Abstract
Context: Clomiphene and insulin sensitizers such as metformin are used to induce ovulation in polycystic ovary syndrome (PCOS), but the ovulatory response is variable, and the causes of this variation are poorly understood.
Objective: Our objective was to identify predictive genetic polymorphisms and other determinants of ovulatory response.
Design: This was a substudy of a multicenter randomized clinical trial.
Setting: This study was performed at academic medical centers and their affiliates.
Participants: A total of 312 women with PCOS were included in the study.
Main Outcome Measures: Historical, biometric, biochemical, and genetic parameters were performed.
Results: We found that the C allele of a single nucleotide polymorphism in the STK11 gene (expressed in liver; also known as LKB1) was associated with a significantly decreased chance of ovulation in PCOS women treated with metformin. In an analysis of ovulation per cycle, the adjusted odds ratio (OR) comparing the C/C genotype to the G/G genotype was 0.30 [95% confidence interval (CI) 0.14, 0.66], and the OR for the C/G genotype vs. the G/G genotype was also 0.30 (95% CI 0.14, 0.66). In an analysis of metformin-treated subjects, we found that the percentage of women who ovulated increased with the number of G alleles present: 48% (10 of 21) of C/C women, 67% (32 of 48) of C/G women, and 79% (15 of 19) of G/G women ovulated. We also found that increased frequency of ovulation was associated with lower body mass index (BMI) [adjusted OR of 2.36 (95% CI 1.65, 3.36) and 2.05 (95% CI 1.46, 2.88), respectively, for comparisons of BMI less than 30 vs. BMI equal to or more than 35, BMI 30–34 vs. BMI equal to or more than 35, in the analysis of ovulation per cycle], a lower free androgen index (FAI) [adjusted OR of 1.59 (95% CI 1.17, 2.18) for FAI < 10 vs. FAI ≥ 10], and a shorter duration of attempting conception [adjusted OR of 1.63 (95% CI 1.20, 2.21) for < 1.5 vs. ≥ 1.5 yr].
Conclusions: We have demonstrated that a polymorphism in STK11, a kinase gene expressed in liver and implicated in metformin action, is associated with ovulatory response to treatment with metformin alone in a prospective randomized trial. The interaction with the effects of changes in modifiable factors (e.g. BMI or FAI) requires further study.
A polymorphism in the hepatic serine-threonine kinase gene STK11, which has been implicated in metformin action, is associated with poor ovulatory response to metformin treatment of polycystic ovary syndrome patients.
Identifying genes that predict or modify response to commonly prescribed medications will allow clinicians to better choose among existing therapies and individualize treatment (1). This is especially important for heterogeneous and poorly understood disorders such as polycystic ovary syndrome (PCOS). PCOS is a common endocrinopathy affecting women, and is characterized by hyperandrogenism, chronic anovulation, and polycystic ovaries, and depending on the diagnostic criteria, affects from 8–15% of the female population (2,3). The best treatment for the disorder is unknown, and there is a paucity of randomized controlled trials to guide treatment (4,5).
Restoring ovulation is pivotal to treating many of the patient complaints, including infertility, dysfunctional, or absent uterine bleeding, and a desire to prevent endometrial cancer. Improving ovulatory frequency may also benefit hyperandrogenic stigmata such as hirsutism, acne, and alopecia through normalization of circulating sex steroid levels.
We recently conducted a randomized double-blind trial [Pregnancy in Polycystic Ovary Syndrome (PPCOS)] in which we examined the effects of clomiphene, metformin, and the combination of the two on live birth in women with PCOS but also studied ovulation rates during the study (6). Despite treatment, a substantial proportion of women, 52 of 209 (24.9%) in the clomiphene group, 93 of 208 (44.7%) in the metformin group, and 35 of 209 (16.7%) in the combined group, experienced no documented ovulations over the course of the study.
In a pharmacogenetics substudy, we obtained DNA from participants, and sought to examine the predictive effect of genetic variation in key genes associated with drug response, alone or in combination with other factors that might modify drug action in women. Single nucleotide polymorphisms (SNPs) in selected candidate genes were tested for association with response to treatment with clomiphene and/or metformin based on previous findings of association with drug response or action: estrogen receptor 1 (ESR1, formerly known as ERa); two cytochrome P450 genes (CYP2C9 and CYP2D6); and a serine-threonine kinase gene expressed in the liver, STK11 (formerly known as LKB1).
Several studies have reported an association between response to hormone replacement therapies and the ESR1 PvuII polymorphism (IVS1–401, SNP rs2234693) (7,8), and variation in two cytochrome P450 genes (CYP2C9 and CYP2D6) has been associated with varied metabolism (9), and response to selective estrogen receptor modulators (SERMs), including tamoxifen (10). Although no specific genetic variation has been associated with response to metformin, a recent study has shown that STK11 is required in the liver to control blood glucose levels (11).
STK11 phosphorylates and activates AMP-activated protein kinase, and is also a tumor suppressor gene implicated in the etiology of the Peutz-Jeghers syndrome (11). The deletion of STK11 (LKB1) in the liver of adult mice resulted in hyperglycemia with increased gluconeogenic and lipogenic gene expression, and its presence was required for metformin efficacy. We also tested a microsatellite marker, D19S884 located in an intron of fibrillin 3 (FBN3), which has been consistently associated with PCOS in family studies (12).
Subjects and Methods
We have previously published the rationale for choosing live birth as the primary outcome (4), the power analysis and main statistical methods (13), the utility of infertility screening in the study (14), and study design and baseline characteristics of the study population (15), and the primary results of the study (6).
