Abstract
Objective: The objective of the study was to assess the prevalence and determinants of elevated apolipoprotein B (apoB) and dense low-density lipoprotein (LDL) in United States youth with type 1 or type 2 diabetes.
Methods: We conducted cross-sectional analyses of apoB concentrations, LDL density, and prevalence of elevated apoB levels and dense LDL from the SEARCH for Diabetes in Youth study, a six-center U.S.-based study of youth with diabetes onset younger than 20 years of age (2657 with type 1 and 345 with type 2).
Results: Among youth with type 1 diabetes, 11% had elevated apoB (≥100 mg/dl, 1.95 mm/liter), 8% had dense LDL (relative flotation rate ≤ 0.237), and 12% had elevated LDL-cholesterol (≥130 mg/dl, 3.36 mm/liter). In contrast, among youth with type 2 diabetes, 36% had elevated apoB, 36% had dense LDL, but only 23% had elevated LDL-cholesterol. Dense LDL and apoB each increased with hemoglobin A1c in both types. Among type 1 diabetics in poor glycemic control (hemoglobin A1c ≥ 9.5%), 28% had elevated apoB, and 18% had dense LDL, whereas 72% of poorly controlled type 2 diabetics had elevated apoB and 62% had dense LDL.
Conclusions: In youth with type 1 diabetes, elevated apoB and dense LDL were not highly prevalent, whereas elevated apoB and dense LDL were common lipoprotein abnormalities in youth with type 2 diabetes. The prevalence of these risk factors substantially increased with poor glycemic control in both groups, stressing the importance of achieving and maintaining an optimal glucose control.
This study of atherogenic lipoprotein abnormalities in young patients with type 1 and 2 diabetes reveals increased apolipoprotein B levels and small, dense low density lipoprotein primarily in those with type 2 diabetes. Poor glycemic control increases the prevalence of these lipoprotein risk factors in both groups.
Cardiovascular disease (CVD) constitutes the main cause of morbidity and mortality in both type 1 (T1) and type 2 (T2) diabetes in adulthood (1). T1 and T2 diabetes is associated with a 10-fold and 2- to 4-fold increase in CVD, respectively, over that of people without diabetes (2,3). Lipoprotein abnormalities contribute significantly to the risk of development of CVD. The early lesions of atherosclerosis that lead to CVD begin early in childhood and are related to the cardiovascular risk factors that precede the development of CVD in adulthood. Both diabetes and lipid and lipoprotein abnormalities have been shown to be important contributors to early atherosclerosis (4,5,6,7). Therefore, the identification and treatment of these risk factors in young persons with diabetes may prevent or delay the development of CVD. Because the prevalence of obesity and diabetes in children and adolescents is increasing dramatically, particularly in the United States, the identification of relevant risk factors and methods of primary prevention is a major public health challenge.
T2 diabetes and insulin resistance in adults are commonly associated with elevated triglycerides, low high-density lipoprotein (HDL) cholesterol levels, dense low-density lipoprotein (LDL), and elevated apolipoprotein B (apoB) (8,9,10,11). Because the occurrence of T2 diabetes in children and adolescents is relatively recent, the specific lipoprotein abnormalities and their determinants associated with T2 diabetes in youth have not been well characterized. Youth with T1 diabetes have been reported to have similar or less dyslipidemia than youth without diabetes (12). Recently, the SEARCH for Diabetes in Youth study reported higher prevalence of having two or more traditional CVD risk factors among youth with T1 diabetes, compared with national estimates for youth without diabetes, whereas the prevalence of adverse CVD risk profile in youth with T2 diabetes was more than 90% (13). Studies focusing only on elevated LDL can miss the identification of individuals with dyslipidemia who have normal LDL levels but have dense LDL and/or elevated apoB. Population-based prevalence and determinants of dense LDL and elevated apoB in youth with diabetes have not been reported. The goal of the present analysis was to assess the prevalence of dense LDL and elevated apoB and identify the determinants of these lipoprotein abnormalities among children and adolescents with T1 or T2 diabetes.
Subjects and Methods
A detailed description of SEARCH study methods has been published elsewhere (14). In brief, SEARCH is a multicenter study that initiated population-based ascertainment of cases of diabetes in patients younger than age 20 yr in 2001. Cases are identified in geographically defined populations in Ohio, Washington, South Carolina, and Colorado. In Hawaii (Hawaii Medical Service Association, MedQuest, Kaiser Permanente Hawaii) and Southern California (Kaiser Permanente Southern California), cases are identified among health plan enrollees. In addition, the Colorado center coordinates identification of cases from the rolls of health service beneficiaries in several American Indian reservation-based populations in Arizona and New Mexico and among participants in the National Institute of Diabetes and Digestive and Kidney Diseases Pima Indian study in Arizona.
SEARCH sought to identify all existing (prevalent) cases of nongestational diabetes in patients younger than 20 yr in 2001 and all cases of nongestational diabetes newly diagnosed at age younger than 20 yr in subsequent calendar years. Cases were considered valid if diagnosed by a health care provider. The type of diabetes was based on a health care provider’s clinical diagnosis. This information was collected from either the health providers at the time of their report to SEARCH or medical records. The clinical type was categorized as type 1 (reported type 1, type 1a, and type 1b) or type 2 (reported type 2 and hybrid). Twenty-four participants had their type reported as either maturity onset diabetes of youth (n = 2), other (n = 7), or unknown (n = 15) and were excluded from the analyses.
The study protocol was reviewed and approved by the local institutional review boards. Written informed consent for the study examination was obtained according to local institutional review board requirements from patients 18 years of age or older or a subject’s parent or guardian if the subject was younger than 18 yr. Written consent was also obtained from patients younger than 18 yr, in accordance with local institutional review board instructions. All centers complied with the privacy requirements of the Health Insurance and Portability Act.
