, radius, or total-body BMD (17,18,19,20) whereas others have reported that BMD of the radius (19,21), femoral neck (22,23,24), and spine (17,22,23,24) declines during the perimenopause. Existing longitudinal studies of menopausal bone loss have important limitations, however. Most previous studies are relatively small, lack ethnic diversity, and measured BMD with insensitive and/or imprecise techniques such as single-photon absorptiometry (SPA) (25) or dual-photon absorptiometry, thereby limiting the ability to asses changes in BMD. In a group of Australian women followed for 2 yr, BMD loss was fastest in women who became postmenopausal, next fastest in women who became late perimenopausal, and undetectable in pre- and early perimenopausal women (24). Two studies have examined changes in BMD across the entire menopause transition (20,23). Ahlborg et al. (20) measured forearm BMD using SPA in 156 Caucasian women every other year from age 48–64 yr. They failed to detect a decline in BMD before the final menstrual period, probably because SPA is not a sensitive technique for detecting bone loss. Recker et al. (23) measured BMD of the spine, femoral neck, and total body annually for an average of 9.5 yr in 75 women who were premenopausal and at least 46 yr old (mean age 49 yr). During the first 3 yr, measurements were made using dual-photon absorptiometry. Subsequently, measurements were made using two different DXA machines. BMD changed in a sigmoid pattern with loss beginning 2–3 yr before the final menstrual period and ending 3–4 yr after the final menstrual period. The small sample size and the changes in measurement technique limit interpretation of these data, however.
After the menopause, some women lose bone much more rapidly than others (3,5,6). For example, forearm bone loss may vary from 0–50% in the first 6–8 yr after menopause (26). It is unclear why some postmenopausal women lose bone rapidly, whereas others lose bone slowly. Several studies have found that higher body mass index and weight are associated with slower rates of peri- and postmenopausal bone loss (3,24,26,27). Weight change has also been associated with changes in BMD (28). Weight was also a powerful determinant of bone loss in our women, and its effect was independent from ethnicity. Based on these results, clinicians should be particularly aware of the risk of bone loss in women who are thin.
The reasons why higher body weight is associated with slower rates of bone loss are unknown. Osteocytes are thought to sense mechanical loading of the skeleton (29). It is possible that in response to greater mechanical loading, osteocytes send signals to other bone cells that either reduce osteoclastic bone resorption, as evidenced by an inverse association between bone turnover and BMI (27), or increase osteoblastic bone formation resulting in an attenuation of bone loss in heavier women. Increased production of estrogens by adipose tissue may also contribute to the association between BMD and body weight. Alternatively, it is possible that the apparent inverse relationship between changes in BMD and body weight is related, at least in part, to technical limitations of DXA technology. Simulations using phantoms suggest that measured BMD is related to the extraosseous soft tissue composition, the mix of red to yellow marrow within the bone being measured, and the homogeneity of soft tissue in the region of interest (30,31). Moreover, these simulations suggest that the degree of DXA artifact is related to the extraosseous soft tissue composition so that BMD will appear to decrease more slowly in subjects with more soft tissue fat and vice versa (30,31). Because estimations of the relationship between BMD and fracture risk have also included these potential artifacts, however, the prognostic value of BMD data for fracture prediction likely remains clinically relevant.
There is a wide disparity in BMD, bone turnover, and fracture incidence across ethnic groups (9,32,33,34,35,36,37,38,39,40,41,42). Ethnic differences in BMD may reflect differences in peak BMD, rates of bone loss, or both. African-American women have higher cortical and trabecular BMD than Caucasians, and this difference may account for their decreased incidence of osteoporotic fractures (32,33,35,36,37,38,43,44). We previously found similar differences in lumbar spine and proximal femur BMD between pre- and early perimenopausal African-American and Caucasian women in SWAN, although these differences were attenuated when women were matched for body weight (9). Like African-Americans, Chinese and Japanese women have lower hip and spine fracture rates than Caucasians (39,40,41,45,46), even though they also have lower BMD if body size is not taken into account (46,47,48,49,50). In SWAN, we also found that unadjusted spine and femoral neck BMDs were higher in Caucasians than in Chinese and Japanese women (9). After adjustment for body weight, however, femoral neck BMD was similar in Asian and Caucasian women, and spine BMD was actually lower in Caucasian women (9). Thus, fracture rates may be higher in Caucasians than in African-American or Asian women because of lower peak premenopausal BMDs for their weight.