Participants
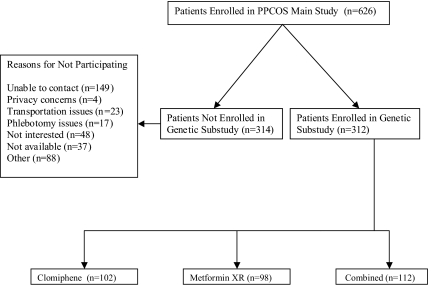
The institutional review board at each center approved the protocol, and all participants gave written informed consent. A total of 312 women infertile women with PCOS participated in this substudy out of 626 who participated in the main randomized study. PCOS was defined as unexplained hyperandrogenic chronic anovulation, and women were randomly assigned to one of three treatment arms by an interactive voice system, and stratified based on study site and prior exposure to either of the study drugs (Fig. 1) (15). Subjects with other causes of infertility, including male factor and tubal factor, were excluded.
Figure 1.
Study flow chart.
Interventions
We performed a double-blind, double-dummy study. Extended-release metformin (Glucophage XR) plus identical placebo were provided by Bristol-Myers Squibb (New York, NY). Overencapsulated clomiphene citrate tablets (Teva Pharmaceuticals, North Wales, PA) and matching placebo capsules were packaged and tested by a commercial pharmacy supply company (Clinical Trial Services, Inc., Research Triangle Park, NC), specifically for this study. Neither manufacturer had another role in the study.
Baseline laboratory testing was performed with subjects in the fasting state. All specimens were analyzed in a core laboratory, which was blinded to treatment allocation (15). Each subject received a monthly medication package consisting of bottle M (metformin in 500-mg tablets or matching placebo) and blister pack C (clomiphene in 50-mg tablets or matching placebo). Both drugs were begun concurrently. Patients gradually increased their dose of bottle M until reaching the maximum dose of four tablets (two tablets twice a day). Patients took 5 d of tablets from blister pack C beginning on d 3 of menses; this dose was maintained if adequate ovulation was documented. However, in non or poor responders, the dose was increased by one tablet a day on a treatment-cycle basis (either after 5-wk anovulation or after a menses until the maximum dose of three tablets per day was reached).
All patients had progesterone levels measured weekly to biweekly in local laboratories to document ovulation (15). If two consecutive elevated progesterone levels (>5 ng/ml) were noted, a weekly pregnancy test was administered until positive or menses occurred. After the baseline visit, subjects returned each month for a more extensive visit, including a limited physical examination, urine pregnancy test, and repeat fasting blood tests (15). A free androgen index (FAI) was calculated from the following formula: FAI = [total testosterone (nmol/liter)/SHBG (nmol/liter)] × 100. Subjects were treated for up to six cycles or 30 wk. All study medication was discontinued upon a positive pregnancy test. The present study was initiated midway through the PPCOS study, and DNA was obtained from blood independently of the study visit in the main PPCOS study.
Genotyping methods
DNA was prepared from whole blood samples using an AutoGen automated nucleic acid extraction system (AutoGen Inc., Holliston, MA) according to the manufacturer’s protocols. Genotypes for six SNPs and the microsatellite marker D19S884 described in Table 1 were obtained by methods described in Stewart et al. (12). D19S884 genotypes were determined using an Applied Biosystems 3130 capillary sequencer (Applied Biosystems, Foster City, CA) and Applied Biosystems GeneMapper software. We designate SNPs by gene name_SNP ID (e.g. STK11_rs8111699). The corresponding genotypes for these SNPs were tested for Hardy-Weinberg proportions in our sample, and none deviated significantly.
Table 1.
SNP and microsatellite markers genotyped in 312 PCOS study participants
| Gene | Marker | Alleles | MAFa | MAFb | Location |
|---|---|---|---|---|---|
| STK11 | rs741765 | C/T | T: 0.15 | T: 0.22 | Intron |
| STK11 | rs8111699 | C/G | C: 0.53 | C: 0.50 | Intron |
| ESR1 | rs2234693 | C/T | C: 0.41 | C: 0.44 | Intron: IVS-401/PvuII (8) |
| CYP2C9 | rs1934963 | C/T | C: 0.15 | C: 0.16 | Intron |
| CYP2C9 | rs1799853 | C/T | T: 0.10 | T: 0.10 | Cys144Arg |
| CYP2D6 | rs3892097 | C/T | T: 0.18 | T: 0.16 | Acceptor splice site: 1846G>A |
| FBN3 | D19S884 | A8/Xc | A8: 0.17 | A8: 0.19 | Intron (12) |
Minor allele frequency (MAF) was taken from dbSNP (http://www.ncbi.nlm.nih.gov/SNP).
Minor allele frequency estimated in the present study.
D19S884 alleles are described by Stewart et al. (12). D19S884-allele 8 (A8) was chosen for analysis because of its association with PCOS. X, any allele other than allele 8.
Data management and statistical analyses
The primary outcome of the trial was the association between SNPs in candidate genes and ovulation rate. Secondary outcomes included the change in response parameters such as FAI, SHBG, insulin, and glucose, which at baseline were associated with an ovulatory response to treatment. All data entry, data management, and analyses were performed at the Data Coordinating Center at the Duke Clinical Research Institute. The study began enrolling patients in November 2002 and completed enrollment in December 2004.
For testing differences between subjects who participated and who did not participate in the genetic substudy, χ2 or Fisher’s exact test was used for categorical variables, and Wilcoxon rank sum tests were used for continuous variables. For descriptive statistics, we examined proportion of ovulated cycles for each individual and compared the rate of at least one ovulated cycles, as well as the mean of the proportion of ovulated cycles between genotypes. The following modeling strategies were used. Generalized estimating equations were used for analysis of ovulation rate per cycle, in which each cycle is an observation unit, to account for correlation of multiple ovulation cycles within a subject. Logistic regression was used for analysis of ovulation rate per patient, in which each patient is an observation unit with an outcome of one if ever ovulated and zero if never ovulated over the course of study. Linear regression was used for modeling the change of response parameters, such as FAI, SHBG, insulin, and glucose. The following steps were used to derive our final models. To examine the interaction of genetic polymorphism with treatment, univariate analyses were performed first with both main and interaction effects of the polymorphism and the treatment group to select genes whose polymorphisms are associated at 0.1 level with the outcome within at least one treatment group. A regression selection procedure was then used to derive a final multivariate model that is adjusted for any potential baseline variables. The genes are retained in the final model if they are associated at the 0.1 level with the outcome within at least one treatment group after adjusting for the significant baseline variables. All analyses were performed with Statistical Analysis System version 8.2 (SAS Institute Inc., Cary, NC).