Eligible for this analysis were prevalent cases from the year 2001 and incident cases from 2002 through 2004 with clinical type of T1 or T2 diabetes who participated in the SEARCH examination; had their blood drawn while fasting for at least 8 h and had no episodes of diabetic ketoacidosis during the previous month; and had measurement of total cholesterol, triglycerides, HDL cholesterol, apoB, lipoprotein(a) [Lp(a)], an assessment of lipoprotein cholesterol distribution after density gradient ultracentrifugation and calculation of the LDL relative flotation rate (Rf), hemoglobin A1c (HbA1c), urine albumin, and creatinine at the SEARCH study central laboratory (Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington). We excluded 39 individuals who were missing all three outcomes [apoB, LDL Rf, and Lp(a)], those with age younger than 3 yr (n = 16), or those who were not fasting (n = 352). Blood specimens were processed locally at the sites and the plasma shipped within 24 h to the central laboratory for analysis.
Measurements of plasma cholesterol, triglycerides, and HDL cholesterol were performed enzymatically on a Hitachi 917 autoanalyzer (Roche Molecular Biochemicals Diagnostics, Indianapolis, IN). LDL cholesterol was calculated by the Friedewald equation for individuals with triglyceride concentration less than 400 mg/dl (4.52 mm/liter) and the BetaQuantification procedure for those with triglycerides of 400 mg/dl or greater (15). HbA1c was measured by a dedicated ion exchange HPLC instrument (TOSOH Bioscience, Inc., San Francisco, CA), urinary albumin was measured by nephelometry (BNII; Behring Diagnostics, Deerfield, IL), and urinary creatinine was measured by the Jaffe method using Roche Diagnostics reagent on the Hitachi 917 autoanalyzer. An albumin to creatinine ratio (ACR) (microgram of albumin to milligram of creatinine) was computed. apoB was measured by a nephelometric system (BNII; Behring Diagnostics) calibrated with the World Health Organization international reference material for apoB (16). The lipoprotein cholesterol distribution was determined by cholesterol measurement of 38 fractions after nonequilibrium density gradient ultracentrifugation using a modification (17) of a previously described technique (18). Lp(a) was determined using a double-monoclonal antibody-based ELISA (19). Because the apoB associated with Lp(a) is metabolically distinct from the bulk of the apoB-containing lipoproteins (20), for some analyses the Lp(a) apoB was subtracted from total plasma apoB (see Results) to obtain plasma apoB not associated with Lp(a). Because each molecule of Lp(a) contains one molecule of apoB (21), Lp(a) in nanomoles/liter × 0.0513 equals Lp(a) apoB in milligrams per deciliter.
Non-HDL cholesterol was calculated from measurements of total cholesterol and HDL. Cutoff points for elevated LDL cholesterol of 130 mg/dl or greater (3.36 mm/liter) and non-HDL-cholesterol of 160 mg/dl or greater (4.14 mm/liter) were taken from targets of treatment as defined by the National Cholesterol Education Program (22).
The average of two weight measurements (electronic scale) and two height measurements (stadiometer) were used to calculate body mass index (BMI; kilograms per square meter). Percentiles for BMI were determined to be specific to sex and month of age using the algorithms of the Centers for Disease Control and Prevention based on the 2000 Centers for Disease Control and Prevention growth charts and used to classify study participants.
Race or ethnicity was based on self-report and categorized as Hispanic ethnicity; non-Hispanic white only, non-Hispanic African-American only, Asian and/or Pacific Islander only, American Indian only, and other (including multiple) race or unknown.
Three blood pressure measurements were obtained after the patient had been sitting for at least 5 min using a portable mercury manometer, and cuffs of five different sizes were used, depending on the size of the arm of the participants. High blood pressure was defined for analyses as systolic blood pressure greater than 120 mm Hg and/or diastolic blood pressure greater than 80 mm Hg.
Statistical analysis
Data from individuals with T1 and T2 diabetes were compared by simple linear regression, using diabetes type as the independent variable. Correlation was assessed using Pearson’s correlation coefficient. Multiple linear regression was used to assess independent associations after adjusting for other variables (see Results for covariate lists). The natural logarithmic transformation of apoB and the square of LDL Rf were used to improve linearity in all regressions. Statistical analyses were performed using SAS Statistical Software (version 9.1; SAS Institute Inc., Cary, NC). Regression results are expressed as β-coefficients and R2. Nonlinear relationships are displayed using locally weighted regression smoothing.
Results
Table 1 shows demographic characteristics along with lipoprotein parameters and HbA1c levels of the participants by diabetes type. There were 2657 youth classified as having T1 diabetes and 345 classified as having T2 diabetes. Females comprised 50% of the T1 participants and 62% of the T2 participants. Non-Hispanic whites represented over 77% of youth with T1, whereas they comprised only 23% of those with T2 diabetes. In contrast, the T2 diabetes group had a substantially greater proportion of non-Hispanic African-Americans, Hispanics, American Indians, and Asians/Pacific Islanders than youth with T1 diabetes (Table 1). As expected, those with T1 diabetes were, in general, younger and leaner than those with T2, with only 12% being overweight (BMI ≥ 95th percentile for age and sex), whereas 71% of youth with T2 diabetes were overweight (Table 1). Very few of the youth with T1 diabetes had elevated triglycerides, whereas 35% of those with T2 had triglycerides of 150 mg/dl or greater. The distribution of HbA1c among the two groups also differed substantially with a greater proportion of those with T2 diabetes having low levels of HbA1c (<7.0%), whereas youth with T1 had a substantially greater proportion with intermediate HbA1c levels (7.0–9.4%) (Table 1).
Table 1.