Although many studies have examined bone loss in Caucasian women, very little is known about changes in BMD in non-Caucasian women. Cross-sectional data suggest that radial BMD may decrease more slowly in postmenopausal African-American than Caucasian women, whereas rates of spine bone loss are similar (37). In contrast, one longitudinal study suggested that bone loss from the hip was actually more rapid in elderly African-American women than in elderly Caucasians (51). Limited longitudinal data suggest that BMD begins to decline in perimenopausal Japanese women (50,52,53,54,55,56). As in Caucasian women, the rate of bone loss in Japanese women is inversely related to body weight (54). No previous studies have directly compared longitudinal rates of bone loss across multiple ethnic groups. The present data demonstrate that apparent ethnic differences in rates of BMD loss during the menopause transition are largely due to ethnic differences in body weight.
Some limitations of this study deserve mention. Although we followed women for up to 5 yr, we could not capture the entire menopause transition in many women. A longer period of follow-up will allow assessment of BMD across the entire menopause transition in most all women in the future. BMD was assessed annually. More frequent measurements would have allowed a more precise and detailed description of patterns of bone loss during the transition.
Our findings may have important clinical implications. Most published guidelines do not recommend routine screening of women for osteoporosis until age 65 (57). However, like BMD (58,59), the rate of bone loss is an independent predictor of fracture risk in postmenopausal women (60,61,62). Because the rate of BMD loss accelerates markedly in the late perimenopause, our data suggest that clinicians should consider measuring BMD once a woman has experienced 3 months of amenorrhea. Intervention may be warranted if a woman has relatively low BMD at the beginning of the menopause transition and is beginning to lose bone rapidly. Clinicians should be particularly wary of the risk for rapid bone loss in late perimenopausal women with low body weight. The finding of accelerated BMD loss in the lightest women adds important support for the recommendation included in several published guidelines that screening bone densitometry should be done at an earlier age in thin women (57). The cost-effectiveness of a broader BMD screening program needs to be assessed.
In conclusion, there is little if any change in BMD in midlife pre- or early perimenopausal women. BMD loss increases substantially in the late perimenopause and remains rapid in the first few postmenopausal years. Body weight is an important determinant of the rates of BMD loss during the menopause transition. These findings suggest that healthcare providers should consider screening for osteoporosis when women enter the late stages of the menopause transition, particularly if they have relatively low body weight.
Supplementary Material
Acknowledgments
We acknowledge the individuals participating at the following clinical centers: University of Michigan, Ann Arbor, MI [MaryFran Sowers, Principal Investigator (PI)]; Massachusetts General Hospital, Boston, MA (Robert M. Neer, PI, 1994–1999; Joel S. Finkelstein, PI, 1999 to present); Rush University Medical Center, Chicago, IL (Lynda Powell, PI); University of California, Davis/Kaiser, Davis, CA (Ellen Gold, PI); University of California, Los Angeles, CA (Gail A. Greendale, PI); University of Medicine and Dentistry New Jersey Medical School, Newark, NJ (Gerson Weiss, PI, 1994–2004; Nanette Santoro, PI, 2004 to present); and the University of Pittsburgh, Pittsburgh, PA (Karen Matthews, PI); at the National Institutes of Health (NIH) Program Office: National Institute on Aging, Bethesda, MD (Marcia Ory, 1994–2001; Sherry Sherman, 1994 to present); National Institute of Nursing Research, Bethesda, MD (Program Officers); Central Laboratory: University of Michigan, Ann Arbor (Daniel McConnell, Central Ligand Assay Satellite Services); and coordinating center: New England Research Institutes, Watertown, MA (Sonja McKinlay, PI, 1995–2001); University of Pittsburgh, Pittsburgh, PA (Kim Sutton-Tyrrell, PI, 2001 to present). We also acknowledge Chris Gallagher and Susan Johnson, Chairs of the Steering Committee.
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
SWAN has grant support from the NIH, Department of Health and Human Services, through the National Institute on Aging, the National Institute of Nursing Research, and the NIH Office of Research on Women’s Health (Grants NR004061, AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, and AG012495). This work was also supported by NIH Grant K24 DK02759 (to J S.F.).