Results
Study population
A total of 312 subjects participated in the substudy, whereas 314 subjects did not participate (Fig. 1). Among nonparticipants, the most common reason for nonparticipation was that that the subjects were unable to be contacted (47.5%; n = 149), followed by other reasons (28.0%; n = 88), not interested (15.3%; n = 48), and not available (11.8%; n = 37). There were no significant differences in treatment groups and baseline variables among subjects who did and did not participate in the substudy (Table 2).
Table 2.
Demographic characteristics and baseline laboratory parameters of randomized patients
| Substudy participants (n = 312) | Substudy nonparticipants (n = 314) | All patients (n = 626) | P value across two patient populations | |
|---|---|---|---|---|
| Treatment | 0.46 | |||
| Clomiphene | 103 (33.0%) | 106 (33.8%) | 209 (33.4%) | |
| Metformin | 98 (31.4%) | 110 (35.0%) | 208 (33.2%) | |
| Combined | 111 (35.6%) | 98 (31.2%) | 209 (33.4%) | |
| Age | ||||
| n | 312 | 314 | 626 | 0.447 |
| Mean (sd) | 28.3 (4.0) | 27.9 (4.1) | 28.1 (4.0) | |
| BMI | ||||
| n | 312 | 313 | 625 | 0.896 |
| Mean (sd) | 35.3 (8.9) | 35.2 (8.4) | 35.2 (8.7) | |
| <30 kg/m2 | 90/312 (28.8%) | 89/313 (28.4%) | 179/625 (28.6%) | 0.993 |
| 30–34 kg/m2 | 67/312 (21.5%) | 68/313 (21.7%) | 135/625 (21.6%) | |
| ≥ 35 kg/m2 | 155/312 (49.7%) | 156/313 (49.8%) | 311/625 (49.8%) | |
| Hirsutism | ||||
| n | 312 | 314 | 626 | 0.152 |
| <8 | 65/312 (20.8%) | 56/314 (17.8%) | 121/626 (19.3%) | 0.136 |
| 8–16 | 138/312 (44.2%) | 124/314 (39.5%) | 262/626 (41.9%) | |
| ≥ 16 | 109/312 (34.9%) | 134/314 (42.7%) | 243/626 (38.8%) | |
| Ethnicity | ||||
| Not Hispanic or Latino | 234/312 (75.0%) | 228/314 (72.6%) | 462/626 (73.8%) | |
| Hispanic or Latino | 78/312 (25.0%) | 86/314 (27.4%) | 164/626 (26.2%) | 0.497 |
| Race | ||||
| White | 214/309 (69.3%) | 221/314 (70.4%) | 435/623 (69.8%) | 0.894 |
| Black or African American | 54/309 (17.5%) | 55/314 (17.5%) | 109/623 (17.5%) | |
| Asian | 8/309 (2.6%) | 9/314 (2.9%) | 17/623 (2.7%) | |
| American Indian or Alaska Native | 37/309 (12.0%) | 35/314 (11.1%) | 72/623 (11.6%) | |
| Native Hawaiian or Other Pacific Islander | 0 | 1/314 (0.3%) | 1/623 (0.2%) | |
| Previous study drug exposure | ||||
| None | 126/312 (40.4%) | 136/314 (43.3%) | 262/626 (41.9%) | 0.774 |
| Metformin only | 28/312 (9.0%) | 26/314 (8.3%) | 54/626 (8.6%) | |
| Clomiphene citrate only | 99/312 (31.7%) | 89/314 (28.3%) | 188/626 (30.0%) | |
| Metformin and clomiphene citrate | 59/312 (18.9%) | 63/314 (20.1%) | 122/626 (19.5%) | |
| Prior parity | 110/312 (35.3%) | 99/314 (31.5%) | 209/626 (33.4%) | 0.323 |
| Ultrasound | ||||
| PCO morphologya | 285/312 (91.3%) | 288/314 (91.7%) | 573/626 (91.5%) | 0.867 |
| Left ovarian volume (cm3) | ||||
| n | 312 | 314 | 626 | 0.308 |
| Mean (sd) | 10.8 (6.1) | 11.4 (6.7) | 11.1 (6.4) | |
| Right ovarian volume (cm3) | ||||
| n | 312 | 314 | 626 | 0.432 |
| Mean (sd) | 12.2 (6.9) | 11.9 (7.1) | 12.0 (7.0) | |
| Testosterone | ||||
| n | 304 | 304 | 608 | 0.063 |
| Mean (sd) | 64.2 (29.7) | 59.9 (27.4) | 62.0 (28.6) | |
| Bioavailable testosterone | ||||
| n | 304 | 304 | 608 | 0.926 |
| Mean (sd) | 9.6 (7.0) | 9.5 (6.5) | 9.5 (6.7) | |
| Glucose | ||||
| n | 306 | 302 | 608 | 0.036 |
| Mean (sd) | 90.6 (18.0) | 87.3 (16.6) | 89.0 (17.4) | |
| Insulin | ||||
| n | 306 | 304 | 610 | 0.648 |
| Mean (sd) | 24.1 (31.7) | 21.8 (20.3) | 23.0 (26.6) | |
| Proinsulin | ||||
| n | 305 | 304 | 609 | 0.757 |
| Mean (sd) | 25.2 (28.0) | 24.6 (23.5) | 24.9 (25.8) | |
| SHBG | ||||
| n | 306 | 304 | 610 | 0.116 |
| Mean (sd) | 30.8 (19.3) | 28.5 (16.7) | 29.7 (18.1) |
Either or both ovaries.