Percentage distribution and mean ± sd concentrations of selected characteristics by diabetes type
| Characteristic | Type 1 (n = 2657) | Type 2 (n = 345) |
|---|---|---|
| Race/ethnicity | ||
| Non-Hispanic white | 2050 (77.2) | 78 (22.6) |
| Hispanic | 282 (10.6) | 70 (20.3) |
| Non-Hispanic African-American | 166 (6.3) | 108 (31.3) |
| Asian/Pacific Islander | 44 (1.7) | 29 (8.4) |
| American Indian | 15 (0.6) | 45 (13.0) |
| Other/unknown | 100 (3.8) | 15 (4.4) |
| Age distribution (yr) | ||
| <10 | 661 (24.9) | 7 (2.0) |
| 10–13 | 876 (33.0) | 65 (18.8) |
| 14–17 | 765 (28.8) | 159 (46.1) |
| 18+ | 355 (13.4) | 114 (33.0) |
| Age (yr, continuous) | 12.6 ± 4.3 | 16.0 ± 3.0 |
| BMI (percentile) | ||
| Normal (<85th) | 1775 (66.8) | 48 (13.9) |
| At risk of overweight (85th to 95th) | 561 (21.1) | 51 (14.8) |
| Overweight (>95th) | 321 (12.1) | 246 (71.3) |
| BMI (kg/m2, continuous) | 21.3 ± 4.6 | 34.1 ± 10.2 |
| Triglyceride levels (mg/dl) | ||
| <150 | 2484 (93.5) | 223 (64.6) |
| 150 to <300 | 144 (5.4) | 84 (24.4) |
| 300+ | 29 (1.1) | 38 (11.0) |
| Triglyceride (mg/dl, continuous) | 77.9 ± 65.4 | 194.6 ± 324.5 |
| HbA1c (%) | ||
| <7.0 | 455 (17.2) | 166 (48.3) |
| 7.0–8.0 | 853 (32.3) | 51 (14.8) |
| 8.1–9.4 | 858 (32.5) | 38 (11.1) |
| ≥9.5 | 475 (18.0) | 89 (25.9) |
| HbA1c (%, continuous) | 8.3 ± 1.6 | 8.0 ± 2.6 |
| ApoB ≥ 100 mg/dl | 269 (10.6) | 122 (36.3) |
| ApoB (mg/dl, continuous) | 76.0 ± 21.6 | 95.4 ± 43.2 |
| Dense LDL (Rf < 0.237) | 201 (7.9) | 120 (36.3) |
| LDL Rf, continuous | 0.279 ± 0.020 | 0.256 ± 0.029 |
| HDL cholesterol (mg/dl, continuous) | 54.9 ± 12.8 | 42.3 ± 10.8 |
| LDL cholesterol (≥130 mg/dl) | 315 (12.0) | 79 (23.1) |
| LDL cholesterol (mg/dl, continuous) | 99.9 ± 26.4 | 107.9 ± 33.4 |
| Non-HDL cholesterol (≥160 mg/dl | 194 (7.4) | 100 (29.2) |
| Non-HDL cholesterol (mg/dl, continuous) | 115.2 ± 31.5 | 141.0 ± 44.6 |
Data are expressed as number (percent) or mean ± sd.
P < 0.0001, P values testing T1 vs. T2 for selected continuous measures (independent samples t tests).
ApoB levels were significantly higher in those with T2 diabetes than those with T1 diabetes before and after adjustment for covariates including age, gender, race or ethnicity, BMI, duration of diabetes, log triglyceride, blood pressure, ACR, and HbA1c (P < 0.001). Thirty-six percent of youth with T2 diabetes had elevated apoB levels (≥100 mg/dl or 1.95 mm/liter), whereas only 11% of youth with T1 diabetes had elevated apoB levels (Table 1). The apoB cutoff point of 100 mg/dl (1.95 mm/liter) corresponds to the LDL cholesterol cutoff point of 130 mg/dl (3.36 mm/liter), the LDL cholesterol cutoff point for cholesterol-lowering therapy as recommended by the National Cholesterol Education Program Adult Treatment Panel III Guidelines, and both cutoff points approximate the 50th percentile for adults (22,23). Among youth with T2 diabetes with elevated apoB, the mean apoB was 130 mg/dl (2.53 mm/liter), and 14% were at risk for being overweight (BMI 85% to < 95%), 75% were overweight (BMI ≥ 95%), and 63% had elevated triglyceride levels (≥150 mg/dl, 1.69 mm/liter) (data not shown). Among youth with T2, 23% had elevated LDL cholesterol and 34% had elevated triglycerides and 29% had elevated non-HDL cholesterol (≥160 mg/dl, 4.14 mm/liter). The proportion of T2 youth with apoB elevation was significantly greater than the proportion of youth with an elevated LDL or non-HDL cholesterol by McNemar’s test (P < 0.001). Among youth with T1, the prevalence of elevated apoB, LDL cholesterol, non-HDL cholesterol, and triglycerides was 11, 12, 7, and 7%, respectively. The ln(apoB) was highly linearly correlated with LDL cholesterol in those with either diabetes type, but the strength of the association was greater for T1 (Pearson correlation coefficient r = 0.890) than for T2 diabetes (r = 0.652). Furthermore, the slope of this relationship was significantly different between the diabetes types [0.00889 (T1) vs. 0.00667 (T2), P < 0.01].
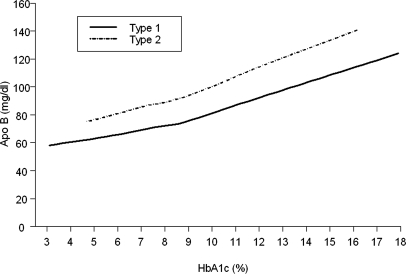
ApoB levels increased with an increase in HbA1c levels (Fig. 1). Although the relationship between apoB and HbA1c was similar in those with either diabetes type, at any given level of HbA1c, apoB was higher on average in those with T2 diabetes than in those with T1 diabetes. Among youth with T2 diabetes with poor glycemic control (HbA1c ≥ 9.5%), 72% had elevated apoB, whereas in those with good glycemic control (HbA1c < 7.0%), only 21% had elevated apoB. This difference represents a relative risk for elevated apoB of 3.5 [95% confidence interval (CI) 2.5, 4.9] for poor vs. good glycemic control. In contrast, only 28% of the youth with T1 diabetes with poor glycemic control had elevated apoB. The relationship between ln(apoB) and HbA1c remained significant in those with either diabetes type after adjustment for covariates (Table 2). Interestingly, the strength of the relationship (r, 95% CI) between ln(apoB) and HbA1c [0.376 (0.342, 0.409) for T1 and 0.471 (0.383, 0.551) for T2] was stronger than the relationship between LDL cholesterol and HbA1c [0.282 (0.246, 0.316) for T1 and 0.312 (0.213, 0.405) for T2] in T1 but not T2. Ln(apoB) increased with the log of triglycerides, and the linear relationship was nearly identical in T1 and T2 diabetes (with slopes of 0.290 and 0.293, respectively). An increase in triglycerides from 150 to 160 mg/dl would result in an increase of apoB of nearly 2 mg/dl. The relationship between apoB and triglycerides also remained significant in both diabetes types after adjustment for covariates.