Disclosure Statement: J.S.F. is on the Speakers Bureau for Merck & Co. and Novartis Pharmaceuticals and has research support from Solvay Pharmaceuticals, AstraZeneca Pharmaceuticals LP, and Ardana Bioscience Ltd. B.E. is a consultant for Procter and Gamble, GlaxoSmithKline, Roche Pharmaceuticals, and Barr Pharmaceuticals; has received lecture fees from Eli Lilly & Co. and Barr Pharmaceuticals; and has research support from Procter and Gamble. J.A.C. is a consultant for Eli Lilly & Co. and Novartis Pharmaceuticals, is on the Speaker’s Bureau for Merck & Co., and has received research support from Merck & Co., Eli Lilly & Co., Pfizer Inc., and Novartis Pharmaceuticals. M.R.S. has received lecture fees from Wyeth Pharmaceuticals. The remaining authors have nothing to disclose.
First Published Online December 26, 2007
Abbreviations: BMD, Bone mineral density; DXA, dual-energy x-ray absorptiometry; SPA, single-photon absorptiometry; SWAN, Study of Women’s Health Across the Nation.
References
- Finkelstein JS 2004 Osteoporosis. In: Goldman L, Ausiello D, eds. Cecil textbook of medicine. 22nd ed. Philadelphia: Saunders; 1547–1555 [Google Scholar]
- Riggs BL, Melton III LJ 1992 The prevention and treatment of osteoporosis. N Engl J Med 327:620–627 [DOI] [PubMed] [Google Scholar]
- Reeve J, Walton J, Russell LJ, Lunt M, Wolman R, Abraham R, Justice J, Nicholls A, Wardley-Smith B, Green JR, Mitchell A 1999 Determinants of the first decade of bone loss after menopause at spine, hip and radius. QJM 92:261–273 [DOI] [PubMed] [Google Scholar]
- Ravn P, Hetland ML, Overgaard K, Christiansen C 1994 Premenopausal and postmenopausal changes in bone mineral density of the proximal femur measured by dual-energy x-ray absorptiometry. J Bone Miner Res 9:1975–1980 [DOI] [PubMed] [Google Scholar]
- Falch JA, Sandvik L 1990 Perimenopausal appendicular bone loss: a 10-year prospective study. Bone 11:425–428 [DOI] [PubMed] [Google Scholar]
- Hansen MA, Overgaard K, Riis B, Christiansen C 1991 Role of peak bone mass and bone loss in postmenopausal osteoporosis: 12 year study. BMJ 303:961–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers MF, Crawford S, Sternfeld B, Morganstein D, Gold EB, Greendale G, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J 2000 Design, survey, sampling and recruitment methods of SWAN: a multi-center, multi-ethnic, community based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus M, eds. Menopause: biology and pathobiology. San Diego: Academic Press; 175–188 [Google Scholar]
- Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE 1992 Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc 92:686–693 [PubMed] [Google Scholar]
- Finkelstein JS, Lee ML, Sowers M, Ettinger B, Neer RM, Kelsey JL, Cauley JA, Huang MH, Greendale GA 2002 Ethnic variation in bone density in premenopausal and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab 87:3057–3067 [DOI] [PubMed] [Google Scholar]
- Marshall D, Johnell O, Wedel H 1996 Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312:1254–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlot ME, Sornay-Rendu E, Garnero P, Vey-Marty B, Delmas PD 1997 Apparent pre- and postmenopausal bone loss evaluated by DXA at different skeletal sites in women: the OFELY cohort. J Bone Miner Res 12:683–690 [DOI] [PubMed] [Google Scholar]
- Buchanon JR, Myers C, Lloyd T, Greer RB 1988 Early vertebral trabecular bone loss in normal premenopausal women. J Bone Miner Res 3:583–587 [DOI] [PubMed] [Google Scholar]
- Block JE, Smith R, Glueer CC, Steiger P, Ettinger B, Genant HK 1989 Models of spinal trabecular bone loss as determined by quantitative computed tomography. J Bone Miner Res 4:249–257 [DOI] [PubMed] [Google Scholar]