Ovulatory rate in the substudy
The rate of ovulation per cycle in the metformin group was significantly lower, 30.5% (n = 164 of 537) compared with the groups receiving clomiphene (54.8% or n = 164 of 537, in clomiphene alone and 60.2% or n = 343 of 570 in combined group) (P < 0.001 for both comparisons). Similar results are found for ovulation rate per patient (63.3% or n = 62 of 98 in metformin-only group compared with 85.4% or n = 88 of 103 in clomiphene alone and 86.5% or n = 96 of 111 in combined group; P < 0.001 for both comparisons). There was a significant advantage to combined therapy over clomiphene only. Over the course of the study, the mean (sd) number of ovulations per subject was 2.68 (1.78) in the clomiphene-only group, 1.67 (1.74) in the metformin-only group, and 3.09 (2.04) in the combination group (P < 0.001 for both comparisons).
Predictors of ovulation
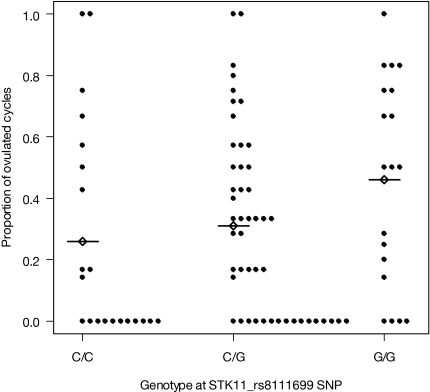
We noted within the metformin-only group a significant negative association between ovulation per cycle and the C allele of STK11_rs8111699 compared with the G/G genotype [Table 3; C/C genotype P = 0.011, and C/G genotype P = 0.002, respectively, for testing that the adjusted odds ratio (OR) = 1] in the analysis of ovulation per cycle. The adjusted OR is 0.30 for both comparisons, whereas the unadjusted OR is 0.41 for C/C vs. C/G and 0.49 for C/G vs. G/G. For descriptive statistics and unadjusted analyses, we also examined individual subjects in the metformin group by STK11 genotype; we note that 48% (10 of 21) of women in the metformin arm with genotype C/C ovulated, 67% (32 of 48) with genotype C/G ovulated, and 79% (15 of 19) with genotype G/G ovulated (Fig. 2) (P = 0.05 for C/C vs. G/G genotype; and P = 0.33 for C/G vs. G/G genotype). The mean (sd) for the proportion of ovulated cycles is 0.26 (0.35), 0.31 (0.30), and 0.46 (0.34) for C/C, C/G, and G/G groups, respectively (P = 0.05 for C/C vs. G/G genotype; and P = 0.09 for C/G vs. G/G genotype). A similar negative association trend was noted in the clomiphene group compared with the G/G genotype (Table 3; C/C genotype P = 0.071, and C/G genotype P = 0.12, respectively, for testing that the adjusted OR = 1.0). We also noted a significant positive association with the C/C genotype of ESRI_rs2234693 (the polymorphic Pvu site) compared with the T/T genotype (P = 0.034), but only in the combination therapy group. These trends were maintained when we examined the analysis results for ovulation per patient.
Table 3.
Adjusted association of genes and clinical parameters with ovulation
| Effect | Ovulation per cycle
|
Ovulation per patient
|
||
|---|---|---|---|---|
| OR (90% CL) | P value | OR (90% CL) | P value | |
| Clomiphene | ||||
| STK11_rs8111699 | ||||
| G/G | 1.0 | 1.0 | ||
| C/C | 0.53 (0.30, 0.95) | 0.071 | 1.05 (0.32, 3.42) | 0.95 |
| C/G | 0.60 (0.35, 1.02) | 0.12 | 0.62 (0.23, 1.70) | 0.43 |
| ESR1_2234693 | ||||
| T/T | 1.0 | 1.0 | ||
| C/C | 0.99 (0.5, 1.94) | 0.99 | 0.89 (0.28, 2.85) | 0.87 |
| C/T | 0.98 (0.57, 1.68) | 0.94 | 1.31 (0.48, 2.56) | 0.65 |
| Metformin | ||||
| STK11_rs8111699 | ||||
| G/G | 1.0 | 1.0 | ||
| C/C | 0.30 (0.14, 0.66) | 0.011 | 0.34 (0.10, 1.23) | 0.17 |
| C/G | 0.30 (0.16, 0.56) | 0.002 | 0.33 (0.11, 0.99) | 0.097 |
| ESR1_2234693 | ||||
| T/T | 1.0 | 1.0 | ||
| C/C | 1.01 (0.51, 2.03) | 0.97 | 0.75 (0.22, 2.53) | 0.70 |
| C/T | 1.00 (0.53, 1.86) | 0.99 | 1.00 (0.39, 2.61) | 0.99 |
| Combined | ||||
| STK11_rs8111699 | ||||
| G/G | 1.0 | 1.0 | ||
| C/C | 0.98 (0.48, 2.00) | 0.97 | 0.72 (0.23, 2.30) | 0.64 |
| C/G | 0.72 (0.40, 1.31) | 0.37 | 0.48 (0.17, 1.38) | 0.87 |
| ESR1_2234693 | ||||
| T/T | 1.0 | 1.0 | ||
| C/C | 2.71 (1.25, 5.85) | 0.034 | 3.31 (0.96, 11.41) | 0.11 |
| C/T | 1.52 (0.86, 2.69) | 0.23 | 2.12 (0.84, 5.33) | 0.18 |
| BMI | ||||
| ≥35 (reference) | 1.0 | 1.0 | ||
| 30–34 | 2.05 (1.46, 2.88) | 0.0005 | 1.54 (0.85, 2.79) | 0.23 |
| <30 | 2.36 (1.65, 3.36) | <0.0001 | 2.31 (1.23, 4.34) | 0.029 |
| Prior loss (vs. no prior loss) | 1.77 (1.26, 2.49) | 0.005 | 1.82 (1.01, 3.28) | 0.097 |
| Baseline FAI (ng/dl) | ||||
| ≥10 (reference) | 1.0 | 1.0 | 1.0 | |
| <10 | 1.59 (1.17, 2.18) | 0.014 | 1.61 (0.96, 2.70) | 0.13 |
| Duration of attempting conception | ||||
| ≥1.5 yr (reference) | 1.0 | 1.0 | ||
| <1.5 yr | 1.63 (1.20, 2.21) | 0.008 | 2.74 (1.60, 4.72) | 0.002 |
Figure 2.