Figure 1.
Relationship of apoB with HbA1c in youth with T1 or T2 diabetes. Curves were determined using locally weighted regression smoothing (Loess).
Table 2.
Simple and multiple linear regressions
| Independent variable | % of variation (R2 × 100) | Simple linear regressions |
Multiple linear regression |
||
|---|---|---|---|---|---|
| β | P value | β | P value | ||
| ln(ApoB), type 1 | R2 × 100 = 38.7 | ||||
| Triglycerides [log (mg/dl)] | 31.9 (32.4) | 0.290 | <0.001 | 0.259 | <0.001 |
| HbA1c (%) | 14.1 (14.0) | 0.062 | <0.001 | 0.034 | <0.001 |
| Age (yr) | 3.4 (3.6) | <0.001 | <0.001 | ||
| 10 to <14 vs. <10 | 0.003 | 0.851 | −0.079 | <0.001 | |
| 14 to <18 vs. <10 | 0.046 | 0.001 | −0.114 | <0.001 | |
| 18+ vs. <10 | 0.147 | <0.001 | −0.089 | <0.001 | |
| HDL cholesterol (mg/dl) | 1.8 (2.0) | −0.003 | <0.001 | 0.001 | 0.102 |
| High blood pressure (yes vs. no) | 1.0 (1.0) | 0.080 | <0.001 | 0.005 | 0.738 |
| Duration of diabetes (months) | 4.1 (4.3) | 0.001 | <0.001 | 0.0003 | 0.008 |
| BMI (percentiles) | 3.1 (2.8) | 0.010 | <0.001 | 0.005 | <0.001 |
| Elevated ACR (yes vs. no) | 1.4 (1.3) | 0.107 | <0.001 | 0.050 | <0.001 |
| Gender (female vs. male) | 1.0 (1.0) | 0.053 | <0.001 | 0.016 | 0.078 |
| Race/ethnicity | 0.5 (0.5) | 0.017 | 0.306 | ||
| API vs. white | 0.038 | 0.357 | −0.031 | 0.372 | |
| African-American vs. white | 0.041 | 0.060 | 0.020 | 0.272 | |
| Hispanic vs. white | 0.016 | 0.353 | −0.004 | 0.792 | |
| American Indian vs. white | 0.177 | 0.010 | −0.029 | 0.599 | |
| Others/unknown vs. white | −0.041 | 0.147 | −0.043 | 0.069 | |
| ln(ApoB), type 2 | R2 × 100 = 54.8 | ||||
| Triglycerides [log (mg/dl)] | 42.4 (44.8) | 0.293 | <0.001 | 0.279 | <0.001 |
| HbA1c (%) | 22.2 (22.6) | 0.062 | <0.001 | 0.028 | <0.001 |
| Age (yr) | 10.0 (10.7) | <0.001 | 0.023 | ||
| 10 to <14 vs. <10 | −0.042 | 0.743 | −0.139 | 0.172 | |
| 14 to <18 vs. <10 | 0.078 | 0.537 | −0.096 | 0.332 | |
| 18+ vs. <10 | 0.251 | 0.048 | −0.005 | 0.960 | |
| HDL cholesterol (mg/dl) | 2.4 (3.1) | −0.005 | 0.005 | 0.003 | 0.091 |
| High blood pressure (yes vs. no) | 3.4 (3.4) | 0.126 | <0.001 | 0.031 | 0.305 |
| Duration of diabetes (months) | 4.1 (4.5) | 0.003 | <0.001 | 0.000 | 0.474 |
| BMI (percentiles) | 0.5 (0.4) | 0.002 | 0.209 | 0.001 | 0.612 |
| Elevated ACR (yes vs. no) | 3.6 (3.4) | 0.166 | <0.001 | −0.031 | 0.419 |
| Gender (female vs. male) | 0.1 (0.1) | 0.020 | 0.609 | 0.020 | 0.514 |
| Race/ethnicity | 1.6 (2.2) | 0.377 | 0.004 | ||
| API vs. white | 0.140 | 0.060 | 0.100 | 0.082 | |
| African-American vs. white | −0.001 | 0.990 | 0.058 | 0.171 | |
| Hispanic vs. white | 0.067 | 0.238 | 0.039 | 0.374 | |
| American Indian vs. white | 0.018 | 0.778 | −0.118 | 0.019 | |
| Others/unknown vs. white | 0.011 | 0.912 | 0.008 | 0.909 | |
API, Asians/Pacific Islanders.
Multiple linear regression included all covariates listed above. R2 × 100 = % of variation.
Numbers in parentheses represent the percent of variation explained by the linear regression of the ln[non-Lp(a) plasma apoB] vs. the given independent variable. The non-Lp(a) plasma apoB equals total plasma apoB minus the apoB in Lp(a).
Overall test among all levels of a variable with greater than two levels.
Systolic blood pressure greater than 120 mm Hg and/or diastolic blood pressure greater than 80 mm Hg.
ACR greater than 30 μg/mg.
Simple linear regression between the ln(apoB) and a series of independent variables indicates that both triglycerides and HbA1c levels explain a significant proportion of the variance in apoB levels in youth with T1 or T2 diabetes, with log triglycerides, HbA1c, age, blood pressure, HDL, and ACR, explaining a greater proportion of the apoB variance in T2 diabetes than T1 diabetes (Table 2). In the multiple regression models, triglycerides, HbA1c, age, duration of diabetes, BMI, and ACR contributed significantly to the variance in apoB in T1 (Table 2), and triglycerides, HbA1c, age, and race contributed significantly to apoB in T2 diabetes (Table 2). In the multiple regression model, these variables explained approximately 55% of the apoB variance in T2 diabetes but only approximately 39% of the variance in T1 diabetes. R2 in the regression of ln(apoB) on a given variable was very similar to that for ln[non-Lp(a)apoB] on that variable.