- Johnston CC, Hui SL, Witt RM, Appledorn R, Baker RS, Longcope C 1985 Early menopausal changes in bone mass and sex steroids. J Clin Endocrinol Metab 61:905–913 [DOI] [PubMed] [Google Scholar]
- Steinberg KK, Freni-Titulaer LW, DePuey EG, Miller DT, Sgoutas DS, Coralli CH, Phillips DL, Rogers TN, Clark RV 1989 Sex steroids and bone density in premenopausal and perimenopausal women. J Clin Endocrinol Metab 69:533–539 [DOI] [PubMed] [Google Scholar]
- Ebeling PR, Atley LM, Guthrie JR, Burger HG, Dennerstein L, Hopper JL, Wark JD 1996 Bone turnover markers and bone density across the menopausal transition. J Clin Endocrinol Metab 81:3366–3371 [DOI] [PubMed] [Google Scholar]
- Pouilles JM, Tremollieres F, Ribot C 1993 The effects of menopause on longitudinal bone loss from the spine. Calcif Tissue Int 52:340–343 [DOI] [PubMed] [Google Scholar]
- Recker RR, Lappe JM, Davies KM, Kimmel DB 1992 Change in bone mass immediately before menopause. J Bone Miner Res 7:857–862 [DOI] [PubMed] [Google Scholar]
- Slemenda C, Hui SL, Longcope C, Johnston CC 1987 Sex steroids and bone mass. A study of changes about the time of menopause. J Clin Invest 80:1261–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlborg HG, Johnell O, Nilsson BE, Jeppsson S, Rannevik G, Karlsson MK 2001 Bone loss in relation to menopause: a prospective study during 16 years. Bone 28:327–331 [DOI] [PubMed] [Google Scholar]
- Sowers MR, Clark MK, Hollis B, Wallace Rb, Jannausch M 1992 Radial bone mineral density in pre- and perimenopausal women: a prospective study of rates and risk factors for loss. J Bone Miner Res 7:647–657 [DOI] [PubMed] [Google Scholar]
- Sowers M, Crutchfield M, Bandekar R, Randolph JF, Shapiro B, Schork MA, Jannausch M 1998 Bone mineral density and its change in pre-and perimenopausal white women: the Michigan Bone Health Study. J Bone Miner Res 13:1134–1140 [DOI] [PubMed] [Google Scholar]
- Recker R, Lappe J, Davies K, Heaney R 2000 Characterization of perimenopausal bone loss: a prospective study. J Bone Miner Res 15:1965–1973 [DOI] [PubMed] [Google Scholar]
- Guthrie JR, Ebeling PR, Hopper JL, Barrett-Connor E, Dennerstein L, Dudley EC, Burger HG, Wark JD 1998 A prospective study of bone loss in menopausal Australian-born women. Osteoporos Int 8:282–290 [DOI] [PubMed] [Google Scholar]
- Abrahamsen B, Stilgren LS, Hermann AP, Tofteng CL, Barenholdt O, Vestergaard P, Brot C, Nielsen SP 2001 Discordance between changes in bone mineral density measured at different skeletal sites in perimenopausal women: implications for assessment of bone loss and response to therapy: the Danish Osteoporosis Prevention Study. J Bone Miner Res 16:1212–1219 [DOI] [PubMed] [Google Scholar]
- Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L 1995 A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas 21:103–113 [DOI] [PubMed] [Google Scholar]
- Ravn P, Cizza G, Bjarnason NH, Thompson D, Daley M, Wasnich RD, McClung M, Hosking D, Yates AJ, Christiansen C 1999 Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. Early Postmenopausal Intervention Cohort (EPIC) study group. J Bone Miner Res 14:1622–1627 [DOI] [PubMed] [Google Scholar]
- Macdonald HM, New SA, Campbell MK, Reid DM 2005 Influence of weight and weight change on bone loss in perimenopausal and early postmenopausal Scottish women. Osteoporos Int 16:163–171 [DOI] [PubMed] [Google Scholar]
- Seeman E, Delmas PD 2006 Bone quality: the material and structural basis of bone strength and fragility. N Engl J Med 354:2250–2261 [DOI] [PubMed] [Google Scholar]