Proportion of ovulated cycles for patients in the metformin group (each dot represents a subject) by genotype at STK11_rs8111699. The mean (sd, no.) proportion of ovulated cycles is 0.26 (0.35, 21), 0.31 (0.30, 48), and 0.46 (0.34, 19) for C/C, C/G, and G/G groups, respectively. Mean proportion is indicated by a diamond with line.
Other factors associated with increased ovulation per cycle were decreased body mass index (BMI), a shorter history of infertility, a lower baseline FAI, and a history of a prior pregnancy loss (Table 4). We examined changes in the FAI over the study that were associated with improved ovulation in our larger study population. We noted no significant associations between alleles of STK11_rs8111699 (and their genotypes) and these par-ameters. We found in the metformin group that the adjusted change of FAI is −0.51 for subjects with genotype C/G STK11_rs741765, whereas the adjusted change is 1.97 for subjects with genotype C/T (P = 0.045). We also found significant differences between genotypes C/C, C/T, and T/T of CYP2C9_rs1934963, in which the change of FAI is positive for genotype C/C, whereas the change is negative for genotypes of C/T and T/T. In the clomiphene group, we observed significant differences in adjusted insulin change between genotypes of C/C, C/T, and T/T of CYP2C9_rs1934963, and in adjusted glucose change between genotypes of C/C, C/T, and T/T of CYP2D6_rs3892097. In the combined group, we found significant differences in adjusted SHBG change between genotypes of C/C, C/T, and T/T of CYP2D6, in adjusted insulin change between genotypes of C/C, C/T, and T/T of CYP2C9_rs1934963, and in adjusted glucose change between genotypes of C/C, C/T, and T/T of CYP2D6_rs3892097. The adjusted changes are not consistent for the same genotype between the clomiphene and combined groups, suggesting the possibility of drug-drug interaction in the combined group.
Table 4.
Adjusted mean change of FAI, SHBG, insulin, or glucose (last value − baseline) by gene and treatment (last value = value before pregnancy or at last visit)
| Gene polymorphism | Clomiphene adjusted change (n) | Within group P value | Metformin adjusted change (n) | Within group P value | Combined adjusted change (n) | Within group P value |
|---|---|---|---|---|---|---|
| Outcome: FAI change | ||||||
| STK11_rs741765 | ||||||
| C/C | −4.66 (55) | Reference | −0.51 (53) | Reference | −4.33 (55) | Reference |
| C/T | −4.95 (26) | 0.81 | 1.97 (25) | 0.045 | −4.55 (40) | 0.84 |
| T/T | −5.72 (6) | 0.63 | −4.11 (2) | 0.32 | −2.31 (5) | 0.39 |
| CYP2C9_rs1934963 | ||||||
| C/C | −5.88 (2) | 0.66 | 4.14 (4) | 0.018 | −3.72 (6) | 0.91 |
| C/T | −5.16 (24) | 0.49 | −4.73 (16) | 0.060 | −3.98 (26) | 0.92 |
| T/T | −4.30 (66) | Reference | −2.05 (60) | Reference | −3.50 (68) | Reference |
| Outcome: SHBG change | ||||||
| CYP2D6_rs3892097 | ||||||
| C/C | 12.87 (67) | Reference | 6.85 (60) | Reference | 19.50 (78) | Reference |
| C/T | 11.35 (25) | 0.78 | 3.03 (24) | 0.48 | 29.86 (19) | 0.063 |
| T/T | 23.02 (2) | 0.48 | 3.79 (3) | 0.80 | 19.51 (5) | 1.0 |
| D19S884 | ||||||
| A8/X | 20.86 (63) | Reference | 5.30 (50) | Reference | 17.93 (58) | Reference |
| X/X | 10.63 (21) | 0.061 | 3.81 (28) | 0.76 | 27.98 (38) | 0.020 |
| Outcome: insulin change | ||||||
| CYP2C9_rs1934963 | ||||||
| C/C | 30.45 (2) | 0.29 | 6.31 (4) | 0.98 | 42.82 (6) | 0.004 |
| C/T | 30.06 (25) | 0.001 | −7.30 (16) | 0.18 | 1.77 (26) | 0.83 |
| T/T | 3.98 (66) | Reference | 5.94 (60) | Reference | 0.07 (68) | Reference |
| Outcome: glucose change | ||||||
| CYP2D6_rs3892097 | ||||||
| C/C | 1.19 (67) | Reference | 0.88 (60) | Reference | −1.31 (78) | Reference |
| C/T | 11.31 (25) | 0.021 | 0.03 (24) | 0.85 | 0.14 (19) | 0.76 |
| T/T | 13.25 (2) | 0.37 | −1.41 (3) | 0.84 | 15.56 (5) | 0.050 |
The following significant baseline variables were adjusted for the specified outcomes: baseline BMI, history of smoking, prior loss and baseline FAI for FAI change; baseline BMI and baseline SHBG for SHBG change; baseline BMI, hirsutism, and baseline insulin for insulin change; and baseline glucose for glucose change. D19S884 alleles are described in Stewart et al. (12). X, Any allele other than allele 8.