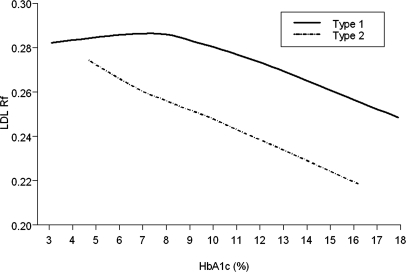
Youth with T2 diabetes had lower LDL Rf or denser LDL than youth with T1 diabetes before and after adjustment for covariates including age, gender, race or ethnicity, BMI, duration of diabetes, log triglycerides, blood pressure, ACR, and HbA1c (P < 0.0001). Thirty-six percent of youth with T2 but only 8% of those with T1 diabetes had dense LDL (LDL Rf ≥ 0.237) (Table 1). Glycemic control, as expressed by the levels of HbA1c, had less of an effect on LDL density in youth with T1 diabetes than youth with T2 diabetes (Fig. 2). In those with T1 diabetes, buoyant LDL shifted toward dense LDL only when the HbA1c was above 8%, whereas in T2 diabetes LDL density increased linearly with an increase in HbA1c. This suggests that a certain degree of hyperglycemia is necessary before LDL density increases in T1 diabetes, but this is not necessary in T2 diabetes. In youth with T2 diabetes in poor glycemic control (HbA1c ≥ 9.5%), 62% had dense LDL (LDL Rf ≤ 0.237), whereas the corresponding percentage for those in good glycemic control (HbA1c < 7.0%) was only 22%. This difference represents a relative risk for dense LDL of 2.8 (95% CI 2.0, 3.9) for poor vs. good control. Only 18% of youth with T1 diabetes with poor glycemic control had dense LDL. The association between LDL Rf and HbA1c remained significant in both those with T1 and T2 diabetes after adjustment for covariates (Table 3).
Figure 2.
Relationship of LDL Rf with HbA1c in youth with T1 or T2 diabetes. Curves were determined using locally weighted regression smoothing (Loess).
Table 3.
Simple and multiple linear regressions
| Independent variable | Variation (%) (R2 × 100) | Simple linear regressions |
Multiple linear regression |
||
|---|---|---|---|---|---|
| β | P value | β | P value | ||
| (LDL Rf)2 type 1 | R2 × 100 = 35.7 | ||||
| Triglycerides [log (mg/dl)] | 23.8 | −0.010 | <0.001 | −0.007 | <0.001 |
| HbA1c (%) | 7.4 | −0.002 | <0.001 | −0.001 | <0.001 |
| Age (yr) | 6.7 | <0.001 | <0.001 | ||
| 10 to <14 vs. <10 | 0.000 | 0.927 | 0.002 | <0.001 | |
| 14 to <18 vs. <10 | −0.004 | <0.001 | 0.001 | 0.066 | |
| 18+ vs. <10 | −0.008 | <0.001 | −0.001 | 0.404 | |
| HDL cholesterol (mg/dl) | 15.9 | 0.0003 | <0.001 | 0.0002 | <0.001 |
| High blood pressure (yes vs. no) | 2.4 | −0.005 | <0.001 | −0.002 | 0.011 |
| Duration of diabetes (months) | 3.8 | −0.00004 | <0.001 | 0.000 | 0.056 |
| BMI (percentiles) | 4.5 | −0.001 | <0.001 | 0.000 | 0.929 |
| Elevated ACR (yes vs. no) | 0.6 | −0.003 | <0.001 | −0.001 | 0.159 |
| Gender (female vs. male) | 0.6 | 0.002 | <0.001 | 0.002 | <0.001 |
| Race/ethnicity | 1.2 | <0.001 | 0.132 | ||
| API vs. white | −0.004 | 0.032 | −0.001 | 0.465 | |
| African-American vs. white | 0.000 | 0.782 | −0.001 | 0.321 | |
| Hispanic vs. white | −0.002 | 0.001 | −0.001 | 0.041 | |
| American Indian vs. white | −0.011 | <0.001 | −0.004 | 0.063 | |
| Others/unknown vs. white | 0.001 | 0.237 | 0.000 | 0.787 | |
| (LDL Rf)2 type 2 | R2 × 100 = 61.5 | ||||
| Triglycerides [log (mg/dl)] | 50.9 | −0.015 | <0.001 | −0.010 | <0.001 |
| HbA1c (%) | 14.3 | −0.002 | <0.001 | −0.001 | <0.001 |
| Age (yr) | 7.8 | <0.001 | 0.015 | ||
| 10 to <14 vs. <10 | 0.000 | 0.943 | 0.001 | 0.746 | |
| 14 to <18 vs. <10 | −0.008 | 0.131 | −0.003 | 0.454 | |
| 18+ vs. <10 | −0.011 | 0.053 | −0.005 | 0.241 | |
| HDL cholesterol (mg/dl) | 19.8 | 0.001 | <0.001 | 0.0004 | <0.001 |
| High blood pressure (yes vs. no) | 2.7 | −0.005 | 0.003 | −0.001 | 0.468 |
| Duration of diabetes (months) | 2.3 | 0.000 | 0.143 | 0.000 | 0.938 |
| BMI (percentiles) | 0.4 | 0.000 | 0.285 | 0.000 | 0.996 |
| Elevated ACR (yes vs. no) | 4.2 | −0.008 | <0.001 | −0.001 | 0.379 |
| Gender (female vs. male) | 1.6 | 0.004 | 0.021 | 0.000 | 0.691 |
| Race/ethnicity | 9.1 | <0.001 | 0.023 | ||
| API vs. white | 0.000 | 0.936 | −0.003 | 0.238 | |
| African-American vs. white | 0.008 | <0.001 | 0.003 | 0.068 | |
| Hispanic vs. white | −0.002 | 0.451 | −0.001 | 0.645 | |
| American Indian vs. white | −0.003 | 0.227 | 0.002 | 0.257 | |
| Others/unknown vs. white | −0.003 | 0.528 | −0.004 | 0.133 | |
API, Asians/Pacific Islanders.