- Bolotin HH 2001 Inaccuracies inherent in dual-energy x-ray absorptiometry in vivo bone mineral densitometry may flaw osteopenic/osteoporotic interpretations and mislead assessment of antiresorptive therapy effectiveness. Bone 28:548–555 [DOI] [PubMed] [Google Scholar]
- Bolotin HH, Sievanen H, Grashuis JL 2003 Patient-specific DXA bone mineral density inaccuracies: quantitative effects of nonuniform extraosseous fat distributions. J Bone Miner Res 18:1020–1027 [DOI] [PubMed] [Google Scholar]
- Trotter M, Broman GE, Peterson RR 1960 Densities of bone of white and Negro skeletons. J Bone Joint Surg 42A:50–58 [PubMed] [Google Scholar]
- Farmer ME, White LR, Brody JA, Bailey KR 1984 Race and sex differences in hip fracture incidence. Am J Public Health 74:1374–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein JS, Sowers M, Greendale GA, Lee ML, Neer RM, Cauley JA, Ettinger B 2002 Ethnic variation in bone turnover in pre- and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab 87:3051–3056 [DOI] [PubMed] [Google Scholar]
- Liel Y, Edwards J, Shary J, Spicer KM, Gordon L, Bell NH 1988 The effects of race and body habitus on bone mineral density of the radius, hip, and spine in premenopausal women. J Clin Endocrinol Metab 66:1247–1250 [DOI] [PubMed] [Google Scholar]
- Luckey MM, Meier DE, Mandeli JP, DaCosta MC, Hubbard ML, Goldsmith SJ 1989 Radial and vertebral bone density in white and black women: evidence for racial differences in premenopausal bone homeostasis. J Clin Endocrinol Metab 69:762–770 [DOI] [PubMed] [Google Scholar]
- Meier DE, Luckey MM, Wallenstein S, Lapinski RH, Catherwood B 1992 Racial differences in pre- and postmenopausal bone homeostasis: association with bone density. J Bone Miner Res 7:1181–1189 [DOI] [PubMed] [Google Scholar]
- Kleerekoper M, Nelson DA, Peterson EL, Flynn MJ, Pawluszka AS, Jacobsen G, Wilson P 1994 Reference data for bone mass, calciotropic hormones, and biochemical markers of bone remodeling in older (55–75) postmenopausal white and black women. J Bone Miner Res 9:1267–1276 [DOI] [PubMed] [Google Scholar]
- Cummings SR, Cauley JA, Palermo L, Ross PD, Wasnich RD, Black D, Faulkner KG 1994 Racial differences in hip axis lengths might explain racial differences in rates of hip fracture. Osteoporosis Int 4:226–229 [DOI] [PubMed] [Google Scholar]
- Ross PD, Norimatsu H, Davis JW, Yano K, Wasnich RD, Fujiwara S, Hosoda Y, Melton LJ 1991 A comparison of hip fracture incidence among native Japanese, Japanese Americans, Mexicans, and Caucasians. Am J Epidemiol 133:801–809 [DOI] [PubMed] [Google Scholar]
- Silverman SL, Madison RE 1988 Decreased incidence of hip fracture in Hispanics, Asians, and Blacks: California hospital discharge data. Am J Public Health 78:1482–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell-Aulet M, Wang J, Thornton JC, Colt EWD, Pierson RN 1993 Bone mineral density and mass in a cross-sectional study of white and Asian women. J Bone Miner Res 8:575–582 [DOI] [PubMed] [Google Scholar]
- Cohn SH, Abesamis C, Yasumura A, Aloia JF, Zanzi I, Ellis KJ 1977 Comparative skeletal mass and radial bone mineral content in black and white women. Metabolism 26:171–178 [DOI] [PubMed] [Google Scholar]
- Ortiz O, Russell M, Daley TL, Baumgartner RN, Waki M, Lichtman S, Wang J, Pierson RN, Heymsfield SB 1992 Differences in skeletal muscle and bone mineral mass between black and white females and their relevance to estimates of body composition. Am J Clin Nutr 55:8–13 [DOI] [PubMed] [Google Scholar]
- Lauderdale DL, Jacobsen SJ, Furner SE 1997 Hip fracture incidence among elderly Asian-American populations. Am J Epidemiol 146:502–509 [DOI] [PubMed] [Google Scholar]