Discussion
We report here a significant negative association between ovulation in women with PCOS on metformin therapy and genotypes of a SNP in STK11, a kinase gene expressed in liver, a site of metformin action. It is plausible that some proportion of nonresponders identified by these genotypes accounts in part for the comparatively poor response of ovulation in the metformin group compared with other groups in the study. We found limited association between ovulation and polymorphisms of the estrogen receptor or with enzymes involved in drug and sex steroid metabolism. A microsatellite marker in the FBN3 gene, associated with PCOS (12), was not associated with response to the medications used in this study.
The association between polymorphisms of STK11 and metformin action has not previously been studied in humans. Our study was conducted prospectively in a large cohort of participants, and examined candidate genes that had been reported to be associated with drug response in other pharmacogenetics studies, with the exception of the FBN3 and STK11, for which there were no prior reports. It focused on an outcome of clinical relevance (i.e. ovulation in women with PCOS) chosen a priori. In addition, we analyzed the association within a model that considered other factors associated with ovulatory failure because it is unlikely that any one common polymorphism acts independently of other environmental and constitutional factors to influence response to the medication. However, the sample size was small, so the power to detect modest effects was not large. The P values reported are nominal, not adjusted for multiple testing.
Our results suggest an association between STK11 genotypes and ovulatory response, with a stepwise increase in ovulation from C/C to C/G to G/G genotype in the metformin group. However, this association was not found in the other two study groups, though a similar trend in the clomiphene group suggests that the mechanism may not involve metformin metabolism. The mechanisms for this treatment-dependent response are beyond the range of this current study but may involve drug-drug interactions in the combined group. Furthermore, we a priori focused on ovulation per cycle rather than ovulation per patient. We note that the same negative association holds up when we examine the outcome as ovulation per patient. One bias toward the identification of nonresponse genotypes in this study is that successful ovulators were more likely to become pregnant and, therefore, to end participation in the treatment phase of the study.
We elected not to use conception as the study outcome because the sample size for this outcome would be significantly smaller than for ovulatory events. Restoring ovulation is a treatment goal for women with PCOS independent of their desire to conceive because it will control bleeding complaints, reduce the risk for endometrial abnormalities such as polyps or hyperplasia, and may lessen peripheral hyperandrogenic stigmata such as hirsutism. We were also interested in the response to these medications.
Other studies have noted an association between the PvuII polymorphism in the ESR1 gene and individual response to hormone replacement therapy in terms of cholesterol levels and other circulating cardiovascular risk parameters (8,16,17). We did not examine these parameters in our study but found no association between ovulation and this polymorphism, except a significant positive association with the C/C genotype of ESR1_rs2234693 in the combined group, but no effect was noted in the clomiphene group. Again drug-drug interactions may explain these inconsistent findings. Further study of the relationship between this gene and response to other SERMs, such as tamoxifen, a triphenylethylene derivative similar to clomiphene, and raloxifene, a benzothiophene derivative, that are more widely used for other indications such as breast cancer, is needed.
There is more comprehensive literature examining the association between SERM treatment response and polymorphisms of common SERM metabolizing genes. Tamoxifen is metabolized by CYP2D6 into a more active compound, endoxifen, and inhibition of this enzyme ameliorates tamoxifen’s benefit in treating women with breast cancer (18). This study also showed a significantly worse relapse-free time and disease free survival among 223 women with breast cancer and the CYP2D6 *4/*4 genotype treated with tamoxifen (10). The metabolism of clomiphene is complex and less well studied than that of tamoxifen, and the CYP450 system is also involved in the metabolism of sex steroids, which change in response to treatment with clomiphene, and this study is unable to explore these relationships.
We modeled the effect of genes and factors such as BMI and FAI to identify predictors of ovulation. The factors that we identified are very similar to those from another large prospective series that sought predictive factors of ovulatory response to clomiphene among 201 women with World Health Organization type II oligomenorrhea, a category mainly consisting of women with PCOS (19). These authors found that the FAI, BMI, cycle history (oligomenorrhea vs. amenorrhea), serum androgen (testosterone and/or androstenedione) levels, and mean ovarian volume assessed by transvaginal sonography were all significantly different (P < 0.01) in responders from those in nonresponders, and FAI was chosen to be the best predictor in univariate analysis. We similarly found that decreasing FAI was a strong predictor of ovulation along with decreasing BMI, a shorter duration of infertility, and a history of prior pregnancy loss (19). These latter two measures may reflect a less severe history of anovulation, analogous to the relative benefit of baseline oligomenorrhea vs. amenorrhea noted by Imani et al. (19). We did not find any clear change in either FAI or glycemic parameters related to an STK11 genotype to explain the association with anovulation in our study. We did not find circulating glucose, insulin, or androgen levels, other than as part of the FAI, or ovarian volume to be a predictor in our model. Differences in our population and diagnostic criteria between studies may also contribute to varying results.
Studies that have examined predictive factors on ovulatory response to metformin have been smaller with mixed results. Moghetti et al. (20), using a logistic regression model, found that higher insulin levels, lower serum androstenedione, and less severe oligomenorrhea in women with PCOS were associated with improved menstrual frequency in response to metformin. Another study found that insulin resistance, based on homeostatic markers, identified improved ovulatory response of metformin therapy (21). However, other studies have not confirmed these predictive factors (22).