Multiple linear regression included all covariates listed above. R2 × 100 = % of variation.
Overall test among all levels of a categorical variable with greater than two levels.
Systolic blood pressure greater than 120 mm Hg and/or diastolic blood pressure greater than 80 mm Hg.
ACR greater than 30 μg/mg.
Simple linear regression between LDL Rf2 and the same variables used for apoB indicated that many of these variables explained a substantially greater proportion of the variance in LDL Rf in youth with T2 diabetes than youth with T1 diabetes (Table 3). Triglyceride (log), for example, explained 51% of the variance in LDL density in those with T2 diabetes but only 24% of the variance in those with T1 diabetes. LDL Rf2 decreased with ln(triglyceride), but this relationship differed significantly between those with T1 and T2 diabetes (slope for T1 = −0.0105; slope for T2 = −0.0151; P < 0.001). Using the same multiple regression models as used with apoB, the following variables contributed significantly to the variance in LDL density (Table 3): log triglycerides, HbA1c, age, and HDL. Sex and blood pressure also contributed significantly to the variance of LDL density in those with T1, but not T2, diabetes, and race or ethnicity contributed significantly to the variance in those with T2, but not T1, diabetes. Using multiple regression models, these variables explained 62% of the LDL Rf variance in those with T2 diabetes but only 36% of the variance in those with T1 diabetes.
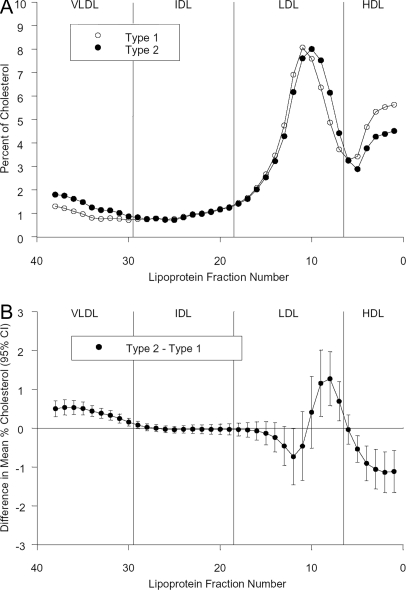
Mean density gradient ultracentrifugation cholesterol profiles for youth with T1 or T2 diabetes are presented in Fig. 3. The difference in mean percent cholesterol between T2 and T1 indicated that individuals with T2 had significantly less HDL cholesterol, more very LDL cholesterol, a greater proportion of their cholesterol in the form of dense LDL, and a lower proportion of the cholesterol in the buoyant LDL subclass (Fig. 3).
Figure 3.
A, Mean lipoprotein cholesterol profiles after density gradient ultracentrifugation of plasma in youth with T1 or T2 diabetes. B, Difference plot of cholesterol profiles, T2 minus T1. Vertical bars are 95% CI for the differences.
Discussion
In this large, multiracial population of youth with diabetes, we found that elevation of apoB and the presence of dense LDL, along with an elevation of triglycerides, are the most prevalent lipoprotein abnormalities in those with T2 diabetes, whereas these risk factors were less common in those with T1 diabetes. Although a significant proportion of adults with T2 diabetes have elevated apoB and dense LDL (10,24,25), the measurement of these nontraditional risk factors in people with diabetes has been sparse, particularly in children and adolescents. The greater proportion of youth with T2 diabetes with elevated apoB, but without elevated LDL cholesterol, can be explained by the increased concentration of triglyceride-rich apoB-containing lipoproteins and by the presence of dense LDL that is enriched in apoB relative to its cholesterol content. Numerous studies have documented that elevated apoB and dense LDL contribute to the increased risk of cardiovascular disease. ApoB is a strong, independent predictor of CVD (26,27) and a report from the Québec Cardiovascular Study has indicated that dense LDL is also a strong and independent predictor of CVD (28). Furthermore, the small dense LDL subclass has been shown to be the strongest predictor of coronary atherosclerosis progression (29,30,31). The impact of dyslipidemia on the development and progression of cardiovascular disease is determined by not only the degree of dyslipidemia but also the duration of exposure. Therefore, it is very likely that the presence of high levels of apoB and dense LDL particles in youth will significantly contribute to cardiovascular morbidity and mortality in adulthood.
Previous data from SEARCH indicated that LDL cholesterol and plasma triglyceride increased significantly with increasing HbA1c in those with either T1 or T2 diabetes (32). The positive association between HbA1c and lipoproteins has been extended in this study to apoB levels and dense LDL. The available cross-sectional analysis strongly supports the notion that poor glycemic control contributes to an increase in apoB levels and an increase in dense LDL. Intensive glucose control in adolescents and adults with T1 diabetes has been shown to decrease apoB levels and shift the cholesterol content of LDL from dense to buoyant particles (17). The effect of intensive diabetes therapy on LDL is complex because small dense LDL has been shown to be related to microalbuminuria in those with T1 diabetes (33) and increases with weight gain in a subset of subjects with T1 diabetes (34). Also, insulin therapy in adults with T2 diabetes has been shown to significantly reduce apoB levels (35).
Dyslipidemia, usually reflected by an elevation of apoB and the presence of dense LDL, outweighs all other cardiovascular risk factors for adults with T2 diabetes, and this is likely to be true for children and adolescents as well. The American Diabetes Association emphasizes improvement in glycemic control, weight loss, and increased activity for treatment of dyslipidemia (36). If this strategy is insufficient to correct the dyslipidemia, then pharmacological therapy should be considered if additional cardiovascular risk factors are present (37). The American Heart Association recommends that the optimal LDL cholesterol concentration in youth be less than 100 mg/dl (38). Because an apoB concentration of 77 mg/dl (1.5 mm/liter) approximates an LDL cholesterol level of 100 mg/dl (23), we recommend that the optimal apoB level in youth be less than 77 mg/dl.