- Ling X, Cummings SR, Mingwei Q, Xihe Z, Xioashu C, Nevitt M, Stone K 2000 Vertebral fractures in Beijing, China: the Beijing Osteoporosis Project. J Bone Miner Res 15:2019–2025 [DOI] [PubMed] [Google Scholar]
- Yano K, Wasnich RD, Vogel JM, Heilbrun LK 1984 Bone mineral measurements among middle aged and elderly Japanese residents in Hawaii. Am J Epidemiol 119:751–764 [DOI] [PubMed] [Google Scholar]
- Yu W, Qin M, Xu L, van Kuijk C, Meng X, Xing X, Cao J, Genant HK 1999 Normal changes in spinal bone mineral density in a Chinese population: assessment by quantitative computed tomography and dual-energy x-ray absorptiometry. Osteoporos Int 9:179–187 [DOI] [PubMed] [Google Scholar]
- Tsai KS, Cheng WC, Sanchez TV, Chen CK, Chieng PU, Yang RS 1997 Bone densitometry of proximal femur in Chinese subjects: gender differences in bone mass and bone areas. Bone 20:365–369 [DOI] [PubMed] [Google Scholar]
- Ito M, Lang TF, Jergas M 1997 Spinal trabecular bone loss and fracture in American and Japanese women. Calcif Tissue Int 61:123–128 [DOI] [PubMed] [Google Scholar]
- Cauley JA, Ensrud KE, Kuller LH, Cummings SR 1996 Hip bone loss increases with age among elderly African American women. The Study of Osteoporotic Fractures. J Bone Miner Res 11:S154 [Google Scholar]
- Iki M, Kajita E, Dohi Y 1996 Age, menopause, bone turnover markers and bone loss in healthy Japanese women. Maturitas 25:59–67 [DOI] [PubMed] [Google Scholar]
- Hagino H, Yamamoto K, Teshima R, Kishimoto H, Kagawa T 1992 Radial bone mineral changes in pre- and postmenopausal healthy Japanese women: cross-sectional and longitudinal studies. J Bone Miner Res 7:147–152 [DOI] [PubMed] [Google Scholar]
- Fujiwara S, Fukunaga M, Nakamura T, Chen JT, Shiraki M, Hashimoto T, Yoh K, Mizunuma H, Tomomitsu T, Kasagi F, Masunari N, Orimo H 1998 Rates of change in spinal bone density among Japanese women. Calcif Tissue Int 63:202–207 [DOI] [PubMed] [Google Scholar]
- Okano H, Mizunuma H, Soda M, Kagami I, Miyamoto S, Ohsawa M, Ibuki Y, Shiraki M, Suzuki T, Shibata H 1998 The long-term effect of menopause on postmenopausal bone loss in Japanese women: results from a prospective study. J Bone Miner Res 13:303–309 [DOI] [PubMed] [Google Scholar]
- Tsunenari T, Yamada S, Kawakatsu M, Negishi H, Tsutsumi M 1995 Menopause-related changes in bone mineral density in Japanese women: a longitudinal study on lumbar spine and proximal femur. Calcif Tissue Int 56:5–10 [DOI] [PubMed] [Google Scholar]
- Morris CA, Cabral D, Cheng H, Katz JN, Finkelstein JS, Avorn J, Solomon DH 2004 Patterns of bone mineral density testing: current guidelines, testing rates, and interventions. J Gen Intern Med 19:783–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DM, Cummings SR, Genant HK, Nevitt MC, Palermo L, Browner W 1992 Axial and appendicular bone density predict fractures in older women. J Bone Miner Res 7:633–638 [DOI] [PubMed] [Google Scholar]
- Cauley JA, Lui LY, Ensrud KE, Zmuda JM, Stone KL, Hochberg MC, Cummings SR 2005 Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA 293:2102–2108 [DOI] [PubMed] [Google Scholar]
- Gnudi S, Malavolta N, Lisi L, Ripamonti C 2001 Bone mineral density and bone loss measured at the radius to predict the risk of nonspinal osteoporotic fracture. J Bone Miner Res 16:1130–1135 [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Center JR, Eisman JA 2005 Femoral neck bone loss predicts fracture risk independent of baseline BMD. J Bone Miner Res 20:1195–1201 [DOI] [PubMed] [Google Scholar]
- Sornay-Rendu E, Munoz F, Duboeuf F, Delmas PD 2005 Rate of forearm bone loss is associated with an increased risk of fracture independently of bone mass in postmenopausal women: the OFELY study. J Bone Miner Res 20:1929–1935 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.