We have demonstrated that a polymorphism of a liver-expressed kinase gene (STK11) implicated in metformin action is associated with a poor ovulatory response to metformin alone in a prospective randomized trial. In this study we performed statistical tests for several SNP genotypes at each of several genes, making the approach in part exploratory. We report nominal P values, recognizing that most of these would not remain significant after correction for multiple testing. Nevertheless, the most significant of these, for STK11, are very suggestive, in view of the prior plausibility of a role for this gene. The impact of this SNP needs to be verified in larger sample sizes in other prospective trials and also in patients receiving metformin for the treatment of type 2 diabetes.
Acknowledgments
* In addition to the authors, other investigators of the National Cooperative Reproductive Medicine Network were as follows: University of Pennsylvania, Philadelphia, PA: K. Barnhart, L. Mastroianni, Jr., L. Martino, and K. Timbers; Duke University, Durham, NC: L. Lambe, R. DeWire, H. Yang, C. Bodine, and D. Mark; Wayne State University, Detroit, MI: E. Puscheck, K. Ginsburg, K. Collins, M. Brossoit, R. Leach, F. Yelian, and M. Perez; Baylor College of Medicine, Houston, TX: J. Buster, P. Amato, and M. Torres; Pennsylvania State University College of Medicine, Hershey, PA: W. C. Dodson, C. Gnatuk, J. Ober, L. Demers, and A. Kunselman; University of Medicine and Dentistry of New Jersey, Newark, NJ: D. Heller, J. Colon, G. Weiss, and A. Solnica; University of Colorado, Denver, CO: K. Gatlin and S. Hahn; University of Texas, Southwestern, Dallas, TX: M. Roark; University of Alabama, Birmingham, AL: R. Blackwell, V. Willis, and L. Love; University of Pittsburgh, Pittsburgh, PA: K. Laychak; Virginia Commonwealth University, Richmond, VA: M. Nazmy and D. Stovall; University of Virginia, Charlottesville, VA: W. Evans; Stanford University, Palo Alto, CA: K. Turner; University of California San Diego, San Diego, CA: J. Chang and P. Malcolm; Denver Health Medical Center, Denver, CO: C. Coddington; and Kaiser Permanente, Denver, CO: K. Faber.
We thank the Ligand Assay and Analysis Core Laboratory at the University of Virginia Center for Research and Reproduction, under the direction of D. Hasenleider, for their substantial contributions. We also thank Dr. Jerry Strauss at Virginia Commonwealth University for the suggestion to investigate the STK11 gene. Finally, we thank members of the Advisory Board: J. Hogan, F. Howard, M. Schiff, J. Wactawski-Wende, and N. Santoro (Chair); and express special gratitude for their careful and devoted oversight of our trial to the Data and Safety Monitoring Committee: E. Thom, J. Peipert, J. Zhang, P. Cato, C. Henderson, and R. Rebar (Chair).
Footnotes
PPCOS, ClinicalTrials.gov number: NCT00068861.
This work was supported by National Institutes of Health/National Institute of Child Health and Human Development Grants U10 HD27049 (to C.C.), U01 HD38997 (to E.R.M.), U10 HD39005 (to M.P.D.), U10 HD27011 (to S.A.C.), U10 HD33172 (to M.P.S.), U10 HD38988 (to B.R.C.), U10 HD38992 (to R.S.L.), U10 HD38998 (to W.D.S.), U10 HD38999 (to P.G.M.), U54 HD34449 National Cooperative Program in Infertility Research (to R.S.S.), U54-HD29834 University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, GCRC Grant MO1RR00056 to the University of Pittsburgh, and MO1RR10732, and construction grant C06 RR016499 to Pennsylvania State University. Glucophage XR and matching placebo were provided by Bristol-Myers Squibb.
Disclosure Summary: R.S.L. has served as a consultant to GlaxoSmithKline and Ferring, and has had lecture fees paid by Serono, meeting support from Abbott, and grant support from Pfizer. J.E.N. has equity ownership/stock options in Bristol Myers Squibb. P.C.L., E.R.M., and H.X.B. had grant support from Tap, H.X.B. also had grant support from Ortho Biotech, and E.R.M. has received research support from Merck and is also a consultant. W.D.S. has grant support from Organon and Wyeth. N.A.C. consults for Organon. P.G.M. has grant support from Ferring and Serono. M.P.D. has grant support from Serono, Tap, Glaxo Smith Klein, and Merck, and has served as a consultant for Tap and Serono. S.A.C., C.C., K.G.E., R.S.S., L.C.G., M.P.S., G.G.G., and B.R.C. have no disclosures.
Present address for P.C.L.: Department of Obstetrics and Gynecology, 100 Trent Drive, 212 Baker House, Duke University Medical Center, Durham, North Carolina 27710.
First Published Online November 13, 2007
Abbreviations: BMI, Body mass index; ESR1, estrogen receptor 1; FAI, free androgen index; FBN3, fibrillin 3; OR, odds ratio; PCOS, polycystic ovary syndrome; PPCOS, pregnancy in PCOS; SERM, selective estrogen receptor modulator; SNP, single nucleotide polymorphism.