The results of our study suggest that the use of LDL or non-HDL cholesterol levels to identify individuals who will benefit from intervention will miss a significant proportion of youth with high apoB and/or dense LDL, thus resulting in an inadequate management of youth with type 2 diabetes and dyslipidemia. Considering that aggressive prevention strategies are essential in these young individuals, serious consideration should be given to complement the determination of the conventional lipid profile with the measurement of other risk factors to maximize the identification of those at high risk for future coronary artery disease. In addition, the results of our study strongly indicate the importance of maintaining an optimal glucose control in youth with both diabetes types.
Acknowledgments
The SEARCH for Diabetes in Youth Study is indebted to the many youth and their families and their health care providers, whose participation made this study possible. The authors acknowledge the involvement of General Clinical Research Centers at the following institutions in the SEARCH for Diabetes in Youth Study: Medical University of South Carolina (Grant M01 RR01070); Cincinnati Children’s Hospital (Grant M01 RR08084); Children’s Hospital and Regional Medical Center and the University of Washington School of Medicine (Grant M01RR00037 and M01RR001271); and Colorado Pediatric General Clinical Research Center (Grant M01 RR00069).
Footnotes
The SEARCH for Diabetes in Youth is funded by the Centers for Disease Control and Prevention (PA no. 00097 and DP-05-069) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Site contract numbers for the following states included: California (U01 DP000246); Colorado (U01 DP000247); Hawaii (U01 DP000245); Ohio (U01 DP000248); South Carolina (U01 DP000254); Washington (U01 DP000244); and the coordinating center (U01 DP000250). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
Disclosure Summary: W.Y.F. is a consultant for Eli Lilly. No other authors have disclosures.
First Published Online December 18, 2007
Abbreviations: ACR, Albumin to creatinine ratio; apoB, apolipoprotein B; BMI, body mass index; CI, confidence interval; CVD, cardiovascular disease; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Lp(a), lipoprotein(a); Rf, relative flotation rate; T1, type 1; T2, type 2.
References
- Libby P, Nathan DM, Abraham K, Brunzell JD, Fradkin JE, Haffner SM, Hsueh W, Rewers M, Roberts BT, Savage PJ, Skarlatos S, Wassef M, Rabadan-Diehl C 2005 Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation 111:3489–3493 [DOI] [PubMed] [Google Scholar]
- Krolewski AS, Kosinski EJ, Warram JH, Leland OS, Busick EJ, Asmal AC, Rand LI, Christlieb AR, Bradley RF, Kahn CR 1987 Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol 59:750–755 [DOI] [PubMed] [Google Scholar]
- Laakso M 2001 Cardiovascular disease in type 2 diabetes: challenge for treatment and prevention. J Intern Med 249:391–393 [DOI] [PubMed] [Google Scholar]
- McGill Jr HC, McMahan CA, Zieske AW, Sloop GD, Walcott JV, Troxclair DA, Malcom GT, Tracy RE, Oalmann MC, Strong JP 2000 Associations of coronary heart disease risk factors with the intermediate lesion of atherosclerosis in youth. The Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb Vasc Biol 20:1998–2004 [DOI] [PubMed] [Google Scholar]
- Järvisalo MJ, Putto-Laurila A, Jartti L, Lehtimäki T, Solakivi T, Rönnemaa T, Raitakari OT 2002 Carotid artery intima-media thickness in children with type 1 diabetes. Diabetes 51:493–498 [DOI] [PubMed] [Google Scholar]
- Krantz JS, Mack WJ, Hodis HN, Liu CR, Liu CH, Kaufman FR 2004 Early onset of subclinical atherosclerosis in young persons with type 1 diabetes. J Pediatrics 145:452–457 [DOI] [PubMed] [Google Scholar]
- McMahan CA, Gidding SS, Fayad ZA, Zieske AW, Malcom GT, Tracy RE, Strong JP, McGill Jr HC 2005 Risk scores predict atherosclerotic lesions in young people. Arch Intern Med 165:883–890 [DOI] [PubMed] [Google Scholar]
- Austin MA, Edwards KL 1996 Small, dense low density lipoproteins, the insulin resistance syndrome and noninsulin-dependent diabetes. Curr Opin Lipidol 7:167–171 [DOI] [PubMed] [Google Scholar]
- Howard BV 1987 Lipoprotein metabolism in diabetes mellitus. J Lipid Res 28:613–628 [PubMed] [Google Scholar]
- Sniderman AD, Lamarche B, Tilley J, Seccombe D, Frolich J 2002 Hypertriglyceridemic hyperapoB in type 2 diabetes. Diabetes Care 25:579–582 [DOI] [PubMed] [Google Scholar]
- Taskinen MR 1990 Hyperlipidaemia in diabetes. Baillieres Clin Endocrinol Metab 4:743–775 [DOI] [PubMed] [Google Scholar]
- Taskinen MR 1992 Quantitative and qualitative lipoprotein abnormalities in diabetes mellitus. Diabetes 41(Suppl 2):12–17 [DOI] [PubMed] [Google Scholar]
- Rodriguez BL, Fujimoto WY, Mayer-Davis EJ, Imperatore G, Williams DE, Bell RA, Wadwa RP, Palla SL, Liu LL, Kershnar A, Daniels SR, Linder B 2006 Prevalence of cardiovascular disease risk factors in U.S. children and adolescents with diabetes: the SEARCH for Diabetes in Youth study. Diabetes Care 29:1891–1896 [DOI] [PubMed] [Google Scholar]
- SEARCH Study Group 2004 SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials 25:458–471 [DOI] [PubMed] [Google Scholar]
- Warnick GR, Nguyen T, Bergelin RO, Wahl PW, Albers JJ 1982 Lipoprotein quantification: an electrophoretic method compared with the Lipid Research Clinics method. Clin Chem 28:2116–2120 [PubMed] [Google Scholar]