References
- Richards JS 2007 Genetics of ovulation. Semin Reprod Med 25:235–242 [DOI] [PubMed] [Google Scholar]
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO 2004 The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 89:2745–2749 [DOI] [PubMed] [Google Scholar]
- Broekmans FJ, Knauff EA, Valkenburg O, Laven JS, Eijkemans MJ, Fauser BC 2006 PCOS according to the Rotterdam consensus criteria: change in prevalence among WHO-II anovulation and association with metabolic factors. BJOG 113:1210–1217 [DOI] [PubMed] [Google Scholar]
- Legro RS, Myers E 2004 Surrogate end-points or primary outcomes in clinical trials in women with polycystic ovary syndrome? Hum Reprod 19:1697–1704 [DOI] [PubMed] [Google Scholar]
- Johnson NP 2006 No more surrogate end-points in randomised trials: the PCOSMIC trial protocol for women with polycystic ovary syndrome using metformin for infertility with clomiphene. Aust N Z J Obstet Gynaecol 46:141–145 [DOI] [PubMed] [Google Scholar]
- Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, Steinkampf MP, Coutifaris C, McGovern PG, Cataldo NA, Gosman GG, Nestler JE, Giudice LC, Leppert PC, Myers ER, Cooperative Multicenter Reproductive Medicine Network 2007 Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 356:551–566 [DOI] [PubMed] [Google Scholar]
- Gennari L, Merlotti D, De Paola V, Calabrò A, Becherini L, Martini G, Nuti R 2005 Estrogen receptor gene polymorphisms and the genetics of osteoporosis: a HuGE review. Am J Epidemiol 161:307–320 [DOI] [PubMed] [Google Scholar]
- Herrington DM, Howard TD, Hawkins GA, Reboussin DM, Xu J, Zheng SL, Brosnihan KB, Meyers DA, Bleecker ER 2002 Estrogen-receptor polymorphisms and effects of estrogen replacement on high-density lipoprotein cholesterol in women with coronary disease. N Engl J Med 346:967–974 [DOI] [PubMed] [Google Scholar]
- Coller JK, Krebsfaenger N, Klein K, Endrizzi K, Wolbold R, Lang T, Nüssler A, Neuhaus P, Zanger UM, Eichelbaum M, Mürdter TE 2002 The influence of CYP2B6, CYP2C9 and CYP2D6 genotypes on the formation of the potent antioestrogen Z-4-hydroxy-tamoxifen in human liver. Br J Clin Pharmacol 54:157–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz MP, Rae JM, Suman VJ, Safgren SL, Ames MM, Visscher DW, Reynolds C, Couch FJ, Lingle WL, Flockhart DA, Desta Z, Perez EA, Ingle JN 2005 Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol 23:9312–9318 [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC 2005 The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310:1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DR, Dombroski BA, Urbanek M, Ankener W, Ewens KG, Wood JR, Legro RS, Strauss 3rd JF, Dunaif A, Spielman RS 2006 Fine mapping of genetic susceptibility to polycystic ovary syndrome on chromosome 19p13.2 and tests for regulatory activity. J Clin Endocrinol Metab 91:4112–4117 [DOI] [PubMed] [Google Scholar]
- Myers ER, Silva SG, Hafley G, Kunselman AR, Nestler JE, Legro RS 2005 Estimating live birth rates after ovulation induction in polycystic ovary syndrome: sample size calculations for the pregnancy in polycystic ovary syndrome trial. Contemp Clin Trials 26:271–280 [DOI] [PubMed] [Google Scholar]
- McGovern PG, Legro RS, Myers ER, Barnhart HX, Carson SA, Diamond MP, Carr BR, Schlaff WD, Coutifaris C, Steinkampf MP, Nestler JE, Gosman G, Leppert PC, Giudice LC, National Institutes for Child Health and Human Development-Reproductive Medicine Network 2006 Utility of screening for other causes of infertility in women with “known” polycystic ovary syndrome. Fertil Steril 87:442–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legro RS, Myers ER, Barnhart HX, Carson SA, Diamond MP, Carr BR, Schlaff WD, Coutifaris C, McGovern PG, Cataldo NA, Steinkampf MP, Nestler JE, Gosman G, Guidice LC, Leppert PC, Reproductive Medicine Network 2006 The Pregnancy in Polycystic Ovary Syndrome study: baseline characteristics of the randomized cohort including racial effects. Fertil Steril 86:914–933 [DOI] [PubMed] [Google Scholar]
- Herrington DM, Howard TD, Brosnihan KB, McDonnell DP, Li X, Hawkins GA, Reboussin DM, Xu J, Zheng SL, Meyers DA, Bleecker ER 2002 Common estrogen receptor polymorphism augments effects of hormone replacement therapy on E-selectin but not C-reactive protein. Circulation 105:1879–1882 [DOI] [PubMed] [Google Scholar]
- Herrington DM, Howard TD 2003 ER-α variants and the cardiovascular effects of hormone replacement therapy. Pharmacogenomics 4:269–277 [DOI] [PubMed] [Google Scholar]
- Goetz MP, Knox SK, Suman VJ, Rae JM, Safgren SL, Ames MM, Visscher DW, Reynolds C, Couch FJ, Lingle WL, Weinshilboum RM, Fritcher EG, Nibbe AM, Desta Z, Nguyen A, Flockhart DA, Perez EA, Ingle JN 2006 The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat 101:113–121 [DOI] [PubMed] [Google Scholar]
- Imani B, Eijkemans MJ, te Velde ER, Habbema JD, Fauser BC 1998 Predictors of patients remaining anovulatory during clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. J Clin Endocrinol Metab 83:2361–2365 [DOI] [PubMed] [Google Scholar]
- Moghetti P, Castello R, Negri C, Tosi F, Perrone F, Caputo M, Zanolin E, Muggeo M 2000 Jan Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab 85:139–146 [DOI] [PubMed] [Google Scholar]
- Eisenhardt S, Schwarzmann N, Henschel V, Germeyer A, von Wolff M, Hamann A, Strowitzki T 2006 Early effects of metformin in women with polycystic ovary syndrome: a prospective randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab 91:946–952 [DOI] [PubMed] [Google Scholar]
- Fleming R, Hopkinson ZE, Wallace AM, Greer IA, Sattar N 2002 Ovarian function and metabolic factors in women with oligomenorrhea treated with metformin in a randomized double blind placebo-controlled trial. J Clin Endocrinol Metab 87:569–574 [DOI] [PubMed] [Google Scholar]