- Marcovina SM, Albers JJ, Kennedy H, Mei JV, Henderson LO, Hannon WH 1994 International Federation of Clinical Chemistry standardization project for measurements of apolipoproteins A-I and B. IV. Comparability of apolipoprotein B values by use of International Reference Material. Clin Chem 40:586–592 [PubMed] [Google Scholar]
- Purnell JQ, Marcovina SM, Hokanson JE, Kennedy H, Cleary PA, Steffes MW, Brunzell JD 1995 Levels of lipoprotein(a), apolipoprotein B, and lipoprotein cholesterol distribution in IDDM. Results from follow-up in the Diabetes Control and Complications Trial. Diabetes 44:1218–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auwerx JH, Marzetta CA, Hokanson JE, Brunzell JD 1989 Large buoyant LDL-like particles in hepatic lipase deficiency. Arteriosclerosis 9:319–325 [DOI] [PubMed] [Google Scholar]
- Marcovina SM, Albers JJ, Gabel B, Koschinsky ML, Gaur VP 1995 Effect of the number of apolipoprotein(a) kringle 4 domains on immunochemical measurements of lipoprotein(a). Clin Chem 41:246–255 [PubMed] [Google Scholar]
- Albers JJ, Cabana VG, Warnick GR, Hazzard WR 1975 Lp(a) lipoprotein: relationship to sinking pre-β lipoprotein hyperlipoproteinemia, and apolipoprotein B. Metabolism 24:1047–1054 [DOI] [PubMed] [Google Scholar]
- Albers JJ, Kennedy H, Marcovina SM 1995 Evidence that Lp(a) contains one molecule of apo(a) and one molecule of apo B: evaluation of amino acid analysis data. J Lipid Res 37:192–196 [PubMed] [Google Scholar]
- National Cholesterol Education Program (NCEP) Expert Panel 2002 Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). NIH publication no. 02–5215. Bethesda, MD: National Institutes of Health [Google Scholar]
- Contois JH, McNamara JR, Lammi-Keefe CJ, Wilson PW, Massov T, Schaefer EJ 1996 Reference intervals for plasma apolipoprotein B determined with a standardized commercial immunoturbidimetric assay: results from the Framingham Offspring Study. Clin Chem 42:515–523 [PubMed] [Google Scholar]
- Wagner AM, Perez A, Calvo F, Bonet R, Castellvi A, Ordonez J 1999 Apolipoprotein(B) identifies dyslipidemic phenotypes associated with cardiovascular risk in normocholesterolemic type 2 diabetic patients. Diabetes Care 22:812–817 [DOI] [PubMed] [Google Scholar]
- Feingold KR, Grunfeld C, Pang M, Doerrier W, Krauss RM 1992 LDL subclass phenotypes and triglyceride metabolism in non-insulin-dependent diabetes. Arterioscler Thromb 12:1496–1502 [DOI] [PubMed] [Google Scholar]
- Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E 2001 High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet 358:2026–2033 [DOI] [PubMed] [Google Scholar]
- Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, Rimm EB 2005 Non-high-density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men. Circulation 112:3375–3383 [DOI] [PubMed] [Google Scholar]
- St. Pierre AC, Cantin B, Dagenais GR, Mauriège P, Bernard PM, Després JP, Lamarche B 2005 Low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men: 13-year follow-up data from the Quebec Cardiovascular Study. Arterioscler Thromb Vasc Biol 25:553–559 [DOI] [PubMed] [Google Scholar]
- Watts GF, Mandalia S, Brunt JN, Slavin BM, Coltart DJ, Lewis B 1993 Independent associations between plasma lipoprotein subfraction levels and the course of coronary artery disease in the St. Thomas’ Atherosclerosis Regression Study (STARS). Metabolism 42:1461–1467 [DOI] [PubMed] [Google Scholar]
- Rosenson RS, Otvos JD, Freedman DS 2002 Relations of lipoprotein subclass levels and low-density lipoprotein size to progression of coronary artery disease in the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC-I) trial. Am J Cardiol 90:89–94 [DOI] [PubMed] [Google Scholar]
- Williams PT, Superko HR, Haskell WL, Alderman EL, Blanche PJ, Holl LG, Krauss RM 2003 Smallest LDL particles are most strongly related to coronary disease progression in men. Arterioscler Thromb Vasc Biol 23:314–321 [DOI] [PubMed] [Google Scholar]
- Petitti DB, Imperatore G, Palla SL, Daniels SR, Dolan LM, Kershnar AK, Marcovina S, Pettitt DJ, Pihoker C; SEARCH for Diabetes in Youth Study Group 2007 Serum lipids and glucose control: the SEARCH for Diabetes in Youth Study. Arch Ped Adolescent Med 161:159–165 [DOI] [PubMed] [Google Scholar]
- Sibley SD, Thomas W, de Boer I, Brunzell JD, Steffes MW 2006 Gender and elevated albumin excretion in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort: role of central obesity. Am J Kidney Dis 47:223–232 [DOI] [PubMed] [Google Scholar]
- Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD 1998 Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. Diabetes Control and Complications Trial. JAMA [Erratum (1998) 280:1484] 280:140–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskinen MR, Kuusi T, Helve E, Nikkila EA, Yki-Jarvinen H 1988 Insulin therapy induces antiatherogenic changes of serum lipoproteins in noninsulin-dependent diabetes. Arteriosclerosis 8:168–177 [DOI] [PubMed] [Google Scholar]
- American Diabetes Association 2000 Type 2 diabetes in children and adolescents. Diabetes Care 23:381–389 [DOI] [PubMed] [Google Scholar]
- American Diabetes Association 2003 Management of dyslipidemia in children and adolescents with diabetes. Diabetes Care 26:2194–2197 [DOI] [PubMed] [Google Scholar]
- Kavey REW, Daniels SR, Lauer RM, Atkins DL, Hayman LL, Tanbertk 2003 American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. Circulation 107:1562–1566 [DOI] [PubMed] [Google Scholar]